Abstract
Age-related increase in monoamine oxidase B (MAO-B) may contribute to CNS neurodegenerative diseases. Moreover, MAO-B inhibitors are used in the treatment of idiopathic Parkinson disease as preliminary monotherapy or adjunct therapy with L-dopa. To date, meager natural sources of MAO-B inhibitors have been identified, and the relative strength, potency and rank of many plants relative to standard drugs such as Selegiline (L-deprenyl, Eldepryl) are not known. In this work, we developed and utilized a high throughput enzyme microarray format to screen and evaluate 905 natural product extracts (0.025–.7 mg/ml) to inhibit human MAO-B derived from BTI-TN-5B1-4 cells infected with recombinant baculovirus. The protein sequence of purified enzyme was confirmed using 1D gel electrophoresis-matrix assisted laser desorption ionization-time-of-flight-tandem mass spectroscopy, and enzyme activity was confirmed by [1] substrate conversion (3-mM benzylamine) to H202 and [2] benzaldehyde. Of the 905 natural extracts tested, the lowest IC50s [<0.07 mg/ml] were obtained with extracts of Amur Corktree (Phellodendron amurense), Bakuchi Seed(Cyamopsis psoralioides), Licorice Root (Glycyrrhiza glabra/uralensis), Babchi (Psoralea corylifolia seed). The data also show, albeit to a lesser extent, inhibitory properties of herbs originating from the mint family (Lamiaceae) and Turmeric, Comfrey, Bringraj, Skullcap, Kava-kava, Wild Indigo, Gentian and Green Tea. In conclusion, the data reflect relative potency information by rank of commonly used herbs and plants that contain human MAO-B inhibitory properties in their natural form.
Keywords: monoamine oxidase, Parkinson’s, herbs, natural medicine, MPTP, dopamine
INTRODUCTION
Monoamine oxidase (MAO, EC 1.4.3.4) is a mitochondrial membrane bound flavin-containing enzyme that catalyzes the oxidative deamination of monoamine neurotransmitters such as dopamine (DA). Due to the function role of MAO in regulation of DAergic neurotransmission, enzyme inhibitors have become of relevant pharmacological interest for treating diseases associated with DAergic malfunction such as Parkinson’s disease (PD) and depression (Drozak and Kozlowski, 2006; Horn et al., 2010). In the case of PD, type B MAO (MAO-B) inhibitors are used in early to advanced disease states, alone or adjunct to DA agonists such as levodopa, pramipexole, ropinirole, rotigotine or entacapone (Rascol et al., 2011). MAO-B inhibitors are primarily used to modulate symptomatic defect in motor control, by augmenting efficacy of Sinemet creating rise in synaptic DA concentrations (Talati et al., 2009) and in some cases providing a time delay for PD patient requirement to commence traditional levodopa treatment (Lohle and Reichmann, 2011).
Much of the literature suggests that MAO-B inhibitors not only modulate DAergic function, but also impart neurological protection (Polanski et al., 2011) and thereby slow the progressive nature of PD (Lohle and Reichmann, 2011; Fedorova et al., 2011; Pagonabarraga and Kulisevsky, 2010). However, the protective effects of MAO-B inhibitors are still in question, with uncertainty as to distinction between symptomatic or etiological relief associated with the inhibition of MAO or other properties of this class of drugs (Kassubek et al., 2010; van Laar et al., 2010). For example, some studies show that the N-propargyl moiety in/or metabolites formed from rasagiline such as 1-(R)-aminoindan antagonize apoptosis, thereby introducing powerful neuroprotective properties. (Weinreb et al., 2010) Independent of MAO inhibition, rasagiline also prevents mitochondrial permeability, cytochrome c release, casapase activation, DNA damage and affords rise in neurotrophic factors (Naoi and Maruyama, 2009) and neurorescue against post- N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-mediated damage in mice. (Mandel et al., 2007)
A rise in capacity or expression of MAO-B is believed to contribute to age-related neurological injury due to aggravated accumulation of 3,4-dihydroxyphenylacetaldehyde, which is readily metabolized to 3,4-dihydroxyphenylacetic acid, free radicals and orthoquinones which provoke oxidative neurological damage. (Anderson et al., 2011; Jinsmaa et al., 2011) Transgenic mice over expressing MAO-B show substantial oxidative stress and inflammation within the SN, with elevated levels of H202 which can oxidize DA to dopaminochrome (a mitochondrial toxin) (Mallajosyula et al., 2008; Siddiqui et al., 2010)
While inhibitors of type B monoamine oxidase (MAO-B) such as rasagiline and L-deprenyl are candidate drugs for treatment of PD, it has also been suggested that natural products as in the case of Banisteriopsis caapi contain MAO-B inhibitory properties. (Wang et al., 2010). With public interest in this enzyme for treatment of PD and neurological diseases associated with aging of the brain, and very little information on standardized screenings for natural MAO-B inhibitors, we screen and rank 905 products sold worldwide. Extracts included diverse range of natural products from sectioned fresh fruits, fresh vegetables, seeds, rinds, herbs, roots cut and dried to cultural spices often used as Chinese and Indian traditional medicines.
METHODS AND MATERIALS
Hanks Balanced Salt Solution, (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES), iodoacetamide, DL-Dithiothreitol (DTT), ethanol, 96 well plates, vanillic acid, 4-aminoantipyrine, general reagents and supplies, MAOb and supplies were all purchased from Sigma Scientific (Sigma, St Louis MO). Natural products were provided by Frontier Natural Products Co-op (Norway, IA), Monterey Bay Spice Company (Watsonville, CA), Mountain Rose Herbs (Eugene, OR), Mayway Traditional Chinese Herbs (Oakland, California), Kalyx Natural Marketplace (Camden, NY), Futureceuticals (Momence, IL), organic fruit vegetable markets and Florida Food Products Inc. (Eustis, FL).
Herbal extraction
Plant and herbal extracts were macerated, diced, chopped and homogenized in 100% ethanol at 50 mg/ml. Samples were placed on a rocker shaker for 24 h and stored in air tight containers at −20°C in the dark. All serial dilutions were made using a diluent consisting of HBSS with 10-mM HEPES adjusted to a pH 7.4.
MALDI MS MS protein identification
Human MAO-B was derived from insect cells (BTI-TN-5B1-4) infected with recombinant baculovirus containing cDNA for human MAO-B (Sigma, St Louis MO). The protein was validated by proteomic analysis using matrix assisted laser desorption ionisation (MALDI) Mass Spec (MS/MS) and analyzed by Mascot ID. Briefly, 10 μg of pure enzyme was solubilized, denatured and subjected to 1 D SDS page gel electrophoresis using a 5–20% Tris-HCL gradient gel with a running buffer 25 mM Tris, 192 mM glycine, 0.1% SDS at 200 V for 35 min. High intensity bands for MAO-B at 60 Kd were excised, followed by in gel digestion of peptides with trypsin following reduction/alkylation with DTT and iodoacetamide respectively. Samples were analyzed using MALDI MS/MS (Applied Biosystems) and protein sequence identified by Mascot analysis.
Monoamine oxidase activity
A continuous MAO-B assay was used to conduct high throughput screenings with slight modifications (Holt and Palcic, 2006). Briefly, MAO-B was prepared in HBSS containing 10-mM HEPES, pH 7.4 at a concentration of 3.5 U/ml HBSS. The enzyme and treatments were distributed in 96-well microplates and incubated at RT for 10 min. The substrate [benzylamine] (final working concentration 3 mM) and chromogen from a 5× stock [5-mM vanillic acid, 2.5-mM 4-aminoantipyrine and 4 U/ml of horseradish peroxidase type II] were added to each well. Addition of the substrate (1:5 v/v) and the chromogenic solution (1:5 v/v) initiated the reaction, which required 18–24 h at RT for completion. Product formation was quantified every 4 h. At time zero, plates were quantified for O.D. at 490 nm to obtain a pre plate reading accounting for background. For data analysis, the post plates readings at time 4, 8,12,16, 20 and 24 h were obtained by subtracting the time zero pre-plate reading to monitor only the continuous product accumulation (increase in O.D at 490 nm).
High throughput design
We designed a model for rapid screening based on the concept used in PCR microarrays for gene amplification. An enzyme microarray format was adopted to where a 96-well plate contained known concentration of enzyme, and 905 treatments of equal concentration dissolved in buffered HBSS were incubated with the enzyme, prior to start of the reaction with the substrate and chromogen. After addition of the substrate, a curve for time-dependent product formation was monitored continuously over 24 h. A first tier investigation was established at a final working concentration of 0.7 mg/ml for each herbal extract. Any compounds that inhibited MAO-B with in the first tier screen below 50% of control were then placed in a second (final concentration = .4 mg/ml), third tier (final concentration =.2 mg/ml) and fourth tier (final concentration =.07 mg/ml). Extracts were ranked for potency, and the most potent were further evaluated for IC50 s along with deprenyl as a positive control. The enzyme microarray format is very rapid, reproducible and validated by triplicates and corroborated by a four tier evaluation process.
Benzaldehyde production
Quantification of benzaldehyde was continuously monitored using a Shimadzu high-performance liquid chromatography (HPLC) system equipped with an SPD-20A UV detector (set at 254 nm), a workstation containing EZSTART version 7.4 software and an SS420X instrument interface docked to a Waters Autosampler Model 717 Plus (Shimadzu Scientific Instruments, Inc. US; Waters Corp., Milford, MA). Benzaldehyde standard curves were established, and samples taken from the enzyme reaction chamber were mixed with 2x distilled water and then 50:50 with methanol prior to injection. The flow rate was isocratic at 1.2 ml/min. The mobile phase consisted of 10-mM sodium phosphate (pH 2.7) mixed at 45:55 with methanol. The column was a TSK-gel ODS-100 V 5 μm 4.6 mm (id) × 15.0 cm (L); (Tosoh Corporation), the run time was 6 mins and injection volume 25 μl.
Determination of H+ concentration
Although we used 10-mM HEPES buffered HBSS (pH 7.4) as a diluent, due to the high number of compounds and potential for pH to contribute to inhibitory effects in the enzyme reaction, in particular for acidic fruits, a high throughput evaluation for pH was conducted in 96-well plates using standard phenol red indicator dye. Briefly, extract matrix blanks for extracts displaying inhibitory properties were evaluated for influence on pH by establishing a pre-plate reading at 550 nm using a Spectra 190-MAX UV spectrophotometric detector (Molecular devices, Sunnydale, CA, USA) and post plate reading after addition of phenol red stock solution (0.2 mg/ml water) in HBSS (15% v/v) to each well. The change in pH was immediately assessed relative to control blanks at 550 nm relative to control blanks. Any extracts that altered the pH of the reaction vessel were buffered to pH 7.2 and re-evaluated at neutral pH for MAO-B inhibition.
Determination of H202 Non enzymatic stoichiometric radical scavenging
Due to the high number of compounds and potential for antioxidant effects to yield a false positive for MAO-B inhibitor identification (an enzyme reaction that monitors continuous production of H2O2), all extracts at concentrations demonstrating MAO-B inhibitor activity were evaluated for ability to scavenge peroxide 30 μM. Briefly, H2O2 was incubated in the presence of experimental compounds at RT for 20 min. A pre-plate reading was obtained where the data was subtracted from the final value to eliminate background interference. The chromogenic reagent (5x stock [5-mM vanillic acid, 2.5-mM 4-aminoantipyrine and 4 U/ml of horseradish per-oxidase type II]) was added to each sample and incubated for 10 min at 37°C. Samples were analyzed at 490 nm using a Spectra 190-MAX UV spectrophotometric detector (Molecular devices, Sunnydale, CA, USA). Extracts showing ability to scavenge peroxide were validated by HPLC via quantification of benzaldehyde.
Data analysis
Statistical analysis was performed using Graph Pad Prism (version 3.0; Graph Pad Software Inc. San Diego, CA, USA) with significance of difference between the groups assessed using a one-way ANOVA, followed by Tukey post hoc means comparison test, a two-way ANOVA or Student’s t test. IC50s were determined by regression analysis using Origin Software (OriginLab, Northampton, MA).
RESULTS
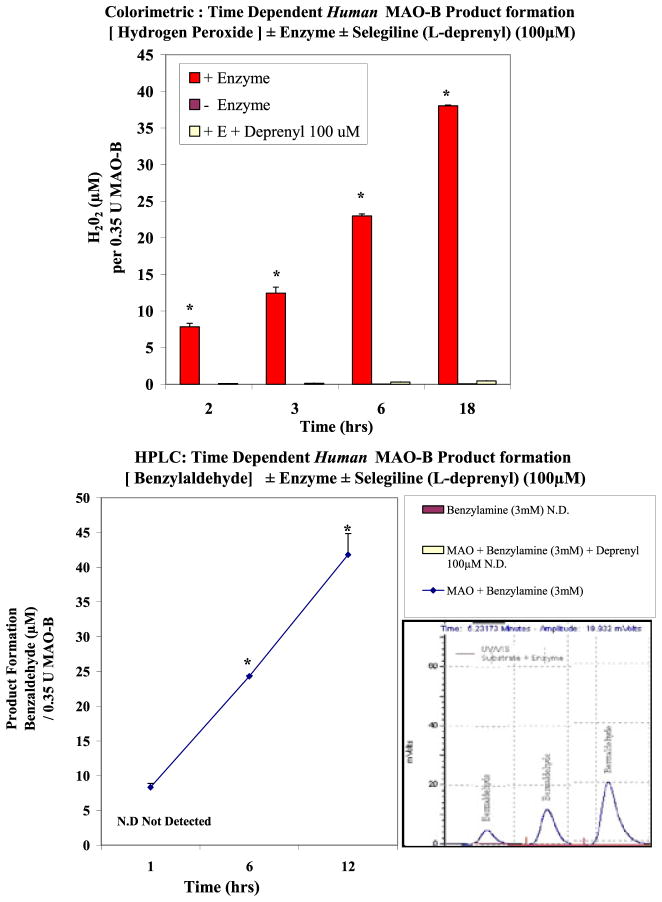
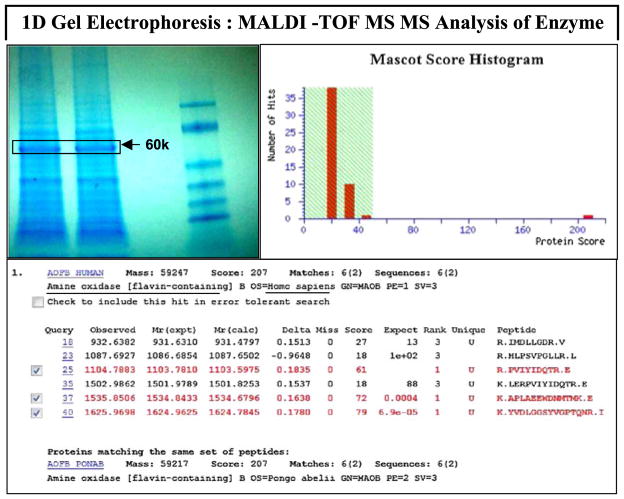
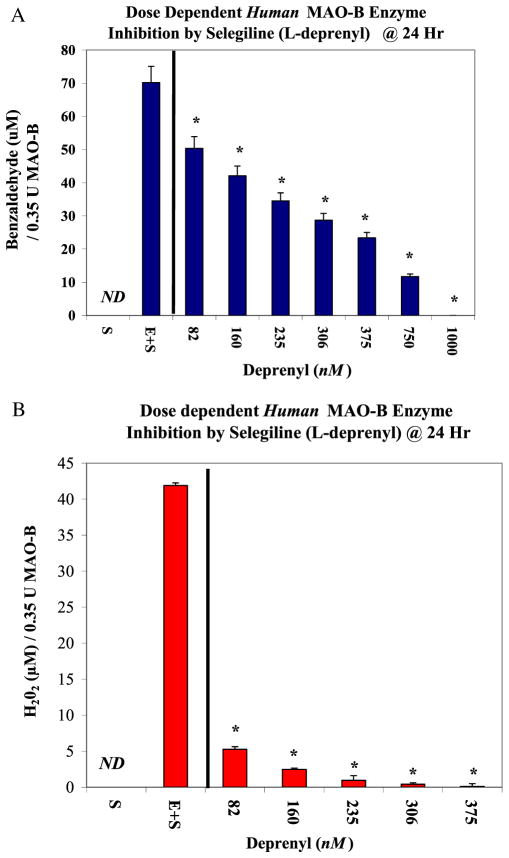
Method validation was established by monitoring the continuous time-dependent product formation in the presence of a substrate (benzylamine 2 mM) ± MAO-B ± Selegiline (L-deprenyl) (100 μM) (Fig. 1a [H202] and Fig. 1b [benzaldehyde]). The data show a slow but steady rate of reaction, resulting in time-dependent product formation with high signal/noise ratio. Protein sequencing using MALDI MS/MS and analysis by Mascot ID showed a positive hit for human MAO-B with a 95% confidence interval for peptide/sequence mass (Fig. 2). MAO-B positive controls were established using a known inhibitor [L-deprenyl] which showed significant potency and a complete loss of product formation [H202] and [benzylamine] at 1 μM (Fig. 3a and 3b). The data reflect a greater sensitivity observed by HPLC quantification of benzaldehyde than color-imetric assessment of H202 despite similar trends in enzyme inhibition.
Figure 1.
A. Human MAO-B activity - Time-dependent H202 product formation from 3-mM benzylamine in the presence or absence of human MAO-B, and in the presence of 3-mM benzylamine + 100 μM of deprenyl. The data represent μM H202 produced from 0–18 h (incubation at RT) and are presented as the Mean ± S.E.M, n=4. Significance of difference for product formation between the Time 0 control versus 2–18 h was determined using a one-way ANOVA followed by a Tukey post hoc test * p< .05. B. Human MAO-B activity - Time-dependent benzaldehyde product formation from 3-mM benzylamine in the presence or absence of human MAO-B, and in the presence of 3-mM benzylamine + 100 μM of deprenyl. The data represent μM benzaldehyde produced from 1–12 h (incubation at RT) and are presented as the Mean ± S.E.M, n=4. Significance of difference for product formation between the time 1 h versus 6 and 12 h was determined using a one-way ANOVA followed by a Tukey post hoc test. * p< .05. This figure is available in colour online at wileyonlinelibrary.com/journal/ptr.
Figure 2.
Mascot results for protein identification by peptide mass fingerprinting of Human MAO-B tryptic digest analyzed by MALDI-TOF/TOF-MS. This figure is available in colour online at wileyonlinelibrary.com/journal/ptr.
Figure 3.
A. Deprenyl inhibitory effects on Human MAO-B. The data represent product formation (μM benzylamine) produced at 24 h (incubation at RT) in the presence or absence of deprenyl (82 – 1000 nM) and are presented as the Mean ± S.E.M, n=4. Significance of difference for product formation between the controls versus deprenyl was determined using a one-way ANOVA followed by a Tukey post hoc test. * p<.05. B. Deprenyl inhibitory effects on Human MAO-B. The data represent product formation (μM H202) produced at 24 h (incubation at RT) in the presence or absence of deprenyl (82 – 375 nM) and are presented as the Mean ± S.E.M, n=4. Significance of difference between the controls versus activity in the presence of deprenyl was determined using a one-way ANOVA followed by a Tukey post hoc test. * p<.05. This figure is available in colour online at wileyonlinelibrary.com/journal/ptr.
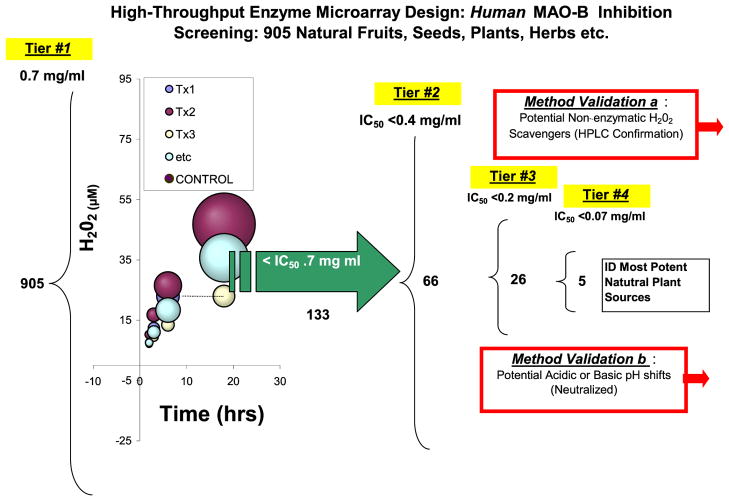
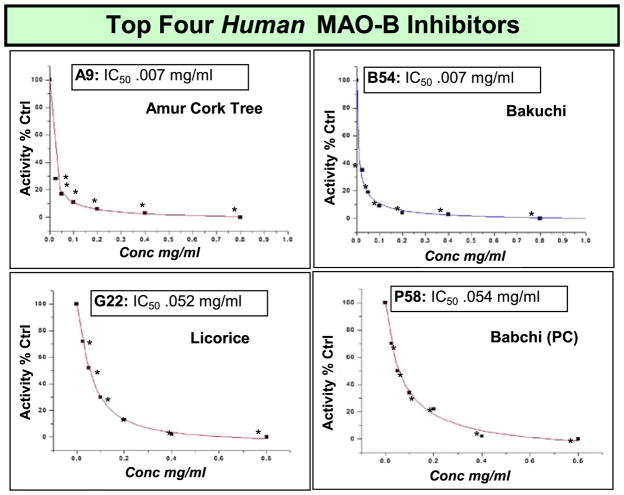
A high throughput enzyme microarray model was developed and used in this work as described in Fig. 4. We designed a model for rapid screening based on a similar concept often applied in microarray analysis for quantitative real-time RT-PCR. However, instead of gene amplification, an enzyme microarray format was adopted to where a 96-well plate contained known concentration of enzyme and 905 treatments of equal concentration dissolved in buffered HBSS were incubated with the enzyme, prior to start of the reaction. After addition of the substrate, a curve for time-dependent product formation was monitored continuously over 24 h. A first tier investigation was established at a final working concentration of 0.7 mg/ml for each herbal extract. Any extract that inhibited MAO-B with in the first tier screen below 50% of control was then subject to a second (final concentration = .4 mg/ml), third tier (final concentration =.2 mg/ml) and fourth tier (final concentration =.07 mg/ml). A total of 905 extracts were evaluated at a final working concentration of .7 mg/ml. Of these, 133 demonstrated an IC50 < 0.7 mg/ml. Of the 133 retested at .4 mg/ml, 66 extracts showed an IC50 <0.4 mg/ml. Of the 66 retested at .2 mg/ml, 26 extracts showed an IC50 <0.2 mg/ml. Of the 26 retested at .2 mg/ml, five extracts showed IC50 <0.07 mg/ml. All extracts showing inhibitory properties were evaluated for potential interfering variable of pH shifts or radical scavenging abilities (which would render false positive based on MAO-B activity based on formation of H202). False positives were eliminated by adjusted pH and confirmed by benzaldehyde product accumulation by HPLC. All inhibitors are listed in Table 1 where extracts demonstrating the greatest potency are listed as Level 1 (strongest) IC50 <.07 mg/ml, followed by Level 2 (strong) IC50 <.2 mg/ml, Level 3 (moderately strong) IC50 >.2<.4 mg/ml, Level 4 (moderate) (IC50>.4 < .7 mg/ml) and Level 5 (weak) IC50=.7 mg/kg. The most potent natural MAO-B inhibitors were identified, confirmed and an IC50 established (Fig. 5). The findings in this study yield evidence to suggest there are a number of natural products commonly used worldwide which have capacity to inhibit human MAO-B enzyme. However, in the therapeutic range, the most likely candidates were Amur Cork tree, Licorice, Psoralea Fruit and Bakuchi.
Figure 4.
A high throughput enzyme experimental microarray design. 905 extracts were evaluated for capacity to inhibit Human MAO-B. A first tier screening was conducted at a final working concentration of 0.7 mg/ml for each herbal extract. Enzyme activity was continuously monitored over a 24-h period. Extracts demonstrating an IC50 <0.7 mg/ml were screened through a tier 2 screening at .4 mg/ml. Extracts demonstrating an IC50 at <0.4 mg/ml were screened through a tier 3 screening at .2 mg/ml. Extracts demonstrating an IC50 at <0.2 mg/ml were screened through a tier 4 screening at .07 mg/ml.. All extracts showing inhibitory properties were evaluated for potential interfering variable of pH shifts or radical scavenging abilities (which would render false positive based on MAO-B activity based on formation of H202). This figure is available in colour online at wileyonlinelibrary.com/journal/ptr.
Table 1.
Human MAO-B inhibitors by potency. Extracts demonstrating the greatest potency are listed as Level 1 (strongest) IC50 <.07 mg/ml, followed by Level 2 (strong) IC50 <.2 mg/ml, Level 3 (moderately strong) IC50 >.2<.4 mg/ml, Level 4 (moderate) (IC50>.4 < .7 mg/ml) and Level 5 (weak) IC50=.7 mg/kg
Natural plant sources of human MAO-B inhibitors
| LEVEL | ID | Common name | Scientific name | Inhibitory conc. |
|---|---|---|---|---|
| LEVEL 1 | A9 | Amur Cork Tree | Phellodendron amurense | IC50 <.07 mg/ml |
| B54 | Bakuchi Seed a | Cyamopsis psoralioides | IC50 <.07 mg/ml | |
| L2 | Licorice Root | Glycyrrhiza glabra | IC50 <.07 mg/ml | |
| G22 | Gan Cao a | Glycyrrhiza uralensis root | IC50 <.07 mg/ml | |
| P58 | Psoralea Fruit Babchi a | Psoralea corylifolia | IC50 <.07 mg/ml | |
| LEVEL 2 | P15 | Beefsteakplant a | Perilla frutescens leaf | IC50 <.2 mg/ml |
| W4 | Black Pepper | Piper nigrum | IC50 <.2 mg/ml | |
| B15 | Bringraj ‘False Daisy’ | Eclipta erecta | IC50 <.2 mg/ml | |
| C34 | Chinese arborvitae a | Truja, Platycladus orientalis twig/leaf | IC50 <.2 mg/ml | |
| C2 | Comfrey | Symphytum officinale leaf | IC50 <.2 mg/ml | |
| C84 | Curry powder | Coriander, turmeric, cumin, fenugreek, red pepper | IC50 <.2 mg/ml | |
| F4 | Figwort | Scrophularia nodosa | IC50 <.2 mg/ml | |
| S54 | Gloryvine Stem | Sargentodoxa cuneata | IC50 <.2 mg/ml | |
| XT3 | Green Tea | Camellia sinensis | IC50 <.2 mg/ml | |
| H20 | Herb de province | Oregano, thyme, savory, lavender, basil, sage, rosemary | IC50 <.2 mg/ml | |
| K8 | Kava Kava | Piper methysticum | IC50 <.2 mg/ml | |
| L45 | Ladys’ Mantle | Alchemilla vulgaris | IC50 <.2 mg/ml | |
| A37 | Lesser Galangal | Alpinia officinarum Root | IC50 <.2 mg/ml | |
| L35 | Red Henna | Lawsonia inermis | IC50 <.2 mg/ml | |
| C53 | Sappanwood | Caesalpinia sappan wood | IC50 <.2 mg/ml | |
| D10 | Thick-stemmed Wooda | Dryopteris crassirhizoma | IC50 <.2 mg/ml | |
| T3 | Turmeric a | Curcuma Longa | IC50 <.2 mg/ml | |
| W13 | Watercress | Nasturtium officinale | IC50 <.2 mg/ml | |
| W3 | Wild Indigo Root | Baptisia tinctoria | IC50 <.2 mg/ml | |
| W10 | Wild lettuce | Lactuca virosa | IC50 <.2 mg/ml | |
| G6 | Yellow Gentian Root | Gentiana lutea | IC50 <.2 mg/ml | |
| LEVEL 3 | A4 | Alfalfa | Medicago sativa | IC50 >.2 <.4 mg/ml |
| A48 | Alpinia | Alpinia katsumadai seed | IC50 >.2 <.4 mg/ml | |
| B48 | Bay Leaf | Laurus nobilis | IC50 >.2 <.4 mg/ml | |
| B13 | Bayberry Root bark a | Morella cerifera | IC50 >.2 <.4 mg/ml | |
| C103 | Cedar Berries | Juniperus monosperma | IC50 >.2 <.4 mg/ml | |
| C101 | Celandine | Chelidonium majus | IC50 >.2 <.4 mg/ml | |
| S56 | Common hedgenettle | Stachys officinales | IC50 >.2 <.4 mg/ml | |
| E3 | Eucalyptus Leaf | Eucalyptus globulus | IC50 >.2 <.4 mg/ml | |
| E6 | Eyebright | Euphrasia officinalis | IC50 >.2 <.4 mg/ml | |
| D18 | Fringed Pink | Dianthus superbus | IC50 >.2 <.4 mg/ml | |
| G31 | Garam Marsala | Garam Marsala | IC50 >.2 <.4 mg/ml | |
| G28 | Gourmet pepper mill | Gourmet pepper mill | IC50 >.2 <.4 mg/ml | |
| G29 | Gunpowder green tea | Camellia sinensis | IC50 >.2 <.4 mg/ml | |
| H22 | Hyssop | Hyssopus officinalis | IC50 >.2 <.4 mg/ml | |
| P89 | Indian long pepper | Piper longum | IC50 >.2 <.4 mg/ml | |
| I2 | Isatis Leaf | Isatis indigotica; Isatis tinctoria | IC50 >.2 <.4 mg/ml | |
| I8 | Italian spice herbal tea | Italian spice herbal tea | IC50 >.2 <.4 mg/ml | |
| K9 | Kola Nut | Cola acuminata | IC50 >.2 <.4 mg/ml | |
| L43 | Lemon Curry | Lemon curry | IC50 >.2 <.4 mg/ml | |
| L49 | Lemon Verbana | Aloysia triphylla | IC50 >.2 <.4 mg/ml | |
| M20 | Lilly Tree | Magnolia denudata flower | IC50 >.2 <.4 mg/ml | |
| L46 | Linden Leaf | Tilia europaea | IC50 >.2 <.4 mg/ml | |
| M38 | Maiden Hair Fern | Adiantum capillus | IC50 >.2 <.4 mg/ml | |
| M40 | Motherwort | Leonurus cardiaca | IC50 >.2 <.4 mg/ml | |
| P4 | Parsley | Petroselinum crispum | IC50 >.2 <.4 mg/ml | |
| R4 | Red Clover | Trifolium pratense | IC50 >.2 <.4 mg/ml | |
| R9 | Red Sandlewood | Pterocarpus santalinus | IC50 >.2 <.4 mg/ml | |
| L33 | Rough bugleweed | Lycopus lucidus | IC50 >.2 <.4 mg/ml | |
| S61 | Sage | Salvia officinalis | IC50 >.2 <.4 mg/ml | |
| S23 | Sassafras | Sassafras Albidum | IC50 >.2 <.4 mg/ml | |
| E21 | Scouringrush horsetail | Equisetum Heimale | IC50 >.2 <.4 mg/ml | |
| S32 | Skull cap a | Scutellaria barbata | IC50 >.2 <.4 mg/ml | |
| S16 | Skull cap a | Scutellaria lateriflora | IC50 >.2 <.4 mg/ml | |
| S64 | Spearmint a | Mentha spicata | IC50 >.2 <.4 mg/ml | |
| S20 | Stevia/Candyleaf | Stevia rebaudiana | IC50 >.2 <.4 mg/ml | |
| S15 | Szechuan pepper | Zanthoxylum bungeanum | IC50 >.2 <.4 mg/ml | |
| W11 | White Sage | Salvia apiana | IC50 >.2 <.4 mg/ml | |
| Y8 | Yohimbe Bark | Corynanthe yohimbe | IC50 >.2 <.4 mg/ml | |
| Y7 | Young Hyson Green Tea | Camellia sinensis | IC50 >.2 <.4 mg/ml | |
| V1 | Zi Hua Di Ding | Viola yedoensis herb | IC50 >.2 <.4 mg/ml | |
| LEVEL 4 | ID | Common Name | Scientific Name | Inhibitory Conc. |
| A67 | Ancho chili pepers | Capsicum annum | IC50 >.4 < .7 mg/ml | |
| B19 | Blackberry leaf | Rubus fruticosus | IC50 >.4 < .7 mg/ml | |
| B12 | Blackberry Root | Rubus fruticosus or Rubus Villosus | IC50 >.4 < .7 mg/ml | |
| F17 | Bladderwrack | Fucus vesiculosus | IC50 >.4 < .7 mg/ml | |
| B26 | Blessed Thistle | Cnicus benedictus | IC50 >.4 < .7 mg/ml | |
| C1 | California Poppy | Eschscholzia californica | IC50 >.4 < .7 mg/ml | |
| C87 | Catnip a | Nepeta cataria | IC50 >.4 < .7 mg/ml | |
| H16 | Chameleon | Houttuynia cordata herb | IC50 >.4 < .7 mg/ml | |
| c80 | Cilantro Leaf | Coriandrum sativum | IC50 >.4 < .7 mg/ml | |
| C39 | Cinnamon Twig | Cinnamomum cassia | IC50 >.4 < .7 mg/ml | |
| C9 | Cleavers | Galium aparine | IC50 >.4 < .7 mg/ml | |
| A49 | Clematis trifoliata | Akebia trifoliata fruit | IC50 >.4 < .7 mg/ml | |
| D1 | Damiana Leaf | Turnera diffusa | IC50 >.4 < .7 mg/ml | |
| E24 | Earlgrey black tea | Earlgrey black tea | IC50 >.4 < .7 mg/ml | |
| E23 | Elder berries | Sambucus nigra | IC50 >.4 < .7 mg/ml | |
| E2 | Epidedium | Epimedium grandiflorum | IC50 >.4 < .7 mg/ml | |
| E15 | Epidedium | Epimedium koreanum | IC50 >.4 < .7 mg/ml | |
| F2 | Feverfew | Tanacetum parthenium | IC50 >.4 < .7 mg/ml | |
| C17 | Garden Chervil | Anthriscus cerefoilium | IC50 >.4 < .7 mg/ml | |
| G30 | German chamomile | Matricaria recutita | IC50 >.4 < .7 mg/ml | |
| G37 | Goats Rue | Galega officinalis | IC50 >.4 < .7 mg/ml | |
| G8 | Great Valley gumweed | Grindelia camporum | IC50 >.4 < .7 mg/ml | |
| L3 | Gromwell root | Lithospermum erythrorhizon root | IC50 >.4 < .7 mg/ml | |
| G4 | Gymnema | Gymnema sylvestre | IC50 >.4 < .7 mg/ml | |
| H3 | Hops, whole | Humulus lupulus | IC50 >.4 < .7 mg/ml | |
| H8 | Horsetail | Equisetum arvense | IC50 >.4 < .7 mg/ml | |
| P14 | Japenese Indigo | Polygonum tinctorium levis | IC50 >.4 < .7 mg/ml | |
| K2 | Kombu | Laminaria setchellii | IC50 >.4 < .7 mg/ml | |
| L6 | Lily of the valley | Convallaria majalis | IC50 >.4 < .7 mg/ml | |
| L23 | Lysimachia | Lysimachia christinae | IC50 >.4 < .7 mg/ml | |
| M32 | Marjoram a | Origanum majorana | IC50 >.4 < .7 mg/ml | |
| M31 | Melilot Herb | Melilotus vulgaris | IC50 >.4 < .7 mg/ml | |
| M34 | Mint | Mentha haplocalyx | IC50 >.4 < .7 mg/ml | |
| M39 | Mistle Toe | Phoradendron flavescens | IC50 >.4 < .7 mg/ml | |
| M8 | Mugwort | Artemisia vulgaris | IC50 >.4 < .7 mg/ml | |
| M36 | Mullein leaf | Verbascum thapsus | IC50 >.4 < .7 mg/ml | |
| P12 | Papaya Leaf | Carica papaya | IC50 >.4 < .7 mg/ml | |
| P5 | Peach leaf | Prunus persica | IC50 >.4 < .7 mg/ml | |
| P81 | Pennyroyal | Mentha pulegium | IC50 >.4 < .7 mg/ml | |
| P56 | Peony | Paeonia suffructicose root - bark | IC50 >.4 < .7 mg/ml | |
| P79 | Peppermint a | Mentha piperita | IC50 >.4 < .7 mg/ml | |
| P88 | Periwinkle | Vinca minor | IC50 >.4 < .7 mg/ml | |
| P3 | Plantain Herb | Plantago asiatica | IC50 >.4 < .7 mg/ml | |
| P85 | Plantain Leaf | Plantago major | IC50 >.4 < .7 mg/ml | |
| R21 | Red rose And Petals | Rosa damascena | IC50 >.4 < .7 mg/ml | |
| R2 | Redroot | Ceanothus americanus | IC50 >.4 < .7 mg/ml | |
| C77 | Senna Leaf | Cassia angustifolia leaf | IC50 >.4 < .7 mg/ml | |
| S19 | Shepards Purse a | Capsella bursa-pastoris | IC50 >.4 < .7 mg/ml | |
| L7 | Spice Bush | Lindera aggregata root | IC50 >.4 < .7 mg/ml | |
| S33 | Spikemoss | Selaginella doederleinii | IC50 >.4 < .7 mg/ml | |
| S60 | Spiralina powder | Arthrospira platensis | IC50 >.4 < .7 mg/ml | |
| S72 | Spirulina | Arthrospira platensis | IC50 >.4 < .7 mg/ml | |
| C40 | Spreading sneezeweed | Centepida mimima | IC50 >.4 < .7 mg/ml | |
| S3 | Strawberry Leaf | Fragaria vesca | IC50 >.4 < .7 mg/ml | |
| S28 | Sweet Grass Braid | Hierochlöe Odorata | IC50 >.4 < .7 mg/ml | |
| T1 | Tansy | Tanacetum vulgare | IC50 >.4 < .7 mg/ml | |
| T5 | Tarragon | Artemisia dracunculus | IC50 >.4 < .7 mg/ml | |
| C95 | Tea | Celyon (black tea) | IC50 >.4 < .7 mg/ml | |
| T2 | Thyme Leaf a | Thymus vulgaris | IC50 >.4 < .7 mg/ml | |
| S68 | Toothache Plant | Spilanthes acmella | IC50 >.4 < .7 mg/ml | |
| S22 | Touch me not | Speranskia tuberculata | IC50 >.4 < .7 mg/ml | |
| T30 | Turkey Rhubarb | Rheum palmatum | IC50 >.4 < .7 mg/ml | |
| V2 | Vervain | Verbena officinalis herb | IC50 >.4 < .7 mg/ml | |
| W16 | White Willow Bark | Salix alba | IC50 >.4 < .7 mg/ml | |
| P73 | Wintergreen | Pyrola calliantha | IC50 >.4 < .7 mg/ml | |
| W8 | Wormwood | Artemisia absinthum | IC50 >.4 < .7 mg/ml | |
| A44 | Wormwood | Artemisia capillaris | IC50 >.4 < .7 mg/ml | |
| LEVEL 5 | A41 | Agrimony | Agrimonia pilosa | IC50≈.7 mg/kg |
| S74 | Alexandrian Senna | Senna alexandrina | IC50≈.7 mg/kg | |
| A6 | Asafoetida | Ferula Assa-Foetida | IC50≈.7 mg/kg | |
| D9 | BAI CAI GAN | Dried Chinese Cabbage | IC50≈.7 mg/kg | |
| B27 | Black Berry Powder | Rubus armeniacus | IC50≈.7 mg/kg | |
| I7 | Black Henna | Indigofera tinctoria | IC50≈.7 mg/kg | |
| C91 | Cayenne powder | Capsicum annuum | IC50≈.7 mg/kg | |
| V6 | Chaste Tree Berry | Vitex agnus-castus | IC50≈.7 mg/kg | |
| C12 | Chickweed | Stellaria media | IC50≈.7 mg/kg | |
| P29 | Chinese Cinquefoil | Potentilla chinensis | IC50≈.7 mg/kg | |
| T24 | Dandelion Root | Taraxacum mongolicum | IC50≈.7 mg/kg | |
| D5 | Dill weed | Anethum graveolens | IC50≈.7 mg/kg | |
| A26 | Dogbane | Apocynum venetum herb | IC50≈.7 mg/kg | |
| F20 | Fennel seed a | Foeniculum vulgare | IC50≈.7 mg/kg | |
| F22 | Fringe Bark Tree | Chionanthus virginicus | IC50≈.7 mg/kg | |
| F23 | Fumitory | Fumaria officinalis | IC50≈.7 mg/kg | |
| G27 | Garcinia cambogia | Garcinia cambogia | IC50≈.7 mg/kg | |
| G23 | Gardenia | Gardenia jasminoides fruit | IC50≈.7 mg/kg | |
| S31 | Japanese Catnip | Schizonepeta tenuifolia | IC50≈.7 mg/kg | |
| P22 | Knotweed Grass | Polygonum aviculare | IC50≈.7 mg/kg | |
| M37 | Malva Flower | Malva sylvestris | IC50≈.7 mg/kg | |
| N11 | Nettle stinging | Urtica dioica | IC50≈.7 mg/kg | |
| O18 | Oatstraw | Avena sativa | IC50≈.7 mg/kg | |
| O14 | Orange peel | Citrus sinensis | IC50≈.7 mg/kg | |
| O12 | Oregano leaf a | Origanum vulgare | IC50≈.7 mg/kg | |
| O2 | Osha Root | Ligusticum porteri | IC50≈.7 mg/kg | |
| P27 | Patrinia | Patrinia villosa herb | IC50≈.7 mg/kg | |
| P28 | Purslane | Portulaca oleracea | IC50≈.7 mg/kg | |
| R8 | Raspberry Leaf | Rubus idaeus | IC50≈.7 mg/kg | |
| H29 | Strawflower | Helichrysum foetidum | IC50≈.7 mg/kg | |
| E25 | Tea | English breakfast black tea | IC50≈.7 mg/kg | |
| I9 | Tea | Irish breakfast green tea | IC50≈.7 mg/kg | |
| S41 | The Holy Herb | Siegesbeckia orientalis | IC50≈.7 mg/kg | |
| P18 | Tongue fern | Pyrrosia Lingua | IC50≈.7 mg/kg | |
| G26 | Wintergreen | Gaultheria procumbens | IC50≈.7 mg/kg |
Botanical Replicates confirmed from various distributors.
Figure 5.
Most potent herbal extract inhibitors of human MAO-B activity. The data represent product formation (H202) as % control produced at 24 h (incubation at RT) in the presence or absence of extracts (.025–.8 mg/ml) and are presented as the Mean, n=4. IC50 concentrations were established from a sigmoidal fit dose–response equation and significance of difference between the controls versus treatment was determined using a one-way ANOVA followed by a Tukey post hoc test * p<.05. This figure is available in colour online at wileyonlinelibrary.com/journal/ptr.
DISCUSSION
In this study, we screened 905 natural product extracts to elucidate potential sources of human MAO-B inhibitors. The data elucidate a number of new potential sources and specify the relatively few that inhibited MAO-B within therapeutic range. The herbal extracts showing greatest potency included Amur Corktree (Phellodendron amurense), Licorice Root (Glycyrrhiza glabra and Glycyrrhiza uralensis), Psoralea Fruit (Psoralea corylifolia (PC)) and Bakuchi Seed (Cyamopsis psoralioides). While it is outside the scope of this paper to review all MAO-B inhibitors identified, we will focus the discussion on the most potent elucidated.
New findings presented from this work show potent MAO-B inhibitory capacity inherent within Bakuchi seed, an otherwise understudied aryurvedic historical medicine most known for treatment of vitiligo, where it is associated with regeneration of melanocytes (Dhanik et al., 2011). It is of interest to note that this plant also contains constituents such as psoralens or hydroxybakuchiol which promote skin pigmentation (Khushboo et al., 2010) augment melanin dispersal (Sultan and Ali, 2011) and augment central DAergic function due to direct inhibition of the DA transporter, making it a likely candidate for treatment of PD (Zhao et al., 2007).
In this study, one of the most interesting findings (also corroborated by others) was the MAO-B inhibitory effects of psoralens or furocoumarins (derived from PC seeds), which impart anti-depressant effects via reduction of stress evoked plasma cortisol (Chen et al., 2005; Chen et al., 2007; Kong et al., 2001) and protect against MPTP-induced DAergic loss within substantia nigra in mice. (Zhao et al., 2009) The anti-depressant properties of PC also correspond to the elevation of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid, as well as rise in striatal DA, effects which are concomittent to reduction of stress-evoked serum corticotropin-releasing factor and adrenal corticotropin-releasing hormone, suggesting a number of neurotransmitter systems are affected. (Yi et al., 2008; Xu et al., 2008).
While future research will have to be initiated on PC and Bakuchi seed, a plethora of existing work demonstrates a range of therapeutic values that exist outside the scope of PD, including a potential to treat cancer and osteoarthritis, (Ming et al., 2011), reduce bone loss (Lim et al., 2009) treat bacterial/viral infections or inflammatory related disease (Szliszka et al., 2011; Yang et al., 2011; Li et al., 2011; Khushboo et al., 2010). In addition, constituents within PC have been well characterized and known to include several classes of compounds; e.g. psoralenoside (benzofuran glycosides), psoralen, isopsoralen, psoralidin (coumarins), bakuchiol (meroterpenes) (Qiu et al., 2011; Song et al., 2011) corylifolean, corylifolin, bakuchicin, isobavachin, bavachinin, bavachalcone, isobavachalcone, corylin, corylidin, bavachromene, astragalin, p-hydroxybenzoic acid stigmasterol, triaconate and β-sitosterol-D-glucoside (Khushboo, Jadhav, 2010).
Glycyrrhiza glabra/uralensis
In a strikingly similar pattern to that of PC, existing research also shows capacity of Glycyrrhiza glabra to reduce stress response in mice through elevating brain concentrations of norepinephrine and DA comparable to that of tricyclic anti-depressant drugs; imipramine and fluoxetine (Dhingra and Sharma, 2006). The data from the current work also corroborate the effects of glycyrrhiza uralensis and glabra roots to have MAO inhibitor capacity with reported IC50 at .03 mg/ml (Tanaka et al., 1987; Hatano et al., 1991a) where the same Hatano et al. also define a number of licorice constituents such as licopyranocoumarin licocoumarone and glycyrrhisoflavone being responsible for MAO inhibition. (Hatano et al., 1991b).
Licorice is a highly studied natural medicine, with therapeutic values ranging from anti-inflammatory properties in microglial BV2 cells, neuroprotective properties against 6-hydroxydopamine cytotoxicity/MPTP nigrostriatal DAergic degeneration in mice (Kim et al., in press) and reduction of glutamate-mediated excitotoxicity associated with hippocampal neuronal cell death.(Yang et al., 2012) Many of these therapeutic properties appear to enhance the case for licorice in therapeutic potential application for PD.
Unlike, other herbs referenced in this study, there is meager research on therapeutic effects or characterization of Amur Corktree Phellodendron amurense in relation to any aspect of DAergic neurotransmission. However, recent interest in this plant surrounds its anti-inflammatory and anti-cancer properties, in addition to preventing osteoarticular cartilage and chondrocyte destruction. (Xian et al., 2011; Kim et al., 2011; Ghosh et al., 2010) Future research will be required to further investigate therapeutic aspects of Amur Corktree in processes inherent to degenerative disease specific to PD.
As a note, in the past, we had investigated a number of compounds for MAO inhibitory activity in foods on a very small scale and at that time had eluded to the fact that green tea catechins and curcumin were likely candidate MAO-B inhibitors.(Mazzio et al., 1998) Since then, a number of studies have examined this and shown green tea components such as epigallocatechin gallate and curcumin supplementation exert reduction of brain MAO-B enzyme activity in rats (Lin et al., 2010), effects which are synergist with rasagiline in PD mice models (Ashizawa and Sano, 1990) and administration which has reversed MPTP induced loss of DA. (Rajeswari and Sabesan, 2008) The findings from this current study broaden our view of potential MAO-B candidates yielding a number of herbs potentially more or equally as promising to green tea or turmeric.
The identification of new natural or synthetic MAO-B inhibitors which provide therapeutic advantage in treatment of PD must be viewed in light of differential selectivity from MAO-A, due to risk of hypertensive crisis in the presence of tyramine containing foods. Although, it is estimated that the adverse effects are less than previously believed (Gillman, 2011), in particular for rasagiline selectivity when used at the recommended dose (Chen and Wilkinson, 2012; Isaacson, 2010) (Goren et al., 2010), future potential drug development should include a tyramine challenge control for in vivo animal studies.
In conclusion, the findings from this paper open up new areas for future research specifying specific natural plants with capacity to inhibit MAO-B, that could lead to medicines with greater capacity to treat diseases associated with maladaptive DAergic function with in the CNS.
Acknowledgments
This research was supported by a grant from NIH NCRR RCMI program (G12RR 03020) and the National Institute of Minority Health and Health Disparities, NIH (8G12MD007582-28 and 1P20 MD006738-01).
Footnotes
Conflict of Interest
The authors have declared that there is no conflict of interest.
References
- Anderson DG, Mariappan SV, Buettner GR, Doorn JA. Oxidation of 3,4-dihydroxyphenylacetaldehyde, a toxic dopaminergic metabolite, to a semiquinone radical and an orthoquinone. J Biol Chem. 2011;286:26978–86. doi: 10.1074/jbc.M111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashizawa K, Sano R. Effects of temperature on the immobilization and the initiation of motility of spermatozoa in the male reproductive tract of the domestic fowl, Gallus domesticus. Comp Biochem Physiol A Comp Physiol. 1990;96:297–301. doi: 10.1016/0300-9629(90)90696-p. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Wilkinson JR. The Monoamine Oxidase Type B Inhibitor Rasagiline in the Treatment of Parkinson Disease: Is Tyramine a Challenge? J Clin Pharmacol. 2012;52:620–8. doi: 10.1177/0091270011406279. [DOI] [PubMed] [Google Scholar]
- Chen Y, Kong LD, Xia X, Kung HF, Zhang L. Behavioral and biochemical studies of total furocoumarins from seeds of Psoralea corylifolia in the forced swimming test in mice. J Ethnopharmacol. 2005;96:451–9. doi: 10.1016/j.jep.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang HD, Xia X, Kung HF, Pan Y, Kong LD. Behavioral and biochemical studies of total furocoumarins from seeds of Psoralea corylifolia in the chronic mild stress model of depression in mice. Phytomedicine. 2007;14:523–9. doi: 10.1016/j.phymed.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Dhanik A, Sujatha N, Rai NP. Clinical evaluation of the efficacy of Shvitrahara kashaya and lepa in vitiligo. Ayu. 2011;32:66–9. doi: 10.4103/0974-8520.85731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra D, Sharma A. Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog Neuropsychopharmacol Bol Psychiatr. 2006;30:449–54. doi: 10.1016/j.pnpbp.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Drozak J, Kozlowski M. Monoamine oxidase as a target for drug action. Postepy Hig Med Dosw (Online) 2006;60:498–515. [PubMed] [Google Scholar]
- Fedorova NV, Tekaeva FK, Bel’gusheva ME. The role of the MAO-B inhibitor razagiline in the treatment of Parkinson’s disease. Zh Nevrol Psikhiatr Im S S Korsakova. 2011;111:30–6. [PubMed] [Google Scholar]
- Ghosh R, Graham H, Rivas P, et al. Phellodendron amurense bark extract prevents progression of prostate tumors in transgenic adenocarcinoma of mouse prostate: potential for prostate cancer management. Anticancer Res. 2010;30:857–65. [PubMed] [Google Scholar]
- Gillman PK. Advances pertaining to the pharmacology and interactions of irreversible nonselective monoamine oxidase inhibitors. J Clin Psychopharmacol. 2011;31:66–74. doi: 10.1097/JCP.0b013e31820469ea. [DOI] [PubMed] [Google Scholar]
- Goren T, Adar L, Sasson N, Weiss YM. Clinical pharmacology tyramine challenge study to determine the selectivity of the monoamine oxidase type B (MAO-B) inhibitor rasagiline. J Clin Pharmacol. 2010;50:1420–8. doi: 10.1177/0091270010369674. [DOI] [PubMed] [Google Scholar]
- Hatano T, Fukuda T, Liu YZ, Noro T, Okuda T. Phenolic constituents of licorice. IV. Correlation of phenolic constituents and licorice specimens from various sources, and inhibitory effects of licorice extracts on xanthine oxidase and monoamine oxidase. Yakugaku Zasshi. 1991a;111:311–21. doi: 10.1248/yakushi1947.111.6_311. [DOI] [PubMed] [Google Scholar]
- Hatano T, Fukuda T, Miyase T, Noro T, Okuda T. Phenolic constituents of licorice. III. Structures of glicoricone and licofuranone, and inhibitory effects of licorice constituents on monoamine oxidase. Chem Pharm Bull(Tokyo) 1991b;39:1238–43. doi: 10.1248/cpb.39.1238. [DOI] [PubMed] [Google Scholar]
- Holt A, Palcic MM. A peroxidase-coupled continuous absorbance plate-reader assay for flavin monoamine oxidases, copper-containing amine oxidases and related enzymes. Nat Protoc. 2006;1:2498–505. doi: 10.1038/nprot.2006.402. [DOI] [PubMed] [Google Scholar]
- Horn PJ, Reti I, Jayaram G. Transdermal selegiline in patients receiving electroconvulsive therapy. Psychosomatics. 2010;51:176–8. doi: 10.1176/appi.psy.51.2.176. [DOI] [PubMed] [Google Scholar]
- Isaacson SH. Selective MAO-B inhibitors have low potential for the tyramine effect. Mov Disord. 2010;25:123–4. doi: 10.1002/mds.22334. [DOI] [PubMed] [Google Scholar]
- Jinsmaa Y, Florang VR, Rees JN, et al. Dopamine-derived biological reactive intermediates and protein modifications: Implications for Parkinson’s disease. Chem Biol Interact. 2011;192:118–21. doi: 10.1016/j.cbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassubek J, Buttner T, Reichmann H, et al. On the role of MAO B inhibitors and NMDA antagonists in the therapy of Parkinson’s disease. Fortschr Neurol Psychiatr. 2010;78(Suppl 1):S34–6. doi: 10.1055/s-0029-1245166. [DOI] [PubMed] [Google Scholar]
- Khushboo PS, Jadhav VM, Kadam VJ, Sathe NS. Psoralea corylifolia Linn.-“Kushtanashini”. Pharmacognosy Rev. 2010;4:69–76. doi: 10.4103/0973-7847.65331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Huh JE, Baek YH, Lee JD, Choi DY, Park DS. Effect of Phellodendron amurense in protecting human osteoarthritic cartilage and chondrocytes. J Ethnopharmacol. 2011;134:234–42. doi: 10.1016/j.jep.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Kim SS, Lim J, Bang Y, et al. Licochalcone E activates Nrf2/antioxidant response element signaling pathway in both neuronal and microglial cells: therapeutic relevance to neurodegenerative disease. J Nutr Biochem. doi: 10.1016/j.jnutbio.2011.07.012. in press. [DOI] [PubMed] [Google Scholar]
- Kong LD, Tan RX, Woo AY, Cheng CH. Inhibition of rat brain monoamine oxidase activities by psoralen and isopsoralen: implications for the treatment of affective disorders. Pharmacol Toxicol. 2001;88:75–80. doi: 10.1034/j.1600-0773.2001.d01-86.x. [DOI] [PubMed] [Google Scholar]
- Li X, Lee YJ, Kim YC, et al. Bakuchicin induces vascular relaxation via endothelium-dependent NO-cGMP signaling. Phytother Res. 2011;25:1574–8. doi: 10.1002/ptr.3478. [DOI] [PubMed] [Google Scholar]
- Lim SH, Ha TY, Kim SR, Ahn J, Park HJ, Kim S. Ethanol extract of Psoralea corylifolia L. and its main constituent, bakuchiol, reduce bone loss in ovariectomised Sprague-Dawley rats. Br J Nutr. 2009;101:1031–9. doi: 10.1017/S0007114508066750. [DOI] [PubMed] [Google Scholar]
- Lin SM, Wang SW, Ho SC, Tang YL. Protective effect of green tea (−)-epigallocatechin-3-gallate against the monoamine oxidase B enzyme activity increase in adult rat brains. Nutrition. 2010;26:1195–200. doi: 10.1016/j.nut.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Lohle M, Reichmann H. Controversies in neurology: why monoamine oxidase B inhibitors could be a good choice for the initial treatment of Parkinson’s disease. BMC Neurol. 2011;11:112. doi: 10.1186/1471-2377-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallajosyula JK, Kaur D, Chinta SJ, et al. MAO-B elevation in mouse brain astrocytes results in Parkinson’s pathology. PLoS One. 2008;3:e1616. doi: 10.1371/journal.pone.0001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel SA, Sagi Y, Amit T. Rasagiline promotes regeneration of substantia nigra dopaminergic neurons in post-MPTP-induced Parkinsonism via activation of tyrosine kinase receptor signaling pathway. Neurochem Res. 2007;32:1694–9. doi: 10.1007/s11064-007-9351-8. [DOI] [PubMed] [Google Scholar]
- Mazzio EA, Harris N, Soliman KF. Food constituents attenuate monoamine oxidase activity and peroxide levels in C6 astrocyte cells. Planta Med. 1998;64:603–6. doi: 10.1055/s-2006-957530. [DOI] [PubMed] [Google Scholar]
- Ming LG, Cheng KM, Ge BF, Ma HP, Zai YK. Effect of isopsoralen on the proliferation and differentiate of osteoblasts in vitro. Zhong yao cai. 2011;34:404–8. [PubMed] [Google Scholar]
- Naoi M, Maruyama W. Functional mechanism of neuroprotection by inhibitors of type B monoamine oxidase in Parkinson’s disease. Expert Rev Neurother. 2009;9:1233–50. doi: 10.1586/ern.09.68. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga J, Kulisevsky J. Rasagiline: effectiveness and protection in Parkinson’s disease. Rev Neurol. 2010;51:535–41. [PubMed] [Google Scholar]
- Polanski W, Reichmann H, Gille G. Stimulation, protection and regeneration of dopaminergic neurons by 9-methyl-beta-carboline: a new anti-Parkinson drug? Expert Rev Neurother. 2011;11:845–60. doi: 10.1586/ern.11.1. [DOI] [PubMed] [Google Scholar]
- Qiu RL, Li L, Zhu MH, Liu J. Study on the chemical constituents of Psoralea corylifolia Zhong yao cai = Zhongyaocai = J Chinese Medicinal Mater. 2011;34:1211–3. [PubMed] [Google Scholar]
- Rajeswari A, Sabesan M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson’s disease induced by MPTP neurodegeneration in mice. Inflammopharmacology. 2008;16:96–9. doi: 10.1007/s10787-007-1614-0. [DOI] [PubMed] [Google Scholar]
- Rascol O, Lozano A, Stern M, Poewe W. Milestones in Parkinson’s disease therapeutics. Mov Disord. 2011;26:1072–82. doi: 10.1002/mds.23714. [DOI] [PubMed] [Google Scholar]
- Siddiqui A, Mallajosyula JK, Rane A, Andersen JK. Ability to delay neuropathological events associated with astrocytic MAO-B increase in a Parkinsonian mouse model: implications for early intervention on disease progression. Neurobiol Dis. 2010;40:444–8. doi: 10.1016/j.nbd.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Qi A, Wang Y, Jing Y, Chai X, Liu Y. Variation of 4 kinds of compounds in Psoralea corylifolia processed by different methods. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China. J Chinese Materia Medica. 2011;36:2071–5. [PubMed] [Google Scholar]
- Sultan T, Ali SA. Psoralea corylifolia extracts stimulate cholinergic-like psoralen receptors of tadpole-tail melanophores, leading to skin darkening. J Recept Signal Transduct Res. 2011;31:39–44. doi: 10.3109/10799893.2010.508164. [DOI] [PubMed] [Google Scholar]
- Szliszka E, Skaba D, Czuba ZP, Krol W. Inhibition of inflammatory mediators by neobavaisoflavone in activated RAW264.7 macrophages. Molecules. 2011;16:3701–12. doi: 10.3390/molecules16053701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talati R, Reinhart K, Baker W, White CM, Coleman CI. Pharmacologic treatment of advanced Parkinson’s disease: a meta-analysis of COMT inhibitors and MAO-B inhibitors. Parkinsonism Relat Disord. 2009;15:500–5. doi: 10.1016/j.parkreldis.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kuwai Y, Tabata M. Isolation of monoamine oxidase inhibitors from Glycyrrhiza uralensis roots and the structure-activity relationship. Planta Med. 1987;53:5–8. doi: 10.1055/s-2006-962604. [DOI] [PubMed] [Google Scholar]
- van Laar T, Boon AJ, Bloem BR. Rasagiline is not for all Parkinson disease patients: the ADAGIO study. Ned Tijdschr Geneeskd. 2010;154:A2496. [PubMed] [Google Scholar]
- Wang YH, Samoylenko V, Tekwani BL, et al. Composition, standardization and chemical profiling of Banisteriopsis caapi, a plant for the treatment of neurodegenerative disorders relevant to Parkinson’s disease. J Ethnopharmacol. 2010;128:662–71. doi: 10.1016/j.jep.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb O, Amit T, Bar-Am O, Youdim MB. Rasagiline: a novel anti-Parkinsonian monoamine oxidase-B inhibitor with neuroprotective activity. Prog Neurobiol. 2010;92:330–44. doi: 10.1016/j.pneurobio.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Xian YF, Mao QQ, Ip SP, Lin ZX, Che CT. Comparison on the anti-inflammatory effect of Cortex Phellodendri Chinensis and Cortex Phellodendri Amurensis in 12-O-tetradecanoyl-phorbol-13-acetate-induced ear edema in mice. J Ethnopharmacol. 2011;137:1425–30. doi: 10.1016/j.jep.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Xu Q, Pan Y, Yi LT, et al. Antidepressant-like effects of psoralen isolated from the seeds of Psoralea corylifolia in the mouse forced swimming test. Biol Pharm Bull. 2008;31:1109–14. doi: 10.1248/bpb.31.1109. [DOI] [PubMed] [Google Scholar]
- Yang EJ, Min JS, Ku HY, et al. Isoliquiritigenin isolated from Glycyrrhiza uralensis protects neuronal cells against glutamate-induced mitochondrial dysfunction. Biochem Biophys Res Commun. 2012;421:658–64. doi: 10.1016/j.bbrc.2012.04.053. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Youn H, Seong KM, et al. Psoralidin, a dual inhibitor of COX-2 and 5-LOX, regulates ionizing radiation (IR)-induced pulmonary inflammation. Biochem Pharmacol. 2011;82:524–34. doi: 10.1016/j.bcp.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Yi LT, Li YC, Pan Y, et al. Antidepressant-like effects of psoralidin isolated from the seeds of Psoralea Corylifolia in the forced swimming test in mice. Prog Neuropsychopharmacol Bol Psychiatr. 2008;32:510–9. doi: 10.1016/j.pnpbp.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Zhao G, Li S, Qin GW, Fei J, Guo LH. Inhibitive effects of Fructus Psoraleae extract on dopamine transporter and noradrenaline transporter. J Ethnopharmacol. 2007;112:498–506. doi: 10.1016/j.jep.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Zhao G, Zheng XW, Qin GW, Gai Y, Jiang ZH, Guo LH. In vitro dopaminergic neuroprotective and in vivo antiparkinsonian-like effects of Delta 3,2-hydroxybakuchiol isolated from Psoralea corylifolia (L.) Cell Mol Life Sci. 2009;66:1617–29. doi: 10.1007/s00018-009-9030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]