Abstract
Objective
To determine the frequency and predictors of cardiac stress testing prior to elective noncardiac surgery in Medicare patients with no indications for cardiovascular evaluation.
Background
American College of Cardiology/American Heart Association guidelines indicate that patients without class I (AHA high risk) or class II cardiac conditions (clinical risk factors) should not undergo cardiac stress testing prior to elective noncardiac, non-vascular surgery.
Methods
We used 5% Medicare inpatient claims data (1996–2008) to identify patients aged ≥ 66 years who underwent elective general surgical, urologic, or orthopedic procedures (N=211,202). We examined use of preoperative stress testing in the subset of patients with no diagnoses consistent with cardiac disease (N=74,785). Bivariate and multivariate analyses were used to identify predictors of preoperative cardiac stress testing.
Results
Of the patients with no cardiac indications for preoperative stress testing, 3.75% (N= 2,803) received stress testing in the 2 months before surgery. The rate of preoperative stress testing increased from 1.72% in 1996 to 6.44% in 2007 (p<0.0001). Multivariate analysis adjusting for patient and hospital characteristics showed a significant increase in preoperative stress testing over time. Female gender (OR=1.11, 95% CI=1.02–1.21), presence of other comorbidities (OR=1.22, 95% CI=1.09–1.35), high risk procedure (OR=2.42, 95% CI=2.04–2.89), and larger hospital size (OR=1.17, 95% CI=1.03–1.32) were positive predictors of stress testing. Patients living in regions with greater Medicare expenditures (OR=1.24, 95% CI=1.05–1.45) were more likely to receive stress tests.
Conclusions
In a 5% sample of Medicare claims data, 2,803 patients underwent preoperative stress testing without any indications. When these results are applied to the entire Medicare population, we estimate that there are over 56,000 patients who underwent unnecessary preoperative stress testing. The rate of testing in patients without cardiac indications increased significantly over time.
INTRODUCTION
Over the next 30 years, the number of noncardiac surgical procedures being performed on older Americans is expected to nearly double, from the current 6 million to almost 12 million per year.1 Cardiac complications are the leading cause of death among patients undergoing elective noncardiac surgery2, 3 and increase hospital stay by an estimated 11 days.4 The purpose of preoperative evaluation of patients with cardiac risk factors is to detect cardiac conditions in order to guide management and decrease risk to the patient.
In 2007, The American College of Cardiology (ACC) and American Heart Association (AHA) Task Force on Practice Guidelines published current guidelines regarding preoperative cardiovascular evaluation for individuals undergoing noncardiac surgery.1 The task force classified noncardiac surgical procedures into the following categories based on incidence of cardiac death and nonfatal myocardial infarction: vascular (cardiac risk > 5%); intermediate risk (cardiac risk 1–5%); and low risk (cardiac risk < 1%). The task force determined that patients with an active cardiac condition (AHA class I condition—high risk), including unstable or severe angina, recent myocardial infarction (MI), decompensated heart failure, significant arrhythmias, or severe valvular disease, should undergo preoperative stress testing.1 Additionally, patients with no active cardiac conditions who are undergoing vascular or intermediate risk surgery, with at least one clinical risk factor (AHA Class II condition) and a functional capacity of 4 metabolic equivalents (METS) or less (assessed by activities of daily living), may require cardiac stress testing if the testing will change management. The presence of multiple minor risk factors (e.g., advanced age, abnormal ECG, uncontrolled systemic hypertension) might lead to higher suspicion of coronary artery disease but is not incorporated into the recommendations for testing. Finally, stress testing is not recommended in the following groups: patients who require emergency noncardiac surgery, patients with no active cardiac conditions (AHA class I conditions) or clinical risk factors (AHA class II conditions) who are undergoing intermediate risk surgery, patients with no active cardiac conditions (AHA class I conditions) who are undergoing low risk surgery, and asymptomatic patients with a functional capacity of 4 METS or more.1 In the 1996 and 2002 ACC/AHA guidelines, the recommendations for patients without active cardiac conditions or clinical risk factors (AHA classes I and II) were similar to 2007 guidelines.5, 6
Patterns of cardiac stress testing prior to surgical procedures have not been systematically studied in older adults. The objectives of our study were to determine the frequency of cardiac stress testing prior to elective noncardiac surgery in a sample of Medicare beneficiaries with no identifiable cardiac risk factors (AHA class I or II conditions), and to determine predictors for overutilization of preoperative stress testing in this population. We identified preoperative stress tests in Medicare beneficiaries who underwent elective, inpatient surgical procedures (cholecystectomy, colectomy, pancreatectomy, mastectomy, radical prostatectomy, transurethral resection of the prostate, radical nephrectomy, back surgery, knee replacement, hip replacement) between 1996 to 2008 who did not have active cardiac conditions or clinical risk factors (AHA class I or II conditions) based on ACC/AHA guidelines. We evaluated time trends in cardiac stress testing and identified patient, hospital, and area characteristics that predicted utilization of preoperative stress testing.
METHODS
This study was approved by the Institutional Review Board at the University of Texas Medical Branch at Galveston.
Data Source
The 5% national Medicare sample Research Identifiable Files provided a random sample of Medicare beneficiaries for the years 1995–2008. The Medicare data files used for this study included the Denominator file, the Medicare Provider Analysis and Review files (MEDPAR), the Physician/Supplier Standard Analytical File (SAF), Outpatient SAF, and the Provider of Services (POS) file. The MEDPAR files contain information on hospital admissions, including type of admission, diagnoses, and procedures. The Physician/Supplier SAF contains Medicare part B claims from service providers that are not facilities and the Outpatient SAF contains part B claims for facilities. The POS file contains information on Medicare-approved facilities (e.g., hospitals), including address, total number of beds, medical school affiliation, and metropolitan size. In addition, United States Census data from the year 2000 and the American Medical Association (AMA) Physician Masterfile were linked to the 5% Medicare sample to provide zip code level population data and physician information.
Cohort Selection
Using MEDPAR inpatient claims data from 1996 to 2008, we selected Medicare beneficiaries who underwent the following surgical procedures for any diagnosis, according to International Classification of Disease-9-Clinical Modification (ICD-9-CM) Procedure codes: cholecystectomy (51.21–51.24), colectomy (45.7–45.79, 45.8), pancreatectomy (52.6, 52.7, 52.51–52.53, 52.59), mastectomy (85.41, 85.43, 84.45, 85.47), radical prostatectomy (60.5), transurethral resection of the prostate (60.2, 60.21, 60.29), radical nephrectomy (55.5, 55.51), back surgery (03.0, 03.02, 03.09, 03.6, 78.50, 78.59, 78.60, 78.69, 78.90, 78.99, 80.5, 80.50–80.52, 80.59, 81.0, 81.00–81.09), knee replacement (81.41, 81.54), and hip replacement (81.51, 81.59). After the initial cohort selection, the following inclusion criteria were applied: 1) patients undergoing elective admissions only based on the Medicare “Type of Admission” code, 2) patients aged 66 years and older, and 3) patients enrolled in Medicare parts A and B without HMO for 12 months before the surgical procedure. This process yielded 259,632 surgical procedures performed on 211,202 patients between 1996 and 2008. We selected the first surgical procedure for each patient, yielding a cohort of 211,202 patients.
From this cohort, we identified a subset of patient with no active cardiac conditions or clinical risk factors in the year prior to surgery (see list of conditions in Table 1). In addition to these diagnoses, we excluded patients who had undergone coronary artery bypass grafting, coronary angioplasty, coronary stent placement, or cardiac catheterization in the year prior to the elective noncardiac surgery. With these exclusions, the final sample size of the cohort was 74,785.
Table 1.
Active Cardiac Conditions and Clinical Risk Factors Used to Exclude Patients from the Study Cohort
| Class I Conditions/Active Cardiac Conditions | Class II Conditions/Clinical Risk Factors | Additional Conditions |
|---|---|---|
| Intermediate coronary syndrome Acute myocardial infarction (MI) Congestive heart failure (CHF) – Unspecified Left heart failure (HF) Systolic HF Diastolic HF Combined systolic and diastolic HF HF – unspecified Hypertensive heart disease with H Mobitz II atrioventricular (AV) block 3rd degree AV block Atrial fibrillation Paroxysmal supraventricular tachycardia Paroxysmal ventricular tachycardia Paroxysmal ventricular tachycardia – unspecified SA node dysfunction – other Rheumatic mitral stenosis Rheumatic mitral insuffiency Rheumatic mitral stenosis with insufficiency Other and unspecified rheumatic mitral valve disease Mitral valve disorders Diseases of mitral and aortic valves Ventricular fibrillation Ventricular flutter |
Acute coronary occlusion without MI Acute coronary insufficiency Subendocardial ischemia Old MI Angina decubitus Prinzmetal angina Other and unspecified angina pectoris Other forms chronic ischemic heart disease Arteriosclerotic cardiovascular disease Diabetes mellitus Acute renal failure Chronic kidney disease Hypertensive heart disease and chronic kidney disease Renal failure unspecified Cerebrovascular disease |
Conduction disorders Dysrhythmias Tricuspid valve disorders Nonrheumatic, pulmonic valve disorders Rheumatic diseases of other endocardial structures Other rheumatic heart diseases Sinus bradycardia |
Definition of Stress Test
We identified patients who had a stress test in the two months prior to elective noncardiac surgery. Stress tests were identified using the CPT codes for stress echocardiography (93350), exercise treadmill or pharmacological stress test (93015–93018), and myocardial nuclear imaging (78460–78465, 78472–78496).
Study Variables
Patient demographic characteristics included age at the time of surgery (65–69, 70–74, 75–79, 80–84, 85+ years), sex, and race (white, black, other). Race was obtained from the Medicare denominator file, which uses Social Security Administration demographic data. Patient comorbidities were ascertained using ICD-9-CM diagnosis and procedure codes from the inpatient and outpatient claims in the year prior to the date of surgery and classified using the Charlson comorbidity index.7, 8 The majority of patients had zero comorbidities; therefore, the comorbidity index was categorized into a dichotomous variable (≥ 1 versus none).
We classified surgical procedures as “high risk” (colectomy, pancreatectomy, radical prostatectomy, radical nephrectomy), “intermediate risk” (cholecystectomy, back surgery, knee replacement, hip replacement), and “low risk” (mastectomy, transurethral resection of the prostate) based on risk of morbidity and mortality. AHA guidelines classify surgical procedures based on incidence of cardiac death and nonfatal myocardial infarction. The AHA categories, in order of decreasing risk, are vascular, intermediate risk, and low risk. All of the surgical procedures included in this study are nonvascular and therefore qualify as intermediate to low risk surgery in terms of cardiac risk. Diagnosis codes from the surgery admission were used to identify cardiac complications following surgery. A subset of Class I and Class II cardiac conditions were selected to indicate cardiac complications. These conditions are listed in Table 2. As described above, the analytic cohort was restricted to patients with no diagnoses for Class I or Class II cardiac conditions in inpatient or outpatient claims in the year prior to date of surgery. Therefore, patients with diagnoses on the surgical claim for any conditions from the subset of Class I or Class II cardiac conditions (Table 2) were considered to have had postoperative cardiac complications. Limitations of this approach are described in the discussion section. In addition to cardiac complications, we examined 30-day mortality in patients following surgery.
Table 2.
Cardiac Conditions and Diagnostic Codes Used to Identify Postoperative Cardiac Complications in Medicare Patients Following Elective Noncardiac Surgery
| Cardiac condition | ICD-9 code |
|---|---|
| Acute myocardial infarction (MI) | 410.0 – 410.92 |
| Acute coronary occlusion without MI | 411.81 |
| Acute coronary insufficiency or subendocardial ischemia | 411.89 |
| Angina | 413.0, 413.1, 413.9 |
| Congestive heart failure, unspecified | 428.0 |
| Left heart failure | 428.1 |
| Systolic heart failure, unspecified or acute | 428.2, 428.20, 428.21 |
| Diastolic heat failure, unspecified or acute | 428.3, 428.30, 428.31 |
| Hypertensive heart disease with heart failure | 402.0, 402.01, 402.11, 402.91 |
| Mobitz II atrioventricular block | 426.12 |
| 3rd degree atrioventricular block | 426.0 |
| Atrial fibrillation | 427.31 |
| Paroxysmal supraventricular tachycardia | 427.0 |
| Paroxysmal ventricular tachycardia | 427.1 |
Hospital characteristics included hospital size (number of beds), type (nonprofit, profit, government), and medical school affiliation. Cardiologist visits prior to surgery were identified using Evaluation and Management codes in the Carrier file. The size of the metropolitan statistical area (MSA) where the hospital was located was categorized as < 250,000; 250,000–1,000,000; and ≥ 1,000,000. Each patient was linked to a Dartmouth Health Atlas Hospital Referral Region (HRR) based on zip code of residence. The number of cardiologists per 100,000 persons in the HRR was included as a measure of physician availability using Dartmouth Atlas data. This variable was estimated as the average of the number of cardiologists in the HRR in 1996 and 2006 and stratified into quartiles. Total Medicare reimbursements (Parts A and B) per enrollee in the HRR in the year of surgery were included to measure Medicare expenditures for each HRR. Expenditures were adjusted to 2006 U.S. dollars using the medical care component of the consumer price index and stratified into quartiles. Expenditure data for operations performed in 2008 were unavailable; 2007 data for each HRR were used as an estimate of 2008 expenditure data and adjusted to 2006 U.S. dollars. Sensitivity analyses showed that imputed results were substantively equivalent to those excluding 2008 expenditure data.
Statistical Analysis
Descriptive characteristics were provided for the overall cohort. The rate of stress testing was compared across groups using chi-square tests. Changes in stress testing over the study period were evaluated using the Cochran-Armitage trend test.9, 10 The increase in testing over time was not linear; therefore, year of surgery was included as a categorical variable in multivariate models, with 1996 as the referent group. The rate of testing was also compared across U.S. regions, cardiologist availability in the HRR, and Medicare expenditures in the HRR using chi-square tests.
A generalized estimating equation model with a binomial distribution, logit link, and exchangeable correlation structure was used to identify independent predictors of preoperative cardiac stress testing. Model parameters and standard errors were estimated using generalized estimating equations to account for clustering of patients within hospitals and HRRs.11 The hospital identifier nested within the HRR identifier were specified as the cluster level variables. The sample size for multivariate analyses was 74,117 because 670 patients were unable to be linked to a hospital or HRR. All other patients had complete covariate data. The final multivariate model included sex, year of surgery, comorbidity, size of the MSA, risk level of the surgical procedure, U.S. region of residence, hospital bed size, and cardiologist availability and Medicare expenditures in the HRR. Models were developed by entering candidate covariates associated with stress testing in bivariate analyses into the model. Variables with an adjusted p value of < 0.10 were retained in the multivariate model. Model fit was assessed using the quasi-likelihood under the Independence Criterion (QIC);12 a lower QIC value indicates a better model fit. A P- value of < 0.05 was considered statistically significant for all analyses. SAS 9.2 statistical software (Cary, NC) was used for data management and statistical analysis.
RESULTS
A total of 211,202 patients undergoing elective noncardiac, nonvascular surgery from 1996 to 2008 were identified from 259,632 Medicare operative claims. Of these patients, 74,785 had no diagnoses consistent with cardiac conditions (Table 1) in the year prior to surgery. The overall study cohort is described in Table 3. Patients undergoing elective surgery with no cardiac indications for stress testing were mostly female (60.3%) and mostly white (93.1%). Fifty-nine percent (59.2%) of patients were age 65–74. The majority of the study cohort had a Charlson comorbidity score of zero (88.1%). Most patients in the cohort were undergoing intermediate risk surgery (64.3%) with a smaller portion of patients undergoing high risk (18.9%) or low risk (16.8%) procedures. Only 2.23% of patients had cardiac complications and 0.53% died within 30 days of surgery. The majority of patients underwent surgery in nonprofit hospitals and hospitals with no medical school affiliation.
Table 3.
Characteristics of Medicare Patients Undergoing Elective Noncardiac Surgery With No Indications for Stress Testing (N=74,785)
| Patient Characteristics | N (%) |
|---|---|
| Age | |
| 65–69 | 21,276 (28.45) |
| 70–74 | 23,024 (30.79) |
| 75–79 | 17,090 (22.85) |
| 80–84 | 9,505 (12.71) |
| 85+ | 3,890 (5.20) |
| Sex | |
| Male | 29,675 (39.68) |
| Female | 45,110 (60.32) |
| Race | |
| White | 69,658 (93.14) |
| Black | 3,369 (4.50) |
| Other | 1,758 (2.35) |
| Charlson comorbidity | |
| 0 | 65,895 (88.11) |
| ≥1 | 8,890 (11.89) |
| Cardiac complications | 1,666 (2.23) |
| 30-day mortality | 400 (0.53) |
| Surgical procedure | |
| High risk | 14,141 (18.91) |
| Intermediate risk | 48,062 (64.27) |
| Low risk | 12,582 (16.82) |
| US region | |
| Northeast | 12,247 (16.38) |
| Midwest | 22,992 (30.74) |
| South | 26,469 (35.39) |
| Mountain West | 5,215 (6.97) |
| Pacific West | 7,860 (10.51) |
| Size MSA | |
| <250,000 | 17,979 (24.04) |
| 250,000–1,000,000 | 21,426 (28.65) |
| 1,000,000+ | 35,378 (47.31) |
| Medical School Affiliation | |
| Yes | 28,793 (38.50) |
| No | 45992 (61.50) |
| Hospital Type | |
| Nonprofit | 56,759 (75.90) |
| Profit | 9,198 (12.30) |
| Government | 8,826 (11.80) |
| Hospital size | |
| <200 beds | 21,726 (29.05) |
| 200–324 beds | 16,503 (22.07) |
| 325–500 beds | 17,994 (24.06) |
| >500 beds | 18,562 (24.82) |
| Cardiologists per 100,000 population | |
| Q1 (0–5) | 18147 (24.48) |
| Q2 (5–<6) | 18607 (25.10) |
| Q3 (6–<7) | 18558 (25.04) |
| Q4 (≥ 7) | 18807 (25.37) |
| Medicare expenditures in HRR (2006$) | |
| Q1 ($0–$6,806) | 18326 (24.73) |
| Q2 ($6,806–7,579) | 18596 (25.09) |
| Q3 ($7,579–8,528) | 18558 (25.04) |
| Q4 (> $8528) | 18639 (25.15) |
MSA = metropolitan statistical area; HRR = hospital referral region;
Out of 74,785 patients with no indications for stress testing, 2,803(3.75%) received stress tests in the two months prior to surgery. The median time interval between testing and surgery was 13 days. Only 31% of patients who underwent stress testing visited a cardiologist prior to testing. Table 4 illustrates the characteristics of patients who received preoperative stress testing with no indications. The rate of stress testing was higher for females (4.07%) than males (3.26%, p<0.0001). Patients with a Charlson comorbidity score ≥ 1 had higher rates of stress testing (4.39%) than patients with a Charlson comorbidity of zero (3.66%, p=0.001). The risk of the surgical procedure also impacted rate of stress testing. Patients who underwent high risk procedures had higher rates of stress testing (4.30%) than patients undergoing low risk procedures (1.57%, p<0.0001). The rate of preoperative stress testing was higher in patients with post-operative cardiac complications (5.22%) compared to patients without cardiac complications (3.70%, p=0.001), but there was no difference in stress testing by 30-day mortality (p=0.430).
Table 4.
Stress Tests Among Patients Undergoing Elective Noncardiac Surgical Procedures with No Indications for Preoperative Stress Testing
| Stress Test N (%) | P value | |
|---|---|---|
| Total | 2,803 (3.75) | |
| Age | ||
| 65–69 | 789 (3.71) | 0.358 |
| 70–74 | 839 (3.64) | |
| 75–79 | 655 (3.83) | |
| 80–84 | 385 (4.05) | |
| 85+ | 135 (3.47) | |
| Sex | ||
| Male | 966 (3.26) | <0.0001 |
| Female | 1,837 (4.07) | |
| Race | ||
| White | 2,593 (3.72) | 0.0704 |
| Black | 150 (4.45) | |
| Other | 60 (3.41) | |
| Charlson comorbidity | ||
| 0 | 2413 (3.66) | 0.001 |
| ≥1 | 390 (4.39) | |
| Surgical procedure | ||
| High risk | 608 (4.30) | <0.0001 |
| Intermediate risk | 1,997 (4.16) | |
| Low risk | 198 (1.57) | |
| Cardiac complications | ||
| Yes | 87 (5.22) | 0.001 |
| No | 2,720 (3.70) | |
| 30-day mortality | 0.430 | |
| Yes | 12 (3.00) | |
| No | 2,791 (3.75) | |
| Size MSA | <0.0001 | |
| < 250,000 | 521 (2.90) | |
| 250,000–1,000,000 | 721 (3.37) | |
| 1,000,000+ | 1,561 (4.41) | |
| Medical School Affiliation | ||
| Yes | 1,182 (4.11) | <0.0001 |
| No | 1,621 (3.52) | |
| Hospital Type | ||
| Nonprofit | 2,220 (3.91) | 0.0002 |
| Profit | 292 (3.17) | |
| Government | 291 (3.30) | |
| Hospital size | ||
| <200 beds | 734 (3.38) | <0.0001 |
| 200–324 beds | 538 (3.26) | |
| 325–500 beds | 715 (3.97) | |
| >500 beds | 816 (4.40) | |
| US region | <0.0001 | |
| Northeast | 477 (3.89) | |
| Midwest | 1,075 (4.68) | |
| South | 865 (3.27) | |
| Mountain West | 174 (3.34) | |
| Pacific West | 212 (2.70) | |
| Cardiologists per 100,000 population | <0.0001 | |
| Q1 (0–5) | 570 (3.14) | |
| Q2 (5–<6) | 599 (3.22) | |
| Q3 (6–<7) | 787 (4.24) | |
| Q4 (≥ 7) | 832 (4.42) | |
| Medicare expenditures in HRR (2006$) | <0.0001 | |
| Q1 ($0–$6,806) | 520 (2.84) | |
| Q2 ($6,806–7,579) | 721 (3.88) | |
| Q3 ($7,579–8,528) | 736 (3.97) | |
| Q4 (> $8528) | 811 (4.35) |
MSA = metropolitan statistical area; HRR = hospital referral region
Patients from a medical service area with > 1 million people were more likely to receive stress testing (4.41%) than patients from a medical service area with 250,000 to 1 million (3.37%) or <250,000 (2.90%, p<0.0001). Patients who underwent surgery in a hospital affiliated with a medical school were more likely to receive stress testing (4.11%) compared those who underwent surgery in a hospital with no medical school affiliation (3.52%, p<0.0001). Additionally, nonprofit hospitals had higher rates of testing (3.90%) compared with profit (3.17%) and government hospitals (3.30%) (p=0.0002). Hospital size was also associated with rates of stress testing. Hospitals with >500 beds had higher rates of testing compared to smaller hospitals (p<0.0001) (Table 4).
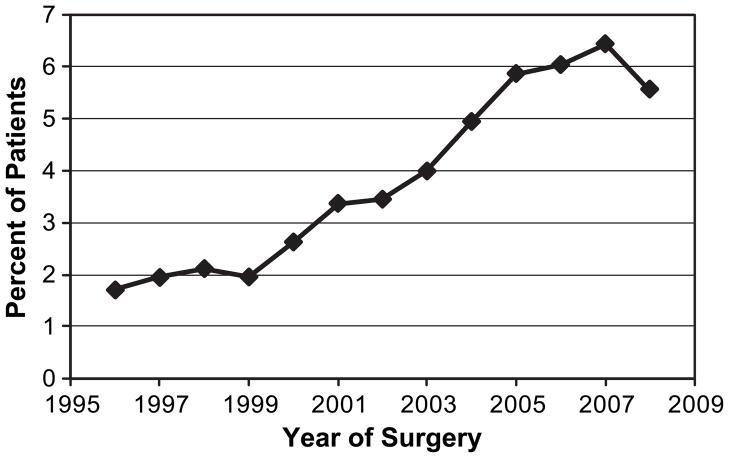
There were significant temporal trends in rate of stress testing, with an increase from 1.72% in 1996 to 6.44% in 2007 (Figure 1, p<0.0001). Following the 2007 ACC/AHA guidelines, the use of stress testing declined by 0.89 percentage points to 5.55% in 2008 (p = 0.064; Figure 1). There was geographic variation in the use of stress testing, with rates ranging from 2.70% in the Pacific West to 4.68% in the Midwest. In addition, patients living in areas with more cardiologists per 100,000 population were also more likely to undergo preoperative stress testing (p<0.0001), with rates ranging from 4.42% in the highest quartile to 3.14% in the lowest quartile. Stress testing rates were positively correlated with Medicare expenditures, with higher rates of testing in patients from areas with the highest expenditures per enrollee (4.35%) compared to areas with lowest expenditures per enrollee (2.84%, p<0.0001).
Figure 1.
Use of preoperative cardiac stress testing in Medicare patients with no active cardiac conditions or clinical risk factors who underwent elective noncardiac, nonvascular surgical procedures, 1996–2008.
The multivariate model (Table 5) showed that female gender (OR 1.11; 95% CI, 1.02–1.21), Charlson comorbidity score ≥ 1 (OR 1.22; 95% CI, 1.09–1.35) and hospital size > 500 beds (OR 1.17; 95% CI 1.03–1.32) were positive predictors of preoperative stress testing. Surgical procedure risk was also a significant predictor of stress testing with patients undergoing high (OR 2.42; 95% CI 2.04–2.89) or intermediate-risk surgery (OR 2.21; 95% CI, 1.88–2.58) being much more likely to have preoperative stress testing compared to patients undergoing low-risk surgery. Patients from the Northeast, Midwest, and Mountain West were also more likely to receive stress testing than patients in the Pacific West. Preoperative testing significantly increased in later years of surgery compared to 1996 (p <0.0001). Patients who lived in smaller MSAs were significantly less likely to undergo stress testing. Patients living in HRRs with higher cardiologist availability were somewhat more likely to have preoperative stress testing, though the association was of marginal statistical significance (p =0.06). Patients living in HRRs with the highest Medicare expenditures were more likely to undergo stress testing (OR= 1.24; 95% CI= 1.05–1.45) compared to patients living in HRRs with the lowest average expenditures.
Table 5.
Predictors of Preoperative Cardiac Stress Testing (N = 74,117)
| Odds Ratio | 95% CI | P value | |
|---|---|---|---|
| Female | 1.11 | 1.02–1.21 | 0.013 |
| Year of surgery | <0.0001 | ||
| 1997 (ref. = 1996) | 1.11 | 0.88–1.42 | |
| 1998 | 1.16 | 0.91–1.48 | |
| 1999 | 1.06 | 0.82–1.38 | |
| 2000 | 1.48 | 1.15–1.89 | |
| 2001 | 1.95 | 1.54–2.47 | |
| 2002 | 1.97 | 1.56–2.49 | |
| 2003 | 2.26 | 1.81–2.83 | |
| 2004 | 2.74 | 2.20–3.42 | |
| 2005 | 3.19 | 2.57–3.97 | |
| 2006 | 3.34 | 2.69–4.15 | |
| 2007 | 3.51 | 2.84–4.34 | |
| 2008 | 2.97 | 2.38–3.70 | |
| Charlson comorbidity | 0.001 | ||
| ≥1 (ref.= 0) | 1.22 | 1.09–1.35 | |
| Surgical procedure | <0.0001 | ||
| High risk (ref. =low) | 2.42 | 2.04–2.89 | |
| Intermediate risk | 2.21 | 1.88–2.58 | |
| Size MSA | <0.0001 | ||
| < 250,000 | 0.76 | 0.67–0.87 | |
| 250,000–1,000,000 | 0.84 | 0.75–0.95 | |
| 1,000,000+ | (Ref) | ||
| US region | <0.0001 | ||
| Northeast (ref. = Pacific West) | 1.36 | 1.13–1.65 | |
| Midwest | 1.82 | 1.53–2.15 | |
| South | 1.15 | 0.97–1.37 | |
| Mountain West | 1.37 | 1.08–1.73 | |
| Hospital size | 0.021 | ||
| 200–324 beds (ref.= <200) | 0.97 | 0.85–1.10 | |
| 325–500 beds | 1.10 | 0.97–1.25 | |
| >500 beds | 1.17 | 1.03–1.32 | |
| Cardiologists per 100,000 population | 0.061 | ||
| Q2 (5.1–<5.9) (ref. = Q1) | 1.02 | 0.89–1.17 | |
| Q3 (5.9–<6.9) | 1.19 | 1.03–1.38 | |
| Q4 (≥ 6.9) | 1.16 | 0.99–1.37 | |
| Medicare expenditures in HRR (2006$) | 0.070 | ||
| Q2 ($6,806–7,579) (ref. = Q1) | 1.13 | 0.99–1.29 | |
| Q3 ($7,579–8,528) | 1.12 | 0.97–1.28 | |
| Q4 (> $8528) | 1.24 | 1.05–1.45 |
MSA=metropolitan statistical area; HRR= hospital referral region
DISCUSSION
Implications
We found that approximately 4% of Medicare patients with no cardiac risk factors had a cardiac stress test in the two months prior to elective noncardiac, nonvascular surgery. These 2,803 patients from our 5% national Medicare sample represent more than 56,000 Medicare patients who received unnecessary cardiac stress testing during our study period. We selected a restricted cohort of patients for whom preoperative cardiac stress testing did not appear to be indicated; therefore, our estimates represent an underestimate of unnecessary testing. ACC/AHA guidelines also stipulate that cardiac stress testing is not indicated in patients with one risk factor and good functional capacity; however, we did not attempt to estimate stress testing use in these patients because Medicare data do not include functional capacity. We expect that rates of cardiac stress testing would be higher in such patients. Medicare reimbursement for cardiac stress tests in 2011 ranged from $92.42 for an exercise stress test with interpretation and report to $341.12 for a myocardial perfusion imaging stress test.13 Unnecessary stress testing could represent significant expense to Medicare. Myocardial perfusion imaging (CPT 78465 during the study period), which includes imaging stress tests, was ranked 14th in the list of Medicare leading CPT procedure codes in 2009, with more than $978 million in allowed charges.14
Physicians need to be informed about indications for preoperative stress testing in order to mitigate costs to the healthcare system and to patients. While testing may be beneficial, there are potential harms. Unnecessary testing may lead to further testing and surgical delay or cancellation.15 We could not assess surgical delays or cancellations with this dataset, as our cohort was defined by identifying patients undergoing the given surgical procedures. While evidence on the downstream harm of testing in asymptomatic patients is lacking, it is estimated that 0.6% to 2.9% of asymptomatic patients undergoing exercise stress testing also undergo angiography and 1% undergo cardiac revascularization, despite the risks and lack of proven benefit in asymptomatic patients.16–19 In our study, 44 (1.6%) patients underwent angiography or revascularization following stress testing. Such patients are exposed to the potential harms related to those procedures, including bleeding and contrast and radiation exposure. In low risk patients with no clinical risk factors, preoperative stress testing has not been shown to improve postoperative outcomes.15, 16
Predictors of Stress Testing
We identified several predictors of preoperative stress testing in patients with no cardiac risk factors. Patients with one or more comorbidities were significantly more likely to undergo preoperative stress testing. Physicians may be overly cautious and order stress testing during preoperative evaluation for patients with conditions that are not considered to be clinically significant risk factors for perioperative cardiac events. Patients who underwent “high risk” procedures were also more likely to undergo preoperative stress testing, indicating that physicians order stress tests for procedures with increased risk of morbidity and mortality, even in patients with no clinical risk factors for perioperative cardiac events. ACC/AHA guidelines indicate that preoperative stress testing should be reserved for patients with clinically significant risk factors.1, 15, 16 Minor risk factors including advanced age, abnormal ECG(left ventricular hypertrophy, left bundle-branch block), atrial fibrillation, low functional capacity, and uncontrolled systemic hypertension have not been shown to independently predict perioperative cardiac events.1, 20–22 However, there has been little research investigating the impact of combinations of these conditions. The presence of multiple minor risk factors increases suspicion of coronary artery disease but is not incorporated into the AHA/ACC testing recommendations.
Our results show somewhat higher rates of stress testing in regions with greater Medicare expenditures and cardiologist availability. This supports previous findings that there is increased diagnostic testing, treatment, and healthcare spending in areas with relatively more medical specialists.23 Previous research has demonstrated wide regional variation in utilization of diagnostic testing24, 25 and treatment,26–28 which directly correlates to regional variability in healthcare spending.23 One study found that the proportion of Medicare patients who undergo echocardiography in the same year varies as much as three-fold between states.29 Our results also showed variation in preoperative stress testing by U.S. region. Regional differences in patient composition may contribute to variation in cardiac stress testing. Use of cardiac stress testing was significantly lower among patients in the Pacific west. It is possible that patients in this region have lower body mass index (BMI) than those in other regions, which could contribute to some of the regional differences. Unfortunately, BMI is not well-captured in Medicare data.
There was a dramatic increase over the study period in the utilization of preoperative cardiac stress testing in patients with no indications for testing. In 1992, only 1% of patients underwent preoperative testing, and this rate increased to 6% in 2007. These results are consistent with previous studies that show increased use of cardiac stress testing in elderly patients over this time period.30, 31 In the context of rising healthcare costs and public concerns over the federal deficit, this is an alarming trend. On the other hand, our data also showed a slight decline in the use of preoperative cardiac stress testing in 2008. It is unclear whether this finding represents a change in trend or simply data fluctuations. Shaw et al. have also reported that high annual growth rates for echocardiography and myocardial perfusion SPECT attenuated after 2005.32 The change in trend may reflect the results of efforts by CMS and the Government Accountability Office to reduce the growth in Medicare Part B spending on imaging services.33
The ACC/AHA guidelines for perioperative cardiovascular evaluation were revised twice during our study period—in 1996 and 2002. However, the recommendations for patients without active cardiac conditions and clinical risk factors were similar for each set of guidelines.1, 5, 6 In 2002, renal insufficiency was added to the list of intermediate clinical risk factors, and in 2007, history of cerebrovascular disease was determined to be an intermediate clinical risk factor. Patients with these conditions were excluded from our study cohort, so these changes should not have impacted our results.
Strength of the Evidence
The ACC/AHA guidelines are developed by the ACC/AHA Task Force on Practice Guidelines. In developing the guidelines, the Task Force performs an extensive literature review, weighs the strength of the evidence, and estimates the expected health outcomes where data exist. The guidelines are reviewed annually and updated as appropriate, and as such, they are the best guidelines currently available. Despite this, the ACC/AHA guidelines for perioperative evaluation may not apply in all circumstances, and physicians are expected to use their best clinical judgment when making decisions regarding patient care. We were unable to determine whether a stress test for any given patient was appropriate or inappropriate. A variety of clinical circumstances may justify the use of preoperative stress testing, and these circumstances are not always well captured in administrative data.
However, we can provide a description at the population level. The patterns and time trends we observed suggest that physician practice patterns, more than patient clinical characteristics, are driving the use of preoperative testing in patients with no cardiac risk factors. Even after controlling for patient characteristics, there was a sharp increase in use over time in our study. In addition, stress testing use was correlated with high Medicare expenditures, with patients living in HRRs with the highest Medicare expenditures having increased odds of undergoing stress testing. As mentioned earlier, other studies evaluating stress testing utilization in other settings have shown similar patterns. Regional variation in rates of stress testing are strongly correlated with rates of coronary angiography and revascularization procedures.29
Limitations
We performed a retrospective, observational study using administrative data. We excluded patients with diagnostic codes that most closely represent the active cardiac conditions and clinical risk factors defined by the ACC/AHA guidelines34, 35; however, the codes were generally less specific than the conditions in the guidelines. Additionally, postoperative cardiac events are difficult to capture using administrative data that were not collected for research purposes. The validity of using ICD-9 diagnosis codes to identify complications following surgery is uncertain because diagnostic codes fail to distinguish between pre-existing comorbid conditions and acute complications.36–39 Investigators have developed screening algorithms to identify hospital complications using discharge data,38–40 but these screening methods may still identify cases where the condition was present on admission rather than occurring in-hospital.38, 40 Nevertheless, some screens have shown good validity for identifying complications in surgical cases.38 In our study, we restricted the sample to patients with no diagnoses for cardiac conditions or clinical risk factors. As recommended by Finlayson and colleagues,37 we used inpatient and outpatient claims prior to the surgical admission to identify patient comorbidities. We limited our analytic cohort to patients with no diagnoses for cardiac conditions or clinical risk factors in the year prior to surgery and attempted to identify “incident” cardiac complications based on the presence of diagnoses for cardiac conditions on the surgical claim. Some patients may have had pre-existing cardiac conditions that were not captured by diagnosis codes on inpatient or outpatient visits in the 12 months prior to surgery; therefore, this approach may have misclassified previously undiagnosed pre-existing conditions as postoperative cardiac complications for some patients. We found that patients who had cardiac complications were more likely to have had a stress test. This may be a due to misclassification of pre-existing conditions as complications or to unmeasured selection for stress testing. Patients chosen for stress testing may have had a higher cardiac risk based on factors unmeasured in our study such as family history, symptoms (chest pain, shortness of breath, etc.), or obesity. However, there is not strong evidence, nor can we conclude from our study, that preoperative stress testing reduced complications in this group of patients.
While we were able to describe trends and identify independent predictors of stress testing in patients with no clinical risk factors, our results may be limited by patient selection bias that may influence the likelihood of undergoing surgery as well as receiving stress testing preoperatively. Another study limitation is that our sample excluded, by design, patients who underwent preoperative testing and, on the basis of their stress test results, did not have surgery or underwent coronary angiography or coronary revascularization. We chose a 2 month time frame to identify stress tests that were likely ordered as part of a preoperative cardiac evaluation, rather than other indications such as chest pain. The rate of preoperative stress testing was similar (4.1%) in sensitivity analyses using a 90 day window. Finally, the absolute numbers of potentially unnecessary preoperative tests are low, corresponding to approximately 4,667 tests per year in the national population of Medicare patients undergoing elective noncardiac, nonvascular surgery with no cardiac risk factors.
Conclusion
This study provided a description of overutilization of preoperative cardiac stress testing in Medicare patients who underwent elective noncardiac, nonvascular surgery. We excluded from the sample patients with any cardiac conditions or clinical risk factors. Our results represent a conservative estimate of overutilization. Approximately 4% of patients received a stress test when it was not indicated, and the rate of stress testing increased over the study period. Our results indicate the inappropriate use of preoperative cardiac stress testing is a strong area for additional investigation and direct clinical validation using better sources of data. Our study specifically looks at overuse in a very carefully selected cohort of patients with no cardiac comorbidities. Further studies can evaluate inappropriate use, including potential overuse and underuse, in patients with specific risk factors.
References
- 1.Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Journal of the American College of Cardiology. 2007 Oct 23;50(17):e159–241. doi: 10.1016/j.jacc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Mangano DT. Perioperative Cardiac Morbidity. Anesthesiology. 1990 Jan;72(1):153–184. doi: 10.1097/00000542-199001000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Mangano DT, Goldman L. Current concepts - Preoperative assessment of patients with known or suspected coronary disease. New England Journal of Medicine. 1995 Dec 28;333(26):1750–1756. doi: 10.1056/NEJM199512283332607. [DOI] [PubMed] [Google Scholar]
- 4.Fleischmann KE, Goldman L, Young B, Lee TH. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med. 2003 Nov;115(7):515–520. doi: 10.1016/s0002-9343(03)00474-1. [DOI] [PubMed] [Google Scholar]
- 5.Eagle KA, Brundage BH, Chaitman BR, et al. Guidelines for perioperative cardiovascular evaluation for noncardiac surgery. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Journal of the American College of Cardiology. 1996 Mar 15;27(4):910–948. doi: 10.1016/0735-1097(95)99999-x. [DOI] [PubMed] [Google Scholar]
- 6.Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Journal of the American College of Cardiology. 2002 Feb 6;39(3):542–553. doi: 10.1016/s0735-1097(01)01788-0. [DOI] [PubMed] [Google Scholar]
- 7.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 8.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000 Dec;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 9.Cochran WG. Some methods for strengthening the common chi-square tests. Biometrics. 1954;10:417–451. [Google Scholar]
- 10.Armitage P. Tests for linear trends in proportions and frequencies. Biometrics. 1955;11:375–386. [Google Scholar]
- 11.Liang KY, Zeger SL. Longitudinal Data-Analysis Using Generalized Linear-Models. Biometrika. 1986 Apr;73(1):13–22. [Google Scholar]
- 12.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001 Mar;57(1):120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 13.AMA Code Manager 2011. American Medical Association; [accessed July 10, 2011]. Available from: https://ocm.ama-assn.org/OCM/CPTRelativeValueSearchResults.do?locality=1&keyword=stress+test. [Google Scholar]
- 14.CMS Centers for Medicare & Medicaid Services. [accessed July 1, 2011];Medicare utilization for Part B. 2011 Apr 5; Available from: https://www.cms.gov/MedicareFeeforSvcPartsAB/04_MedicareUtilizationforPartB.asp.
- 15.Grayburn PA, Hillis LD. Cardiac events in patients undergoing noncardiac surgery: shifting the paradigm from noninvasive risk stratification to therapy. Ann Intern Med. 2003 Mar 18;138(6):506–511. doi: 10.7326/0003-4819-138-6-200303180-00017. [DOI] [PubMed] [Google Scholar]
- 16.Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A. Non-invasive cardiac stress testing before elective major non-cardiac surgery: population based cohort study. BMJ. 2010;340:b5526. doi: 10.1136/bmj.b5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aktas MK, Ozduran V, Pothier CE, Lang R, Lauer MS. Global risk scores and exercise testing for predicting all-cause mortality in a preventive medicine program. JAMA. 2004 Sep 22;292(12):1462–1468. doi: 10.1001/jama.292.12.1462. [DOI] [PubMed] [Google Scholar]
- 18.Chou R, Arora B, Dana T, Fu R, Walker M, Humphrey L. Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011 Sep 20;155(6):375–385. doi: 10.7326/0003-4819-155-6-201109200-00006. [DOI] [PubMed] [Google Scholar]
- 19.Cournot M, Taraszkiewicz D, Galinier M, et al. Is exercise testing useful to improve the prediction of coronary events in asymptomatic subjects? Eur J Cardiovasc Prev Rehabil. 2006 Feb;13(1):37–44. doi: 10.1097/01.hjr.0000198447.26613.3d. [DOI] [PubMed] [Google Scholar]
- 20.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999 Sep 7;100(10):1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 21.Lette J, Waters D, Bernier H, et al. Preoperative and long-term cardiac risk assessment. Predictive value of 23 clinical descriptors, 7 multivariate scoring systems, and quantitative dipyridamole imaging in 360 patients. Ann Surg. 1992 Aug;216(2):192–204. doi: 10.1097/00000658-199208000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. New England Journal of Medicine. 1990 Dec 27;323(26):1781–1788. doi: 10.1056/NEJM199012273232601. [DOI] [PubMed] [Google Scholar]
- 23.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003 Feb 18;138(4):273–287. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 24.Lucas FL, Wennberg DE, Malenka DJ. Variation in the use of echocardiography. Eff Clin Pract. 1999 Mar-Apr;2(2):71–75. [PubMed] [Google Scholar]
- 25.Song Y, Skinner J, Bynum J, Sutherland J, Wennberg JE, Fisher ES. Regional variations in diagnostic practices. New England Journal of Medicine. 2010 Jul 1;363(1):45–53. doi: 10.1056/NEJMsa0910881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn EM, Hartz AJ, Baras M. Correlation of rates of coronary artery bypass surgery, angioplasty, and cardiac catheterization in 305 large communities for persons age 65 and older. Health Services Research. 1995 Aug;30(3):425–436. [PMC free article] [PubMed] [Google Scholar]
- 27.Wennberg JE, Cooper MM. The Dartmouth Atlas of Health Care 1998. Chicago, IL: American Hospital Publishing, Inc; 1998. [PubMed] [Google Scholar]
- 28.Wennberg DE, Birkmeyer JD, Lucas FL, et al. The Dartmouth Atlas of Cardiovascular Health Care. Chicago, IL: American Hospital Association Press; 1999. [Google Scholar]
- 29.Wennberg DE, Kellett MA, Dickens JD, Malenka DJ, Keilson LM, Keller RB. The association between local diagnostic testing intensity and invasive cardiac procedures. JAMA. 1996 Apr 17;275(15):1161–1164. [PubMed] [Google Scholar]
- 30.Alter DA, Stukel TA, Newman A. Proliferation of cardiac technology in Canada: a challenge to the sustainability of Medicare. Circulation. 2006 Jan 24;113(3):380–387. doi: 10.1161/CIRCULATIONAHA.105.560466. [DOI] [PubMed] [Google Scholar]
- 31.Lucas FL, DeLorenzo MA, Siewers AE, Wennberg DE. Temporal trends in the utilization of diagnostic testing and treatments for cardiovascular disease in the United States, 1993–2001. Circulation. 2006 Jan 24;113(3):374–379. doi: 10.1161/CIRCULATIONAHA.105.560433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw LJ, Marwick TH, Zoghbi WA, et al. Why all the focus on cardiac imaging? JACC Cardiovasc Imaging. 2010 Jul;3(7):789–794. doi: 10.1016/j.jcmg.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 33.GAO. U.S. Goverment Accountability Office. Medicare Part B Imaging Services. U.S. Government Accountability Office; [accessed July 1, 2011]. Rapid Spending Growth and Shift to Physician Offices Indicate Need for CMS to Consider Additional Management Practices. Available from: http://www.gao.gov/new.items/d08452.pdf. [Google Scholar]
- 34.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Medical Care. 2005 May;43(5):480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 35.Elixhauser A, Steiner C, Harris DR, Coffey RN. Comorbidity measures for use with administrative data. Medical Care. 1998 Jan;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Romano PS, Schembri ME, Rainwater JA. Can administrative data be used to ascertain clinically significant postoperative complications? American Journal of Medical Quality. 2002 Jul-Aug;17(4):145–154. doi: 10.1177/106286060201700404. [DOI] [PubMed] [Google Scholar]
- 37.Finlayson EVA, Birkmeyer JD, Stukel TA, Siewers AE, Lucas FL, Wennberg DE. Adjusting surgical mortality rates for patient comorbidities: More harm than good? Surgery. 2002 Nov;132(5):787–794. doi: 10.1067/msy.2002.126509. [DOI] [PubMed] [Google Scholar]
- 38.Lawthers AG, McCarthy EP, Davis RB, Peterson LE, Palmer RH, Iezzoni LI. Identification of in-hospital complications from claims data - Is it valid? Medical Care. 2000 Aug;38(8):785–795. doi: 10.1097/00005650-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Iezzoni LI, Daley J, Heeren T, et al. Identifying Complications of Care Using Administrative Data. Medical Care. 1994 Jul;32(7):700–715. doi: 10.1097/00005650-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Naessens JM, Huschka TR. Distinguishing hospital complications of care from pre-existing conditions. International Journal for Quality in Health Care. 2004 Apr;16:I27–I35. doi: 10.1093/intqhc/mzh012. [DOI] [PubMed] [Google Scholar]