Abstract
Aortic valve calcification without outflow obstruction (stenosis) is common in the elderly and increases the risk of cardiovascular morbidity and mortality. Although high blood pressure (BP) measured at the doctor’s office is known to be associated with aortic valve calcification, little is known about the association between 24-hour ambulatory BP (ABP) and aortic valve calcification. Our objective was to clarify the association between ABP variables and aortic valve calcification. The study population consisted of 737 patients (mean age 71±9 years) participating in the Cardiovascular Abnormalities and Brain Lesions (CABL) study who underwent 24-hour ABP monitoring. Each aortic valve leaflet was graded on a scale of 0 (normal) to 3 (severe calcification). A total valve score (values 0–9) was calculated as the sum of all leaflets’ score. Advanced aortic valve calcification (score ≥4) was present in 77 subjects (10.4%). All systolic ABP variables (except systolic BP nocturnal decline) and mean asleep diastolic BP were positively associated with advanced calcification, while normal dipping status and diastolic BP nocturnal decline were negatively associated. Multiple regression analysis indicated that mean awake diastolic BP (OR 1.31, 95% CI 1.01–1.71) and asleep diastolic BP (OR 1.34, 95% CI 1.04–1.72) remained independently associated with advanced calcification after adjustment for age, sex, cigarette smoking, diabetes mellitus, hypercholesterolemia, hypertension, serum creatinine, and any degree of aortic insufficiency. Diastolic ABP is independently associated with advanced calcification. This finding may have important implications in gaining further insight into the mechanism of aortic valve calcification.
Keywords: ambulatory blood pressure, aortic valve calcification
Introduction
Aortic valve calcification (AVC), which is defined as focal areas of increased echogenicity and thickening of the aortic valve leaflets without any resultant outflow obstruction (stenosis), is a subclinical abnormality that is common in the elderly1, 2, and has traditionally been considered a relatively benign degenerative process. However, this view has recently been challenged, as AVC has been found to be associated with cardiovascular morbidity and mortality.3 AVC is an active process 4 related to the traditional risk factors for atherosclerosis, including male sex 5, hypertension 5–8, hypercholesterolemia7, 8, smoking 5, and diabetes7, 8, as well as hyperparathyroidism.9 Local hemodynamic factors, such as low shear stress and high tensile stress on the aortic leaflets, can also facilitate endothelial injury and are associated with early atherosclerotic changes including AVC.10, 11
Ambulatory blood pressure monitoring (ABPM) provides information that is not obtained from conventional office blood pressure (BP) readings, and is also more reliable and reproducible than office BP measurements12. Several studies have demonstrated that target organ damage13, 14 and prognosis15 are more closely associated with ambulatory BP than with office BP. Moreover, ABPM provides data on possibly reduced nocturnal BP decline (“non-dipping”) and increased 24-hour BP variability, which are associated with subsequent cardiovascular events and cardiovascular mortality16, 17. However, the association between ABPM variables and AVC has not been addressed in a properly sized clinical study.
The aim of the present study was therefore to assess the relationship between AVC and ABPM variables (24-hour/awake/asleep mean values, variability and nocturnal decline) in an unselected sample of the general population.
Methods
Study population
This study was conducted at Columbia University Medical Center. The study sample was derived from the National Institutes of Neurological Disorders and Stroke (NINDS)-sponsored Cardiovascular Abnormalities and Brain Lesions (CABL) study, whose aim is to assess the relationship between subclinical cardiovascular disease and silent brain infarctions in a community-based cohort. Participants in CABL were drawn from the Northern Manhattan Study (NOMAS), an epidemiologic study carried out in New York City. Extensive details about the population and enrollment of NOMAS have been published previously18. Briefly, subjects were eligible if they (1) had never been diagnosed with stroke, (2) were aged ≥50 years, and (3) resided in northern Manhattan for ≥3 months in a household with a telephone. Cardiovascular risk factors were ascertained through direct examination and interviews conducted by trained research assistants. Among the variables used in the analysis, hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg (mean of two readings taken in a research clinic setting) or a patient’s self-reported history of hypertension or antihypertensive medication use. Diabetes mellitus was defined by the patient’s self-report of history and/or current use of insulin or hypoglycemic agents, or a fasting glucose of >126 mg/dl, tested on at least two occasions in each participant. Smoking status was defined as cigarette smoking at any time in the past or present. Hypercholesterolemia was defined as total serum cholesterol >240 mg/dl, a patient’s self-report of hypercholesterolemia or use of lipid-lowering medications.
The study was approved by the institutional review board of Columbia University Medical Center, and informed consent was obtained from all study participants.
Detection of aortic valve calcification
Two-dimensional transthoracic images of the aortic valve were obtained by a registered cardiac sonographer following a standardized protocol with a commercially available system (iE33; Philips Medical Systems, Andover, MA) equipped with a 2.5-MHz to 3.5-MHz transducer. All the tests were stored on digital media for subsequent analysis. AVC was defined as bright dense echoes more than 1 mm in size on one or more cusps19. Each valve leaflet was graded on a scale of 0 (normal) to 3 (severe calcification). A total valve score (values 0–9) was calculated as the sum of the scores of all leaflets1. A score ≥4 was defined as advanced AVC, which means presence of at least one moderate calcified deposit and two mild calcified deposits in three leaflets (Figure 1). We excluded 18 subjects with bicuspid aortic valve or aortic stenosis (defined as peak flow velocity ≥ 2.5m/s).
Figure 1.
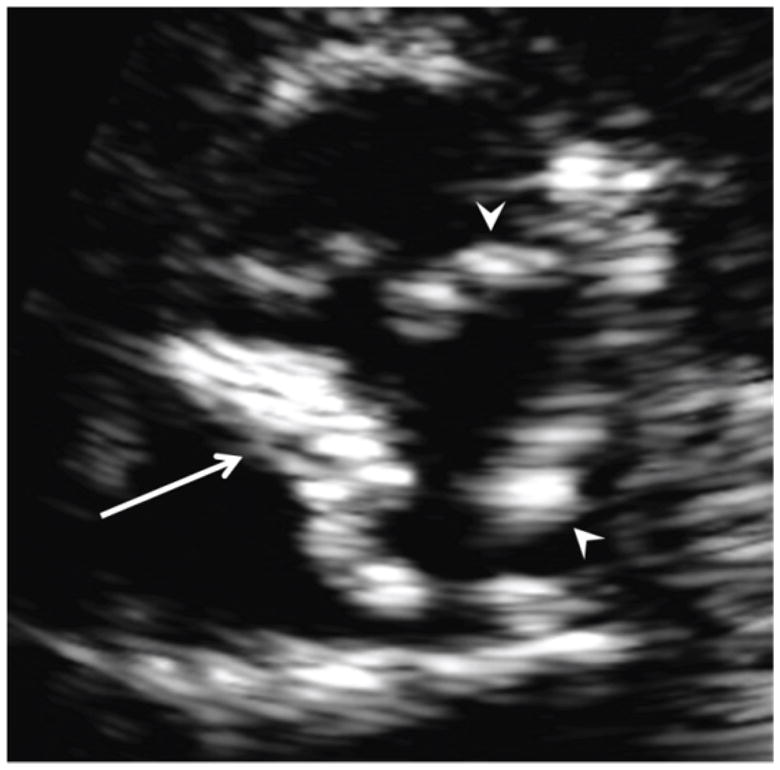
Two-dimensional image (parasternal short-axis view) of the aortic valve showing an example of calcification score determination. The calcification of the non-coronary cusp (arrow) was graded as severe (score=3) and the calcifications of right and left coronary cusps (arrowheads) were graded as mild (score=1). The total valve calcification score, obtained from the sum of the individual scores, was therefore 5.
All images were interpreted by a single experienced echocardiographer (SI) blinded to subject’s characteristics and risk factors.
Ambulatory Blood Pressure Assessment
An ambulatory BP monitor (SpaceLabs Model 90207, Redmond, WA) was used to assess 24-hour BP as the subjects performed their normal activities. The accuracy and reliability of the device have been previously validated according to the Association for the Advancement of Medical Instrumentation criteria 20. ABPM was performed according to previously published protocols with a BP cuff appropriately sized to arm circumference and placed on the subject’s non-dominant arm. The monitor was set to automatically record BP at 15-minute intervals during awake hours and 30-minute intervals during sleep hours. Before use, the device was calibrated against a reference mercury manometer; the criterion for target agreement was within ± 5 mmHg. Recordings were uploaded, stored, and exported with the aid of ambulatory BP report management system software (SpaceLabs Systems, 2004). The mean SBP and DBP obtained by ambulatory BP assessment were calculated for a 24-hour period and separately for awake and sleep periods, which were determined using subjects’ diary reports of actual sleep and awake times. BP variability was calculated as the SDs of mean awake and asleep SBP and DBP. The percent nocturnal decline in SBP was calculated as (awake SBP - asleep SBP) × 100/ awake SBP; nocturnal decline in DBP was similarly calculated17. The management of the ABPM data and computation of summary measures was supervised by one of the authors (JES). Office BP was assessed as the average of two measurements in sitting position taken by a research assistant using a mercury sphygmomanometer and BP cuff appropriately sized to arm circumference.
Statistical analysis
Continuous variables are expressed as mean value ± SD, categorical variables as percentages. The distribution of ABPM variables and potential covariates was evaluated in the overall population and among advanced aortic valve calcification (Score ≥4) versus the rest (Score ≤3). Comparison between groups was assessed using unpaired Student’s t-test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. A multiple logistic regression analysis was carried out to identify those ABPM variables that were associated with advanced AVC adjusting for age, male sex, cigarette smoking, hypercholesterolemia, diabetes mellitus, hypertension, serum creatinine, and any degree of aortic insufficiency. (model 1). In addition to the above covariates, we adjusted for anti-hypertensive medication use and anti-hypertensive medication class (model 2), and further for anti-hypercholesterolemic medication (model 3). We also adjusted for awake/asleep mean SBP/DBP levels in the analyses involving BP variability, and for 24-hour mean BP in the analyses involving nocturnal BP decline. A p value of < 0.05 was considered statistically significant for all tests. Statistical analysis was performed using SAS software version 9.2 (SAS Institute Inc, Cary, NC).
Results
Study Cohort
A total of 1004 participants were enrolled in CABL, but 169 did not have ABPM because of refusal (n=155) or inability to complete the test (n=14). This left 835 participants with ABPM data, out of which incomplete information on AVC or insufficient ABPM data points were observed in 98, leaving a final sample size of 737. The distribution of calcification score was Score 0 (n=18), Score 1 (n=105), Score 2 (n=389), Score 3(n=148), Score 4 (n=57), Score 5 (n=16), Score 6 (n=3), Score 7 (n=1). Advanced AVC (score ≥4) was present in 77 subjects (10.4%). Clinical characteristics of the study cohort and ABPM findings according to the aortic valve calcification score are shown in Table 1. Office SBP, but not office DBP, tended to be associated with advanced AVC. Age, male sex, hypertension, serum creatinine, any degree of aortic insufficiency and all SBP variables (except SBP nocturnal decline, which was of borderline significance) were positively associated with advanced AVC. Normal dipping status and DBP nocturnal decline were inversely associated with advanced AVC. Mean asleep DBP was positively associated with advanced AVC.
Table 1.
Baseline characteristics and ABPM according to the aortic valve calcification score
| Characteristics | Overall population N=737 | Calcification Score (≤3) N=660 | Calcification Score (≥4) N=77 | P value |
|---|---|---|---|---|
| Age | 71±9 | 70±9 | 78±8 | <0.0001 |
| Male,% | 40% | 38% | 55% | 0.01 |
| Race/ethnicity | 0.02 | |||
| White,% | 13% | 12% | 14% | |
| Black, % | 17% | 15% | 27% | |
| Hispanic,% | 71% | 72% | 58% | |
| Cigarette smoking (ever), % | 54% | 54% | 55% | 0.90 |
| Diabetes mellitus, % | 30% | 29% | 31% | 0.79 |
| Hypertension, % | 78% | 77% | 88% | 0.03 |
| Hypercholesterolemia, % | 68% | 67% | 75% | 0.20 |
| Serum creatinine, mg/dl | 0.9±0.3 | 0.9±0.3 | 1.1±0.5 | 0.002 |
| Left ventricular mass index, g/m2 | 103±25 | 102±25 | 110±30 | 0.03 |
| Left atrial dimension, mm | 39±5 | 39±5 | 42±5 | <0.001 |
| Aortic insufficiency (any degree), % | 32% | 29% | 55% | <0.001 |
| Moderate or severe aortic insufficiency, % | 2% | 2% | 3% | 0.65 |
| Left ventricular ejection fraction, % | 63±8 | 63±8 | 62±8 | 0.22 |
| Anti-hypertensive medication use, % | 72% | 71% | 79% | 0.14 |
| ACEI/ARB | 18% | 15% | 22% | 0.38 |
| βblocker | 12% | 13% | 5% | 0.06 |
| Ca blocker | 20% | 19% | 27% | 0.18 |
| Diuretic | 14% | 13% | 21% | 0.13 |
| Other medication | 11% | 12% | 6% | 0.21 |
| Anti-hypercholesterolemic medication use, % | 57% | 55% | 72% | 0.01 |
| Aspirin use, % | 69% | 68% | 78% | 0.06 |
| 24 hr mean HR, bpm | 71.8±9.1 | 71.8±9.1 | 72.0±9.0 | 0.80 |
| Mean awake HR, bpm | 75.1±10.1 | 75.1±10.2 | 75.0±9.3 | 0.94 |
| Mean asleep HR, bpm | 65.9±8.9 | 65.8±8.9 | 66.8±9.2 | 0.39 |
| Office SBP, mmHg | 135.6±17.5 | 135.2±17.4 | 139.2±18.1 | 0.06 |
| Office DBP, mmHg | 78.6±9.4 | 78.5±9.2 | 79.3±11.0 | 0.47 |
| 24 hr mean SBP, mmHg | 124.7±14.4 | 124.1±14.4 | 130.5±12.8 | 0.002 |
| 24 hr mean DBP, mmHg | 71.5±8.5 | 71.3±8.5 | 72.9±8.7 | 0.12 |
| SBP nocturnal decline, % | 7.4±7.3 | 7.5±7.4 | 5.9±6.4 | 0.07 |
| DBP nocturnal decline, % | 10.6±8.3 | 10.9±8.3 | 8.3±8.1 | 0.01 |
| Mean awake SBP, mmHg | 128.3±14.7 | 127.7±14.7 | 133.5±13.5 | <0.001 |
| Mean awake DBP, mmHg | 74.3±9.0 | 74.1±9.0 | 75.4±9.1 | 0.23 |
| Mean asleep SBP, mmHg | 118.7±16.3 | 117.9±16.3 | 125.5±14.8 | <0.001 |
| Mean asleep DBP, mmHg | 66.3±9.4 | 66.0±9.3 | 68.9±10.2 | 0.01 |
| SBP awake variability, mmHg | 12.5±3.4 | 12.4±3.3 | 13.4±3.9 | 0.02 |
| DBP awake variability, mmHg | 9.0±2.3 | 9.0±2.3 | 9.0±2.5 | 0.84 |
| SBP asleep variability, mmHg | 10.9±3.4 | 10.8±3.4 | 11.6±3.5 | 0.05 |
| DBP asleep variability, mmHg | 8.0±2.5 | 8.0±2.5 | 8.2±2.7 | 0.58 |
| Normal BP dipping*, % | 37% | 38% | 25% | 0.03 |
= more than 10% decline in SBP from awake to asleep mean values
ABPM variables associated with advanced AVC - Multivariable analysis
Table 2 shows the ABPM variables associated with the presence of advanced AVC (score ≥4) and their adjusted standardized odds ratio obtained by multiple logistic regression analysis. Awake SBP were associated with a borderline increase in risk of advanced AVC. Awake (Odds Ratios 1.31 per 1 standard deviation increase, 95% CI 1.01 to 1.71, p<0.05), asleep (OR 1.34 per 1 standard deviation increase, 95% CI 1.04 to 1.72, p<0.05), mean DBP remained independently associated with advanced AVC after adjustment for age, sex, smoking status, diabetes mellitus, hypercholesterolemia, hypertension, serum creatinine, and the presence of aortic insufficiency (model 1). Additional adjusted models including anti-hypertensive medications and their classes (model 2) and anti-hypercholesterolemic medication use (model 3) did not significantly affect the results, with the exception that 24-hour mean DBP became significantly associated with advanced AVC in model 3. Nocturnal BP non-dipping, SBP and DBP variability were not associated with advanced AVC in the models and when we used the coefficient of variation instead that was not different in results from SD.
Table 2.
ABPM variables associated with advanced aortic valve calcification (score ≥4mm) -Multivariate analysis
| BP variables | Advanced calcification | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | |
| Office SBP | 1.03 | 0.79–1.34 | 0.85 | 1.00 | 0.77–1.32 | 0.98 | 1.08 | 0.82–1.43 | 0.59 |
| Office DBP | 1.18 | 0.91–1.53 | 0.22 | 1.17 | 0.90–1.52 | 0.24 | 1.21 | 0.91–1.59 | 0.19 |
| Mean 24 h SBP | 1.24 | 0.96–1.60 | 0.11 | 1.24 | 0.96–1.61 | 0.10 | 1.24 | 0.94–1.62 | 0.12 |
| Mean 24 hr DBP | 1.29 | 0.99–1.67 | 0.06 | 1.29 | 0.99–1.68 | 0.06 | 1.42 | 1.06–1.89 | 0.02 |
| Mean awake SBP | 1.27 | 0.99–1.63 | 0.06 | 1.28 | 1.00–1.65 | 0.053 | 1.30 | 1.00–1.69 | 0.053 |
| Mean awake DBP | 1.31 | 1.01–1.71 | 0.04 | 1.32 | 1.01–1.72 | 0.04 | 1.45 | 1.09–1.94 | 0.01 |
| Mean asleep SBP | 1.21 | 0.93–1.57 | 0.15 | 1.22 | 0.94–1.58 | 0.14 | 1.23 | 0.94–1.62 | 0.13 |
| Mean asleep DBP | 1.34 | 1.04–1.72 | 0.02 | 1.35 | 1.04–1.74 | 0.02 | 1.49 | 1.13–1.97 | 0.005 |
| SBP awake variability | 1.02 | 0.79–1.31 | 0.91 | 1.02 | 0.79–1.32 | 0.87 | 1.03 | 0.78–1.35 | 0.85 |
| SBP awake variability † | 0.94 | 0.72–1.23 | 0.66 | 0.95 | 0.73–1.24 | 0.69 | 0.95 | 0.71–1.26 | 0.72 |
| DBP awake variability | 0.93 | 0.71–1.22 | 0.61 | 0.92 | 0.70–1.21 | 0.54 | 0.96 | 0.72–1.29 | 0.81 |
| DBP awake variability † | 0.84 | 0.63–1.12 | 0.24 | 0.83 | 0.62–1.11 | 0.21 | 0.86 | 0.63–1.17 | 0.32 |
| SBP asleep variability | 1.01 | 0.79–1.30 | 0.94 | 1.02 | 0.79–1.31 | 0.89 | 0.98 | 0.74–1.28 | 0.86 |
| SBP asleep variability † | 0.96 | 0.74–1.25 | 0.76 | 0.97 | 0.74–1.25 | 0.80 | 0.92 | 0.70–1.22 | 0.56 |
| DBP asleep variability | 0.96 | 0.75–1.22 | 0.71 | 0.96 | 0.75–1.22 | 0.74 | 0.99 | 0.76–1.30 | 0.95 |
| DBP asleep variability † | 0.89 | 0.69–1.14 | 0.36 | 0.89 | 0.69–1.14 | 0.35 | 0.90 | 0.68–1.19 | 0.47 |
| SBP nocturnal decline | 1.01 | 0.97–1.04 | 0.80 | 1.01 | 0.97–1.04 | 0.78 | 1.00 | 0.96–1.04 | 0.94 |
| SBP nocturnal decline ‡ | 1.01 | 0.97–1.05 | 0.69 | 1.01 | 0.97–1.05 | 0.68 | 1.00 | 0.97–1.05 | 0.83 |
| DBP nocturnal decline | 0.98 | 0.95–1.01 | 0.20 | 0.98 | 0.95–1.01 | 0.20 | 0.97 | 0.94–1.01 | 0.11 |
| DBP nocturnal decline ‡ | 0.98 | 0.95–1.01 | 0.23 | 0.98 | 0.95–1.01 | 0.22 | 0.98 | 0.94–1.01 | 0.15 |
| Normal dipping status | 0.87 | 0.48–1.58 | 0.64 | 0.89 | 0.49–1.61 | 0.69 | 0.79 | 0.41–1.51 | 0.47 |
Each row is a separate analysis;
Model 1: adjusts for age, male sex, cigarette smoking, hypercholesterolemia, diabetes mellitus, hypertension, serum creatinine, and the presence of aortic insufficiency.
Model 2: as in model 1 plus anti-hypertensive medication use and anti-hypertensive medication classes.
Model 3: as in model 2 plus anti-hypercholesterolemic medication use. All odds ratios are for 1 standard deviation change in the variable.
In addition to the general adjustments, this analysis was further adjusted for awake/asleep mean systolic/diastolic BP.
In addition to the general adjustments, this analysis was further adjusted for 24-hour BP.
Only 46 subjects had LV ejection fraction less than 50% (41, or 6% in the lower AVC group; 5, or 7%, in the higher AVC group; P=0.92). Their exclusion from the analyses did not significantly affect the associations observed.
AVC and BP control
Among hypertensives (N=577), the proportion of advanced AVC was significantly lower in subjects with better controlled BP by ABPM (daytime mean SBP≤135 and daytime mean DBP≤85 than in those less well controlled (9.5% vs. 15.9%; P=0.02).
Discussion
In the present study, we observed that all systolic ABPM variables (except SBP nocturnal decline) were associated with the presence of advanced AVC. However, only 24-hour/awake/asleep mean DBP remained significantly associated with advanced calcification after adjustment for accepted atherosclerotic risk factors. SBP variables and a blunted nocturnal decline in both systolic and diastolic BP were not found to be associated with advanced calcification in the multivariable analyses. These results suggest that DBP may be more important than SBP for early-stage atherosclerotic process in the aortic valve. To the best of our knowledge, this is the first study to demonstrate that 24-hour/awake/asleep mean DBP are associated with advanced AVC in the general population.
AVC without outflow obstruction is a subclinical abnormality that is common in the elderly1, 2. Once AVC has occurred, it causes less effective stress sharing among the leaflets and facilitates injury to leaflet endothelial cells 21. In general, this is an irreversible process that may lead to clinically significant stenosis and consequent poor clinical outcome3, 22,23. Therefore, the identification of factors involved in the subclinical stages of valve calcification may provide therapeutic targets to inhibit or slow down the progression towards aortic stenosis.
There is growing evidence that hypertension is associated with calcific aortic valve disease1, 6–8. However, most studies on the topic have also included aortic valve stenosis, making the separate analysis of subclinical valvular abnormalities more difficult or not feasible. Moreover, no studies to date have explored the separate impact of SBP and DBP on AVC using 24-hour ABPM values. The finding that subclinical AVC is more strongly associated with DBP than with SBP may provide insight into the pathogenesis of AVC, which is not completely understood. Early alterations occur on the aortic side of the leaflet that is exposed to low shear stress and elevated stretch, suggesting local hemodynamic factors may be the initiating factors in valvular calcification 24. Shear stress, the drag force acting on the leaflets that results from blood flow, plays an important role in the development of endothelial dysfunction and atherosclerosis25,11. Shear stress acting along the endothelium on the aortic side of the leaflet is lower than that on the ventricular side of the leaflet 25 and influenced by end-diastolic pressure. Aortic cusps are stretched (cyclic stretch) to overcome backward pressure, i.e. end-diastolic pressure, from the blood in the aorta during diastole. Elevated cyclic stretch causes matrix remodeling in the aortic valve leaflets10. DBP, rather than SBP, is assumed to affect the blood flow characteristics on the aortic side of leaflet, increasing the effects of low shear stress and cyclic stretch. Consequently, elevated 24-hour/awake/asleep mean DBP may be both the initiating factor of an atherosclerotic process in the aortic valve and a therapeutic target to slow its progression. In our study, participants with well controlled ABPM values had significantly less frequency of advanced AVC, suggesting a role for BP control in preventing advanced AVC.
In our study, 24-hour/awake/asleep mean DBP were associated with AVC, but office DBP was not. This is consistent with previous studies showing the superiority of ABPM over office BP in predicting hypertensive vascular damage. A previously published study conducted in older subjects (mean age of 82.4 years) reported that 24-hour mean SBP, but not office SBP, was associated with white matter hyperintensity volume, which is a marker of microvascular brain damage 26. We have previously reported that asleep systolic BP variability, which can only be obtained from ABPM, is associated with large arch atherosclerotic plaque in the same study population as the present report9. The mechanisms behind the different results (SBP associated with aortic atherosclerosis, DBP associated with aortic valve calcification) are unclear, but may reflect regional differences in the atherosclerotic process. For example, the aortic valve, which is a mobile structure that regulates high velocity blood flow, might be more resistant to the effects of shear stress (and therefore SBP) than to cyclic stretch.
The Oulu Project Elucidating Risk of Atherosclerosis (OPERA) study reported that BP non-dipping status was associated with carotid intima-media thickness, a marker of early atherosclerosis27. In our study, neither BP dipping status (a binary variable) nor nocturnal BP decline (a continuous measure) was associated with AVC. In the same cohort, we previously showed that BP non-dipping status was not associated with aortic arch atherosclerosis 9. The role of BP dipping status as a risk factor for early atherosclerosis remains to be clarified, and BP non-dipping may not be involved in early valvular atherosclerotic changes.
Our study has some limitations. First, cross-sectional data cannot provide evidence of causality. Prospective studies would be necessary to assess whether BP variables indeed predict progression of aortic calcification to aortic stenosis. Second, given the relatively small number of advanced calcifications, we could not compare the relationship between BP and aortic calcification across different race/ethnic groups. Third, although we adjusted our analyses for the most pertinent variables that may affect aortic valve calcification, our study was not designed as a case-control, and the possibility exists that some confounding factors may have been incompletely adjusted for.
Perspectives
Awake, asleep, and 24-hour mean DBP, but not office DBP or any SBP variables, were independently associated with advanced aortic valve calcification, which carries a greater risk of cardiac events. This finding implies that DBP may be more important than SBP in the onset and progression of aortic valve calcification. From a practical standpoint, this finding supports the hypothesis that tight DBP control may provide clinical benefit for preventing or slowing the progression of calcified aortic disease. Further investigation is needed to confirm this hypothesis.
Novelty and Significance.
What is New
This is the first study to assess the relationship between ABPM variables (24-hour/awake/asleep mean values, variability and nocturnal decline) and aortic valve calcification
This is also the first study to demonstrate that 24-hour/awake/asleep mean DBP values, but not SBP values, are associated with advanced aortic valve calcification
What Is Relevant?
Awake, asleep, and 24-hour mean DBP, but not office DBP, are independently associated with advanced aortic valve calcification, and may identify individuals at increased risk
Lowering DBP values may avoid or delay the progression of AV calcification towards aortic stenosis
Summary.
Awake, asleep, and 24-hour mean DBP but not office DBP or SBP values, are independently associated with advanced aortic valve calcification. This finding supports the hypothesis that DBP control may provide clinical benefit in preventing or slowing the progression of calcified aortic disease.
Acknowledgments
Sources of Finding
This study was supported by R01 NS36286 and R37 NS29993 from the National Institute of Neurological Disorders and Stroke (NINDS).
Abbreviations
- AVC
aortic valve calcification
- ABPM
ambulatory blood pressure monitoring
- BP
blood pressure
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
Footnotes
Disclosure
None
References
- 1.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular health study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 2.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: An echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 3.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 4.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the national heart and lung and blood institute aortic stenosis working group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens DS, Katz R, Takasu J, Kronmal R, Budoff MJ, O’Brien KD. Incidence and progression of aortic valve calcium in the multi-ethnic study of atherosclerosis (mesa) Am J Cardiol. 2010;105:701–708. doi: 10.1016/j.amjcard.2009.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindroos M, Kupari M, Valvanne J, Strandberg T, Heikkila J, Tilvis R. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J. 1994;15:865–870. doi: 10.1093/oxfordjournals.eurheartj.a060602. [DOI] [PubMed] [Google Scholar]
- 7.Boon A, Cheriex E, Lodder J, Kessels F. Cardiac valve calcification: Characteristics of patients with calcification of the mitral annulus or aortic valve. Heart. 1997;78:472–474. doi: 10.1136/hrt.78.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronow WS, Schwartz KS, Koenigsberg M. Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am J Cardiol. 1987;59:998–999. doi: 10.1016/0002-9149(87)91144-1. [DOI] [PubMed] [Google Scholar]
- 9.Iwata S, Walker MD, Di Tullio MR, Hyodo E, Jin Z, Liu R, Sacco RL, Homma S, Silverberg SJ. Aortic valve calcification in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2012;97:132–137. doi: 10.1210/jc.2011-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balachandran K, Sucosky P, Jo H, Yoganathan AP. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: Implications for degenerative aortic valve disease. Am J Physiol Heart Circ Physiol. 2009;296:H756–764. doi: 10.1152/ajpheart.00900.2008. [DOI] [PubMed] [Google Scholar]
- 11.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 12.James GD, Pickering TG, Yee LS, Harshfield GA, Riva S, Laragh JH. The reproducibility of average ambulatory, home, and clinic pressures. Hypertension. 1988;11:545–549. doi: 10.1161/01.hyp.11.6.545. [DOI] [PubMed] [Google Scholar]
- 13.Mancia G, Parati G. Ambulatory blood pressure monitoring and organ damage. Hypertension. 2000;36:894–900. doi: 10.1161/01.hyp.36.5.894. [DOI] [PubMed] [Google Scholar]
- 14.Iwata S, Jin Z, Schwartz JE, Homma S, Elkind MS, Rundek T, Sacco RL, Di Tullio MR. Relationship between ambulatory blood pressure and aortic arch atherosclerosis. Atherosclerosis. 2012;221:427–431. doi: 10.1016/j.atherosclerosis.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohkubo T, Hozawa A, Nagai K, Kikuya M, Tsuji I, Ito S, Satoh H, Hisamichi S, Imai Y. Prediction of stroke by ambulatory blood pressure monitoring versus screening blood pressure measurements in a general population: The ohasama study. J Hypertens. 2000;18:847–854. doi: 10.1097/00004872-200018070-00005. [DOI] [PubMed] [Google Scholar]
- 16.Sander D, Kukla C, Klingelhofer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: A 3-year follow-up study. Circulation. 2000;102:1536–1541. doi: 10.1161/01.cir.102.13.1536. [DOI] [PubMed] [Google Scholar]
- 17.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: The ohasama study. J Hypertens. 2002;20:2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 18.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and hispanics: The northern manhattan study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 19.Wang AY, Woo J, Wang M, Sea MM, Ip R, Li PK, Lui SF, Sanderson JE. Association of inflammation and malnutrition with cardiac valve calcification in continuous ambulatory peritoneal dialysis patients. J Am Soc Nephrol. 2001;12:1927–1936. doi: 10.1681/ASN.V1291927. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien E, Coats A, Owens P, Petrie J, Padfield PL, Littler WA, de Swiet M, Mee F. Use and interpretation of ambulatory blood pressure monitoring: Recommendations of the british hypertension society. BMJ. 2000;320:1128–1134. doi: 10.1136/bmj.320.7242.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deck JD. Endothelial cell orientation on aortic valve leaflets. Cardiovasc Res. 1986;20:760–767. doi: 10.1093/cvr/20.10.760. [DOI] [PubMed] [Google Scholar]
- 22.Faggiano P, Antonini-Canterin F, Erlicher A, Romeo C, Cervesato E, Pavan D, Piazza R, Huang G, Nicolosi GL. Progression of aortic valve sclerosis to aortic stenosis. Am J Cardiol. 2003;91:99–101. doi: 10.1016/s0002-9149(02)03011-4. [DOI] [PubMed] [Google Scholar]
- 23.Rosenhek R, Klaar U, Schemper M, Scholten C, Heger M, Gabriel H, Binder T, Maurer G, Baumgartner H. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J. 2004;25:199–205. doi: 10.1016/j.ehj.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg EJ, Mack PJ, Schoen FJ, Garcia-Cardena G, Kaazempur Mofrad MR. Hemodynamic environments from opposing sides of human aortic valve leaflets evoke distinct endothelial phenotypes in vitro. Cardiovasc Eng. 2010;10:5–11. doi: 10.1007/s10558-009-9089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White WB, Wolfson L, Wakefield DB, Hall CB, Campbell P, Moscufo N, Schmidt J, Kaplan RF, Pearlson G, Guttmann CR. Average daily blood pressure, not office blood pressure, is associated with progression of cerebrovascular disease and cognitive decline in older people. Circulation. 2011;124:2312–2319. doi: 10.1161/CIRCULATIONAHA.111.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasunta RL, Kesaniemi YA, Ylitalo A, Ukkola O. Nondipping pattern and carotid atherosclerosis in a middle-aged population: Opera study. Am J Hypertens. 2012;25:60–66. doi: 10.1038/ajh.2011.159. [DOI] [PubMed] [Google Scholar]


