Abstract
Nanopreparations such as liposomes, micelles, polymeric and inorganic nanoparticles, and small molecule/nucleic acid/protein conjugates have demonstrated various advantages versus “naked” therapeutic molecules. These nanopreparations can be further engineered with functional moieties to improve their performance in terms of circulation longevity, targetability, enhanced intracellular penetration; carrier-mediated enhanced visualization, and stimuli-sensitivity. The idea of application of a stimulus-sensitive drug or imaging agent delivery system for tumor targeting is based on the fact of the significant abnormalities in the tumor microenvironment and its cells, such as an acidic pH, altered redox potential, up-regulated proteins and hyperthermia. These internal conditions as well as external stimuli such as magnetic field, ultrasound and light, can be used to modify the behavior of the nanopreparations that control drug release, improve drug internalization, control the intracellular drug fate and even allow for certain physical interactions, resulting in an enhanced tumor targeting and antitumor effect. This article provides a critical view of current stimulus-sensitive drug delivery strategies and possible future directions in tumor targeting with primary focus on the combined use of stimulus-sensitivity with other strategies in the same nanopreparation, including multifunctional nanopreparations and theranostics.
1. Introduction
During the past few decades, many efforts have been made to improve anticancer diagnosis and therapy. Lack of clinical effectiveness and side-effects of anti-tumor drugs which are caused mainly by their low tumor targeting, insufficient cellular drug uptake or local drug concentration have always been the scientists’ headache. Various nanopreparations have been developed for the experimental and clinical delivery of therapeutic and diagnostic agents, to improve their tumor targeting. Commonly, the application and therapeutic outcome of a nanopreparation are determined by the properties of the nanocarriers, while the properties of loaded molecules or reagents are negligible since they are low in quantity and can be isolated or surrounded by carrier matrices 1. The most commonly used diagnostic/therapeutic nanopreparations include nano-particulate delivery systems such as liposomes, micelles, dendrimers, nanospheres, nanocapsules, and inorganic nanoparticles (e.g. gold nanoparticles, carbon nanotubes, and quantum dots) and macromolecular delivery systems such as antibody-drug conjugates (ADC) and drug-polymer conjugates (e.g. PEG-protein conjugates and PEG-siRNA conjugates). 2
Besides benefiting from their nanoscopic scale3 , the architecture and components of nanocarriers/nanopreparations provide extra opportunities for engineering and modification, aimed at controlling their biological properties in a desirable fashion to allow them to perform simultaneously various therapeutic or diagnostic functions. These result in the increased stability, long blood circulation half-life, higher bioavailability, enhanced targetability, as well as the minimization of undesirable protein binding and biodistribution, immunogenicity, and other side-effects 4. To improve therapeutics’and diagnostics’ anti-tumor specificity and bioavailability, a variety of delivery strategies have been investigated, including decrease of non-specific protein binding and increase of in vivo longevity by PEGylation, improved tumor/tumor cells-specific targetability using targeting ligands, controlling cellular drug uptake or drug release using stimulus-responsive moieties, and enhancing a nanocarrier’s internalization in target cells by intracellular delivery moieties.5 Among these strategies, the stimulus-sensitive strategies are the focus of this review.
2. Delivery barriers and strategies for tumor targeting
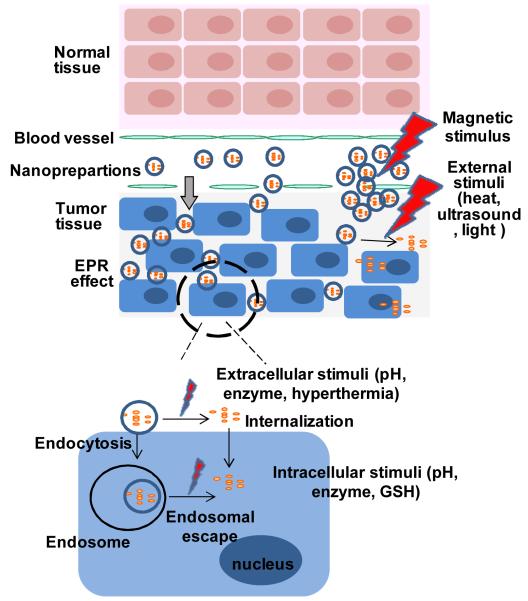
In general, the physical barriers including endothelium, extra cellular matrix (ECM), cellular and subcellular membranes, the digesting enzymes and efflux receptors of target cells, the host immune systems like the reticuloendothelial system (RES) and macrophages, as well as the excretory organs like liver and kidney, significantly influence the nanopreparation’s tumor targetability and resultant clinical outcomes. Therefore, delivery of exogenous molecules into biological systems that have developed natural barriers and adopted a defense system during evolution can be extremely difficult with the unmodified drug molecules and imaging reagents themselves. In addition to these barriers, however, several “abnormalities” in tumor tissues may also play an important role in the targeted delivery of drugs and imaging reagents to tumor cells. These abnormal changes can be obstacles as well as opportunities for tumor-specific drug delivery. One significant physiological change in tumor tissues is its neovascularization or angiogenesis.6 However, the neovascularization is not evenly distributed in the tumor tissue, especially in solid tumors. Solid tumors are highly irregular in their architecture that includes a relatively loose outer part with a rich vasculature and a dense “core” with an insufficient oxygen (hypoxia) and nutrient supply, which can be “normalized” using the proper treatments.7 It is also well known that chronic inflammation is always associated with the tumor, resulting in an increased local vascular permeability and recruitment of leukocytes. Indeed, tumor neovascularization is imperfect with larger endothelial fenestrae than that of normal tissues, and so facilitates the extravasation of nanoparticles.8 This effect combined with a weak lymphatic drainage contributes to the enhanced permeability and retention (EPR) in solid tumor tissues, allowing for passive tumor targeting.9 The EPR effect has been extensively explored in tumor diagnosis and therapy.9 Other significant alterations include the lowered pH, elevated temperature, elevated redox potential, and up-regulated protein/enzyme levels of the tumor microenvironment. 5, 10 These internal physiopathological alterations as well as the applied external changes including heat, ultrasound, magnetic field, and light, have been used as stimuli for enhanced tumor targeting. Since these delivery systems in response to the local stimuli result in controlling drug release and/or cellular drug uptake, influencing intracellular drug fate, and even allowing for certain physical actions in tumor microenvironment, they are said to be “smarter” compared to the traditional delivery systems. These “smart” tumor targeting strategies will be discussed in detail.
3. Use of stimulus-responsive nanopreparations for tumor targeting
To construct a stimulus-responsive nanopreparation, stimulus-sensitive polymers or lipids which can undergo either conformational change or cleavage in their structure in response to corresponding stimuli are required. These allow either exposing other functional moieties or collapse of the nanocarrier that results in drug internalization or drug release in the tumor cell or its microenvironment. The strategies for use of stimulus-sensitivity will be discussed individually below and are summarized in Figure 1.
Figure 1.
Stimulus-responsive delivery strategies for tumor targeting.
3.1 pH-sensitive systems
The lowered extracellular/interstitial pH is a hallmark of tumor malignancy, especially in solid tumors, which is caused by excessive metabolite, mainly lactic acid and CO2 11 as well as the increased expression and activity of vacuolar-type (V-type) H(+)-ATPases (proton pumps) 12. The tumor’s extracellular pH may drop to 6.5 or less compared to about pH 7.4 in normal blood or most normal tissues. The abnormal pH gradient continues from extracellular microenvironment to intracellular organelles, such as endosomes (5.5) and lysosomes (below 5.5). This pH gradient is one of the reasons for the multidrug resistance, especially for weakly basic chemotherapeutic drugs. 13 However, this pH gradient of about 2 pH unit decrease between the extracellular matrix and intracellular endosome is a great local stimulus. The acidity of the tumor microenvironment and endosomes has been the most utilized stimulus for the design of the stimulus-sensitive nanopreparations.
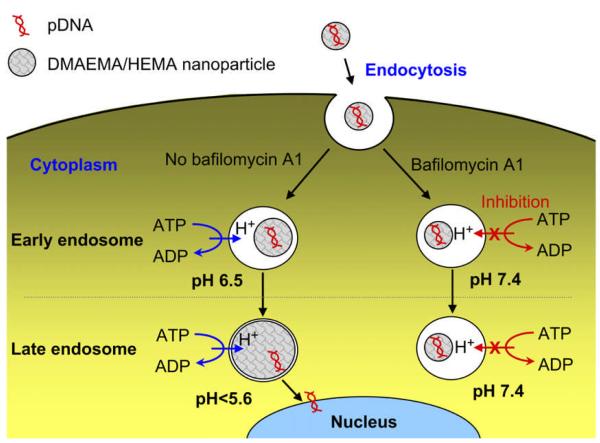
One strategy is that the pH-responsive components of nanopreparations can be protonated at acidic pH, leading to destabilization of the nanostructure and release of the loaded drugs. The hydrophobic poly L-histidine protonates at low pH, resulting in the increase of its hydrophilicity. This property has been utilized to design pH-responsive amphiphilic polymers such as PEGylated poly L-histidine. By adjusting the molecular weights (length) of the two blocks, a nanoscaled micellar system can be obtained, which is fairly stable in the aqueous environment at a pH of above 6.5, while the acidity of the tumor microenvironment and endosomes protonates the poly(L-His) “core” and increases the hydrophicility of the copolymer. This change elevates the critical micelle concentration (CMC) and destabilizes the “core-shell” structure of micelles, resulting in the rapid drug release.14 The dramatically increased drug concentration in the tumor microenvironment or cytoplasm may overcome the efflux receptor (e.g. P-glycoprotein) or other mechanism-driven drug resistance.15 Other types of cationic nanocarriers such as dimethylaminoethyl methacrylate (DMAEMA)/ 2-hydroxyethyl methacrylate (HEMA)-based nanocarriers could dramatically swell themselves and efficiently escape from the endosomes (Figure 2) in response to the acidic pH, resulting in the high exogenous gene expression. 16
Figure 2.
Schematic representation of pH-sensitive DMAEMA/HEMA nanoparticle-mediated gene transfection with and without bafilomycin A1 as a V ATPase inhibitor (Reproduced with permission from ref.16b).
For the endocytosed drugs, especially for macromolecules such as nucleic acids, the fate of these molecules relies on the efficiency of endosomal escape to a significant extent. After endocytosis, DNAs or oligonucleotides (ODNs) must reach the nucleus for therapeutic activity, while the target for RNA interference (RNAi) molecules is in the cytoplasm.2c To avoid premature degradation by late endosomes or lysosomes, the pH-sensitive moieties with the ability to destabilize the endosomal membrane or rupture the endosomes can be used to modify nanopreparations that facilitate endosomal escape. Dioleoylphosphatidylethanolamine (DOPE), a fusogenic lipid, is frequently used in pH-sensitive liposomal formulations. The fusogenicity of DOPE comes from its strong propensity to form a non-lamellar structure/phase (hexagonal phase) due to its cone-shape geometry and its weak acidity which stabilizes its lamellar phase at neutral pH but becomes partially protonated at low pH resulting in destabilization of the lamellar phase. Therefore, upon endocytosis of DOPE-containing formulations, the acidic environment triggers DOPE’s fusion with the endosomal membrane leading to its destabilization.17 Incorporation of fusogenic DOPE in the cationic liposomes significantly improves the endosomal escape by membrane fusion and destabilization 18, resulting in the higher transfection efficiency/transgene expression 19 and gene down-regulation20. Oleyl alcohol, a nonionic unsaturated lipidic surfactant, renders pH-sensitivity as well as serum resistance to the liposomes, however, by a mechanism other than lipid fusion. 17 A new class of lipids, PEG-diortho ester-lipid conjugate (POD), with an acid-labile diortho ester linker between the hydrophilic headgroup (PEG) and the hydrophobic tail (distearoyl glycerol), was synthesized and showed rapid degradation in the mildly acidic pH range (5-6) but with good stability at neutral pH and high content of serum, when used as liposomal components.21
Several microorganisms such as influenza22 and herpes simplex viruses23 have evolved endosomal escape strategies that take advantage of the lowered pH in host’s endosomes. The proteins and peptides derived from these viruses showed fusogenicity. A 100% membrane fusion was observed when the wild-type fusogenic peptide from glycoprotein H of herpes simplex virus was mixed with lipids at the ratio of 0.005:1 (peptide:lipid) at pH 4.5.23 Physical mixing of this peptide with lipoplexes increased the transgene expression up to 30-fold in human cancer cells.23 In another study, The recombinant TATp-HA2 peptide (The HA2 peptide from the influenza virus hemagglutinin protein was bioengineered to TAT peptide.) enhanced gene transduction by HA2-mediated endosomal disruption.22
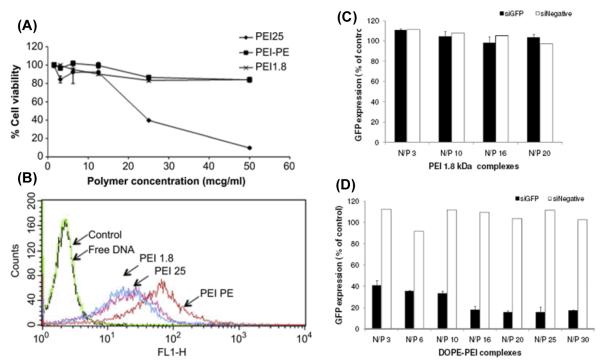
Another important pH-sensitive strategy utilizes the superior buffering capacity of polyethylenimine (PEI), a most widely used cationic polymer for gene delivery. PEI’s buffering capacity comes from its different types of amine groups with different pKa values, which have potential for protonation at different levels at the given pH.2c Upon endocytosis, the protonation of amines significantly increases the influx of protons and chloride ions as well as water into the endosome resulting in swelling and rupture of the endosome (a “proton sponge” effect), by which the endosomal contents are released24. The buffering capacity as well as the positive charge has made PEI a universal vector for both in vitro and in vivo nucleic acid delivery 24a, 25. Researchers have increasingly realized the advantages of combination use of lipids and PEI. 26 To target delivery of siRNA to prostate cancer, an “all in one” nanocarrier containing hydrophobic hexadecylated polyethylenimine (H-PEI) and solid lipids (cholesterol, PE and PEGylated PE) was designed. This hybrid system showed higher stability of loaded siRNAs, higher prostate cancer targeting, and a significant reduction in both acute and delayed carrier-caused toxicity, and most significantly, with the sustained intracellular siRNA release for more than one week.26c Another strategy to incorporate lipids with PEI is the direct conjugation of lipid to PEI, such as PEI-cholesterol, a water-soluble lipopolymer with buffering capacity similar as PEI. 26a More recently, DOPE conjugated with small molecular PEI (1800 Da) significantly improved the performance of PEI in gene 26d and siRNA 26b delivery in terms of the stability of the polyplexes, the delivery efficiency, and the carrier-mediated toxicity (Figure 3). This is understandable because the resultant PEI-DOPE conjugate possesses the advantages of both components including a positive charge, buffering capacity, and fusogenicity. In addition to these advantages, the amphiphilicity promotes PEI-DOPE self-assembly in an aqueous environment and form micellar nanocarriers which are easy for cells to take up. This promising lipopolymer is currently being investigated for more applications by our group.
Figure 3.
Cytotoxicity of PEI (125KDa and 1800Da) and PEI(1800)-PE in B16F10 cells (A) ( Reproduced with permission from ref. 26d); Cellular uptake of DNA from different complexes [PEI(1800) N/P 16, PEI (25K)N/P 4, PEI(1800) -PE N/P 16] in B16F10 cells after 4h (B) ( Reproduced with permission from ref. 26d); GFP down-regulation by GFP siRNA/PEI (1800) (C) and siRNA/PEI(1800)-PE (D) in C166-GFP cells (Reproduced with permission from ref.26b).
PEGylation is well known to stabilize nanoparticles, protect otherwise fragile molecules, prevent protein binding, and prolong drug and carrier circulation time.26d, 27 Therefore, most current nanopreparations have a PEG modification either in their surface or in their backbone. However, recent data has shown that the stable PEGylation of nanocarriers may not always be beneficial for drug delivery. It has been reported that the PEG “corona” on the surface of liposomes interfered with the interaction between nanocarrier and cell membrane resulting in the low cellular uptake of PEGylated liposomes 4, 27b. Additionally, flexible hydrophilic PEG can also interfere with intracellular events, such as endosomal escape.28 Ideally, the protective effect of PEG should be avoided by the target cells during internalization and the endocytic processes. A variety of ester and hydrazone moieties have been successfully covalently incorporated between the PEG and the nanocarrier since these bonds are fairly stable at a neutral pH in blood, but easily hydrolyzed at a pH of 6 or below that is typical of a tumor mass or endocytic vacuole. 4, 27a, 27c These designs assure the long blood circulation time as well as the deshielding of PEG chains by cleavage of the pH-sensitive bonds within the acidic targets. The pH-sensitive PEG-hydrazone-phosphatidylethanolamine (PEG-Hz-PE)-based micelles and liposomes designed have rapid and effective detachment of the PEG at a pH of 5-6 compared to pH 8.0, resulting in a cellular uptake similar to PEG-free liposomes.4 Similarly, the macromolecular prodrugs, such as the galactose-PEG-oligonucleotide conjugate with an ester bond between PEG and its load, was fairly stable when incubated with blood serum, but the loaded oligonucleotides were quickly released when incubated with a pH 5.5 buffer. 27a
3.2 Redox potential-sensitive systems
Glutathione (GSH), an abundant reducing agent in living cells, has an intracellular concentration of about 2-10 mM especially in certain organelles such as cytosol, mitochondria, and cell nucleus, while it is just 1/100 to 1/1000 the concentration (about 2-20 μM) of the intracellular glutathione in blood and extracellular matrix. 29 However, in a tumor mass, the glutathione concentration is also significantly higher (100-fold) than the extracellular level of glutathione in normal tissue.5 This high redox potential difference between normal tissue and tumor tissue and between intra- and extracellular environments caused by glutathione, cysteine and other reducing agents provides a rationale for a tumor targeting strategy as well as intracellular drug delivery.
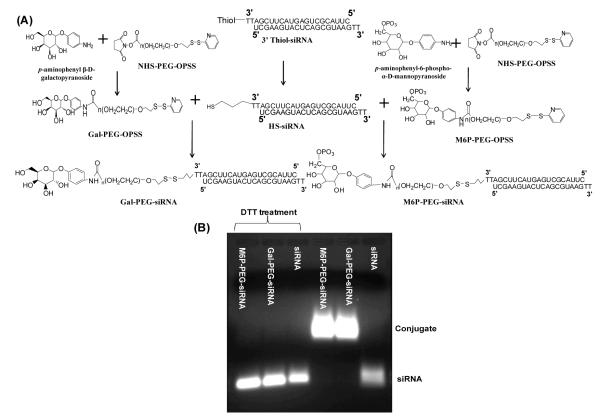
The disulfide bond has been widely used as the cleavable/reversible linker in nanocarriers to render redox potential sensitivity to nanopreparations due to the disulfide-to-thiol reduction reaction. To increase the efficiency of intracellular gene delivery, the bio-reducible cationic lipids/ polymers containing disulfide bonds, such as poly(disulfide amine) 30, disulfide-based poly(amido amine) polymers 31 and dendrimers32, have been synthesized. Plasmids or siRNAs can be condensed to polyplexes by these polymers and released from the complexes after cleavage of disulfide bonds in the reductive environment after internalization. Unlike complexation with the loaded macromolecules, the block copolymers, PEG-SS-PPS, with the hydrophilic PEG block, the hydrophobic poly(propylene sulfide) (PPS) block, and disulfide bonds between them, were found to self-assemble into polyersomes, which were disrupted and released their payloads within 10 min of exposure with cells due to the intracellular redox potential. 33 To decrease the cytotoxicity and non-specific protein binding/biodistribution caused by the positive charges, anti-luciferase siRNAs have been directly conjugated to galactose- or M6P-coupled PEG conjugates via a disulfide bond (Figure 4).34 The resultant macromolecular targeted delivery systems efficiently released intact siRNAs in the presence of reducing agents, resulting in the gene down-regulation of reporter (luciferase) genes as well as therapeutic (TGF-beta 1) genes in both HepG2 and hepatic stellate cells. 34 Conjugation of anti-GFP siRNA to lipids such as DOPE via the disulfide bond introduced hydrophobicity to the resultant siRNA conjugate, which could incorporate in the non-cationic polymeric micelles (PEG2000-PE). The nanosized micelles released siRNA in the presence of a 10 mM GSH solution, leading to a 30-fold GFP gene down-regulation compared to free siRNA. 35
Figure 4.
Synthesis scheme (A) and siRNA release (B) of Gal-PEG-siRNA and M6P-PEG-siRNA (Reproduced with permission from ref.34).
3.3 Enzyme-sensitive systems
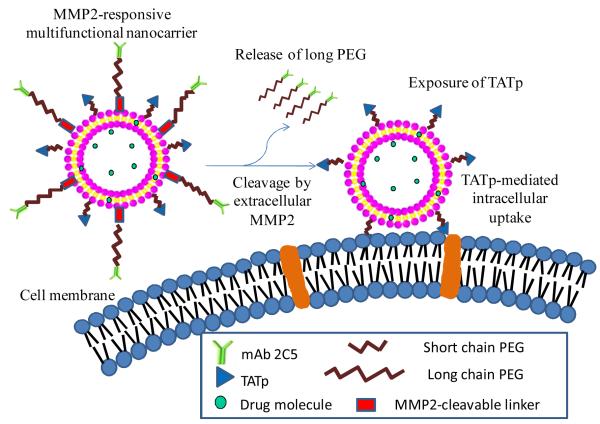
The alteration in the composition and expression level of local enzymes such as matrix metalloproteinases (MMPs) which have been considered as biomarkers for diagnostics and prognostics in many types and stages of cancer, also provide an opportunity for delivery of drug molecules and imaging agents to pathological sites via an enzyme-triggered mechanism.36 In the MMP family, overexpressed MMP2 and MMP9 have been widely recognized as involved in the invasion, progression and metastasis of most human tumors. 37 MMP-sensitive substrates including proteins, peptides and polymers have been designed and used for drug and imaging reagent delivery.27b, 38 An activatable cell penetrating peptide containing a polycation, a polyanion and a MMP-cleavable peptide in between has been designed and shown significant tumor targeting when incorporated in the imaging systems.38c-e The 100nm quantum dot gelatin nanoparticles(QDGelNP) composed of a gelatin core with amino-PEG QDs conjugated to the surface were designed to respond to the presence of gelatinases (MMPs) in the tumor mass resulting in the collapse of naoparticles and release of 10nm quantum dots. This “shrunken” particle size significantly improved the nanoparticles’ diffusion in an in vitro collagen diffusion experiment after pretreatment with MMP2. This novel design allowed passive tumor targeting at 100nm via EPR effect as well as fast and efficient diffusion in the tumor ECM after the nanoparticles’ extravasation due to the decreased particle size to 10nm.38a In another study, an MMP2-cleavable lipopolymer was synthesized and incorporated into targeted liposomes to endow enzyme-sensitivity for the delivery of N4-octadecyl-1-β-D-arabinofuranosylcytosine (NOAC) to tumor cells. 39 More recently, the multifunctional liposomal nanocarrier containing a MMP2-responsive moiety (maleimide-PEG-cleavable peptide-DOPE) was designed.27b The MMP2-sensitivity of this functional polymer as well as the nanocarrier was well characterized. The combination of MMP2-sensitive moiety with other functional moieties significantly increased the tumor targetability as well as tumor cell internalization of the nanocarrier (Figure 5).27b Cancer-associated proteases (CAPs), another up-regulated enzyme, have recently become attractive for tumor targeting. A polymer-caged liposome encapsulating drugs in a hypertonic buffer was prepared by cross-linking a protease-cleavable block polymer (cholesterol-peptide-polyacrylic acid). After cleavage by proteases, the restriction of the polymer cage disappeared and the high internal osmotic pressure burst the liposomes resulting in the rapid drug release. 40
Figure 5.
Enhanced tumor targeting using a MMP2-responsive liposomal multifunctional nanocarrier. Reproduced with permission from ref.27b.
3.4 Thermosensitive systems
Although the idea of thermosensitive systems came from the observation of local hyperthermia in certain diseases such as inflammation and cancer, the actual temperature difference between normal and diseased tissues is still not different enough for the most thermosensitive nanopreparations. Commonly, an external heating source/device to precisely control the local temperature, such as a water bath41, electromagnet 42, laser43 or high intensity focused ultrasound44, is required to utilize the thermosensitivity of the nanopreparations. The lipids with the proper gel-to-liquid phase transition temperature (Tg) can be used to prepare the liposomal nanopreparations for the purpose of thermo response. The most commonly used thermosensitive lipid with a safe Tg (41°C) for most tissues is dipalmitoylphosphatidylcholine (DPPC). The liposomes composed of DPPC and cholesterol (67:33, mass ratio) have been reported to release more than 80% of their loaded methotrexate within 30min when the temperature increased from 37°C to 41°C while 60% remained inside the liposomes for up to 24 h at 37°C. 41 This non-linear temperature-dependent drug release combined with a magnetic response resulted in a high drug accumulation in the target tissue in a mouse model. 41 The thermosensitive nanopreparations can also be made using the temperature sensitive polymers which display a lower critical solution temperature (LCST). At the LCST, the polymer undergoes a sharp phase transition/conformational change. The most commonly used thermosensitive polymer is poly(N-isopropylacrylamide) (PNIPAM), which changes its conformation from a hydrophilic “coil” below its LCST (32°C) to a hydrophobic “globule” above its LCST in the aqueous environment. The LCST of PNIPAM can be tuned by variation of the hydrophilic or hydrophobic content. 45 Grafting the copolymer composed of vinylpyrrolidinone and N-isopropylacryamide (NIPAM) on PEI enhanced the accumulation of the thermosensitive PEI conjugate in hyperthermia (42°C)-treated tumors, resulting in up to a 10-fold increase of DNA deposition compared to non-hyperthermia-treated tumor.46 The thermosensitive polymer-modified liposomes have also been widely studied. A comprehensive review is available in 47. Recently, a novel dendrimer-PE conjugate modified with the poly(oligo((ethylene glycol) methacrylate)s (poly(OEGMA)s), a safe thermosensitive moiety, has been shown to possess a tunable thermosensitivity (LCST) ranging from 20 to 60°C as well as a “core-shell” structure suitable for loading of poorly soluble anticancer drugs.48
3.5 Magnetically sensitive systems
To use external magnetic field for tumor targeting, the nanopreparations have to be magnetized either by incorporation of magnetite or by direct modification of the magnetized metal particles with biocompatible polymers. Magnetite, known as ferrimagnetic particles with the size of less than 10nm, such as Fe3O4 or γ-Fe2O3, have been extensively explored in many different biomedical applications including drug delivery, magnetic resonance imaging (MRI), magnetic hyperthermia, magnetic transfection and manipulation of cells and proteins.5 Unlike ferromagnetic materials, magnetite has a lower stray magnetic field intensity which reduces the potential health risks caused by magnetic fields, and a lower chance of agglomeration due to magnetostatic interactions during preparation and storage of magnetic nanoparticles.41 Due to their superior properties and nanoscale size, they are also referred to as superparamagnetic iron oxide nanoparticles (SPION). In order to reduce the agglomeration caused by its hydrophobic nature and increase its biocompatibility, SPION can be surface-modified with biocompatible compounds, such as glutamic acid 41, dextran3, pullulan 49, citric acid50 and silane 51 .
To achieve magnetic tumor targeting, SPION can be loaded into nanocarriers such as liposomes41, micelles52 or solid nanoparticles53. Methotrexate and 10nm of glutamic acid-chelated γ-Fe2O3 have been successfully co-loaded into the aqueous core of liposomes using a reverse phase evaporation method. The resultant magnetic carrier significantly increased methotrexate accumulation (AUC) by more than 5-fold in the target tissue when exposed to a magnetic field compared to the same formulation without an external magnetic stimulus in a mouse model.41 Due to the hydrophobicity of the “plain” SPION, these nanoparticles were also encapsulated within polymeric micelles formed by PEG-DSPE using a lipid film rehydration method. 52a In this case, the PEG chain provided the longevity 54 and the possibility for further modification with other functional moieties, such as monoclonal antibody 52a, to facilitate precise tumor targeting. Direct conjugation of an anti-cancer drug to SPION is another strategy to make a magnetically sensitive nanopreparation. Paclitaxel conjugated on the surface of PEGylated SPION via an ester bond can be released in a pH-dependent manner through the hydrolysis of the ester bond between PEG and paclitaxel, resulting in in vitro cytotoxicity comparable to Taxol. The higher in vivo tumor targeting of this conjugate observed using magnetic resonance imaging (MRI), led to better tumor growth inhibition than Taxol in an in vivo model.52b, 55 Based on the same idea, a more complicated conjugate containing cRGD, doxorubicin and 64Cu-labeled SPION was prepared to simultaneously perform tumor targeting, drug delivery as well as imaging. 56
3.6 Ultrasound-sensitive systems
Ultrasound imaging, such as visualization of internal tissues and organs, and ultrasound therapies, such as tumor ablation and kidney stone disruption, have been used in clinics for many years. To achieve better resolution of the image, contrast reagents, including lipid nano-/micro-bubbles were used to enhance ultrasound effect via the echoes coming form the interaction between sound waves and the stabilized gas bubbles. 57 Ultrasound, however, can destroy the nanocarrier’s structure and cause drug leakage as well with ultrasound-generated energy input (referred to as “sonoporation”). Thus, ultrasound can be used to control/trigger drug targeting and release from the ultrasound-sensitive nanopreparations. The most commonly proposed ultrasound-sensitive nanocarriers are liposomes and lipid nanobubbles encapsulating air or perfluorated hydrocarbon. Liposomes composed of hydrogenated soybean phosphatidylcholine (HSPC), cholesterol and PEG2000-DSPE (51:44:5 molar ratio) responded to the low-frequency ultrasound (LFUS) and released nearly 80% of loaded drug (cisplatin) within 3min via a transient introduction of porelike defects in the liposome membrane. 58 This efficient ultrasound response led to the tumor-specific release of nearly 70% of loaded cisplatin within 2min compared to less than 3% in those formulations without LFUS in a lymphoma -bearing mouse model after intraperitoneal injection. 59 The best therapeutic effect of this formulation was also observed in the colon adenocarcinoma bearing mice after i.v. injection.59 Modification of lipid formulations with targeting ligands imparted tumor targeting to the ultrasound-sensitive nanocarriers. The sgc8c aptamer-conjugated nanobubbles showed CCRF-CEM cell (a human acute lymphoblastic leukemia cell) - specific targeting.60 To further improve the tumor targetability, a triple-targeted microbubble coupling three antibodies against mouse αVβ-integrin, P-selectin and vascular endothelial growth factor receptor 2 was prepared and showed a 50% increase of binding affinity to mouse SVR angiosarcoma endothelial cells compared to dual-targeted microbubbles and a 40% increase in tumor image intensity compared to single- and dual-targeted microbubbles in a breast cancer-bearing mouse model. 61
3.7 Light-sensitive systems
Light activation/irritation by the adjustment of parameters, such as wavelength, intensity, pulse duration and cycle, is a promising tool for many biomedical applications. Although visible, UV and near-infrared (NIR) light have been widely used in clinics, 62 only NIR light penetrates into deeper tissues and therefore is more desirable for tumor targeting.
One promising light-sensitive strategy for tumor targeting is photodynamic therapy (PDT) which involves in the use of a photosensitizing agent (PSA) such as porphyrin derivatives, chlorins, phthalocyanines and porphycenes. 63 Light-mediated activation of PSA results in the generation of radical oxygen species (ROS) which destroy the targeted tumor cells. Since most PSA are hydrophobic, nanopreparations such as liposomes and micelles are used for stabilization and tumor targeting. Conjugation of Ala-Pro-Arg-Pro-Gly (a ligand for angiogenic endothelial cells) to PEGylated liposomes containing benzoporphyrin derivative monoacid ring A (a photosensitizer) led to tumor cell-specific targeting as well as the light-sensitivity, resulting in the higher tumor growth inhibition.64 A brief review regarding PDT is available. 63
To impart the light-sensitivity to nanopreparations, the light-sensitive polymers can be used to build or modify the drug delivery carriers. Light-sensitive lipids have been designed to facilitate photo-triggered structural changes, leading to direct interaction of lipid-based nanocarriers such as liposomes with the target cells via membrane fusion, photo-isomerism, photofragmentation or photopolymerization. As a result, the nanocarriers undergo leakage or collapse. This issue has been well discussed in a recent article. 65 In addition to photosensitive liposomes, other nanocarriers have been imparted with light-sensitivity. A novel photosensitive amphiphilic dendritic conjugate (glucose/lactose-D3-PCL-DNQ(NIR-sensitive diazonaphthoquinone)) has been synthesized, by which a photo-sensitive micellar nanocarrier can be assembled in an aqueous environment. The hydrophobic DNQ groups in the core were designed to load the poorly water-soluble doxorubicin and respond to external NIR light resulting in micelle destabilization and rapid drug release. The outer layer of the sugar residue was used as the targeting ligand to improve the targetability of the nanocarriers. 66 In another study, a NIR light-responsive drug delivery platform composed of a DNA cross-linked hydrogel shell and an Au-Ag nanorod core was designed. This novel system responded to NIR light and generated heat at its metal core, resulting in the solid-gel transition which facilitated rapid drug release. 43b
4. Control of a nanocarrier’s functionalities with stimulus-sensitive moieties for enhanced tumor targeting
Many studies have shown that better clinical outcomes could be achieved by combination of a stimulus-sensitive moiety with other compatible drug delivery strategies in the same nanopreparation 4, 27a, 34, 41, 43b, 67. In these nanopreparations, the stimulus-sensitive moieties “switch” the functionalities of the nanocarrier between “on” and “off” in response to corresponding stimuli, resulting in the drug release and/or drug internalization. PEGylation combined with a pH-sensitive moiety showed long blood circulation and reduced nonspecific cellular uptake as well as pH-dependent drug release in both macromolecular 27a and particulate 4 systems. Combined use of a disulfide bond with PEGylation and sugar residue ligands showed liver cell-specific targeting and reduction-triggered siRNA release into the cytoplasm. 34 Even combined use of different stimulus-sensitive strategies has also improved targetability. A pH (ester) and reduction (disufide) dual-sensitive co-polymeric micellar system has been designed and found to be more effective for the release of loaded Doxorubicin in the presence of both pH and reduction stimuli compared to only one stimulus. 68 Thermosensitive magnetoliposomes enhanced magnetic targeting as well as temperature-dependent drug release, leading to significant improvement of a drug’s PK parameters. 41 More recently, sophisticated multifunctional nanocarriers and theranostics, have been proposed, especially for tumor-specific therapy and diagnosis, in which the more functional moieties including longevity, stimulus sensitivity, targetability, intracellular penetration, and visualization, are combined together and coordinated in an optimal fashion to achieve maximal tumor targeting. 69 A novel multifunctional liposomal nanocarrier, composed of a protective long chain PEG, an antitumor monoclonal antibody (mAb 2C5), an intracellular penetrating moiety (TAT peptide), and a matrix metalloproteinase 2 (MMP2)-sensitive PEG-lipid conjugate, has been designed to enhance tumor targeting. (Figure 5) 27b The functions of the nanocarrier include (i) long circulation time and reduced nonspecific interactions due to PEGylation; (ii) passive tumor targeting via the EPR effect due to its nanoscale size; (iii) active tumor targeting due to mAb 2C5; (iv) removal of the protective long PEG chains due to the tumoral extracellular MMP2-triggered cleavage between PEG and lipid; and (v) TATp triggering of the enhanced intracellular delivery of the system after long-chain PEG removal and exposure of the previously hidden surface-attached TATp. A similar strategy has also been used to prepare the pH-sensitive nanocarriers including liposomes and micelles to improve their tumor targetability.4
5. Conclusions
Compared to the conventional drug delivery systems, the stimulus-sensitive nanopreparations show a superior ability for control and adjustment of the location and time of drug release and internalization in the tumor cells and their microenvironment by responding to local stimuli. However, the stimulus-sensitivity of the current nanopreparations is still imperfect. The challenges include the off-tumor-targeting caused by non-specific stimuli such as up-regulation of stimulus proteins in drug- or harsh condition-stimulated non-cancer cells 70 or imprecise local stimuli such as uncontrolled depth of magnetic field and heating 41, insufficient stimulus-responsiveness such as lower pH (≤ 5.0) used in some studies 4, 71 compared to the actual tumoral pH (5.5-6), the high concentration/activity of enzymes/reductive agents used in vitro compared to the mild in vivo ones 34, 68, and the slower stimulus-response 27a, 38a. Therefore, to design a stimulus-sensitive nanopreparation, in addition to knowing the drug delivery barriers and the desired clinical outcomes, the properties of the stimuli as well as the stimulus-sensitive polymers/lipids have to be fully understood including the rate and extent of the stimulus-sensitivity, the distribution and activity of internal stimuli, and the properties of external stimuli. Besides the above mentioned stimuli, there will likely be more stimuli found for drug delivery purposes along with the progress in biology, chemistry, physics, and other related fields. The combined use of stimulus-sensitivity with other strategies is of great interest for enhanced tumor targeting. The use of “smart” multi-componential theranostics makes the tumor diagnosis and therapy more controllable and efficient. Although significant progress regarding tumor targeting has been made over the past few decades, further work is needed to make the dream of a “magic bullet” more of a reality.
Insight, innovation, integration.
Stimulus-responsiveness is a natural characteristic of living organisms used to avoid damage from the environment. To mimic this strategy, nanopreparations designed to respond to particular stimuli such as acidity, redox potential, enzymes, hyperthermia, magnetic field, light, and ultrasound indicate the “smartness” in their antitumor applications. The development of a stimulus-responsive nanopreparation requires a comprehensive understanding of cancer biology, nanotechnology, conjugation chemistry, physics and other related science and a rational approach for their integration. This article provides a critical review of the popularly used drug and imaging agent delivery strategies as well as of the newly emerging multifunctional nanopreparations and theranostics in terms of their stimuli-sensitivity.
Acknowledgement
The authors thank Drs. William C. Hartner and Federico Perche for their help in manuscript preparation. This work was supported by the NIH Grant 1R01CA121838 to Vladimir P. Torchilin.
Reference
- 1.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–22. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 2 (a).Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10:3223–30. doi: 10.1021/nl102184c. DOI: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res. 2011;17:6437–47. doi: 10.1158/1078-0432.CCR-11-0762. DOI: 10.1158/1078-0432.CCR-11-0762. [DOI] [PubMed] [Google Scholar]; (c) Zhu L, Mahato RI. Lipid and polymeric carrier-mediated nucleic acid delivery. Expert Opin Drug Deliv. 2010;7:1209–26. doi: 10.1517/17425247.2010.513969. DOI: 10.1517/17425247.2010.513969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang FH, Kim DK, Yoshitake T, Johansson SM, Bjelke B, Muhammed M, Kehr J. Diffusion and clearance of superparamagnetic iron oxide nanoparticles infused into the rat striatum studied by MRI and histochemical techniques. Nanotechnology. 2011;22:015103. doi: 10.1088/0957-4484/22/1/015103. DOI: 10.1088/0957-4484/22/1/015103. [DOI] [PubMed] [Google Scholar]
- 4.Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. “SMART” drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjug Chem. 2006;17:943–9. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torchilin V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur J Pharm Biopharm. 2009;71:431–44. doi: 10.1016/j.ejpb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 7.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. DOI: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 8.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annual review of medicine. 2012;63:185–98. doi: 10.1146/annurev-med-040210-162544. DOI: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 9.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 10.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10:671–84. doi: 10.1038/nrd3504. DOI: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 11.Helmlinger G, Sckell A, Dellian M, Forbes NS, Jain RK. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin Cancer Res. 2002;8:1284–91. [PubMed] [Google Scholar]
- 12.De Milito A, Iessi E, Logozzi M, Lozupone F, Spada M, Marino ML, Federici C, Perdicchio M, Matarrese P, Lugini L, Nilsson A, Fais S. Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res. 2007;67:5408–17. doi: 10.1158/0008-5472.CAN-06-4095. [DOI] [PubMed] [Google Scholar]
- 13.Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm. 2011;8:2032–8. doi: 10.1021/mp200292c. DOI: 10.1021/mp200292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee ES, Shin HJ, Na K, Bae YH. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J Control Release. 2003;90:363–74. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- 15 (a).Kim D, Lee ES, Oh KT, Gao ZG, Bae YH. Doxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal pH. Small. 2008;4:2043–50. doi: 10.1002/smll.200701275. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kim D, Gao ZG, Lee ES, Bae YH. In vivo evaluation of doxorubicin-loaded polymeric micelles targeting folate receptors and early endosomal pH in drug-resistant ovarian cancer. Mol Pharm. 2009;6:1353–62. doi: 10.1021/mp900021q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16 (a).You JO, Auguste DT. Nanocarrier cross-linking density and pH sensitivity regulate intracellular gene transfer. Nano Lett. 2009;9:4467–73. doi: 10.1021/nl902789s. DOI: 10.1021/nl902789s. [DOI] [PubMed] [Google Scholar]; (b) You JO, Auguste DT. The effect of swelling and cationic character on gene transfection by pH-sensitive nanocarriers. Biomaterials. 2010;31:6859–66. doi: 10.1016/j.biomaterials.2010.04.048. DOI: 10.1016/j.biomaterials.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 17.Sudimack JJ, Guo W, Tjarks W, Lee RJ. A novel pH-sensitive liposome formulation containing oleyl alcohol. Biochim Biophys Acta. 2002;1564:31–7. doi: 10.1016/s0005-2736(02)00399-1. [DOI] [PubMed] [Google Scholar]
- 18.Farhood H, Serbina N, Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim Biophys Acta. 1995;1235:289–95. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- 19.Zhu L, Lu Y, Miller DD, Mahato RI. Structural and formulation factors influencing pyridinium lipid-based gene transfer. Bioconjug Chem. 2008;19:2499–512. doi: 10.1021/bc8004039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spagnou S, Miller AD, Keller M. Lipidic carriers of siRNA: differences in the formulation, cellular uptake, and delivery with plasmid DNA. Biochemistry. 2004;43:13348–56. doi: 10.1021/bi048950a. [DOI] [PubMed] [Google Scholar]
- 21.Guo X, Szoka FC., Jr. Steric stabilization of fusogenic liposomes by a low-pH sensitive PEG--diortho ester--lipid conjugate. Bioconjug Chem. 2001;12:291–300. doi: 10.1021/bc000110v. [DOI] [PubMed] [Google Scholar]
- 22.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–5. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 23.Tu Y, Kim JS. A fusogenic segment of glycoprotein H from herpes simplex virus enhances transfection efficiency of cationic liposomes. J Gene Med. 2008;10:646–54. doi: 10.1002/jgm.1184. [DOI] [PubMed] [Google Scholar]
- 24 (a).Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sonawane ND, Szoka FC, Jr., Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278:44826–31. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 25.Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12:461–6. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- 26 (a).Wang DA, Narang AS, Kotb M, Gaber AO, Miller DD, Kim SW, Mahato RI. Novel branched poly(ethylenimine)-cholesterol water-soluble lipopolymers for gene delivery. Biomacromolecules. 2002;3:1197–207. doi: 10.1021/bm025563c. [DOI] [PubMed] [Google Scholar]; (b) Navarro G, Sawant RR, Biswas S, Essex S, Tros de Ilarduya C, Torchilin VP. P-glycoprotein silencing with siRNA delivered by DOPE-modified PEI overcomes doxorubicin resistance in breast cancer cells. Nanomedicine (Lond) 2012;7:65–78. doi: 10.2217/nnm.11.93. DOI: 10.2217/nnm.11.93. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Xue HY, Wong HL. Solid lipid-PEI hybrid nanocarrier: an integrated approach to provide extended, targeted, and safer siRNA therapy of prostate cancer in an all-in-one manner. ACS Nano. 2011;5:7034–47. doi: 10.1021/nn201659z. DOI: 10.1021/nn201659z. [DOI] [PubMed] [Google Scholar]; (d) Sawant RR, Sriraman SK, Navarro G, Biswas S, Dalvi RA, Torchilin VP. Polyethyleneimine-lipid conjugate-based pH-sensitive micellar carrier for gene delivery. Biomaterials. 2012;33:3942–51. doi: 10.1016/j.biomaterials.2011.11.088. DOI: 10.1016/j.biomaterials.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27 (a).Zhu L, Ye Z, Cheng K, Miller DD, Mahato RI. Site-specific delivery of oligonucleotides to hepatocytes after systemic administration. Bioconjug Chem. 2008;19:290–8. doi: 10.1021/bc070126m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano. 2012;6:3491–8. doi: 10.1021/nn300524f. DOI: 10.1021/nn300524f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Poon Z, Chang D, Zhao X, Hammond PT. Layer-by-layer nanoparticles with a pH-sheddable layer for in vivo targeting of tumor hypoxia. ACS Nano. 2011;5:4284–92. doi: 10.1021/nn200876f. DOI: 10.1021/nn200876f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83:97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- 29.Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Control Release. 2011;152:2–12. doi: 10.1016/j.jconrel.2011.01.030. DOI: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH, Jeong JH, Kim TI, Kim SW, Bull DA. VEGF siRNA delivery system using arginine-grafted bioreducible poly(disulfide amine) Mol Pharm. 2009;6:718–26. doi: 10.1021/mp800161e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vader P, van der Aa LJ, Engbersen JF, Storm G, Schiffelers RM. Disulfide-based poly(amido amine)s for siRNA delivery: effects of structure on siRNA complexation, cellular uptake, gene silencing and toxicity. Pharm Res. 2011;28:1013–22. doi: 10.1007/s11095-010-0344-y. DOI: 10.1007/s11095-010-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurtoglu YE, Navath RS, Wang B, Kannan S, Romero R, Kannan RM. Poly(amidoamine) dendrimer-drug conjugates with disulfide linkages for intracellular drug delivery. Biomaterials. 2009;30:2112–21. doi: 10.1016/j.biomaterials.2008.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerritelli S, Velluto D, Hubbell JA. PEG-SS-PPS: reduction-sensitive disulfide block copolymer vesicles for intracellular drug delivery. Biomacromolecules. 2007;8:1966–72. doi: 10.1021/bm070085x. [DOI] [PubMed] [Google Scholar]
- 34.Zhu L, Mahato RI. Targeted delivery of siRNA to hepatocytes and hepatic stellate cells by bioconjugation. Bioconjug Chem. 2010;21:2119–27. doi: 10.1021/bc100346n. DOI: 10.1021/bc100346n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musacchio T, Vaze O, D’Souza G, Torchilin VP. Effective stabilization and delivery of siRNA: reversible siRNA-phospholipid conjugate in nanosized mixed polymeric micelles. Bioconjug Chem. 2010;21:1530–6. doi: 10.1021/bc100199c. DOI: 10.1021/bc100199c. [DOI] [PubMed] [Google Scholar]
- 36 (a).Meers P. Enzyme-activated targeting of liposomes. Adv Drug Deliv Rev. 2001;53:265–72. doi: 10.1016/s0169-409x(01)00205-8. [DOI] [PubMed] [Google Scholar]; (b) Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. Febs J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. DOI: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 37.Mansour AM, Drevs J, Esser N, Hamada FM, Badary OA, Unger C, Fichtner I, Kratz F. A new approach for the treatment of malignant melanoma: enhanced antitumor efficacy of an albumin-binding doxorubicin prodrug that is cleaved by matrix metalloproteinase 2. Cancer Res. 2003;63:4062–6. [PubMed] [Google Scholar]
- 38 (a).Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A. 2011;108:2426–31. doi: 10.1073/pnas.1018382108. DOI: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dettin M, Muncan N, Bugatti A, Grezzo F, Danesin R, Rusnati M. Chemoselective surface immobilization of proteins through a cleavable peptide. Bioconjug Chem. 2011;22:1753–7. doi: 10.1021/bc200254u. DOI: 10.1021/bc200254u. [DOI] [PubMed] [Google Scholar]; (c) Olson ES, Jiang T, Aguilera TA, Nguyen QT, Ellies LG, Scadeng M, Tsien RY. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc Natl Acad Sci U S A. 2010;107:4311–6. doi: 10.1073/pnas.0910283107. DOI: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Nguyen QT, Olson ES, Aguilera TA, Jiang T, Scadeng M, Ellies LG, Tsien RY. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc Natl Acad Sci U S A. 2010;107:4317–22. doi: 10.1073/pnas.0910261107. DOI: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Aguilera TA, Olson ES, Timmers MM, Jiang T, Tsien RY. Systemic in vivo distribution of activatable cell penetrating peptides is superior to that of cell penetrating peptides. Integrative biology : quantitative biosciences from nano to macro. 2009;1:371–81. doi: 10.1039/b904878b. DOI: 10.1039/b904878b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terada T, Iwai M, Kawakami S, Yamashita F, Hashida M. Novel PEG-matrix metalloproteinase-2 cleavable peptide-lipid containing galactosylated liposomes for hepatocellular carcinoma-selective targeting. J Control Release. 2006;111:333–42. doi: 10.1016/j.jconrel.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 40.Basel MT, Shrestha TB, Troyer DL, Bossmann SH. Protease-sensitive, polymer-caged liposomes: a method for making highly targeted liposomes using triggered release. ACS Nano. 2011;5:2162–75. doi: 10.1021/nn103362n. DOI: 10.1021/nn103362n. [DOI] [PubMed] [Google Scholar]
- 41.Zhu L, Huo Z, Wang L, Tong X, Xiao Y, Ni K. Targeted delivery of methotrexate to skeletal muscular tissue by thermosensitive magnetoliposomes. Int J Pharm. 2009;370:136–43. doi: 10.1016/j.ijpharm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Pradhan P, Giri J, Rieken F, Koch C, Mykhaylyk O, Doblinger M, Banerjee R, Bahadur D, Plank C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2010;142:108–21. doi: 10.1016/j.jconrel.2009.10.002. DOI: 10.1016/j.jconrel.2009.10. [DOI] [PubMed] [Google Scholar]
- 43 (a).Sherlock SP, Tabakman SM, Xie L, Dai H. Photothermally enhanced drug delivery by ultrasmall multifunctional FeCo/graphitic shell nanocrystals. ACS Nano. 2011;5:1505–12. doi: 10.1021/nn103415x. DOI: 10.1021/nn103415x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kang H, Trondoli AC, Zhu G, Chen Y, Chang YJ, Liu H, Huang YF, Zhang X, Tan W. Near-infrared light-responsive core-shell nanogels for targeted drug delivery. ACS Nano. 2011;5:5094–9. doi: 10.1021/nn201171r. DOI: 10.1021/nn201171r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44 (a).Ranjan A, Jacobs GC, Woods DL, Negussie AH, Partanen A, Yarmolenko PS, Gacchina CE, Sharma KV, Frenkel V, Wood BJ, Dreher MR. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J Control Release. 2012;158:487–94. doi: 10.1016/j.jconrel.2011.12.011. DOI: 10.1016/j.jconrel.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) de Smet M, Heijman E, Langereis S, Hijnen NM, Grull H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: an in vivo proof-of-concept study. J Control Release. 2011;150:102–10. doi: 10.1016/j.jconrel.2010.10.036. DOI: 10.1016/j.jconrel.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 45.de Las Heras Alarcon C, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chem Soc Rev. 2005;34:276–85. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- 46.Schwerdt A, Zintchenko A, Concia M, Roesen N, Fisher K, Lindner LH, Issels R, Wagner E, Ogris M. Hyperthermia-induced targeting of thermosensitive gene carriers to tumors. Hum Gene Ther. 2008;19:1283–92. doi: 10.1089/hum.2008.064. [DOI] [PubMed] [Google Scholar]
- 47.Kono K. Thermosensitive polymer-modified liposomes. Adv Drug Deliv Rev. 2001;53:307–19. doi: 10.1016/s0169-409x(01)00204-6. [DOI] [PubMed] [Google Scholar]
- 48.Sun G, Z. G. Synthesis and Investigation of Core-Shell Dendritic Nanoparticles with Tunable Thermosensitivity. Macromolecules. 2010;43:9668–9673. [Google Scholar]
- 49.Gupta AK, Gupta M. Cytotoxicity suppression and cellular uptake enhancement of surface modified magnetic nanoparticles. Biomaterials. 2005;26:1565–73. doi: 10.1016/j.biomaterials.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Maver U, Bele M, Makovec D, Čampelj S, Jamnik J, Gaberšček M. Incorporation and release of drug into/from superparamagnetic iron oxide nanoparticles. Journal of Magnetism and Magnetic Materials. 2009;19:3187–3192. [Google Scholar]
- 51.Zhang C, Wangler B, Morgenstern B, Zentgraf H, Eisenhut M, Untenecker H, Kruger R, Huss R, Seliger C, Semmler W, Kiessling F. Silica- and alkoxysilane-coated ultrasmall superparamagnetic iron oxide particles: a promising tool to label cells for magnetic resonance imaging. Langmuir. 2007;23:1427–34. doi: 10.1021/la061879k. [DOI] [PubMed] [Google Scholar]
- 52 (a).Sawant RM, Sawant RR, Gultepe E, Nagesha D, Papahadjopoulos-Sternberg B, Sridhar S, Torchilin VP. Nanosized cancer cell-targeted polymeric immunomicelles loaded with superparamagnetic iron oxide nanoparticles. Journal of Nanoparticle Research. 2009;11:1777–1785. [Google Scholar]; (b) Liao C, Sun Q, Liang B, Shen J, Shuai X. Targeting EGFR-overexpressing tumor cells using Cetuximab-immunomicelles loaded with doxorubicin and superparamagnetic iron oxide. Eur J Radiol. 2011;80:699–705. doi: 10.1016/j.ejrad.2010.08.005. DOI: 10.1016/j.ejrad.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 53 (a).Lee JH, Jang JT, Choi JS, Moon SH, Noh SH, Kim JW, Kim JG, Kim IS, Park KI, Cheon J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat Nanotechnol. 2011;6:418–22. doi: 10.1038/nnano.2011.95. DOI: 10.1038/nnano.2011.95. [DOI] [PubMed] [Google Scholar]; (b) Xie J, Liu G, Eden HS, Ai H, Chen X. Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Acc Chem Res. 2011;44:883–92. doi: 10.1021/ar200044b. DOI: 10.1021/ar200044b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee H, Lee E, Kim do K, Jang NK, Jeong YY, Jon S. Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. J Am Chem Soc. 2006;128:7383–9. doi: 10.1021/ja061529k. [DOI] [PubMed] [Google Scholar]
- 55.Liu D, Wu W, Chen X, Wen S, Zhang X, Ding Q, Teng G, Gu N. Conjugation of paclitaxel to iron oxide nanoparticles for tumor imaging and therapy. Nanoscale. 2012;4:2306–10. doi: 10.1039/c2nr11918h. DOI: 10.1039/c2nr11918h. [DOI] [PubMed] [Google Scholar]
- 56.Yang X, Hong H, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, Xiao Y, Yang Y, Zhang Y, Nickles RJ, Cai W, Steeber DA, Gong S. cRGD-functionalized, DOX-conjugated, and (6)(4)Cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging. Biomaterials. 2011;32:4151–60. doi: 10.1016/j.biomaterials.2011.02.006. DOI: 10.1016/j.biomaterials.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids. 2009;162:1–16. doi: 10.1016/j.chemphyslip.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Schroeder A, Avnir Y, Weisman S, Najajreh Y, Gabizon A, Talmon Y, Kost J, Barenholz Y. Controlling liposomal drug release with low frequency ultrasound: mechanism and feasibility. Langmuir. 2007;23:4019–25. doi: 10.1021/la0631668. [DOI] [PubMed] [Google Scholar]
- 59.Schroeder A, Honen R, Turjeman K, Gabizon A, Kost J, Barenholz Y. Ultrasound triggered release of cisplatin from liposomes in murine tumors. J Control Release. 2009;137:63–8. doi: 10.1016/j.jconrel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 60.Wang CH, Huang YF, Yeh CK. Aptamer-conjugated nanobubbles for targeted ultrasound molecular imaging. Langmuir. 2011;27:6971–6. doi: 10.1021/la2011259. DOI: 10.1021/la2011259. [DOI] [PubMed] [Google Scholar]
- 61.Warram JM, Sorace AG, Saini R, Umphrey HR, Zinn KR, Hoyt K. A triple-targeted ultrasound contrast agent provides improved localization to tumor vasculature. J Ultrasound Med. 2011;30:921–31. doi: 10.7863/jum.2011.30.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katz JS, Burdick JA. Light-responsive biomaterials: development and applications. Macromol Biosci. 2010;10:339–48. doi: 10.1002/mabi.200900297. DOI: 10.1002/mabi.200900297. [DOI] [PubMed] [Google Scholar]
- 63.Robertson CA, Evans DH, Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol B. 2009;96:1–8. doi: 10.1016/j.jphotobiol.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 64.Oku N, Ishii T. Antiangiogenic photodynamic therapy with targeted liposomes. Methods Enzymol. 2009;465:313–30. doi: 10.1016/S0076-6879(09)65016-3. [DOI] [PubMed] [Google Scholar]
- 65.Yavlovich A, Smith B, Gupta K, Blumenthal R, Puri A. Light-sensitive lipid-based nanoparticles for drug delivery: design principles and future considerations for biological applications. Mol Membr Biol. 2010;27:364–81. doi: 10.3109/09687688.2010.507788. DOI: 10.3109/09687688.2010.507788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun L, Yang Y, Dong CM, Wei Y. Two-photon-sensitive and sugar-targeted nanocarriers from degradable and dendritic amphiphiles. Small. 2011;7:401–6. doi: 10.1002/smll.201001729. DOI: 10.1002/smll.201001729. [DOI] [PubMed] [Google Scholar]
- 67.Yudina A, de Smet M, Lepetit-Coiffe M, Langereis S, Van Ruijssevelt L, Smirnov P, Bouchaud V, Voisin P, Grull H, Moonen CT. Ultrasound-mediated intracellular drug delivery using microbubbles and temperature-sensitive liposomes. J Control Release. 2011;155:442–8. doi: 10.1016/j.jconrel.2011.06.006. DOI: 10.1016/j.jconrel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 68.Chen J, Qiu X, Ouyang J, Kong J, Zhong W, Xing MM. pH and reduction dual-sensitive copolymeric micelles for intracellular doxorubicin delivery. Biomacromolecules. 2011;12:3601–11. doi: 10.1021/bm200804j. DOI: 10.1021/bm200804j. [DOI] [PubMed] [Google Scholar]
- 69.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58:1532–55. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 70 (a).Spallarossa P, Altieri P, Garibaldi S, Ghigliotti G, Barisione C, Manca V, Fabbi P, Ballestrero A, Brunelli C, Barsotti A. Matrix metalloproteinase-2 and -9 are induced differently by doxorubicin in H9c2 cells: The role of MAP kinases and NAD(P)H oxidase. Cardiovasc Res. 2006;69:736–45. doi: 10.1016/j.cardiores.2005.08.009. [DOI] [PubMed] [Google Scholar]; (b) Cheng YC, Chen LM, Chang MH, Chen WK, Tsai FJ, Tsai CH, Lai TY, Kuo WW, Huang CY, Liu CJ. Lipopolysaccharide upregulates uPA, MMP-2 and MMP-9 via ERK1/2 signaling in H9c2 cardiomyoblast cells. Mol Cell Biochem. 2009;325:15–23. doi: 10.1007/s11010-008-0016-y. [DOI] [PubMed] [Google Scholar]
- 71.Deng Z, Zhen Z, Hu X, Wu S, Xu Z, Chu PK. Hollow chitosan-silica nanospheres as pH-sensitive targeted delivery carriers in breast cancer therapy. Biomaterials. 2011;32:4976–86. doi: 10.1016/j.biomaterials.2011.03.050. DOI: 10.1016/j.biomaterials.2011.03.050. [DOI] [PubMed] [Google Scholar]