Abstract
In 1994 the first heat shock protein 90 (Hsp90) inhibitor was identified and Hsp90 was reported to be a target for anticancer therapeutics. In the past 18 years there have been 17 distinct Hsp90 inhibitors entered into clinical trial, and the small molecule Hsp90 inhibitors have been highly valuable as probes of the role of Hsp90 and its client proteins in cancer. Although no Hsp90 inhibitor has achieved regulatory approval, recently there has been significant progress in Hsp90 inhibitor clinical development, and in the past year RECIST responses have been documented in HER2-positive breast cancer and EML4-ALK-positive non-small cell lung cancer. All of the clinical Hsp90 inhibitors studied to date are specific in their target, i.e. they bind exclusively to Hsp90 and two related heat shock proteins. However, Hsp90 inhibitors are markedly pleiotropic, causing degradation of over 200 client proteins and impacting critical multiprotein complexes. Furthermore, it has only recently been appreciated that Hsp90 inhibitors can, paradoxically, cause transient activation of the protein kinase clients they are chaperoning, resulting in initiation of signal transduction and significant physiological events in both tumor and tumor microenvironment. An additional area of recent progress in Hsp90 research is in studies of the posttranslational modifications of Hsp90 itself and Hsp90 co-chaperone proteins. Together, a picture is emerging in which the impact of Hsp90 inhibitors is shaped by the tumor intracellular and extracellular milieu, and in which Hsp90 inhibitors impact tumor and host on a microenvironmental and systems level. Here we review the tumor intrinsic and extrinsic factors that impact the efficacy of small molecules engaging the Hsp90 chaperone machine.
Keywords: Heat shock protein 90, host, postranslational, phosphorylation, reactive oxygen species, stroma, systems
1. INTRODUCTION
Hsp90 was identified as a molecular target for anticancer therapeutics in 1994 [1]. Five years later the first Hsp90 inhibitor entered clinical trial. To date over a thousand patients have been enrolled in clinical trials of 17 distinct Hsp90 inhibitors, but as yet no Hsp90 inhibitor has achieved FDA approval [2-5]. Recently, however, several studies have demonstrated promising clinical activity and registration strategies are becoming apparent. Not surprisingly, patient selection based on tumor molecular characterization indicating heightened dependence on Hsp90 and sensitivity to Hsp90 inhibition is proving fruitful, as exemplified by RECIST responses in a recent clinical trial of NSCLC patients with EML4-ALK mutations treated with Hsp90 inhibitor STA-9090 monotherapy, and HER2-positive breast cancer patients progressing on trastuzmab treated with the Hsp90 inhibitor 17-AAG plus trastuzumab [6-9]. Another strategy that has shown promising results is the combination of Hsp90 inhibitor with the proteasome inhibitor bortezomib in multiple myeloma [10, 11], where the therapeutic effect may be due at least in part to induction of severe proteotoxic stress [12-14].
For approximately the first 12 years after discovery of Hsp90 as a molecular target a significant number of Hsp90 publications were devoted to identification of individual client proteins, of which there are now well over 200 (for a continually updated list see http://www.picard.ch/downl-loads), and to use of an Hsp90 inhibitor to probe individual client protein function. More recently, it has become apparent that Hsp90 is a key regulator of cell biology at the systems level [15, 16], and new functions for Hsp90 are being discovered, including, for example, the role of HSC70/Hsp90 chaperone machinery in RNA-induced silencing complex (RISC) assembly [17, 18], which may intersect with other global regulatory networks [19]. Moreover, although Hsp90 clients have been generally defined as proteins whose dissociation from Hsp90 results in their degradation, it is now appreciated that some Hsp90 interactors (e.g. heat shock factor 1 [HSF1]) are not degraded upon drug-induced dissociation from Hsp90 (see later in this review and [5] for further details). In the case of some Hsp90 clients, including steroid hormone receptors and some kinases, loss of signaling activity in response to Hsp90 inhibition precedes protein degradation, suggesting that measurement of steady state client protein expression alone may not be the most sensitive or accurate indicator of drug response [5].
In some important respects, Hsp90 is a compelling and potentially ideal target for anticancer drug development. Small molecules that bind to the Hsp90 N-terminal ATP/ADP binding site, including all of the clinical Hsp90 inhibitors, cause degradation of virtually all proteins chaperoned by Hsp90, albeit with vastly differing kinetics. The list of Hsp90 client proteins that are degraded in response to an Hsp90 inhibitor include many oncogenic fusion proteins, mutated and activated serine/threonine protein kinases, tyrosine kinases, and transcription factors with oncogenic activity [2, 20-22]. Many of the compensatory mechanisms used by tumors to overcome targeted and cytotoxic therapy are vulnerable to Hsp90 inhibition [5]. This review addresses some of the potential reasons why Hsp90 inhibitors were not more rapidly successful in the clinic, and how advances in our understanding of the remarkably complex Hsp90 machinery can promote development of this class of therapeutics.
1.1. The Hsp90 Family
In humans there are two Hsp90 isoforms, stress-induced Hsp90α and constitutively expressed Hsp90β. The two isoforms, which have 85% sequence identity, are thought to be the result of a gene duplication event that occurred over a million years ago. Hsp90 functions as a dimer. Although both Hsp90α and Hsp90β are highly expressed in all cell types investigated, in the systems studied thus far it appears that Hsp90 tends to form homodimers. Little is known about functional differences between Hsp90α and Hsp90β. Interestingly, as discussed later, Hsp90α and Hsp90β differ in posttranslational modifications, in some cases even when the same amino acid is present in both isoforms. The very recent availability of a number of antibodies that recognize posttranslational modifications of specific Hsp90α and Hsp90β residues is providing critical tools for revealing α- and β-specific functions. In yeast it has been possible to knockout Hsp90 and replace it with either Hsp90α or Hsp90β. Certain clients were found to be more efficiently activated with Hsp90α, while expression of Hsp90β as the sole isoform was associated with increased sensitivity to the Hsp90 inhibitor radicicol [23]. Data suggest that changes in the ratio of Hsp90α and Hsp90β, such as may be seen after stress or malignant transformation, may also regulate sensitivity to Hsp90 inhibitor therapy [23].
In humans there are two additional members of the Hsp90 family: TNF receptor-associated protein 1 (TRAP1), which is a mitochondrial chaperone [24], and glucose-regulated protein 94 (GRP94), an endoplasmic reticulum (ER)-localized chaperone. It is thought that the clinical Hsp90 inhibitors do not enter mitochondria and thus do not appreciably affect TRAP1 in vivo, although this has only been rigorously evaluated for the benzoquinone ansamycin class of drugs [25]. In contrast, Hsp90 associates with the cytoplasmic domains of the two kinases that act as sensors of ER stress, IRE1 and PERK, and this interaction is required for IRE1 and PERK stability. Hsp90 inhibitor treatment induces the unfolded protein response (UPR), and this induction is dependent on binding to the Hsp90 paralog GRP94. Due to accompanying Hsp90-mediated degradation of IRE1 and PERK, the UPR induction is transient, but it can be sufficient to initiate significant signaling [26].
1.2. Hsp90 Structure, Co-chaperones, and the Hsp90 Chaperone Cycle
Hsp90 is composed of an N-terminal domain of ~ 25 kDa, containing a nucleotide/drug binding site, sites for association of a subset of co-chaperones, and part of a bipartite ATPase. An unstructured, charged-linker of species-specific length that plays a role in Hsp90 secretion and chaperone function bridges the N-terminal domain to the ~ 40 kDa middle domain, which contains sites for association of co-chaperones and client proteins and the second part of the bipartite ATPase motif. Lastly, a C-terminal domain of ~ 12 kDa, contains the dimerization domain, a second nucleotide binding site, and a MEEVD pentapeptide that is a tetratricopeptide repeat (TPR) motif recognition site engaged in binding co-chaperones including p60Hop, PP5, Chip, and the immunophilins FKBP51 and FKBP52 [27-31].
Hsp90 and associated nucleotides, co-chaperones and clients, which together are known as the Hsp90 machine, cycle through a variety of conformational states [32, 33]. ATP binds when the N-termini are in an undimerized, open conformation. ATP binding promotes repositioning of an N-terminal lid segment, which leads to transient dimerization of the N domains. Subsequent conformational changes bring together the two parts of the ATPase motif to create a functional unit associated with a closed and twisted conformation of the Hsp90 dimer. Upon ATP hydrolysis, ADP is subsequently released and the Hsp90 dimer returns to the open or apo conformation [34].
For the Hsp90 machine to perform its function in regulating protein folding and stability, active cycling through various open and closed conformational states and ATP binding and hydrolysis are required. The N-terminal Hsp90 inhibitors disrupt the chaperone cycle by replacing ATP in the N-terminal nucleotide-binding pocket. A number of co-chaperones influence Hsp90 conformational equilibrium, or the dwell time in each conformational state. The co-chaperone activator of Hsp90 ATPase 1 (Aha1) enhances the rate of ATP hydrolysis by increasing the rate of conformational alterations that result in a functional ATPase. In contrast, the co-chaperones Hsp70-Hsp90 organizing protein (Hop) also known as STIP1 or STI1 (stress inducible protein 1), and cell division cycle 37 homolog (Cdc37) exert opposing activity to Aha1 by preventing the initial structural changes required for N-terminal dimerization, while the co-chaperone prostaglandin E synthase 3 (PTGES3 or p23) stabilizes the closed and twisted conformation committed to ATP hydrolysis and slows the ATPase cycle [35-48].
Some co-chaperones can function as adaptors that deliver specific clients to Hsp90, including Cdc37, which delivers protein kinase clients, and Hop, which participates in delivery of steroid hormone receptor clients. Steroid hormone receptor clients are associated with additional co-chaperones, including FKBP51 and FKBP52. There are Hsp90 co-chaperones that have catalytic functions, including the serine/threonine phosphatase PP5, the peptidyl prolyl isomerases FKBP51, FKBP52 and cyp40, and the E3 ubiquitin ligase carboxyl terminus of the Hsc70-interacting protein (CHIP). CHIP-dependent ubiquitination of clients such as HER2 directs the client to the proteasome for degradation [49]. CHIP was the first E3 ubiquitin ligase reported to be associated with Hsp90 [50]. Subsequently the Cullin family protein Cul5 was shown to act as an Hsp90-associated E3 promoting degradation of clients HER2 and HIF-1α [51]. Many Hsp90 co-chaperones have other roles that do not require association with Hsp90, such as CHIP, which can regulate Hsp70 induction and degradation in response to stress, and ubiquitinate and sort newly synthesized Hsp90-unchaperoned clients to the proteasome [52], and Cdc37, which can function as a molecular chaperone in the absence of Hsp90 [40, 53].
2. HSP90 AND COCHAPERONE POSTRANSLATIONAL MODIFICATIONS AND IMPACT ON HSP90 INHIBITOR RESPONSE
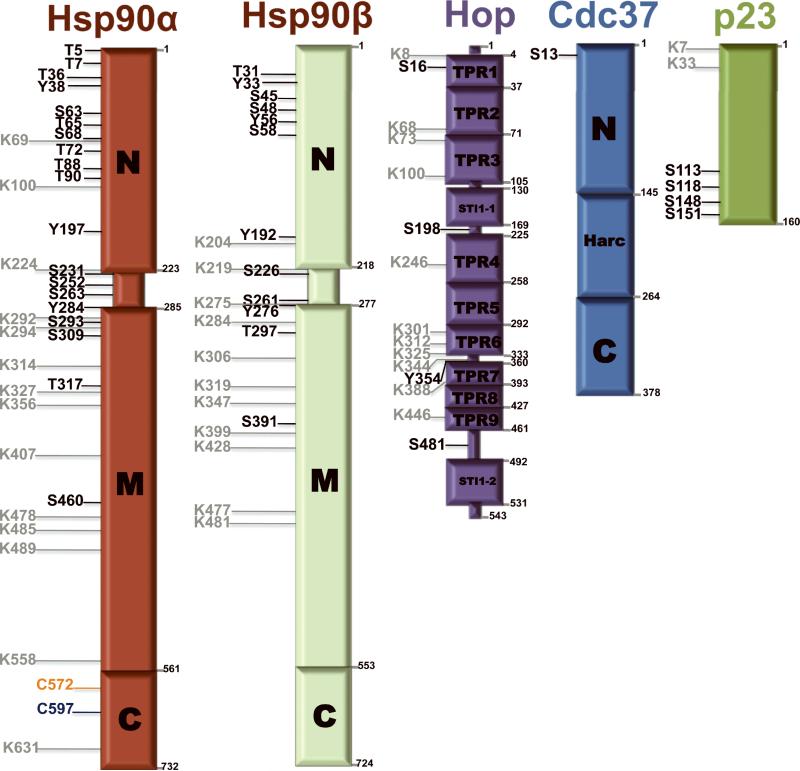
Hsp90 is posttranslationally modified by serine-, threonine- and tyrosine-phosphorylation, lysine acetylation, ubiquitination and methylation, and several cysteine modifications including oxidation and S-nitrosylation [54]. The amino acid residues on Hsp90α modified by each of these posttranslational events are shown in Figure 1. Hsp90β is also the substrate for an array of posttranslational modifications, some overlapping with Hsp90α and some distinct (Fig. 1). Many Hsp90 co-chaperones are also heavily post-translationally modified (see Fig. 1). The great majority of these site-specific modifications in components of the Hsp90 machine have been identified in the past 5 years by proteomic analyses, and functional consequences of these modifications have been reported for only a minority of sites. However, a number of in depth studies of individual modifications have demonstrated important modulation of Hsp90 activity in response to site-specific posttranslational events and have led to the recognition that posttranslational modifications provide an additional layer of regulation of Hsp90 function in eukaryotes.
Figure 1.
Post-translationally modified residues on Hsp90 and co-chaperones, HOP, Cdc37and p23. Phosphorylation of serine (S), theronine (T) and tyrosine (Y) sites are denoted in black, acetylated lysine (K) residues are denoted in gray, S-nitrosylated cysteine (C) residues are denoted in blue, and cysteine oxidation sites are denoted in orange. These residues are based on published data.
2.1. Hsp90 Phosphorylation
The first report of a posttranslational modification of Hsp90 was in the 1981 study of Brugge et al., which identified phosphate residues in eight tryptic peptides of chick Hsp90 [55]. In 1985 Anderson and colleagues reported that double-stranded DNA PK induced phosphorylation of threonine 5 and 7 of Hsp90α, and in 1989 they reported phosphorylation of S231 and S264 catalyzed by casein kinase II [56-58]. A long interval followed these studies before further site-specific phosphorylations were identified. Recently, several Hsp90 phosphosite-specific antibodies have become available, which will accelerate advances in the field. The impact of phosphorylation has been reviewed recently [54], and here we provide an overview of studies that have identified regulated Hsp90 phosphorylation and assessed its functional significance.
2.1.1 Hsp90 Serine/Threonine Phosphorylation
An early study of the impact of the serine/threonine phosphatase inhibitor okadaic acid demonstrated that okadaic acid induces Hsp90 hyperphosphorylation and loss of avidity for its client kinase p60vsrc, suggesting a role for Hsp90 phosphorylation in regulating its chaperone activity [59]. Consistent with this observation, PP5, a serine/threonine phosphatase that contains a TPR domain and binds to the Hsp90 C-terminal EEVD sequence, dephosphorylates Hsp90 in vitro and regulates its chaperone function [60].
In aortic endothelial cells of hyperglycemic animals protein kinase A phosphorylates Hsp90α at T90, which is associated with Hsp90 translocation to the plasma membrane. This has been proposed to limit the availability of Hsp90 for chaperoning eNOS, providing a mechanism for the low levels of nitric oxide associated with diabetes mellitus [61]. PKA-mediated T90 phosphorylation and PP5-catalyzed T90 dephosphorylation have also been shown to regulate Hsp90 secretion [62], as well as other aspects of Hsp90 function [63].
2.1.2 Hsp90 Tyrosine Phosphorylation
Budding yeast possess only one true tyrosine kinase, Swe1, which is the homologue of human Wee1, a key regulator of the G2/M transition. The expression of only one tyrosine kinase facilitated discovery that Swe1 and Wee1 phosphorylate Hsp90 at a single residue (yeast Y24/human Y38), that this phosphorylation enhances Hsp90 binding and stability of Swe1/Wee1 and a subset of other protein kinase clients but not nuclear receptor clients, and that Y24/Y38 tyrosine phosphorylation status affects Hsp90 inhibitor sensitivity [64]. These data suggest a strategy for enhancing efficacy of Hsp90 inhibitors, particularly in the setting of activated Wee1, is combination therapy with a Wee1 inhibitor [65].
Vascular endothelial growth factor receptor-2 (VEGFR-2) activation stimulates c-Src-catalyzed phosphorylation of Hsp90β Y300. This results in enhanced association of Hsp90 and eNOS, eNOS activation, and increased production and release of nitric oxide, which contributes to VEGF proangiogenic activity [66].
2.2. Hsp90 Lysine Acetylation
Hsp90 is acetylated in response to various histone deacetylase inhibitors (HDACi) including trichostatin A and the clinical HDACi romidepsin and panobinostat. Although all of the histone acetyltransferases and histone deacetylases that can modulate Hsp90 acetylation have not been definitively defined, extensive data indentify HDAC6 as an Hsp90 deacetylase, while other studies indicate HDACs 1 and 10 also have Hsp90 deacetylase activity [54]. Hsp90 acetylation decreases ATP binding to Hsp90 and affects interaction with and stability of various Hsp90 clients, including HER2, mutant p53, Raf-1, and androgen receptor [67, 68]. Thus, HDACi that cause hyperacetylation of Hsp90 mimic features of Hsp90 inhibitors, and may exert part of their therapeutic activity via inhibition of Hsp90 chaperone activity [69].
2.3. Hsp90 S-Nitrosylation
Cysteine-597 in the C-terminal domain of Hsp90 is a target for modification by S-nitrosylation, a covalent attachment of nitrogen monoxide to the cysteine thiol side chain [70]. In endothelial cells nitric oxide (NO) causes S-nitrosylation of Hsp90α and inhibits its chaperone activity. NO is produced in endothelial cells by eNOS (endothelial cell nitric oxide synthase), which is an Hsp90 client and requires Hsp90 for activity. It has been proposed that NO-associated S-nitrosylation and inactivation of Hsp90 may serve as a feedback control of NO production in situations such as shear stress where overproduction of NO may be harmful to endothelial cells [70].
S-nitrosylation inhibits Hsp90 ATPase, demonstrating that an environmental cue such as NO production can exert an effect in a distant Hsp90 domain [70, 71]. This propagation of signaling between the C-terminal and N-terminal regions has also been observed using fluorescence-based single molecule assays that demonstrated that nucleotides bound to the N-terminal domain modulate opening and closing of the C-terminal dimer, which had previously been thought to be maintained in a non-dynamic, dimerized state [31].
2.4. Hsp90 Oxidation
Treatment of the breast cancer cell line MDA-MB-231 with the withanolide tubocapsenolide causes direct thiol oxidation of Hsp90, forming intermolecular disulfide bonds and inactivating Hsp90 chaperone activity, leading to client protein degradation and apoptosis [72].
Oxidative stress-induced lipid peroxidation leads to increased concentrations of thiol-reactive aldehydes including 4-hydroxy-2-nonenal (4-HNE) and 4-oxo-2-nonenal (4-ONE). Proteomic analysis of liver samples after ethanol-induced oxidative stress demonstrated modification of Hsp90 with 4-HNE and 4-ONE and inactivation Hsp90 chaperone activity. Liquid chromatography/tandem mass spectrometry analysis of Hsp90 trypic digests identified cysteine 572 as a site for 4-HNE-associated thiol modification and chaperone dysregulation [73].
2.5. Hsp90 Ubiquitination
The ubiquitin ligase C-terminus of Hsc70 interacting protein (CHIP) associates with both Hsp70 and Hsp90. It interacts with the TPR acceptor site of Hsp90, incorporates into Hsp90 heterocomplexes, and induces ubiquitination of a subset of Hsp90 clients. CHIP remodels Hsp90 complexes to favor protein degradation, and thus modulates triage of proteins among mature and functional, improperly folded, and targeted for degradation. Hsp90 itself is ubiquitinated on multiple residues by CHIP and targeted to the proteasome [74]. Lysine residues ubiquitinated by CHIP on Hsp90 were mapped and 13 sites were identified, (K107, K204, K219, K275, K284, K347, K399, K477, K481, K538, K550, K607, and K623). CHIP forms polyubiquitin chains on Hsp70 and Hsp90 linked via K6, K11, K48 and K63 [75].
The photodynamic signal transduction inhibitor and anticancer drug hypericin induces Hsp90 client protein degradation, similarly to other Hsp90 inhibitors. However, unlike N-terminal Hsp90 inhibitors hypericin causes increased cell volume, multinucleation, and mitotic cell death. Examination of the mechanism of action of this drug revealed that hypericin causes Hsp90 ubiquitination and degradation [76].
The anticancer drug docetaxol has antiangiogenic activity associated with inhibition of VEGF signaling. At lower concentrations than required for cell cycle arrest or apoptosis, docetaxol inhibits VEGF-stimulated endothelial cell migration and VEGF-stimulated phosphorylation and activation of three Hsp90 clients that contribute to angiogenesis, focal adhesion kinase, Akt and eNOS. The inhibition of Hsp90 chaperone activity is associated with docetaxol-stimulated Hsp90 ubiquitination and degradation [77].
Finally, Wee1-mediated Hsp90 tyrosine phosphorylation in yeast leads to Hsp90 polyubiquitination and subsequent proteasomal degradation, emphasizing that cross-talk between two distinct Hsp90 post-translational modifications can affect Hsp90 function [64].
2.6. Hsp90 Methylation
Hsp90 interacts with the TPR domain of the lysine methyltransferases SMYD (SET and MYND domain) 2 and SMYD3. SMYD2 methylates Hsp90 K209, in the nucleotide binding domain, and K615 in the dimerization domain [78, 79]. Each of these sites, although methylated by the same methyltransferase, is regulated independently by co-chaperones, pH, and the demethylase LSD1. Numerous SMYD proteins interact with Hsp90, suggesting the possibility that Hsp90 may contribute to regulation of the cell methylome [78].
2.7. Co-chaperone Phosphorylation
Although co-chaperones regulate Hsp90 function, they also are subject to post-translational modification, including acetylation and phosphorylation (see Fig. 1). These modifications provide an additional layer of control over Hsp90 activity. The co-chaperones Cdc37 and PP5/Ppt1 form complexes with Hsp90 in yeast and in human tumor cells. The protein phosphatase PP5/Ppt1 dephosphorylates Ser-13 on Cdc37 in vivo, directly affecting its interaction with Hsp90 and negatively impacting the chaperoning of numerous kinase clients by the Hsp90-Cdc37 complex [48]. Serine phosphorylation of Cdc37, mediated by casein kinase 2 (CK2), is necessary for it to chaperone numerous kinase clients including CK2 itself [80]. Cdc37 phosphorylation status also affects the cellular toxicity of GA [48], and over-expression of PP5/Ppt1 in yeast is synthetically lethal with GA treatment. In cancer cells, PP5 over-expression correlated with reduced Cdc37 phosphorylation, reduced Raf-1 protein (an Hsp90 client) expression, and reduced activity of the MAP kinase pathway.
CK2 has been also reported to phosphorylate the co-chaperone p23 on Ser-113 and Ser-118 [81]. This phosphorylation is required for the formation of a tertiary complex between p23, CK2 and Hsp90. Finally, CK2 and pp90rsk phosphorylate murine Sti1 (the co-chaperone Hop) on Ser-189, affecting its interaction with Hsp90 [82]. The kinase Cdc2 is also able to phopshorylate mSti1 on Thr-198, suggesting that mSti1 phosphorylation may play a role in cell cycle regulation [83].
3. DEREGULATION OF HSP90 AND CO-CHAPERONE EXPRESSION
Although Hsp90 is one of the most highly expressed genes in malignant and non-malignant cells, there is evidence that Hsp90 and its co-chaperones can be deregulated at the level of gene amplification or gene deletion in cancer. In 1997 it was shown that the A549 non-small cell lung cancer cell line contains two amplified regions, one at 7p12-7p13 containing the epidermal growth factor receptor, and one at 6p12 containing Hsp90β. Fluorescence in situ hybridization demonstrated that both genes were actively transcribed within the sites of amplification [84].
A genome-wide screen of chromosomal copy number changes affecting gene expression in resected tumor samples from stage I and II non-small cell lung cancer patients identified, in 44% of tumor samples, a deletion on 14q32.2-33 that encompassed Hsp90α and affected Hsp90α expression. Patients whose tumors expressed lower levels of Hsp90α survived longer than did patients whose tumors expressed higher amounts of the protein (confirmed in three independent validation sets) [85]. Polymorphisms in Hsp90α and β have been identified [86], but an impact of polymorphisms on chaperone function has not been reported.
Proteomic examination of 17 human tumors for Hsp90α and β, co-chaperones Aha1, Cdc37, p23, and Tpr2, as well as the heat shock response element-binding transcription factor HSF1 demonstrated significant upregulation of at least one Hsp90 isoform or Hsp90 co-chaperone in 10 out of 17 tumors [87].
4. HSP90 INHIBITORS
The clinical development of Hsp90 inhibitors is reviewed in depth elsewhere in this issue (Whitesell et al., this issue). This section contains a brief summary of this area to provide background for the sections on tumor-intrinsic signaling affecting Hsp90 inhibitor response.
4.1 Clinical Hsp90 Inhibitors
4.1.1 Ansamycins
The benzaquinoid ansamycin geldanamycin was identified in 1970 (see Fig. 2 for a timeline). It was thought for many years that the primary activity of geldanamycin was src kinase inhibition. However, in 1992 it was shown that geldanamycin had tumoricidal activity unrelated to inhibition of src kinase [88]. Two years later, Hsp90 was identified as the molecular target of geldanamycin, and Hsp90 was recognized as a molecular target for anticancer therapeutics [1]. At the time Hsp90 was a protein of particular interest in endocrinology because it was known to bind to steroid hormone receptors including glucocorticoid, progesterone, estrogen and androgen receptors, and regulate their hormone sensitivity and nuclear transport [89]. Geldanamycin and all subsequent N-terminal Hsp90 inhibitors (see Fig. 2 for a current list) not only inhibit Hsp90, but, importantly, cause degradation of all proteins that require Hsp90 for maturation, activation, and/or stabilization. Thus Hsp90 inhibitors have served as both drug candidates and bioprobes, revealing which proteins are Hsp90 clients and helping to elucidate their function, while simultaneously serving as lead compounds to develop agents for targeting Hsp90 in the clinic.
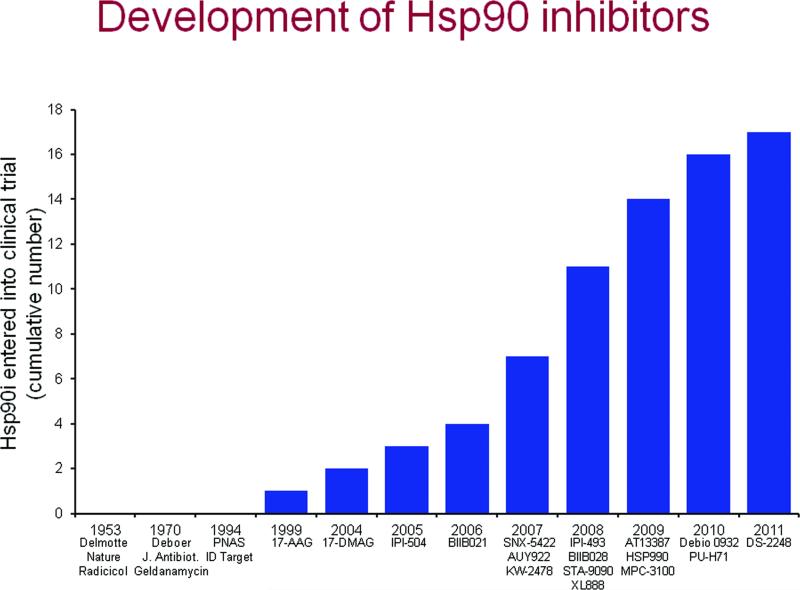
Figure 2.
A timeline of Hsp90 inhibitor identification and clinical development.
Geldanamycin proved to be too poorly soluble and too toxic in preclinical testing and was replaced by 17-allyl-17-demothoxygeldanamycin (17-AAG), which though difficult to formulate, retained activity and had an acceptable toxicity profile. In a phase II study of women with HER2+ breast cancer refractory to trastuzumab, with a primary endpoint of response rate by RECIST criteria, addition of 17-AAG resulted in significant clinical responses. The overall response rate was 22% and the clinical benefit rate (CR + PR + SD) was 59%. This was the first phase II study in solid tumors to show definitive RECIST responses to 17-AAG [8]. The effectiveness of this combination may relate to an ability of Hsp90 inhibition to overcome mechanisms of trastuzumab resistance, as discussed in Section 5.
Promising results were also observed in phase I/II and phase II studies of 17-AAG plus bortezomib in relapsed/refractory multiple myeloma [11]. Anti-tumor activity was seen in bortezomib-naive, -pretreated and -refractory patients. Further, the combination demonstrated a decreased rate and severity of bortezomib-induced peripheral neuropathy, and a phase III trial in multiple myeloma was planned. However, despite signs of activity in HER2+ breast cancer and multiple myeloma [90], Bristol-Myers-Squibb, which acquired 17-AAG in its purchase of Kosan Biosciences in 2008, terminated the ansamycin program, potentially due to considerations of patent life and difficulty of 17-AAG synthesis and competition from second-generation Hsp90 inhibitors [8, 91]. The negative impact of this decision on patients with trastuzumab-resistant breast cancer was highlighted in a recent commentary [6].
A more water soluble derivative of 17-AAG, 17-desmethoxy-17-N,N-dimethylaminoethylaminogeldanamycin (17-DMAG) was synthesized by the NCI and Kosan and entered clinical trial in 2005. Thus, as shown in Figure 2, there were 5 years between identification of Hsp90 as a therapeutic target and the first Hsp90 inhibitor trial, and 6 years before a second inhibitor entered phase I trials. Various schedules were explored in phase I studies and 1 CR in castrate-resistant prostate cancer and 1PR in melanoma were reported [92]. In a phase I study of 17-DMAG and trastuzumab in 28 metastatic breast and ovarian cancer patients there was 1CR and 1PR in patients with HER2+ breast cancer and 1 CR in a patient with ovarian cancer of unknown HER2 status [93]. In a phase I study in acute myeloid leukemia 17-DMAG was well-tolerated and showed signs of clinical activity (CR in 3 of 17 evaluable patients) [94]. As indicated above, however, Bristol Myers ended their ansamycin program, which included 17-DMAG.
IPI-504 (17-allylamino-17-demethoxygeldananmycin hydroquinone hydrochloride; retaspimycin), developed by Infinity Pharmaceuticals, is a water-soluble salt of 17-AAG. IPI-504 and 17-AAG exist in vivo in a redox equilibrium between the hydroquinone (IPI-504) and the quinone (17-AAG). The quinone has been reported to be a source of 17-AAG-related hepatotoxicity, and reduction of the quinone appears to result in a less toxic and more potent inhibitor. The reader is referred to the article by Whitesell et al. in this issue for more information on this compound.
4.1.2 Purines
The first reported synthetic Hsp90 inhibitor, PU3, was a purine compound optimized for binding to the distinctive ATP/ADP binding site present in Hsp90 and closely related proteins, known as the Bergerat fold [7]. A second purine scaffold-based inhibitor, BIIB021, was discovered by Conforma Therapeutics and later developed by Biogen Idec. In a phase I study of BIIB021 plus trastuzumab in HER2+ metastatic breast cancer, of 30 patients enrolled there were 2 RECIST PR and 10 SD. Biogen Idec developed additional Hsp90 inhibitors, had entered BIIB028 in phase I trial, and was planning a phase II of BIIB021 plus Aromasin, but the company has terminated its oncology program, including the further development of Hsp90 inhibitors for cancer indications.
Myrexis and its parent company Myriad Pharmaceuticals has completed a phase I of the purine-based compound MPC-3100. Due to poor solubility and bioavailability Myrexis has halted development of MPC-3100 and plans to begin clinical development of the prodrug MPC-0767.
Curis developed a purine like derivative, CUDC-305, which was licensed by Debiopharm and entered clinical trial in 2010 as Debio 0932 in a phase I dose-escalation study.
PU-H71, developed at Memorial Sloan Kettering and licensed to Samus Therapeutics, is in phase I trial in two dosing schedules, initiated in 2011. In addition, 124I-PU-H71 is being evaluated as a non-invasive tool for determining intratumoral drug concentration and kinetics.
4.1.3 Resorcinols
Radicicol is an antifungal antibiotic isolated in 1953 from mold found in a sample of soil from what was then the Belgian Congo [95]. Radicicol, which has Hsp90-inhibitory activity, is not active in vivo, but it is highly active in vitro, and the resorcinol moiety has been incorporated in several clinical Hsp90 inhibitor candidates.
STA-9090 (ganetespib) is a resorcinol developed by Synta Pharmaeuticals [96]. In a phase I trial of 35 patients with advanced solid tumors treated weekly, 3 weeks out of 4, there was one PR in a patient with rectal cancer. In a second phase I evaluating twice weekly ganetespib, 3 weeks out of 4, there was a PR in a patient with metastatic melanoma. A phase II study was performed in NSCLC patients enrolled on the basis of EGFR and KRAS mutational status. The EGFR and KRAS wild type cohort reached expansion criteria with 1 PR and 7 SD>16 weeks. In a subset of patients mutational analysis was performed on BRAF, PIK3CA, MET and HER2 and FISH analysis was performed for EML4-ALK rearrangement. Six of 8 patients identified as EML4-ALK positive were crizotinib naive and had tumor shrinkage, and an additional patient with crizotinib-refractory disease responded to ganetespib monotherapy. Eight of 13 patients with mutant KRAS had tumor shrinkage. Synta has initiated a phase IIb/III trial of ganetespib plus docetaxel as second line therapy in advanced NSCLC.
AUY922 was identified by Cancer Research UK and Vernalis, and is being developed by Novartis. In a phase I trial of 96 patients, 9 patients had a partial metabolic response on FDG-PET and 16 patients had SD. In a phase II expansion in patients with ER+ or HER2+ metastatic breast cancer assessed respectively by bevacizumab and trastuzumab PET there were 2 partial metabolic responses in HER2+ patients and 1 of these patients had a confirmed PR by RECIST [97]. A phase II trial of AUY922 is open in GIST [98] and a phase II trial has been approved but is not yet active for patients with diffuse large B-cell lymphoma or peripheral T-cell lymphoma [99]. Novartis is also evaluating HSP990, an orally available follow-on to AUY922 [100] in phase I trials in the US, Korea and Japan.
Kyowa Hakko Kirin Pharma has developed KW-2478 which has been evaluated in a phase I dose escalation trial in CLL, relaspsed/refractory multiple myeloma, and B-cell non-Hodgkin's lymphoma, and is in a phase I/II study in combination with bortezomib in relapsed/refractory multiple myeloma [101].
AT13387 was developed by Astex Pharmaceuticals and is now being evaluated in phase I trials with different schedules and in a phase II study with or without imatinib in GIST [102].
4.1.4 Pyrazoles
SNX-5422, an orally available prodrug of SNX2112 identified by Serenex [103], entered phase I clinical trial in 2007 in solid tumors and lymphoma on a schedule of every other day for 21 days in a 28-day cycle. No DLTs were observed among 11 patients. In 2008 Pfizer Inc. acquired Serenex and was developing the drug in phase I dose-escalation studies examining daily and twice weekly schedules. The twice weekly study had escalated to the tenth dose level, 177 mg/m2, with one DLT (nonseptic arthritis) and no MTD had been reached when ocular toxicity on the daily schedule, recapitulated in animal studies, led Pfizer to discontinue development of SNX-5422. A report of the twice-weekly schedule was published, which demonstrated no objective responses in 32 evaluable patients, stable disease in 15 patients, and progressive disease in 17 patients. This was the first published study of a second generation, non-ansamycin Hsp90 inhibitor. Pharmacokinetics and pharmacodynamics (Hsp70 in PBMC) demonstrated, for the first time among reported Hsp90 trials, to have a statistically significant PK/PD relationship, which was seen across all dose levels [104].
4.1.5 Additional Hsp90 N-Domain Inhibitors
Three additional Hsp90 inhibitors have entered clinical trial. XL-888 is from Exelixis, which has terminated its phase I tolerability, safety, and kinetics study [105]. DS-2248 from Daiichi Sankyo, and the imidazopyridine Debio 0932 from Curis licensed to Debiopharm SA, are both in phase I trial at this writing.
4.2. Hsp90 C-Domain Inhibitors
All clinical Hsp90 inhibitors target the N-terminal ATP binding site. The Hsp90 C-terminus also contains a nucleotide-binding site [106]. Novobiocin, the first identified C-domain Hsp90 inhibitor, has served as the prototype for development of new Hsp90 inhibitors with greatly enhanced potency and specificity [107]. Inhibitors of the C-terminal site do not bind or inhibit the N-terminal site, nor do N-terminal inhibitors bind or inhibit the C-terminal site. C-terminal inhibitors in development cause client protein degradation, have moderate anti-proliferative activity, and are being moved toward clinical evaluation. N-terminal Hsp90 inhibitors uniformly cause activation of heat shock factor 1, resulting in increased expression of Hsp70, Hsp90 Hsp40 and Hsp27, all of which have been associated with anti-apoptotic activity and drug resistance. In contrast, C-terminal inhibitors do not induce a heat shock response, which may be an advantage in various oncology indications [108].
4.3. Difficulty in Assessing Target Engagement In Vivo
The ability to assess the impact of a drug on its target in the patient provides an important guide for drug development. For a number of reasons intrinsic to Hsp90 inhibitors, it has been difficult to successfully perform pharmacodynamic (PD) analyses. The great majority of PD analyses in Hsp90 inhibitor clinical trials have been performed on peripheral blood mononuclear cells (PBMC), largely because PBMC are a readily obtainable surrogate, while tumor biopsies pre- and post-therapy are frequently not available. Most investigators have attempted to find an Hsp90 client that was degraded in response to drug administration. However, client degradation in PBMC has been highly variable and not dose-dependent. The most reliable indicator of Hsp90 inhibitor target engagement in clinical trials thus far has been the induction of Hsp70, which occurs after Hsp90 inhibitor treatment due to release of heat shock factor 1 from Hsp90 and transcriptional activation of genes with heat shock factor response elements such as Hsp70 [109]. A statistically significant Hsp90 inhibitor PD response was first reported in a phase I trial of 17-DMAG administered twice weekly in patients with advanced solid malignancies [110], and a statistically significant response across all dose levels was seen in a phase II trial of the Hsp90 inhibitor PF-04929113 (SNX-5422) [104]. In both studies, the PD response was obtained by measuring Hsp70 induction in PBMC.
Amongst the clinical trials published thus far the paucity of clinical responses to Hsp90 inhibitor monotherapy has not made it possible to compare client protein degradation with clinical benefit. Several intrinsic properties of Hsp90 inhibitors that contribute to their value as therapeutics also contribute to the difficulty of using a non-tumor surrogate for PD studies. Firstly, Hsp90 inhibitors accumulate selectively in tumor versus plasma or non-tumor tissue [111, 112], and Hsp90 in tumor has been reported to have a higher affinity for Hsp90 inhibitors than does Hsp90 from non-cancerous cells [113]. Thus, inhibitor accumulation and client protein degradation in peripheral blood mononuclear cells is unlikely to be representative of drug levels and activity in tumor. Secondly, the most sensitive Hsp90 inhibitor targets are frequently not expressed in PBMC, i.e. oncogenic fusion proteins such as EML4-Alk or an overexpressed client such as HER2. Thirdly, facets of the intracellular milieu that can contribute to Hsp90 inhibitor sensitivity, such as overload of the protein degradation apparatus and proteotoxic stress, are selectively present in tumor versus non-tumor tissue.
To address some of these issues PET probes have been developed to assess Hsp90 inhibitor PD activity. Hsp90 is an essential chaperone of HIF proteins critical for VEGF transcription and tumor angiogenesis. 89Zr-bevacizumab PET was shown to provide a sensitive noninvasive measure of Hsp90 inhibition in small animal studies [114] and this agent is being evaluated in a clinical trial of the Hsp90 inhibitor AUY922 (NCT01081613). 68Ga-DOTA-F(Ab')2 Herceptin has been developed to monitor HER2 degradation in response to Hsp90 inhibition in a breast cancer xenograft model using small animal PET [115], and this probe was shown to be superior to 18F-FDG in detecting response to Hsp90 inhibitor therapy in microPET studies [116]. As mentioned earlier, 124I-PU-H71 is being used in a pilot study to monitor PU-H71 levels and kinetics in tumor [117], in the hope that information gained will assist in developing an optimal dose and schedule for this drug.
5. CELLS RESISTANT TO THERAPY TARGETING HSP90 CLIENTS REMAIN SENSITIVE TO HSP90 INHIBITORS
As described below, there are numerous examples of tumor cells exhibiting de novo or acquired resistance to targeted therapy directed at Hsp90 client proteins. In nearly all cases, these Hsp90 clients display equivalent or increased sensitivity to Hsp90 inhibitors. For example, acquired resistance to imatinib mesylate in patients with advanced Philadelphia chromosome-positive leukemia is commonly associated with reactivation of Bcr-Abl due to mutations in the kinase domain or gene amplification. These cells maintain or increase their dependence on Hsp90 and sensitivity to Hsp90 inhibitors [118].
In tumor cells harboring MET gene amplification, c-Met tyrosine kinase inhibitors cause only transient inhibition of c-Met-dependent downstream signaling, including Akt, Erk and/or STAT3 signaling and EGFR family activation, despite sustained c-Met inhibition. In contrast, in MET-amplified tumor cells, 17-AAG destabilizes c-Met protein and induces sustained suppression of c-Met-dependent signaling and substantial apoptosis [119]. The SNX-2122 prodrug SNX-5422 displays antitumor activity in mice bearing MET-amplified tumor xenografts that are both sensitive and resistant to c-Met tyrosine kinase inhibitors (TKI) [120].
Non-small cell lung cancer (NSCLC) cells with T790M mutation in EGFR are resistant to the reversible TKIs gefitinib and erlotinib. Irreversible EGFR inhibitors such as CL-387,785 have been employed to circumvent resistance. However, CL-387,785 does not fully suppress activity of other receptor tyrosine kinases that become activated in T790M-expressing cells, resulting in residual Akt and mammalian target of rapamycin (mTOR) activities. Inhibition of Hsp90 causes depletion of EGFR, depletion of other receptor tyrosine kinases and phopho-Akt, inhibits mTOR signaling and causes regression in an in vivo murine lung EGFR L858R tumor model [121]. Cells expressing EGFR with L858R and T790M mutations conferring resistance to EGFR tyrosine kinase inhibitors are also sensitive to the Hsp90 inhibitor SNX-5422 [122].
Mature wild-type EGFR requires high dose, long-term exposure to an Hsp90 inhibitor for degradation. Mutations in EGFR exons 18, 19 and 21 render EGFR dependent on Hsp90 and these mutations in non-small cell lung cancer (NSCLC) have been associated with enhanced sensitivity to EGFR TKI therapy [123]. In contrast, insertions in EGFR exon 20 in NSCLC confer resistance to gefitinib and erlotinib therapy [124]. Gefitinib- and erlotinib-resistant NSCLC harboring exon 20 insertion mutations in either EGFR or HER2 are sensitive to Hsp90 inhibitors. 17-AAG destabilizes these mutants, suppresses Akt and Stat3 signaling, and induces loss of tumor cell viability [124].
The echinoderm microtubule-associated protein-like 4 (EML4)-anaplastic lymphoma kinase (ALK) fusion oncogene has been identified in a subset of NSCLC tumors. The ALK TKI crizotinib has impressive antitumor activity in this patient population. However, resistance develops, typically within one year, and is associated with EML4-ALK gene amplification or L1196M ALK kinase domain mutation. These crizotinib-resistant cells are sensitive to two structurally distinct ALK inibitors NVP-TAE684 and AP26113, and are highly sensitive to the Hsp90 inhibitor 17-AAG [125]. Similarly, a secondary mutation in ALK, F1174L, detected in neuroblastoma and in a patient with inflammatory myofibroblastic tumor harboring a RANBP-ALK translocation, display crizotinib resistance, but tumor cells harboring this mutation demonstrate sensitivity to the ALK inhibitor TAE684 and the Hsp90 inhibitor 17-AAG [126].
The BRAF inhibitor PLX4720 is highly effective in cells bearing mutant BRAF(V600E) alone, but ineffective in cells bearing both BRAFV600E and phosphoinositide-3-kinase catalytic alpha peptide (PIK3CA) H1047R oncogenic mutations. Overcoming resistance to the combined mutations however can be achieved by addition of the apoptosis inducer TNF-related apoptosis-inducing ligand (TRAIL) to PLX4720, or by combination of TRAIL with 17-AAG [127].
Treatment with trastuzumab, a fully humanized monoclonal antibody to HER2 is an effective treatment in women with amplification of the HER2 oncogene. HER2 is one of the most sensitive client proteins to Hsp90 inhibition. Trastuzumab resistance, however, whether de novo or acquired is common and inevitable in all women with metastatic disease. Most trastuzumab-resistant tumors demonstrate continued hyperactive HER2 signaling, with resistance conferred by a variety of mechanisms including truncation of HER2 that removes the trastuzumab-binding epitope, HER2 dimerization and activation with other receptor tyrosine kinases, deletion of downstream tumor suppressors including PTEN, and activating mutations of PI3K. The Hsp90 inhibitor IPI-540 is active against HER2-positive cell lines with intrinsic or acquired trastuzumab resistance and in tumor models with PI3K activating mutation or low levels of PTEN. IPI-504 downregulates HER2 and blocks downstream signaling as evidenced by inhibition of Akt and MAPK phosphorylation [128]. Truncation of HER2 to the p95-HER2 form results in deletion of the trastuzumab binding site and trastuzumab resistance. However, similarly to wild-type HER2, p95-HER2 is dependent on Hsp90 chaperone activity. Treatment of p95-HER2-expressing tumor cells with Hsp90 inhibitor results in sustained loss of p95-HER2 and HER2, inhibition of Akt activation, induction of apoptosis, and complete inhibition of tumor growth in an animal xenograft model [129]. Triple negative breast cancers, which lack expression of HER2, estrogen receptors, and progesterone receptors, and are insensitive to most hormonal, targeted and cytotoxic therapies, are sensitive to the purine Hsp90 inhibitor PU-H71 via downregulation of multiple signaling pathways resulting in anti-proliferative activity, anti-invasive activity and induction of apoptosis in in vitro and in vivo models [130].
5.1. Other Settings In Which Hsp90 Inhibitors Overcome Resistance
5.1.1 Proteasome Inhibitor Resistance
Treatment with the proteasome inhibitor bortezomib has shown promise in mantle cell lymphoma. However, not all patients respond to bortezomib and resistance in responders develops rapidly. Bortezomib resistance is associated with upregulation of the prosurvival chaperone BiP/Grp78, which is dependent on chaperoning by Hsp90. Treatment of resistant cells with the Hsp90 inhibitor IPI-504 together with bortezomib leads to dissociation of the Hsp90/BiP complex, BiP/Grp78 depletion, inhibition of the unfolded protein response, induction of NOXA-mediated mitochondrial depolarization, apoptosis in vitro and growth arrest of tumor xenografts in vivo [131].
5.1.2 Tumors Addicted to Activated Ras
Ras is one of the most highly mutated genes in cancer and despite vigorous efforts there is not yet an approved therapeutic targeting Ras. Recently the combination of Hsp90 inhibition and mTOR inhibition was shown to provide potent antitumor activity in several models of Ras-driven malignancies [132]. The survival and proliferation of Ras-driven tumors requires a finely tuned balance of metabolic activities (see [133]). Ras signaling both induces and requires elevated levels of reactive oxygen species (ROS). Activated Ras directly elevates ERK1/2 levels via the Ras/Raf/Mek/ERK pathway. A moderate level of ERK is required for Ras-driven cell proliferation, but high ERK levels induce growth arrest [134]. Mitochondrially-derived ROS are required to buffer the level of ERK generated by deregulated Ras [135]. Due to hypoxia, mutant protein load and inherent oxidative stress, tumor cells have a constitutively elevated level of endoplasmic reticulum (ER) proteotoxic stress. Unlike the reducing environment of the cytosol, ER maintans an oxidative environment that promotes proper protein disulfide bond formation, a byproduct of which is ROS production. If ROS production is persistently elevated in ER and the ER stress response machinery is successfully inhibited, ER collapse and cell death results. Thus ROS is required to buffer ERK but excess ROS is cytotoxic. To achieve a viable balance of ROS levels cancer cells must maintain sufficient reducing capacity, which they achieve in part by metabolizing glucose via the pentose phosphate pathway and its key enzyme glucose 6-phosphate dehydrogenase, which generates NADPH and reduced glutathione. G6PD expression is upregulated by mTOR, which is activated by the Ras/PI3K/Akt/mTOR pathway. Inhibition of mTOR interferes with the generation of reduced glutathione. Hsp90 inhibitors have a number of salutary effects in preventing Ras-driven tumor cells from employing compensatory mechanisms for their survival: they prevent Hsp90 from buffering ROS, they inhibit mTOR signaling, and they block the ER stress response by inducing degradation of the ER stress regulatory proteins and Hsp90 clients IRE1 and PERK. In fact, these characteristics of Hsp90 inhibitors may underlie the unexpected activity of these drugs in KRAS mutant NSCLC (see earlier in this review). Combining mTOR inhibition with Hsp90 inhibition suppresses the ER stress response and deregulates ROS production, resulting in potent anticancer activity [134]. A clinical trial (NCT01427946) has recently been initiated in KRAS mutant NSCLC combining the Hsp90 inhibitor retaspimycin (IPI-504) and the mTOR inhibitor everolimus [136].
6. HSP90 INHIBITOR RESISTANCE
As discussed earlier in this review, all N-domain Hsp90 inhibitors have the property of inducing a heat shock response that likely limits the anti-tumor efficacy of these drugs and is a major contributor to drug resistance [90]. Although resistance to Hsp90 inhibitors has been previously linked to expression of P-glycoprotein and the multidrug resistant phenotype, the stress response induced by Hsp90-targeted therapy is considered to be a more important resistance mechanism [137]. Heat shock factor 1 (HSF1) is the master transcriptional regulator of the heat shock response and is inactive and localized to the cytoplasm when bound to Hsp90 [138]. Induction of the heat shock response occurs as a result of Hsp90 inhibitor dissociation of HSF1/Hsp90 complexes. One of the anti-apoptotic chaperone proteins induced by Hsp90 inhibitors is clusterin [139]. Inhibition of clusterin expression with the antisense-based drug OGX-011 suppressed clusterin induction by two distinct Hsp90 inhibitors in a prostate cancer model and synergistically enhanced the cytotoxic activity of the Hsp90 inhibitors. Unexpectedly, OGX-011 inhibited the HSF1 activation caused by the Hsp90 inhibitors, suggesting a positive feedback role for clusterin in promoting the heat shock response. In prostate cancer xenograft models the combination of OGX-011 and Hsp90 inhibitors markedly potentiated anti-tumor efficacy and significantly increased survival compared to Hsp90 inhibitor monotherapy [139].
7. HSP90 INHIBITORS MODULATE HOST PHYSIOLOGY
7.1. Antiangiogenic Activity of Hsp90 Inhibitors
Hypoxia inducible factor (HIF) is a potent, proangiogenic transcription factor that upregulates genes favoring survival under hypoxic conditions. HIF expression is a major factor in the angiogenic switch promoting neovascularization and tumor cell survival. HIF-1α is an Hsp90 client and is degraded in response to Hsp90 inhibitors [140, 141]. Hsp90 inhibitors promote HIF degradation via the proteasome, but in an oxygen-independent, HIF prolylhydroxylase-independent manner [51, 142]. This activity likely underlies the antiangiogenic properties of Hsp90 inhibitors.
7.2. Hsp90 inhibitors and the Immune System
Many antitumor drug therapies that induce tumor cell death are immunosuppressive and their direct mechanism of cytotoxicity is non-immune mediated. However, some drugs, such as the proteasome inhibitor bortezomib, induce antitumor immunity via a mechanism that entails drug-induced exposure of Hsp90 on the surface of dying tumor cells, cell-cell contact between dying tumor cells and dendritic cells, and delivery to the dendritic cell of an activating signal leading to an enhanced autologous antitumor T-cell response to primary tumor cells. While the combination of Hsp90 inhibitor and bortezomib leads to greater tumor cell apoptosis, addition of the Hsp90 inhibitor geldanamycin blocks bortezomib-induced antitumor immunity [143]. Thus, an Hsp90 inhibitor may have an unanticipated effect on the host that impairs a sustained antitumor response to combination therapies associated with immunogenic death. Human prostate and ovarian cancer cells and acute lymphoblastic leukemia cells also respond to anthracyclines with an immunogenic cell death associated with rapid translocation of Hsp90 to the tumor cell surface [144], although it has not been determined whether this activity is compromised by concomitant exposure to Hsp90 inhibitor.
Hsp90 inhibitors have been shown to prevent lymphocyte activation in vitro. Geldanamycin abrogates CD28-mediated activation of T lymphocytes, interferes with kinase-dependent signaling events occurring during lymphocyte activation, and suppresses bacterial DNA-mediated stimulation of murine spleen cells and macrophages [145-148].
In contrast to the immunosuppressive activity of geldanamycin in vitro and on immunogenic cell death in myeloma, Hsp90 inhibitors are reported to enhance anti-myeloma immunity by promoting transcription of both major histocompatibility class I chain-related A and B genes (MICA and MICB) in myeloma cells, and MICA and MICB act as activating ligands for natural killer cell receptors that mediate tumor cell recognition and lysis [149].
Hsp90 inhibitors enhance the anti-tumor cytotoxicity of natural killer cells by also abrogating NFkB activation [150]. The mechanism of action involves disruption of the IkappaB kinase signaling pathway [151-154]. Because of their negative impact on NFkB, Hsp90 inhibitors are anti-inflammatory [155] and may provide a novel approach to reduce chronic inflammation, a recognized host factor that contributes to cancer development [156]. Because of their anti-inflammatory activity, Hsp90 inhibitors may be useful for treating autoimmune diseases [157]. Further, Hsp90 inhibition may represent a novel strategy to selectively prevent graft-versus-host disease in hematopoetic stem cell transplant recipients [158].
The receptor tyrosine kinase ephrin receptor A2 (EphA2) was recently identified as an HSP90 client [159, 160]. EphA2 is abundantly expressed in a broad range of cancers and its expression correlates with poor clinical outcome. Tumor EphA2 is recognized as a self protein by the host and CD8+ T cells are poorly competent to recognize EphA2+ tumor cells. Tumor cell recognition can be significantly enhanced by pretreating CD8+ T cells with receptor agonists that promote proteasome-mediated degradation of the kinase and its upregulated expression in class I complexes at the cell surface [161]. Hsp90 inhibitors also enhance tumor cell recognition, suggesting that they may significantly improve the anti-tumor activity of host CD8+ T cells. Finally, the complex nature of the impact of Hsp90 inhibitors on host immunity is demonstrated by the observation that Hsp90 inhibition negatively affects dendritic cell function and antigen cross-presentation, which has a negative impact on tumor recognition by cytotoxic T cells [162-165].
8. HSP90 INHIBITORS AND TUMOR STROMA
Studies of the impact of Hsp90 inhibitors on dsRNA-dependent kinase PKR [166], Akt [167], HER2 [168] and c-Src [169] have demonstrated apparently paradoxical activation of the kinase prior to Hsp90 inhibitor-induced kinase degradation. These observations may relate to the metastable nature of certain Hsp90 clients, facilitating the chaperoning of a protein in a thermodynamically favorable but inactive state, conducive to rapid activation associated with greater instability when the client is released from Hsp90 by ligand or Hsp90 inhibitor. This transient activation is sufficient to initiate downstream signaling, i.e. from Src to insulin-like growth factor and insulin signaling pathways [166, 170], Src to Akt and Erk [169], and Src to HER2 [168], thus activating various proteins with potential oncogenic activity, including proteins that are not themselves clients of Hsp90 such as Erk.
Further, Hsp90 inhibitor-induced activation of signal transduction pathways in tumor stroma may have unpredictable effects on tumor growth in specific microenvironments. Thus, Hsp90 inhibitor-dependent (and c-Src mediated) osteoclast activation in vitro and in vivo results in the enhancement of intra-osseous prostate cancer xenograft growth in mice, while the same drug dose and schedule effectively inhibits the growth of subcutaneous prostate tumor xenografts [171]. These data provide one example of how drug-dependent modulation of the local tumor environment may profoundly affect the antitumor efficacy of Hsp90-directed therapy.
A functional Hsp90 complex plays an evolutionarily conserved role in kinetochore assembly [172, 173]. In yeast, Hsp90 inhibition and heat stress are reported to induce aneuploidy, thus uncovering a form of stress-inducible mutation that is capable of promoting rapid phenotypic evolution and drug resistance [Chen G, Nature, 2012]. Such a role for Hsp90 in regulating the emergence of adaptive traits under environmental stress has been previously proposed [174, 175], raising the possibility that Hsp90 inhibitors could promote further genetic instability and drug resistance in tumor cells. However, such findings in higher metazoans have yet to be reported, while a recent screen of cancer cell lines for aneuploid-selective drugs identified Hsp90 inhibitors as being particularly active [176].
9. PERSPECTIVE
At first glance, Hsp90 represents an ideal molecular target for anticancer therapy. Small molecule inhibitors cause degradation of many Hsp90 chaperoned proteins, and the list of Hsp90 clients efficiently degraded in response to adequate intracellular levels of drug includes major players in each of the 8 pathways identified as critical hallmarks of malignancy. It is perhaps surprising that Hsp90 inhibitors have been in the clinic for eleven years, and no Hsp90 inhibitor has yet been approved. Recent clinical successes indicate that significant hurdles have been overcome and regulatory approval may be forthcoming within a few years [9]. In this review we touched on some of the factors that increase the complexity of achieving success with Hsp90 inhibitors in oncology trials. To summarize why Hsp90i should be efficacious: they cause degradation of pro-oncogenic proteins, they accumulate selectively in tumor versus normal tissue, and therapy-induced mutations, which are a major cause of resistance to other anticancer drugs are less likely to affect Hsp90 inhibitors. Mutation of an Hsp90 client in response to targeted therapy is unlikely to abrogate the requirement of that client for Hsp90-mediated chaperone activity, and the mutant protein may indeed be more dependent on Hsp90 for folding and stability than its wild type counterpart. Another mechanism of resistance to targeted therapy, oncogenic switch, i.e. to a different kinase after targeted tyrosine kinase inhibitor therapy, typically has resulted in a switch to another Hsp90-dependent kinase and, consequently, maintenance of Hsp90i sensitivity [119]. Therefore, combination of an Hsp90i with other targeted therapy, either upfront or after resistance has developed, is likely to be among the most successful strategies for utilizing these drugs, and indeed, this setting is where some of the best clinical responses have been seen.
Studies of the impact of targeted therapy in oncology trials have tended to focus on the predicted effects of the drug on tumor without consideration of the impact of therapy on the host. As discussed here, Hsp90 inhibitors have effects on the host that have systemic impact, i.e anti-angiogenic and immunomodulatory effects, as well as effects on the microenvironment that engage host tissue-specific biology. These systemic effects can either enhance or antagonize the anti-tumor activity of Hsp90 inhibitors in patients.
There has been extraordinary progress in understanding of the regulation of the Hsp90 machine at the molecular level. The structural basis of the client-chaperone machinery interaction is a critically important area of research that needs to be approached not only at the biophysical level, but also ultimately from a systems perspective, incorporating the totality of cell signaling, subsequent posttranslational modifications of client, chaperones and cochaperones, and other events that together constitute the target cell and its mileau. These considerations are of great importance in identifying fruitful indications for Hsp90 inhibitor therapy in oncology, and will be of importance in implementing Hsp90 inhibitor therapy in neurologic, inflammatory, infectious or other indications in the future.
ACKNOWLEDGEMENT
The authors of this work received funding from the NCI Intramural Program, Center for Cancer Research.
References
- 1.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 3.Taldone T, Gozman A, Maharaj R, Chiosis G. Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr Opin Pharmacol. 2008;8:370–374. doi: 10.1016/j.coph.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YS, Alarcon SV, Lee S, et al. Update on Hsp90 inhibitors in clinical trial. Curr Top Med Chem. 2009;9:1479–1492. doi: 10.2174/156802609789895728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arteaga CL. Why is this effective HSP90 inhibitor not being developed in HER2+ breast cancer? Clin Cancer Res. 2011;17:4919–4921. doi: 10.1158/1078-0432.CCR-11-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modi S, Stopeck A, Linden H, et al. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res. 2011;17:5132–5139. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- 9.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson PG, Badros AZ, Jagannath S, et al. Tanespimycin with bortezomib: activity in relapsed/refractory patients with multiple myeloma. Br J Haematol. 2010;150:428–437. doi: 10.1111/j.1365-2141.2010.08264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson PG, Chanan-Khan AA, Lonial S, et al. Tanespimycin and bortezomib combination treatment in patients with relapsed or relapsed and refractory multiple myeloma: results of a phase 1/2 study. Br J Haematol. 2011;153:729–740. doi: 10.1111/j.1365-2141.2011.08664.x. [DOI] [PubMed] [Google Scholar]
- 12.Davenport EL, Moore HE, Dunlop AS, et al. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood. 2007;110:2641–2649. doi: 10.1182/blood-2006-11-053728. [DOI] [PubMed] [Google Scholar]
- 13.Mimnaugh EG, Xu W, Vos M, Yuan X, Neckers L. Endoplasmic reticulum vacuolization and valosin-containing protein relocalization result from simultaneous hsp90 inhibition by geldanamycin and proteasome inhibition by velcade. Mol Cancer Res. 2006;4:667–681. doi: 10.1158/1541-7786.MCR-06-0019. [DOI] [PubMed] [Google Scholar]
- 14.Neznanov N, Komarov AP, Neznanova L, Stanhope-Baker P, Gudkov AV. Proteotoxic stress targeted therapy (PSTT): induction of protein misfolding enhances the antitumor effect of the proteasome inhibitor bortezomib. Oncotarget. 2011;2:209–221. doi: 10.18632/oncotarget.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McClellan AJ, Xia Y, Deutschbauer AM, Davis RW, Gerstein M, Frydman J. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell. 2007;131:121–135. doi: 10.1016/j.cell.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 16.Moulick K, Ahn JH, Zong H, et al. Affinity-based proteomics reveal cancerspecific networks coordinated by Hsp90. Nat Chem Biol. 2011;7:818–826. doi: 10.1038/nchembio.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwasaki S, Kobayashi M, Yoda M, et al. Hsc70/Hsp90 chaperone machinery mediates ATP-dependent RISC loading of small RNA duplexes. Mol Cell. 2010;39:292–299. doi: 10.1016/j.molcel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Wu C, So J, Davis-Dusenbery BN, et al. Hypoxia potentiates microRNA-mediated gene silencing through posttranslational modification of Argonaute2. Mol Cell Biol. 2011;31:4760–4774. doi: 10.1128/MCB.05776-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerji U. Heat shock protein 90 as a drug target: some like it hot. Clin Cancer Res. 2009;15:9–14. doi: 10.1158/1078-0432.CCR-08-0132. [DOI] [PubMed] [Google Scholar]
- 21.Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J. 2008;410:439–453. doi: 10.1042/BJ20071640. [DOI] [PubMed] [Google Scholar]
- 22.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 23.Millson SH, Truman AW, Racz A, et al. Expressed as the sole Hsp90 of yeast, the alpha and beta isoforms of human Hsp90 differ with regard to their capacities for activation of certain client proteins, whereas only Hsp90beta generates sensitivity to the Hsp90 inhibitor radicicol. FEBS J. 2007;274:4453–4463. doi: 10.1111/j.1742-4658.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- 24.Felts SJ, Owen BA, Nguyen P, Trepel J, Donner DB, Toft DO. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J Biol Chem. 2000;275:3305–3312. doi: 10.1074/jbc.275.5.3305. [DOI] [PubMed] [Google Scholar]
- 25.Kang BH, Altieri DC. Compartmentalized cancer drug discovery targeting mitochondrial Hsp90 chaperones. Oncogene. 2009;28:3681–3688. doi: 10.1038/onc.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcu MG, Doyle M, Bertolotti A, Ron D, Hendershot L, Neckers L. Heat shock protein 90 modulates the unfolded protein response by stabilizing IRE1alpha. Mol Cell Biol. 2002;22:8506–8513. doi: 10.1128/MCB.22.24.8506-8513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali MM, Roe SM, Vaughan CK, et al. Crystal structure of an Hsp90-nucleotide-p23/ Sba1 closed chaperone complex. Nature. 2006;440:1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 29.Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci. 2002;59:1640–1648. doi: 10.1007/PL00012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prodromou C, Pearl LH. Structure and functional relationships of Hsp90. Curr Cancer Drug Targets. 2003;3:301–323. doi: 10.2174/1568009033481877. [DOI] [PubMed] [Google Scholar]
- 31.Ratzke C, Mickler M, Hellenkamp B, Buchner J, Hugel T. Dynamics of heat shock protein 90 C-terminal dimerization is an important part of its conformational cycle. Proc Natl Acad Sci U S A. 2010;107:16101–16106. doi: 10.1073/pnas.1000916107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hessling M, Richter K, Buchner J. Dissection of the ATP-induced conformational cycle of the molecular chaperone Hsp90. Nat Struct Mol Biol. 2009;16:287–293. doi: 10.1038/nsmb.1565. [DOI] [PubMed] [Google Scholar]
- 33.Mickler M, Hessling M, Ratzke C, Buchner J, Hugel T. The large conformational changes of Hsp90 are only weakly coupled to ATP hydrolysis. Nat Struct Mol Biol. 2009;16:281–286. doi: 10.1038/nsmb.1557. [DOI] [PubMed] [Google Scholar]
- 34.Southworth DR, Agard DA. Species-dependent ensembles of conserved conformational states define the Hsp90 chaperone ATPase cycle. Mol Cell. 2008;32:631–640. doi: 10.1016/j.molcel.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbas-Terki T, Briand PA, Donze O, Picard D. The Hsp90 co-chaperones Cdc37 and Sti1 interact physically and genetically. Biol Chem. 2002;383:1335–1342. doi: 10.1515/BC.2002.152. [DOI] [PubMed] [Google Scholar]
- 36.Chang HC, Nathan DF, Lindquist S. In vivo analysis of the Hsp90 cochaperone Sti1 (p60). Mol Cell Biol. 1997;17:318–325. doi: 10.1128/mcb.17.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson JL, Halas A, Flom G. Nucleotide-dependent interaction of Saccharomyces cerevisiae Hsp90 with the cochaperone proteins Sti1, Cpr6, and Sba1. Mol Cell Biol. 2007;27:768–776. doi: 10.1128/MCB.01034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee P, Shabbir A, Cardozo C, Caplan AJ. Sti1 and Cdc37 can stabilize Hsp90 in chaperone complexes with a protein kinase. Mol Biol Cell. 2004;15:1785–1792. doi: 10.1091/mbc.E03-07-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lotz GP, Lin H, Harst A, Obermann WM. Aha1 binds to the middle domain of Hsp90, contributes to client protein activation, and stimulates the ATPase activity of the molecular chaperone. J Biol Chem. 2003;278:17228–17235. doi: 10.1074/jbc.M212761200. [DOI] [PubMed] [Google Scholar]
- 40.MacLean M, Picard D. Cdc37 goes beyond Hsp90 and kinases. Cell Stress Chaperones. 2003;8:114–119. doi: 10.1379/1466-1268(2003)008<0114:cgbhak>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer P, Prodromou C, Liao C, et al. Structural basis for recruitment of the ATPase activator Aha1 to the Hsp90 chaperone machinery. EMBO J. 2004;23:1402–1410. doi: 10.1038/sj.emboj.7600141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panaretou B, Siligardi G, Meyer P, et al. Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone aha1. Mol Cell. 2002;10:1307–1318. doi: 10.1016/s1097-2765(02)00785-2. [DOI] [PubMed] [Google Scholar]
- 43.Picard D. Intracellular dynamics of the Hsp90 co-chaperone p23 is dictated by Hsp90. Exp Cell Res. 2006;312:198–204. doi: 10.1016/j.yexcr.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Richter K, Muschler P, Hainzl O, Reinstein J, Buchner J. Sti1 is a non-competitive inhibitor of the Hsp90 ATPase. Binding prevents the N-terminal dimerization reaction during the atpase cycle. J Biol Chem. 2003;278:10328–10333. doi: 10.1074/jbc.M213094200. [DOI] [PubMed] [Google Scholar]
- 45.Siligardi G, Panaretou B, Meyer P, et al. Regulation of Hsp90 ATPase activity by the co-chaperone Cdc37p/p50cdc37. J Biol Chem. 2002;277:20151–20159. doi: 10.1074/jbc.M201287200. [DOI] [PubMed] [Google Scholar]
- 46.Song Y, Masison DC. Independent regulation of Hsp70 and Hsp90 chaperones by Hsp70/Hsp90-organizing protein Sti1 (Hop1). J Biol Chem. 2005;280:34178–34185. doi: 10.1074/jbc.M505420200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan WP, Owen BA, Toft DO. The influence of ATP and p23 on the conformation of hsp90. J Biol Chem. 2002;277:45942–45948. doi: 10.1074/jbc.M207754200. [DOI] [PubMed] [Google Scholar]
- 48.Vaughan CK, Mollapour M, Smith JR, et al. Hsp90-dependent activation of protein kinases is regulated by chaperone-targeted dephosphorylation of Cdc37. Mol Cell. 2008;31:886–895. doi: 10.1016/j.molcel.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu W, Marcu M, Yuan X, Mimnaugh E, Patterson C, Neckers L. Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc Natl Acad Sci U S A. 2002;99:12847–12852. doi: 10.1073/pnas.202365899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ballinger CA, Connell P, Wu Y, et al. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ehrlich ES, Wang T, Luo K, et al. Regulation of Hsp90 client proteins by a Cullin5-RING E3 ubiquitin ligase. Proc Natl Acad Sci U S A. 2009;106:20330–20335. doi: 10.1073/pnas.0810571106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsukahara F, Maru Y. Bag1 directly routes immature BCR-ABL for proteasomal degradation. Blood. 2010;116:3582–3592. doi: 10.1182/blood-2009-10-249623. [DOI] [PubMed] [Google Scholar]
- 53.Mandal AK, Lee P, Chen JA, et al. Cdc37 has distinct roles in protein kinase quality control that protect nascent chains from degradation and promote posttranslational maturation. J Cell Biol. 2007;176:319–328. doi: 10.1083/jcb.200604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mollapour M, Neckers L. Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbamcr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brugge JS, Erikson E, Erikson RL. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981;25:363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- 56.Lees-Miller SP, Anderson CW. Two human 90-kDa heat shock proteins are phosphorylated in vivo at conserved serines that are phosphorylated in vitro by casein kinase II. J Biol Chem. 1989;264:2431–2437. [PubMed] [Google Scholar]
- 57.Lees-Miller SP, Anderson CW. The human double-stranded DNA-activated protein kinase phosphorylates the 90-kDa heat-shock protein, hsp90 alpha at two NH2-terminal threonine residues. J Biol Chem. 1989;264:17275–17280. [PubMed] [Google Scholar]
- 58.Walker AI, Hunt T, Jackson RJ, Anderson CW. Double-stranded DNA induces the phosphorylation of several proteins including the 90 000 mol. wt. heat-shock protein in animal cell extracts. EMBO J. 1985;4:139–145. doi: 10.1002/j.1460-2075.1985.tb02328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mimnaugh EG, Worland PJ, Whitesell L, Neckers LM. Possible role for serine/threonine phosphorylation in the regulation of the heteroprotein complex between the hsp90 stress protein and the pp60v-src tyrosine kinase. J Biol Chem. 1995;270:28654–28659. doi: 10.1074/jbc.270.48.28654. [DOI] [PubMed] [Google Scholar]
- 60.Wandinger SK, Suhre MH, Wegele H, Buchner J. The phosphatase Ppt1 is a dedicated regulator of the molecular chaperone Hsp90. EMBO J. 2006;25:367–376. doi: 10.1038/sj.emboj.7600930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lei H, Venkatakrishnan A, Yu S, Kazlauskas A. Protein kinase A-dependent translocation of Hsp90 alpha impairs endothelial nitric-oxide synthase activity in high glucose and diabetes. J Biol Chem. 2007;282:9364–9371. doi: 10.1074/jbc.M608985200. [DOI] [PubMed] [Google Scholar]
- 62.Wang X, Song X, Zhuo W, et al. The regulatory mechanism of Hsp90alpha secretion and its function in tumor malignancy. Proc Natl Acad Sci U S A. 2009;106:21288–21293. doi: 10.1073/pnas.0908151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Lu XA, Song X, et al. Thr90 phosphorylation of Hsp90alpha by protein kinase A regulates its chaperone machinery. Biochem J. 2012;441:387–397. doi: 10.1042/BJ20110855. [DOI] [PubMed] [Google Scholar]
- 64.Mollapour M, Tsutsumi S, Donnelly AC, et al. Swe1Wee1-dependent tyrosine phosphorylation of Hsp90 regulates distinct facets of chaperone function. Mol Cell. 2010;37:333–343. doi: 10.1016/j.molcel.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mollapour M, Tsutsumi S, Neckers L. Hsp90 phosphorylation, Wee1 and the cell cycle. Cell Cycle. 2010;9:2310–2316. doi: 10.4161/cc.9.12.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duval M, Le Boeuf F, Huot J, Gratton JP. Src-mediated phosphorylation of Hsp90 in response to vascular endothelial growth factor (VEGF) is required for VEGF receptor-2 signaling to endothelial NO synthase. Mol Biol Cell. 2007;18:4659–4668. doi: 10.1091/mbc.E07-05-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scroggins BT, Robzyk K, Wang D, et al. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell. 2007;25:151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Y, Rao R, Shen J, et al. Role of acetylation and extracellular location of heat shock protein 90alpha in tumor cell invasion. Cancer Res. 2008;68:4833–4842. doi: 10.1158/0008-5472.CAN-08-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu X, Guo ZS, Marcu MG, et al. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J Natl Cancer Inst. 2002;94:504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- 70.Martinez-Ruiz A, Villanueva L, Gonzalez de Orduna C, et al. S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci U S A. 2005;102:8525–8530. doi: 10.1073/pnas.0407294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Retzlaff M, Stahl M, Eberl HC, et al. Hsp90 is regulated by a switch point in the C-terminal domain. EMBO Rep. 2009;10:1147–1153. doi: 10.1038/embor.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen WY, Chang FR, Huang ZY, Chen JH, Wu YC, Wu CC. Tubocapsenolide A, a novel withanolide, inhibits proliferation and induces apoptosis in MDA-MB-231 cells by thiol oxidation of heat shock proteins. J Biol Chem. 2008;283:17184–17193. doi: 10.1074/jbc.M709447200. [DOI] [PubMed] [Google Scholar]
- 73.Carbone DL, Doorn JA, Kiebler Z, Ickes BR, Petersen DR. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J Pharmacol Exp Ther. 2005;315:8–15. doi: 10.1124/jpet.105.088088. [DOI] [PubMed] [Google Scholar]
- 74.Connell P, Ballinger CA, Jiang J, et al. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3:93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- 75.Kundrat L, Regan L. Identification of residues on Hsp70 and Hsp90 ubiquitinated by the cochaperone CHIP. J Mol Biol. 2010;395:587–594. doi: 10.1016/j.jmb.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blank M, Mandel M, Keisari Y, Meruelo D, Lavie G. Enhanced ubiquitinylation of heat shock protein 90 as a potential mechanism for mitotic cell death in cancer cells induced with hypericin. Cancer Res. 2003;63:8241–8247. [PubMed] [Google Scholar]
- 77.Murtagh J, Lu H, Schwartz EL. Taxotere-induced inhibition of human endothelial cell migration is a result of heat shock protein 90 degradation. Cancer Res. 2006;66:8192–8199. doi: 10.1158/0008-5472.CAN-06-0748. [DOI] [PubMed] [Google Scholar]
- 78.Abu-Farha M, Lanouette S, Elisma F, et al. Proteomic analyses of the SMYD family interactomes identify HSP90 as a novel target for SMYD2. J Mol Cell Biol. 2011;3:301–308. doi: 10.1093/jmcb/mjr025. [DOI] [PubMed] [Google Scholar]
- 79.Donlin LT, Andresen C, Just S, et al. Smyd2 controls cytoplasmic lysine methylation of Hsp90 and myofilament organization. Genes Dev. 2012;26:114–119. doi: 10.1101/gad.177758.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bandhakavi S, McCann RO, Hanna DE, Glover CV. A positive feedback loop between protein kinase CKII and Cdc37 promotes the activity of multiple protein kinases. J Biol Chem. 2003;278:2829–2836. doi: 10.1074/jbc.M206662200. [DOI] [PubMed] [Google Scholar]
- 81.Kobayashi T, Nakatani Y, Tanioka T, et al. Regulation of cytosolic prostaglandin E synthase by phosphorylation. Biochem J. 2004;381:59–69. doi: 10.1042/BJ20040118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lassle M, Blatch GL, Kundra V, Takatori T, Zetter BR. Stress-inducible, murine protein mSTI1. Characterization of binding domains for heat shock proteins and in vitro phosphorylation by different kinases. J Biol Chem. 1997;272:1876–1884. doi: 10.1074/jbc.272.3.1876. [DOI] [PubMed] [Google Scholar]
- 83.Longshaw VM, Dirr HW, Blatch GL, Lassle M. The in vitro phosphorylation of the co-chaperone mSTI1 by cell cycle kinases substantiates a predicted casein kinase II-p34cdc2-NLS (CcN) motif. Biol Chem. 2000;381:1133–1138. doi: 10.1515/BC.2000.139. [DOI] [PubMed] [Google Scholar]
- 84.Jolly C, Michelland S, Rocchi M, Robert-Nicoud M, Vourc'h C. Analysis of the transcriptional activity of amplified genes in tumour cells by fluorescence in situ hybridization. Hum Genet. 1997;101:81–87. doi: 10.1007/s004390050591. [DOI] [PubMed] [Google Scholar]
- 85.Gallegos Ruiz MI, Floor K, Roepman P, et al. Integration of gene dosage and gene expression in non-small cell lung cancer, identification of HSP90 as potential target. PLoS One. 2008;3:e0001722. doi: 10.1371/journal.pone.0001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Passarino G, Cavalleri GL, Stecconi R, et al. Molecular variation of human HSP90alpha and HSP90beta genes in Caucasians. Hum Mutat. 2003;21:554–555. doi: 10.1002/humu.9141. [DOI] [PubMed] [Google Scholar]
- 87.McDowell CL, Bryan Sutton R, Obermann WM. Expression of Hsp90 chaperone [corrected] proteins in human tumor tissue. Int J Biol Macromol. 2009;45:310–314. doi: 10.1016/j.ijbiomac.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 88.Whitesell L, Shifrin SD, Schwab G, Neckers LM. Benzoquinonoid ansamycins possess selective tumoricidal activity unrelated to src kinase inhibition. Cancer Res. 1992;52:1721–1728. [PubMed] [Google Scholar]
- 89.Pratt WB. Control of steroid receptor function and cytoplasmic-nuclear transport by heat shock proteins. Bioessays. 1992;14:841–848. doi: 10.1002/bies.950141209. [DOI] [PubMed] [Google Scholar]
- 90.Erlichman C. Tanespimycin: the opportunities and challenges of targeting heat shock protein 90. Expert Opin Investig Drugs. 2009;18:861–868. doi: 10.1517/13543780902953699. [DOI] [PubMed] [Google Scholar]
- 91. [December 20, 2011];Bristol-Myers Squibb halts development of tanespimycin. Available at: http://www.myelomabeacon.com/news/2010/07/22/tanespimycin-developmenthalted/)
- 92.Pacey S, Wilson RH, Walton M, et al. A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin Cancer Res. 2011;17:1561–1570. doi: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miller K, Rosen LS, Modi S, et al. Phase I trial of alvespimycin (KOS-1022; 17-DMAG) and trastuzumab (T). ASCO Meeting Abstracts. 2007;25:1115. [Google Scholar]
- 94.Lancet JE, Gojo I, Burton M, et al. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia. 2010;24:699–705. doi: 10.1038/leu.2009.292. [DOI] [PubMed] [Google Scholar]
- 95.Delmotte P, Delmotte-Plaque J. A new antifungal substance of fungal origin. Nature. 1953;171:344. doi: 10.1038/171344a0. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y, Trepel JB, Neckers LM, Giaccone G. STA-9090, a small-molecule Hsp90 inhibitor for the potential treatment of cancer. Curr Opin Investig Drugs. 2010;11:1466–1476. [PubMed] [Google Scholar]
- 97.Schroder CP, Pedersen JV, Chua S, et al. Use of biomarkers and imaging to evaluate the treatment effect of AUY922, an HSP90 inhibitor, in patients with HER2+ or ER+ metastatic breast cancer. ASCO Meeting Abstracts. 2011;29:e11024. [Google Scholar]
- 98. [December 27, 2011];Study of Hsp90 Inhibitor AUY922 for the Treatment of Patients With Refractory Gastrointestinal Stromal Tumor. Available at: http://www.cancer.gov/clinicaltrials/search/view?cdrid=706997&version=HealthProfessional&protocolsearchid=10012955.
- 99. [February 7, 2012];A Study of the HSP90 Inhibitor AUY922. Available at: http://www.cancer.gov/clinicaltrials/search/view?cdrid=719298&version=HealthProfessional&protocolsearchid=10012955.
- 100. [February 7, 2012];Cancer: HSP990. Available at: http://production.investis.com/ver/rdc2/pipeline/hsp990/
- 101. [February 7, 2012];A Study of KW-2478 in Combination With Bortezomib in Subjects With Relapsed and/or Refractory Multiple Myeloma. Available at: http://www.cancer.gov/clinicaltrials/search/view?cdrid=665838&version=HealthProfessional&protocolsearchid=10012955.
- 102. [February 7, 2012];A Study to Investigate the Safety and Efficacy of AT13387, Alone or in Combination With Imatinib, in Patients With GIST. Available at: http://www.cancer.gov/clinicaltrials/search/view?cdrid=695610&version=HealthProfessional&protocolsearchid=10012955.
- 103.Chandarlapaty S, Sawai A, Ye Q, et al. SNX2112, a synthetic heat shock protein 90 inhibitor, has potent antitumor activity against HER kinase-dependent cancers. Clin Cancer Res. 2008;14:240–248. doi: 10.1158/1078-0432.CCR-07-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rajan A, Kelly RJ, Trepel JB, et al. A phase I study of PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas. Clin Cancer Res. 2011;17:6831–6839. doi: 10.1158/1078-0432.CCR-11-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. [February 7, 2012];Safety Study of Pharmacokinetics of XL888 in Adults With Solid Tumors. Available at: http://clinicaltrials.gov/ct2/show/NCT00796484?term=XL-888&rank=1.
- 106.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATPbinding domain in the carboxyl terminus of the chaperone. J Biol Chem. 2000;275:37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 107.Zhao H, Donnelly AC, Kusuma BR, et al. Engineering an antibiotic to fight cancer: optimization of the novobiocin scaffold to produce anti-proliferative agents. J Med Chem. 2011;54:3839–3853. doi: 10.1021/jm200148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duerfeldt AS, Blagg BS. Hsp90 inhibition: elimination of shock and stress. Bioorg Med Chem Lett. 2010;20:4983–4987. doi: 10.1016/j.bmcl.2010.06.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 110.Kummar S, Gutierrez ME, Gardner ER, et al. Phase I trial of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), a heat shock protein inhibitor, administered twice weekly in patients with advanced malignancies. Eur J Cancer. 2010;46:340–347. doi: 10.1016/j.ejca.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Egorin MJ, Lagattuta TF, Hamburger DR, et al. Pharmacokinetics, tissue distribution, and metabolism of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (NSC 707545) in CD2F1 mice and Fischer 344 rats. Cancer Chemother Pharmacol. 2002;49:7–19. doi: 10.1007/s00280-001-0380-8. [DOI] [PubMed] [Google Scholar]
- 112.Eiseman JL, Lan J, Lagattuta TF, et al. Pharmacokinetics and pharmacodynamics of 17-demethoxy 17-[[(2-dimethylamino)ethyl]amino]geldanamycin (17DMAG, NSC 707545) in C.B-17 SCID mice bearing MDA-MB-231 human breast cancer xenografts. Cancer Chemother Pharmacol. 2005;55:21–32. doi: 10.1007/s00280-004-0865-3. [DOI] [PubMed] [Google Scholar]
- 113.Kamal A, Thao L, Sensintaffar J, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 114.Nagengast WB, de Korte MA, Oude Munnink TH, et al. 89Zr-bevacizumab PET of early antiangiogenic tumor response to treatment with HSP90 inhibitor NVP-AUY922. J Nucl Med. 2010;51:761–767. doi: 10.2967/jnumed.109.071043. [DOI] [PubMed] [Google Scholar]
- 115.Smith-Jones PM, Solit DB, Akhurst T, Afroze F, Rosen N, Larson SM. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol. 2004;22:701–706. doi: 10.1038/nbt968. [DOI] [PubMed] [Google Scholar]
- 116.Smith-Jones PM, Solit D, Afroze F, Rosen N, Larson SM. Early tumor response to Hsp90 therapy using HER2 PET: comparison with 18F-FDG PET. J Nucl Med. 2006;47:793–796. [PMC free article] [PubMed] [Google Scholar]
- 117. [February 7, 2012];PET Imaging of Cancer Patients Using 124I-PUH71: A Pilot Study. Available at: http://www.cancer.gov/clinicaltrials/search/view?cdrid=692682&version=HealthProfessional&protocolsearchid=9955666.
- 118.Gorre ME, Ellwood-Yen K, Chiosis G, Rosen N, Sawyers CL. BCR-ABL point mutants isolated from patients with imatinib mesylate-resistant chronic myeloid leukemia remain sensitive to inhibitors of the BCR-ABL chaperone heat shock protein 90. Blood. 2002;100:3041–3044. doi: 10.1182/blood-2002-05-1361. [DOI] [PubMed] [Google Scholar]
- 119.Wang S, Pashtan I, Tsutsumi S, Xu W, Neckers L. Cancer cells harboring MET gene amplification activate alternative signaling pathways to escape MET inhibition but remain sensitive to Hsp90 inhibitors. Cell Cycle. 2009;8:2050–2056. doi: 10.4161/cc.8.13.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bachleitner-Hofmann T, Sun MY, Chen CT, et al. Antitumor activity of SNX-2112, a synthetic heat shock protein-90 inhibitor, in MET-amplified tumor cells with or without resistance to selective MET Inhibition. Clin Cancer Res. 2011;17:122–133. doi: 10.1158/1078-0432.CCR-10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shimamura T, Li D, Ji H, et al. Hsp90 inhibition suppresses mutant EGFR-T790M signaling and overcomes kinase inhibitor resistance. Cancer Res. 2008;68:5827–5838. doi: 10.1158/0008-5472.CAN-07-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rice JW, Veal JM, Barabasz A, et al. Targeting of multiple signaling pathways by the Hsp90 inhibitor SNX-2112 in EGFR resistance models as a single agent or in combination with erlotinib. Oncol Res. 2009;18:229–242. doi: 10.3727/096504009x12596189659240. [DOI] [PubMed] [Google Scholar]
- 123.Shimamura T, Lowell AM, Engelman JA, Shapiro GI. Epidermal growth factor receptors harboring kinase domain mutations associate with the heat shock protein 90 chaperone and are destabilized following exposure to geldanamycins. Cancer Res. 2005;65:6401–6408. doi: 10.1158/0008-5472.CAN-05-0933. [DOI] [PubMed] [Google Scholar]
- 124.Xu W, Soga S, Beebe K, et al. Sensitivity of epidermal growth factor receptor and ErbB2 exon 20 insertion mutants to Hsp90 inhibition. Br J Cancer. 2007;97:741–744. doi: 10.1038/sj.bjc.6603950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Katayama R, Khan TM, Benes C, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A. 2011;108:7535–7540. doi: 10.1073/pnas.1019559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sasaki T, Okuda K, Zheng W, et al. The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res. 2010;70:10038–10043. doi: 10.1158/0008-5472.CAN-10-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oikonomou E, Koc M, Sourkova V, Andera L, Pintzas A. Selective BRAFV600E inhibitor PLX4720, requires TRAIL assistance to overcome oncogenic PIK3CA resistance. PLoS One. 2011;6:e21632. doi: 10.1371/journal.pone.0021632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Scaltriti M, Serra V, Normant E, et al. Antitumor activity of the Hsp90 inhibitor IPI-504 in HER2-positive trastuzumab-resistant breast cancer. Mol Cancer Ther. 2011;10:817–824. doi: 10.1158/1535-7163.MCT-10-0966. [DOI] [PubMed] [Google Scholar]
- 129.Chandarlapaty S, Scaltriti M, Angelini P, et al. Inhibitors of HSP90 block p95-HER2 signaling in Trastuzumab-resistant tumors and suppress their growth. Oncogene. 2010;29:325–334. doi: 10.1038/onc.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Caldas-Lopes E, Cerchietti L, Ahn JH, et al. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc Natl Acad Sci U S A. 2009;106:8368–8373. doi: 10.1073/pnas.0903392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roue G, Perez-Galan P, Mozos A, et al. The Hsp90 inhibitor IPI-504 overcomes bortezomib resistance in mantle cell lymphoma in vitro and in vivo by down-regulation of the prosurvival ER chaperone BiP/Grp78. Blood. 2011;117:1270–1279. doi: 10.1182/blood-2010-04-278853. [DOI] [PubMed] [Google Scholar]
- 132.De Raedt T, Walton Z, Yecies JL, et al. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell. 2011;20:400–413. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu W, Trepel J, Neckers L. Ras, ROS and proteotoxic stress: a delicate balance. Cancer Cell. 2011;20:281–282. doi: 10.1016/j.ccr.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 135.Weinberg F, Hamanaka R, Wheaton WW, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. [February 7, 2012];Phase 1b/2 Study of Retaspimycin HCl (IPI-504) in Combination With Everolimus in KRAS Mutant Non-small Cell Lung Cancer. Available at: http://www.cancer.gov/clinicaltrials/search/view?cdrid=710867&version=HealthProfessional&protocolsearchid=10114616.
- 137.McCollum AK, TenEyck CJ, Stensgard B, et al. P-Glycoprotein-mediated resistance to Hsp90-directed therapy is eclipsed by the heat shock response. Cancer Res. 2008;68:7419–7427. doi: 10.1158/0008-5472.CAN-07-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ali A, Bharadwaj S, O'Carroll R, Ovsenek N. HSP90 interacts with and regulates the activity of heat shock factor 1 in Xenopus oocytes. Mol Cell Biol. 1998;18:4949–4960. doi: 10.1128/mcb.18.9.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lamoureux F, Thomas C, Yin MJ, et al. Clusterin inhibition using OGX-011 synergistically enhances Hsp90 inhibitor activity by suppressing the heat shock response in castrate-resistant prostate cancer. Cancer Res. 2011;71:5838–5849. doi: 10.1158/0008-5472.CAN-11-0994. [DOI] [PubMed] [Google Scholar]
- 140.Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alphadegradative pathway. J Biol Chem. 2002;277:29936–29944. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 141.Mabjeesh NJ, Post DE, Willard MT, et al. Geldanamycin induces degradation of hypoxia-inducible factor 1alpha protein via the proteosome pathway in prostate cancer cells. Cancer Res. 2002;62:2478–2482. [PubMed] [Google Scholar]
- 142.Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL. RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol Cell. 2007;25:207–217. doi: 10.1016/j.molcel.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109:4839–4845. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fucikova J, Kralikova P, Fialova A, et al. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821–4833. doi: 10.1158/0008-5472.CAN-11-0950. [DOI] [PubMed] [Google Scholar]
- 145.Schnaider T, Somogyi J, Csermely P, Szamel M. The Hsp90-specific inhibitor, geldanamycin, blocks CD28-mediated activation of human T lymphocytes. Life Sci. 1998;63:949–954. doi: 10.1016/s0024-3205(98)00352-x. [DOI] [PubMed] [Google Scholar]
- 146.Schnaider T, Somogyi J, Csermely P, Szamel M. The Hsp90-specific inhibitor geldanamycin selectively disrupts kinase-mediated signaling events of T-lymphocyte activation. Cell Stress Chaperones. 2000;5:52–61. doi: 10.1043/1355-8145(2000)005<0052:THSIGS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yorgin PD, Hartson SD, Fellah AM, et al. Effects of geldanamycin, a heat-shock protein 90-binding agent, on T cell function and T cell nonreceptor protein tyrosine kinases. J Immunol. 2000;164:2915–2923. doi: 10.4049/jimmunol.164.6.2915. [DOI] [PubMed] [Google Scholar]
- 148.Zhu FG, Pisetsky DS. Role of the heat shock protein 90 in immune response stimulation by bacterial DNA and synthetic oligonucleotides. Infect Immun. 2001;69:5546–5552. doi: 10.1128/IAI.69.9.5546-5552.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fionda C, Soriani A, Malgarini G, Iannitto ML, Santoni A, Cippitelli M. Heat shock protein-90 inhibitors increase MHC class I-related chain A and B ligand expression on multiple myeloma cells and their ability to trigger NK cell degranulation. J Immunol. 2009;183:4385–4394. doi: 10.4049/jimmunol.0901797. [DOI] [PubMed] [Google Scholar]
- 150.Boll B, Eltaib F, Reiners KS, et al. Heat shock protein 90 inhibitor BIIB021 (CNF2024) depletes NF-kappaB and sensitizes Hodgkin's lymphoma cells for natural killer cell-mediated cytotoxicity. Clin Cancer Res. 2009;15:5108–5116. doi: 10.1158/1078-0432.CCR-09-0213. [DOI] [PubMed] [Google Scholar]
- 151.Lewis J, Devin A, Miller A, et al. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J Biol Chem. 2000;275:10519–10526. doi: 10.1074/jbc.275.14.10519. [DOI] [PubMed] [Google Scholar]
- 152.Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell. 2002;9:401–410. doi: 10.1016/s1097-2765(02)00450-1. [DOI] [PubMed] [Google Scholar]
- 153.Broemer M, Krappmann D, Scheidereit C. Requirement of Hsp90 activity for IkappaB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-kappaB activation. Oncogene. 2004;23:5378–5386. doi: 10.1038/sj.onc.1207705. [DOI] [PubMed] [Google Scholar]
- 154.Pittet JF, Lee H, Pespeni M, O'Mahony A, Roux J, Welch WJ. Stress-induced inhibition of the NF-kappaB signaling pathway results from the insolubilization of the IkappaB kinase complex following its dissociation from heat shock protein 90. J Immunol. 2005;174:384–394. doi: 10.4049/jimmunol.174.1.384. [DOI] [PubMed] [Google Scholar]
- 155.Shimp SK, 3rd, Parson CD, Regna NL, et al. HSP90 inhibition by 17-DMAG reduces inflammation in J774 macrophages through suppression of Akt and nuclear factor-kappaB pathways. Inflamm Res. 2012 doi: 10.1007/s00011-012-0442-x. [DOI] [PubMed] [Google Scholar]
- 156.Salminen A, Paimela T, Suuronen T, Kaarniranta K. Innate immunity meets with cellular stress at the IKK complex: regulation of the IKK complex by HSP70 and HSP90. Immunol Lett. 2008;117:9–15. doi: 10.1016/j.imlet.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 157.Yun TJ, Harning EK, Giza K, et al. EC144, a synthetic inhibitor of heat shock protein 90, blocks innate and adaptive immune responses in models of inflammation and autoimmunity. J Immunol. 2011;186:563–575. doi: 10.4049/jimmunol.1000222. [DOI] [PubMed] [Google Scholar]
- 158.Stuehler C, Mielke S, Chatterjee M, et al. Selective depletion of alloreactive T cells by targeted therapy of heat shock protein 90: a novel strategy for control of graft-versus-host disease. Blood. 2009;114:2829–2836. doi: 10.1182/blood-2009-06-224600. [DOI] [PubMed] [Google Scholar]
- 159.Annamalai B, Liu X, Gopal U, Isaacs JS. Hsp90 is an essential regulator of EphA2 receptor stability and signaling: implications for cancer cell migration and metastasis. Mol Cancer Res. 2009;7:1021–1032. doi: 10.1158/1541-7786.MCR-08-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kawabe M, Mandic M, Taylor JL, et al. Heat shock protein 90 inhibitor 17-dimethylaminoethylamino-17-demethoxygeldanamycin enhances EphA2+ tumor cell recognition by specific CD8+ T cells. Cancer Res. 2009;69:6995–7003. doi: 10.1158/0008-5472.CAN-08-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wesa AK, Herrem CJ, Mandic M, et al. Enhancement in specific CD8+ T cell recognition of EphA2+ tumors in vitro and in vivo after treatment with ligand agonists. J Immunol. 2008;181:7721–7727. doi: 10.4049/jimmunol.181.11.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bae J, Mitsiades C, Tai YT, et al. Phenotypic and functional effects of heat shock protein 90 inhibition on dendritic cell. J Immunol. 2007;178:7730–7737. doi: 10.4049/jimmunol.178.12.7730. [DOI] [PubMed] [Google Scholar]
- 163.Callahan MK, Garg M, Srivastava PK. Heat-shock protein 90 associates with N-terminal extended peptides and is required for direct and indirect antigen presentation. Proc Natl Acad Sci U S A. 2008;105:1662–1667. doi: 10.1073/pnas.0711365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lev A, Takeda K, Zanker D, et al. The exception that reinforces the rule: crosspriming by cytosolic peptides that escape degradation. Immunity. 2008;28:787–798. doi: 10.1016/j.immuni.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ichiyanagi T, Imai T, Kajiwara C, et al. Essential role of endogenous heat shock protein 90 of dendritic cells in antigen cross-presentation. J Immunol. 2010;185:2693–2700. doi: 10.4049/jimmunol.1000821. [DOI] [PubMed] [Google Scholar]
- 166.Donze O, Abbas-Terki T, Picard D. The Hsp90 chaperone complex is both a facilitator and a repressor of the dsRNA-dependent kinase PKR. EMBO J. 2001;20:3771–3780. doi: 10.1093/emboj/20.14.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yun BG, Matts RL. Hsp90 functions to balance the phosphorylation state of Akt during C2C12 myoblast differentiation. Cell Signal. 2005;17:1477–1485. doi: 10.1016/j.cellsig.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 168.Xu W, Yuan X, Beebe K, Xiang Z, Neckers L. Loss of Hsp90 association up-regulates Src-dependent ErbB2 activity. Mol Cell Biol. 2007;27:220–228. doi: 10.1128/MCB.00899-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Koga F, Xu W, Karpova TS, McNally JG, Baron R, Neckers L. Hsp90 inhibition transiently activates Src kinase and promotes Src-dependent Akt and Erk activation. Proc Natl Acad Sci U S A. 2006;103:11318–11322. doi: 10.1073/pnas.0604705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Meares GP, Zmijewska AA, Jope RS. Heat shock protein-90 dampens and directs signaling stimulated by insulin-like growth factor-1 and insulin. FEBS Lett. 2004;574:181–186. doi: 10.1016/j.febslet.2004.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yano A, Tsutsumi S, Soga S, et al. Inhibition of Hsp90 activates osteoclast c-Src signaling and promotes growth of prostate carcinoma cells in bone. Proc Natl Acad Sci U S A. 2008;105:15541–15546. doi: 10.1073/pnas.0805354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stemmann O, Neidig A, Kocher T, Wilm M, Lechner J. Hsp90 enables Ctf13p/Skp1p to nucleate the budding yeast kinetochore. Proc Natl Acad Sci U S A. 2002;99:8585–8590. doi: 10.1073/pnas.082223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Davies AE, Kaplan KB. Hsp90-Sgt1 and Skp1 target human Mis12 complexes to ensure efficient formation of kinetochore-microtubule binding sites. J Cell Biol. 2010;189:261–274. doi: 10.1083/jcb.200910036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jarosz DF, Lindquist S. Hsp90 and environmental stress transform the adaptive value of natural genetic variation. Science. 2011;330:1820–1824. doi: 10.1126/science.1195487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 176.Tang YC, Williams BR, Siegel JJ, Amon A. Identification of aneuploidy-selective antiproliferation compounds. Cell. 2011;144:499–512. doi: 10.1016/j.cell.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]