Abstract
Pro-atherogenic, hyperlipidemic states demonstrate increases in circulating ligands for scavenger receptor CD36 (e.g. oxidized LDL (oxLDL)) and the Na/K-ATPase (e.g. cardiotonic steroids). These factors increase inflammation, oxidative stress, and progression of chronic kidney disease. We hypothesized that diet-induced obesity and hyperlipidemia potentiate a CD36/ Na/K-ATPase -dependent inflammatory paracrine loop between proximal tubule cells (PTC) and their associated macrophages and thereby facilitates development of chronic inflammation and tubulointerstitial fibrosis. ApoE−/− and apoE−/−/cd36−/− mice were fed a high-fat diet (HFD) for up to 32 weeks and examined for physiologic and histologic changes in renal function. Compared to apoE−/−, apoE−/−/cd36−/− mice had improved creatinine clearance and blood pressure which corresponded histologically to less glomerular and tubulointerstitial macrophage accumulation, foam cell formation, oxidant stress, and interstitial fibrosis. Co-IP and a cell surface fluorescence-based crosslinking assay showed CD36 and Na/K-ATPase α-1 co-localized in PTC and macrophages, and this association was increased by oxLDL or the cardiotonic steroid ouabain. OxLDL and ouabain also increased activation of Src and Lyn in PTC. Cell-free conditioned media from PTC treated with oxLDL or ouabain increased macrophage migration. OxLDL, ouabain, or plasma isolated from HFD-fed mice stimulated reactive oxygen species production in PTC which was inhibited by N-acetyl-cysteine, apocynin or Na/K-ATPase α-1 knockdown. These data suggest that ligands generated in hyperlipidemic states activate CD36 and the Na/K-ATPase, and potentiate an inflammatory signaling loop involving PTC and their associated macrophages which facilitates the development of chronic inflammation, oxidant stress, and fibrosis underlying the renal dysfunction common to pro-atherogenic, hyperlipidemic states.
Keywords: CD36, Na/K-ATPase, Obesity, Inflammation, Kidney Disease
INTRODUCTION
Pro-atherogenic hyperlipidemic states not only increase the risk of cardiovascular disease in the chronic kidney disease (CKD) population but also increase the risk of worsening renal function1, 2. Given the implications that accelerating both cardiovascular disease and renal dysfunction have on morbidity and mortality in this population, investigating the mechanisms of renal glomerular and tubulointerstitial injury observed in this setting is a topic of great interest.
Recently, the apoE null mouse has been used as a model of hyperlipidemic renal injury and offers a valuable tool to study such mechanisms3. On this genetic background, we and others have demonstrated that the class B scavenger receptor CD36 is a key molecule in mediating the inflammation, insulin resistance, and atherogenesis involved in pro-atherogenic hyperlipidemic states4–7. CD36 is expressed on a variety of cell types including monocytes and macrophages8 and proximal tubular cells (PTC)9, and recognizes modified lipoproteins such as oxidized LDL (oxLDL) that arise as byproducts during an inflammatory response10. Elevated levels of various forms of oxLDL have been demonstrated in pro-atherogenic hyperlipidemic states such as uremia11, 12, and both human and experimental animal studies suggest an important role for scavenger receptors such as CD36 in the uptake of oxLDL and hyperlipidemic kidney injury that leads to inflammation and interstitial fibrosis in CKD3, 13, 14. Monocyte CD36 expression and oxidant stress are increased in CKD patients versus controls without renal insufficiency15, and increased levels of a circulating form of CD36 in patients with diabetes and CKD predicts cardiovascular mortality in stage 5 CKD patients16. Interestingly, in a hyperlipidemic model of CKD, CD36 null mice had attenuated renal TGF-β signaling and NF-κB activity and displayed significantly reduced macrophage accumulation, oxidative stress and renal fibrosis17.
Binding of oxLDL to CD36 initiates Src family kinase activation and a pro-inflammatory, pro-atherogenic phenotype in multiple cell types, yet the mechanism of regulation of specific Src family kinases, including Fyn and Lyn, by CD36 remains unresolved. Since we previously demonstrated that the Na/K-ATPase regulates Src family kinases,18, 19 and since the signaling fraction of the Na/K-ATPase resides in similar cholesterol-rich, detergent-insoluble lipid raft and caveolar domains as CD364, 20 we reasoned that the Na/K-ATPase may be involved in regulating CD36-dependent signaling events mediated by oxLDL. The association of the Na/K-ATPase with pathogenesis of pro-atherogenic, hyperlipidemic states is not without precedent as Na/K-ATPase expression and activity is significantly reduced in humans with type 2 diabetes 21, 22 and obesity23, suggesting a possible role of Na/K-ATPase in these conditions.
In this report, we tested the hypothesis that diet-induced obesity and hyperlipidemia potentiate a CD36-dependent inflammatory paracrine loop between proximal tubule cells and their associated macrophages and thereby facilitates development of chronic inflammation and tubulointerstitial fibrosis. We found that genetic deletion of CD36 on the apoE null background was protective against pro-atherogenic hyperlipidemic renal injury and identified molecular mechanisms that contributed to this effect.
METHODS
Animals and diets
Littermate derived apoE−/− and apoE−/−/cd36−/− (10x backcrossed to C57Bl/6), mice were maintained as previously described24, 25. Age and sex matched mice were fed a high-fat diet (HFD) (36% wt/wt adjusted calories from fat, Bio-Serv S3282) for up to 32 weeks; controls were maintained on a standard chow diet (Harlan Teklad, TD 2918). All animal procedures were approved by the Institutional Animal Care and Use Committee and more fully described in Supplementary material online.
Reagents, cell culture, and immunoblotting
Tissue culture media, supplements, reagents and immunoblotting material and methods are described as in Supplementary material online.
In vitro adhesion and migration assays
Macrophage migration and adhesion was measured as described in Supplementary material online.
In situ proximity ligation assay (PLA), immunofluorescence staining and confocal microscopy The Doulink™ in situ PLA reagent (Olink Biosciences, Uppsala, Sweden) was used as we have previously described to characterize endogenous protein interactions26 and is described further in Supplementary material online.
Histology and Immunohistochemistry
Histochemical and morphometric analysis was performed on deparafinized rehydrated 4μm serial kidney sections processed for staining with F4/80 (AbD Serotec), Galectin-3/MAC-2 (Cedarlane), 8-hydroxy-2′deoxyguanosine (Abcam), glomerular basement membranes (American MasterTech), and picrosirius red as described in Supplementary material online.
Statistical Analysis
Data are presented as mean ± standard error of the mean and is described in Supplementary material online.
RESULTS
Deletion of CD36 protects the kidney in a pro-atherogenic hyperlipidemic model
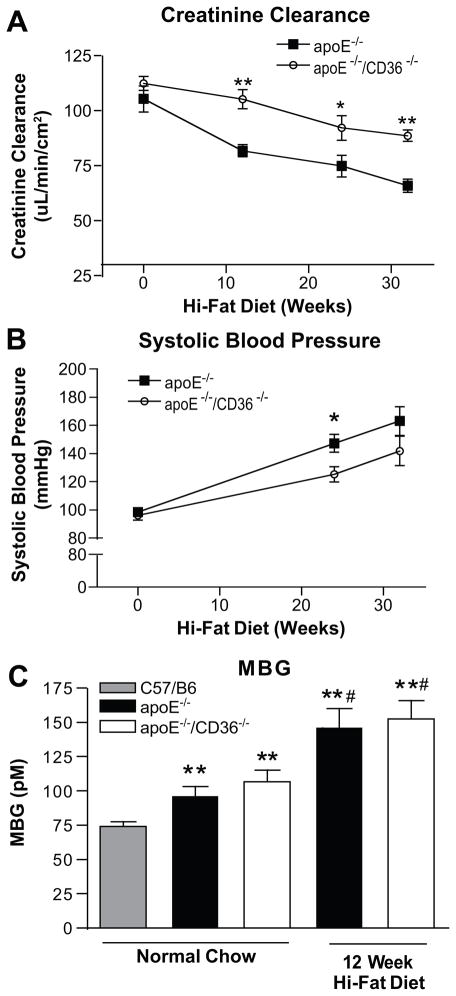
Age-matched apoE−/− and apoE−/−/cd36−/− mice fed HFD demonstrated significant decreases in creatinine clearance as early as 12 wk, but the apoE−/−/cd36−/− mice had significantly better glomerular function at each time point compared to the apoE−/− mice (Figure 1A). Similarly, while both groups displayed increases in systolic blood pressure on the HFD, apoE−/−/cd36−/− mice had significantly smaller increases at 24wk compared to apoE−/− (125±5.4 vs. 147±6.3 mmHg, p<0.05, Figure 1B), although this difference was not seen at 32 wk. Creatinine clearance and systolic blood pressure did not change significantly in normal chow fed mice (Figure S1A, B). Plasma levels of the cardiotonic steroid (CTS) marinobufagenin (MBG), an endogenous Na/K-ATPase ligand which is known to be elevated in hypertension, volume expansion, and renal injury27, 28 were increased by ~30% in normal chow fed apoE−/− and apoE−/−/cd36−/− mice compared to normal chow fed, age matched C57/B6 mice, and both groups showed a further 2-fold increase after 12 wk HFD (p<0.05, Figure 1C). As previously reported, both genotypes had equivalent body weights (Figure S1C, D), adiposity, and lipid profiles on this diet.29
Figure 1. Absence of CD36 improves renal function and blood pressure after high fat diet feeding.
(A) 24 hour creatinine clearance adjusted for Body Surface Area and (B) systolic blood pressure in apoE−/− and apoE−/− /cd36−/− mice after high-fa diet feeding at the indicated times. *p < 0.05, **p < 0.01 vs. apoE−/−, n ≥ 8 mice per group per time point. (C) Plasma MBG levels in wild type C57/B6, apoE−/− and apoE−/− /cd36−/− mice on normal chow or 12 week high-fat diet feeding. **p < 0.01 vs. wild type C57/B6; #p < 0.05 vs. normal chow controls, n ≥ 8 mice/group.
Histological analysis revealed a 3-fold decrease in F4/80+ macrophage staining in apoE−/−/cd36−/− mice compared to apoE−/− after HFD (p<0.05, Figure 2A), and this corresponded to more than a 5-fold reduction of foam cell positive glomeruli in both chow and HFD fed animals at 32 wks (p<0.05, Figure 2B). Galectin-3, a soluble β-galactoside-binding lectin expressed in many cell types including macrophages and renal epithelium, functions as an important regulator of inflammation and promoter of fibrosis in cardiac and renal disease30, 31 and was highly expressed in the kidneys of apoE−/− mice after HFD feeding. Staining intensity was diminished 2-fold in apoE−/−/cd36−/− mice (p<0.05, Figure 2C), indicating attenuation of a pro-inflammatory, pro-fibrotic phenotype. Compared to apoE−/− mice, we found >1.5-fold reduction in staining for 8-hydroxy-2-deoxyguanosine, a DNA adduct that reflects oxidative damage, in both normal chow and HFD fed apoE−/−/cd36−/− animals (p<0.05, Figure 2D). Similarly, HFD fed apoE−/−/cd36−/− mice had more than 2-fold less renal collagen deposition compared to apoE−/− mice as measured by picosiurius red staining (p<0.05, Figure 2E). Further, HFD fed apoE−/− mice had a 23% increase in glomerular basement membrane thickening compared to apoE−/−/cd36−/− animals (Figure 2F). These findings together implicate CD36 as a contributor to the pro-inflammatory, pro-fibrotic phenotype induced by hyperlipidemic renal injury.
Figure 2. CD36 contributes to inflammation, oxidant stress and fibrosis in kidney after high fat diet feeding.
Representative immunohistochemistry, histology, and quantification from apoE−/− and apoE−/− /cd36−/− mouse kidneys after high-fat diet feeding for the indicated time points identifying (A) F4/80+ macrophages (B) glomerular foam cell formation (C) inflammatory marker Galectin-3, (D) oxidative stress marker 8-hydroxy, 2′deoxyguanosine (E) picosirius red collagen staining, and (F) Jones glomerular basement membrane stain and quantification. *p < 0.05, **p < 0.01 vs. apoE−/−, n ≥ 8 mice per group per time point.
OxLDL or ouabain induce Src-family kinase activation in proximal tubule cells and enhance physical association of CD36 and Na/K-ATPase
To probe mechanisms underlying the protective effect of CD36 deletion on HFD-induced renal injury we first investigated whether NO2LDL, a specific CD36 ligand, increased Src family kinase activation in proximal tubular cells similar to the effects that we have reported with the CTS Na/K-ATPase ligand ouabain19. As shown in Figure 3A, we found a > 5-fold increase in phosphorylation of both Src (Tyr416) and Lyn (Tyr396) 30min after addition of NO2LDL to HK-2 cells. We also found by co-immunoprecipitation that addition of NO2LDL to LLC-PK1 cells induced a 6-fold increase in association of CD36 with the Na/K-ATPase α-1 subunit (Figure S2). The effect was seen as early as 5min and was sustained for at least 60min (Figure 3B). Pretreatment of the cells with the anti-oxidant n-acetyl-cysteine significantly attenuated the association (Figure 3C). To demonstrate the interaction of CD36 and the Na/K-ATPase in cells in situ, we used a crosslinking assay which allows direct observation of protein-protein interactions resolved to distances ≤40 nm32. We found constitutive interactions between CD36 and the Na/K-ATPase α-1 subunit (denoted by fluorescent spots) under basal conditions in both HK-2 cells and mouse peritoneal macrophages (Figures 3D–E). The interaction was enhanced by addition of NO2LDL, ouabain, or a combination of both ligands (Figures 3D–E) and not seen with irrelevant IgG (Figure S3). These data suggest that oxLDL and/or CTS could promote renal injury by triggering CD36-Na/K-ATPase interactions and subsequent downstream signaling through activation of Src family kinases.
Figure 3. CD36 interaction with the Na/K-ATPase.
(A) Src (Y416) and Lyn (Y396) phosphorylation in HK-2 cells treated for 30 minutes with ouabain and NO2LDL. (B) Co-immunoprecipitation of CD36 with the Na/K-ATPase α-1 subunit in LLC-PK1 cells is increased by NO2LDL and (C) inhibited by pretreatment with n-acetyl-cysteine (NAC). Confocal images from Proximity Ligation Assay demonstrate CD36 interaction with the Na/K-ATPase α-1 subunit in (D) peritoneal macrophages and (E) HK-2 cells is increased after 60 minute treatment with ouabain, NO2LDL, or a combination of both ligands. Quantitative data in (A–E) summarized from n ≥ 3 separate experiments, *p < 0.05 vs control.
OxLDL Promotes Na/K-ATPase Endocytosis
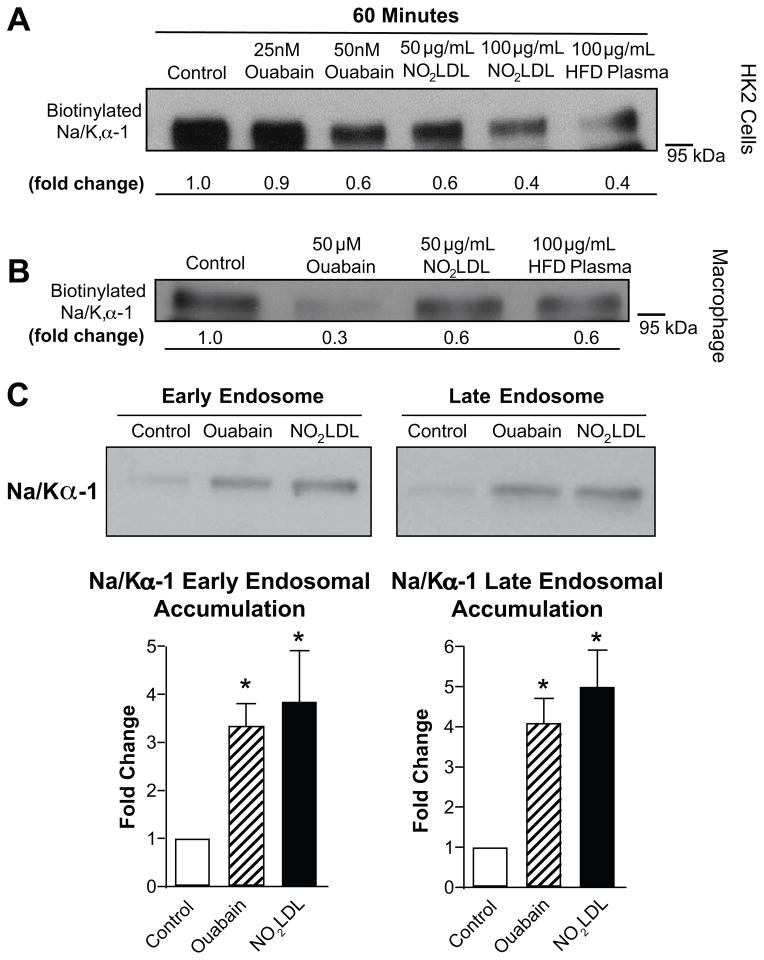
As seen in Figure 4A and 4B using a surface biotinylation technique, we found that the CD36 ligand NO2LDL also induced dose dependent reduction in cell surface Na/K-ATPase α-1 subunit expression in both HK-2 cells and mouse macrophages. Notably, this reduction was also induced by incubating cells with plasma from HFD fed apoE−/ − mice. Immunofluorescence microscopy revealed that co-localization of Na/K-ATPaseα-1 and clathrin in HK-2 cells was increased after 60min incubation with either ouabain or NO2LDL and was diminished by pretreatment with n-acetyl-cysteine (Figure S4). Cell fractionation studies with HK-2 cells showed that ouabain and NO2LDL induced an ~3-fold increase in Na/K-ATPase α-1 subunit accumulation into early endosomes and a ~3.5-fold increase into late endosomes (Figure 4C). Redistribution of Na/K-ATPase α-1 immunoreactivity was also seen in situ in kidney sections from HFD fed mice where we observed a marked redistribution of straining from tubular basolateral membranes to a more diffuse pattern (Figure S5).
Figure 4. CD36 ligands activate Na/K-ATPase signaling pathways.
Biotinylation of both (A) HK-2 cells and (B) peritoneal macrophages demonstrates reductions in cell surface Na/K- ATPase α-1 in response to 60 minute treatment with ouabain, NO2LDL and plasma from high- fat fed mice. (C) Two hour treatment with ouabain and NO2LDL induce similar increases of Na/K-ATPase α-1 in the early and late endosome fraction of HK-2 proximal tubular cells. Quantitative data summarized from n ≥ 3 separate experiments, *p < 0.05 vs control.
CD36 and Na/K-ATPase ligands activate a pro-inflammatory phenotype in macrophages and proximal tubule epithelial cells
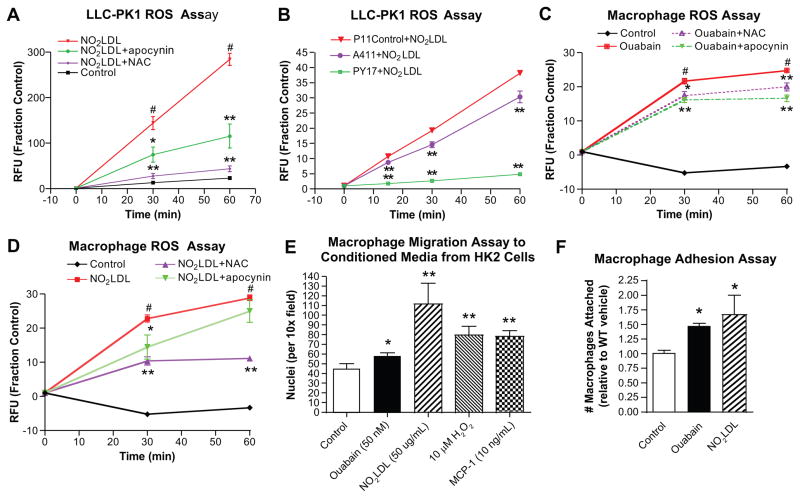
To determine the functional consequences of Na/K-ATPase-CD36 signaling in the kidney we exposed LLC-PK1 cells to NO2LDL and measured ROS production. As shown in Figure 5A, NO2LDL induced a rapid increase in ROS with a 3-fold increase at 60min compared to control cells. ROS production was significantly attenuated by pretreatment with the antioxidants n-acetyl-cysteine (NAC, 84% reduction at 60min) or apocynin (60% reduction at 60min) (p<0.01). ROS production was also dramatically attenuated in a dose dependent fashion by RNAi- mediated knock-down of the Na/K-ATPase α-1 subunit (22% reduction in ROS with 40% knock-down of Na/K α-1, and 87% reduction in ROS with 90% knock-down of Na/K α-1 at 60min, p<0.01, Figure 5B). In addition both ouabain and NO2LDL elicited pro-inflammatory cytokine production in human HK-2 cells (Table S1). Thus, NO2LDL promotes a pro-inflammatory phenotype in proximal tubule cells and this requires expression of Na/K-ATPase.
Figure 5. CD36 and Na/K-ATPase ligands activate pro-inflammatory phenotype in macrophages and proximal tubule cells.
(A) NO2LDL (50 ug/mL) induced ROS in LLC-PK1 cells is attenuated by 30 minute pretreatment with antioxidants (20 mM n-acetyl-cysteine, NAC, or 10 μM apocynin); #p < 0.01 vs. control, *p < 0.05, **p < 0.01 vs. NO2LDL treatment from n ≥ 5 separate experiments. (B) NO2LDL (50 ug/mL) induced ROS in LLC-PK1 cells is attenuated in a dose dependent manner by knockdown of the Na/K-ATPase α-1 in A411 (40% knockdown of Na/K,α-1) and PY17 (90% knockdown of Na/K,α-1) vs P11 empty vector control LLC-PK1cells; **p < 0.01 vs. P11 control from n ≥ 5 separate experiments. (C) Ouabain (50 μM) and (D) NO2LDL (50 ug/mL) induced ROS is attenuated by 30 minute pretreatment with antioxidants (20 mM NAC or 10 μM apocynin) in mouse peritoneal macrophages; #p < 0.01 vs. control, *p < 0.05, **p < 0.01 vs. treatment from n ≥ 5 separate experiments. (E) Macrophage migration is increased to conditioned media from HK-2 cells which had been previously exposed to ouabain and NO2LDL vs. control conditioned media from vehicle treated HK-2 cells. (F) Ouabain and NO2LDL induced macrophage attachment to tissue culture plastic. *p < 0.05, **p < 0.01 vs. control from n ≥ 5 separate experiments.
Because macrophage infiltration and foam cell formation were prominent features in our in vivo studies, we also explored the phenotype of macrophages exposed to both CTS and oxLDL. Treatment with ouabain and NO2LDL increased inflammatory cytokine production (Table S2) as well as ROS production (Figure 5C, 5D). Increases in ROS were attenuated by pretreatment with NAC or apocynin.
We used a modified Boyden chamber migration assay to assess the functional interaction between macrophages and proximal tubule cells. HK-2 cells were exposed to various ligands, washed, and then conditioned media collected and placed in a transwell migration chamber. Conditioned media from cells treated with ouabain or NO2LDL significantly increased macrophage migration compared to media from control HK-2 cells (30% and 200% increase respectively; p<0.05, Figure 5E). These increases were similar to those elicited by conditioned media from HK-2 cells treated with H2O2 or MCP-1. We also found that both ouabain and NO2LDL increased macrophage attachment to shaking tissue culture plastic (≥50% increase, p<0.05, Figure 5F). Taken together, these findings suggest that known Na/K-ATPase and CD36 ligands, such as CTS and oxidized LDL, are capable of initiating key events (ROS and cytokine production, macrophage migration and adhesion) which promote an inflammatory phenotype in cell types important for the development of inflammation, oxidant stress, and fibrosis in the kidney.
DISCUSSION
In this report, we provide multiple lines of in vitro and in vivo evidence supporting a mechanistic link between inflammation, oxidative stress, hyperlipidemia and renal dysfunction mediated by the type 2 scavenger receptor CD36 in agreement with previous evidence from our lab and others25, 29, 33, 34. This work supports the hypothesis that CD36 not only recognizes pathological ligands and removes them in a physiologic manner, but in circumstances of pro-atherogenic, hyperlipidemic states such as obesity, these ligands signal via CD36 to affect pathophysiologic inflammatory signaling pathways in the kidney. Our findings further suggest a novel role for the Na/K-ATPase in mediating a pro-inflammatory signaling loop between macrophages and proximal tubule cells that may contribute to renal fibrosis in hyperlipidemic settings.
CD36−/− mice on the apoE−/− background were protected from renal inflammation, oxidant stress, and fibrosis induced by HFD feeding: they demonstrated significantly lower levels of renal foam cell formation, as well as improved creatinine clearance and blood pressure compared to apoE−/− mice, despite equivalent levels of the cardiotonic steroid MBG. Although the blood pressure reduction in CD36−/− mice was not sustained for the duration of the study, it is possible it accounts for some of the reduced renal injury and inflammation as well. In this same HFD feeding model, we have previously demonstrated that macrophages from CD36−/− mice secreted less pro-inflammatory cytokines and ROS, had increased arginase activity and decreased expression of key inflammatory mediators29. Further, in the current study, conditioned media from HK-2 proximal tubule cells stimulated with oxLDL and CTS had important functional consequences related to inflammation: conditioned media from HK-2 cells treated with these ligands contained elevated levels of pro-inflammatory cytokines and also enhanced macrophage migration, suggesting that soluble factors released from PTC’s in response to CD36 signaling may play an important role in macrophage recruitment and inflammation in the kidney as observed in in vivo studies.
CD36 signaling partners in the kidney: Src kinases and the Na/K-ATPase
Our understanding of the mechanisms by which CD36 can activate multiple signaling pathways in multiple cell types despite its short cytoplasmic domains and the lack of requisite signaling features such as scaffolding domains, intrinsic kinase or phosphatase activity, or links to GTPases remains a topic of intense research. While it is clear that a common theme in CD36 signal transduction is activation of Src family kinases and MAPKs35, the mechanisms underlying this interaction are unknown. CD36 has been shown to coprecipitate with Src kinases and upstream MAPK kinases (MAPKKs) in multiple cell types, and engagement with ligands such as oxLDL increases the amount of activated Src kinases in the precipitates36, 37. These studies suggest that CD36 associates with and participates in assembly of a dynamic signaling complex essential to downstream functions.
Given the regulation of Src family kinases by the Na/K-ATPase18, 19, as well as its residence in similar cholesterol-rich, detergent-insoluble lipid raft and caveolar domains as CD364, 20, we hypothesized that the Na/K-ATPase may be involved in regulating the CD36-dependent signaling events mediated by oxLDL. Indeed, using both conventional co-immunoprecipitation as well as a novel proximity ligation assay, we confirmed the association of CD36 with the Na/K-ATPase in both PTC’s as well as macrophages. This association was enhanced by prototypical ligands for both of these receptors, and attenuated with the addition of the antioxidant NAC. Furthermore, reduction of Na/K-ATPase α-1 in PTC’s resulted dose dependent reductions in ROS generation in response to a specific oxLDL ligand for CD36. These data suggest that CD36 may partner with the Na/K-ATPase in these cells types and that ROS are important second messengers in the crosstalk, potentiating a pro-inflammatory feedback loop between macrophages and PTCs.
Implications of Na/K-ATPase signaling in obesity and CKD
While initially discovered as an ion pump, cumulative studies from multiple laboratories have shown that the Na/K-ATPase is also a signal transducer involved in regulation of several gene-regulatory second messengers and pathways38. Na/K-ATPase-mediated signaling directs a number of important cellular functions including protein trafficking, gene expression, cell growth, cardiac and renal fibrosis and ROS production (reviewed in38, 39).
Obesity is a volume expanded state which increases risk for progressive CKD and end-stage renal disease1, 40 and is accompanied by increases in CTS, endogenous ligands for the Na/K-ATPase41. Increases in the circulating concentrations of, CTS, has been postulated in CKD as an adaptive response to volume expansion. Here, volume may be reduced when CTS induce endocytosis of PTC Na/K-ATPase, removing it from the basolateral membrane, reducing the vectorial transport of sodium from the tubular lumen to the blood compartment, and thus increasing sodium excretion38, 39.
In the current study we identified elevated levels of the CTS MBG in the hyperlipidemic apoE−/− model. Further, we found that both NO2LDL and plasma from hyperlipidemic mice induced dose-dependent reductions in cell surface Na/K-ATPase in PTC’s similar to the effects of the CTS ouabain. Interestingly, in kidneys from HFD fed mice, we also noted an apparent redistribution of the Na/K-ATPase α-1 from basolateral membranes in chow fed mice to a more diffuse tubular distribution after HFD. This endocytosis was also evidenced by accumulation of the plasmalemmal Na/K-ATPase in early and late endosomes and by colocalization studies of Na/K-ATPase α-1 and clathrin in HK-2 PTC’s. Together, these findings indicate that similar to known effects of CTS, endocytosis of the plasmalemmal Na/K-ATPase also occurs in response to oxLDL and may represent an adaptive mechanism to volume expansion in the setting of hyperlipidemic states such as obesity.
Perspectives
In the present study we provide evidence supporting the role of CD36 as a mediator of oxLDL induced inflammation in the kidney which provides a potential signaling pathway to be targeted by therapeutics. This is particularly relevant as pro-atherogenic, hyperlipidemic states such as obesity significantly increase circulating levels of oxLDL and are associated with increased cardiovascular morbidity and mortality. Multiple human and experimental animal models support a causal relationship between hyperlipidemia and renal injury3, 42, 43. In the RENAAL study, increased total and LDL cholesterol and triglycerides were associated with an increased risk of doubling of serum creatinine, ESRD, and all-cause death2. Patients with hyperlipidemia are at increased risk for progressive renal disease40, 44, while elevated cholesterol and obesity have been associated with glomerular structural changes and end-stage renal disease1, 45 Furthermore, lipid lowering therapy has shown promise in attenuating renal injury in this population46.
These data suggest that ligands generated in volume expanded, hyperlipidemic states such as obesity activate CD36 and the Na/K-ATPase, both of which involve activation of Src family kinases and ROS generation. We propose that CD36 and the Na/K-ATPase act synergistically through shared ligands and/or downstream molecular cross-talk in the kidney to potentiate an inflammatory paracrine loop between PTC and their associated macrophages. The paracrine effects of elevated ROS and cytokines in these facilitates the development of chronic inflammation, oxidant stress, and fibrosis underlying the renal dysfunction common to pro-atherogenic, hyperlipidemic states (Figure 6).
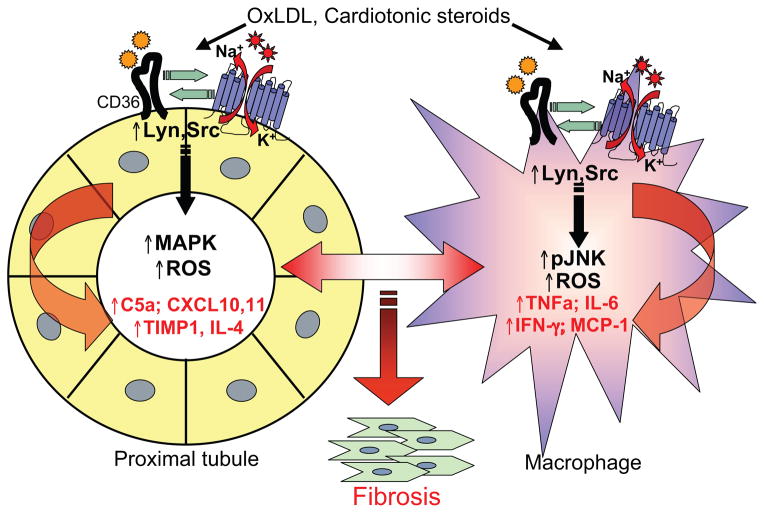
Figure 6. Proposed mechanism a CD36-Na/K-ATPase signaling loop which leads to inflammation and oxidant stress in proximal tubules cells and macrophages and contributes to renal interstitial fibrosis.
Ligands generated in volume expanded, hyperlipidemic states such as obesity activate CD36 (oxLDL) and the Na/K-ATPase (CTS), both of which involve activation of Src family kinases (Fig. 3A) and ROS generation (Fig. 5A–C). We propose that CD36 and the Na/K-ATPase act synergistically (Fig. 3B–E) through shared ligands and/or downstream molecular cross-talk in the kidney to potentiate an inflammatory paracrine loop between PTC and their associated macrophages (Figs. 2, 5D–E and Tables S1,2). The paracrine effects of ROS and cytokine release may facilitate the development of chronic inflammation, oxidant stress, and fibrosis underlying the renal dysfunction common to pro-atherogenic, hyperlipidemic states. This proposed model is further supported by data in macrophages demonstrating that oxLDL activates Lyn, JNK, ROS, and inflammatory cytokines25, 29, 37, 47 and in proximal tubule cells that demonstrating that cardiotonic steroids activate Lyn, MAPK, and ROS18, 19, 39.
Supplementary Material
Novelty and Significance.
1) What is new?
This work demonstrates a functional role for the type 2 scavenger receptor CD36 on proximal tubule cells and shows for the first time that CD36 and the Na/K ATPase function together to respond to endogenous ligands generated in circumstances of obesity and hyperlipidemia to trigger a pro-inflammatory signaling pathway in the kidney.
2) What is relevant?
These findings provide a mechanistic connection between pro-atherogenic, hyperlipidemic states and the renal glomerular and tubulointerstitial injury observed in this setting.
3) Summary
The present study supports a mechanistic link between pro-atherogenic hyperlipidemic states such as obesity and renal inflammation, oxidative stress, and fibrosis mediated by the type 2 scavenger receptor CD36. This work supports the hypothesis that CD36 not only recognizes pathological ligands and removes them, a physiologic role, but in circumstances of obesity and hyperlipidemia, these ligands signal via CD36 in cooperation with the Na/K ATPase to affect pro-inflammatory signaling pathways in a pathophysiologic response. This newly recognized role of CD36 has the potential to be targeted by therapeutics.
Acknowledgments
Some of this data was presented in abstract form at the 2011 American Society of Nephrology Meeting and the 2012 Arteriosclerosis, Thrombosis, and Vascular Biology Meeting.
FUNDING:
This work was supported by the National Institutes of Health [P01 HL087018, P01 HL46403, and HL072942, R.L.S.; R01 HL09015, J.I.S. and Z.X.]; the American Heart Association Great Rivers Affiliate [0825685D, D.J.K.]; and the Lerner Research Institute’s David and Lindsay Morgenthaler Endowed Fellowship [D.J.K.].
Footnotes
CONFLICT OF INTEREST:
None.
References
- 1.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 2.Appel GB, Radhakrishnan J, Avram MM, DeFronzo RA, Escobar-Jimenez F, Campos MM, Burgess E, Hille DA, Dickson TZ, Shahinfar S, Brenner BM. Analysis of metabolic parameters as predictors of risk in the renaal study. Diabetes Care. 2003;26:1402–1407. doi: 10.2337/diacare.26.5.1402. [DOI] [PubMed] [Google Scholar]
- 3.Wen M, Segerer S, Dantas M, Brown PA, Hudkins KL, Goodpaster T, Kirk E, LeBoeuf RC, Alpers CE. Renal injury in apolipoprotein e-deficient mice. Lab Invest. 2002;82:999–1006. doi: 10.1097/01.lab.0000022222.03120.d4. [DOI] [PubMed] [Google Scholar]
- 4.Kincer JF, Uittenbogaard A, Dressman J, Guerin TM, Febbraio M, Guo L, Smart EJ. Hypercholesterolemia promotes a cd36-dependent and endothelial nitric-oxide synthase-mediated vascular dysfunction. J Biol Chem. 2002;277:23525–23533. doi: 10.1074/jbc.M202465200. [DOI] [PubMed] [Google Scholar]
- 5.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, Khoury JE, Golenbock DT, Moore KJ. Cd36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy DJ, Kashyap SR. Pathogenic role of scavenger receptor cd36 in the metabolic syndrome and diabetes. Metab Syndr Relat Disord. 2011;9:239–245. doi: 10.1089/met.2011.0003. [DOI] [PubMed] [Google Scholar]
- 7.Silverstein RL, Febbraio M. Cd36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. Cd36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 9.Okamura DM, Lopez-Guisa JM, Koelsch K, Collins S, Eddy AA. Atherogenic scavenger receptor modulation in the tubulointerstitium in response to chronic renal injury. Am J Physiol Renal Physiol. 2007;293:F575–585. doi: 10.1152/ajprenal.00063.2007. [DOI] [PubMed] [Google Scholar]
- 10.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28:1717–1725. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 11.Apostolov EO, Shah SV, Ok E, Basnakian AG. Quantification of carbamylated ldl in human sera by a new sandwich elisa. Clin Chem. 2005;51:719–728. doi: 10.1373/clinchem.2004.044032. [DOI] [PubMed] [Google Scholar]
- 12.Ok E, Basnakian AG, Apostolov EO, Barri YM, Shah SV. Carbamylated low-density lipoprotein induces death of endothelial cells: A link to atherosclerosis in patients with kidney disease. Kidney Int. 2005;68:173–178. doi: 10.1111/j.1523-1755.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 13.Abrass CK. Cellular lipid metabolism and the role of lipids in progressive renal disease. Am J Nephrol. 2004;24:46–53. doi: 10.1159/000075925. [DOI] [PubMed] [Google Scholar]
- 14.Baines RJ, Brunskill NJ. Tubular toxicity of proteinuria. Nat Rev Nephrol. 2011;7:177–180. doi: 10.1038/nrneph.2010.174. [DOI] [PubMed] [Google Scholar]
- 15.Chmielewski M, Bryl E, Marzec L, Aleksandrowicz E, Witkowski JM, Rutkowski B. Expression of scavenger receptor cd36 in chronic renal failure patients. Artif Organs. 2005;29:608–614. doi: 10.1111/j.1525-1594.2005.29097.x. [DOI] [PubMed] [Google Scholar]
- 16.Chmielewski M, Bragfors-Helin AC, Stenvinkel P, Lindholm B, Anderstam B. Serum soluble cd36, assessed by a novel monoclonal antibody-based sandwich elisa, predicts cardiovascular mortality in dialysis patients. Clin Chim Acta. 2010;411:2079–2082. doi: 10.1016/j.cca.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Okamura DM, Pennathur S, Pasichnyk K, Lopez-Guisa JM, Collins S, Febbraio M, Heinecke J, Eddy AA. Cd36 regulates oxidative stress and inflammation in hypercholesterolemic ckd. J Am Soc Nephrol. 2009;20:495–505. doi: 10.1681/ASN.2008010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. Binding of src to na+/k+-atpase forms a functional signaling complex. Molecular Biology of the Cell. 2006;17:317–326. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor cross-talk between the na+/k+-atpase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem. 2002;277:18694–18702. doi: 10.1074/jbc.M111357200. [DOI] [PubMed] [Google Scholar]
- 20.Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: Implications for human disease. J Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Djurhuus MS, Vaag A, Klitgaard NA. Muscle sodium, potassium, and [(3)h]ouabain binding in identical twins, discordant for type 2 diabetes. J Clin Endocrinol Metab. 2001;86:859–866. doi: 10.1210/jcem.86.2.7239. [DOI] [PubMed] [Google Scholar]
- 22.Umeda F, Noda K, Hashimoto T, Yamashita T, Nawata H. Effect of aldose reductase inhibitor (ponalrestat) on erythrocyte na, k-atpase activity in non-insulin-dependent diabetic patients with polyneuropathy. Diabetes Res. 1989;12:125–129. [PubMed] [Google Scholar]
- 23.De Luise M, Blackburn GL, Flier JS. Reduced activity of the red-cell sodium-potassium pump in human obesity. N Engl J Med. 1980;303:1017–1022. doi: 10.1056/NEJM198010303031801. [DOI] [PubMed] [Google Scholar]
- 24.Febbraio M, Guy E, Silverstein RL. Stem cell transplantation reveals that absence of macrophage cd36 is protective against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2333–2338. doi: 10.1161/01.ATV.0000148007.06370.68. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy DJ, Kuchibhotla SD, Guy E, Park YM, Nimako G, Vanegas D, Morton RE, Febbraio M. Dietary cholesterol plays a role in cd36-mediated atherogenesis in ldlr-knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:1481–1487. doi: 10.1161/ATVBAHA.109.191940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W, Febbraio M, Silverstein RL. Cd9 tetraspanin interacts with cd36 on the surface of macrophages: A possible regulatory influence on uptake of oxidized low density lipoprotein. PLoS One. 2012;6:e29092. doi: 10.1371/journal.pone.0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonick HC, Ding Y, Vaziri ND, Bagrov AY, Fedorova OV. Simultaneous measurement of marinobufagenin, ouabain, and hypertension-associated protein in various disease states. Clin Exp Hypertens. 1998;20:617–627. doi: 10.3109/10641969809053240. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, Kahaleh MB, Xie Z, Malhotra D, Kolodkin NI, Lakatta EG, Fedorova OV, Bagrov AY, Shapiro JI. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47:488–495. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy DJ, Kuchibhotla S, Westfall KM, Silverstein RL, Morton RE, Febbraio M. A cd36-dependent pathway enhances macrophage and adipose inflammation and impairs insulin signaling. Cardiovasc Res. 2011;89:604–613. doi: 10.1093/cvr/cvq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, Sethi T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, Andre S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 32.Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 33.Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, Alvarez-Leite JI, de Winther MP, Tabas I, Freeman MW. Loss of sr-a and cd36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Fuentes P, Civeira F, Recalde D, Garcia-Otin AL, Jarauta E, Marzo I, Cenarro A. Individual variation of scavenger receptor expression in human macrophages with oxidized low-density lipoprotein is associated with a differential inflammatory response. J Immunol. 2007;179:3242–3248. doi: 10.4049/jimmunol.179.5.3242. [DOI] [PubMed] [Google Scholar]
- 35.Huang MM, Bolen JB, Barnwell JW, Shattil SJ, Brugge JS. Membrane glycoprotein iv (cd36) is physically associated with the fyn, lyn, and yes protein-tyrosine kinases in human platelets. Proc Natl Acad Sci U S A. 1991;88:7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen K, Febbraio M, Li W, Silverstein RL. A specific cd36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ Res. 2008;102:1512–1519. doi: 10.1161/CIRCRESAHA.108.172064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A cd36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Xie ZJ. The sodium pump and cardiotonic steroids-induced signal transduction protein kinases and calcium-signaling microdomain in regulation of transporter trafficking. Biochim Biophys Acta. 2010;1802:1237–1245. doi: 10.1016/j.bbadis.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Kennedy DJ, Yan Y, Shapiro JI. Reactive oxygen species modulation of na/k-atpase regulates fibrosis and renal proximal tubular sodium handling. International Journal of Nephrology. 2012;2012:1–14. doi: 10.1155/2012/381320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, Rogers NL, Teschan PE. Predictors of the progression of renal disease in the modification of diet in renal disease study. Kidney Int. 1997;51:1908–1919. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 41.Giampietro O, Clerico A, Penno G, Gregori G, Del Chicca MG, Cionini R, Volpe L, Navalesi R. Endogenous digitalis-like factors (edlf) in obese individuals: Preliminary results. J Nucl Biol Med. 1992;36:41–45. [PubMed] [Google Scholar]
- 42.Miyahara Y, Nishimura S, Watanabe M, Ito K, Nakashima H, Saito T. Scavenger receptor expressions in the kidneys of mice with lipoprotein glomerulopathy. Clin Exp Nephrol. 2012;16:115–121. doi: 10.1007/s10157-011-0554-6. [DOI] [PubMed] [Google Scholar]
- 43.Kasiske BL, O’Donnell MP, Garvis WJ, Keane WF. Pharmacologic treatment of hyperlipidemia reduces glomerular injury in rat 5/6 nephrectomy model of chronic renal failure. Circ Res. 1988;62:367–374. doi: 10.1161/01.res.62.2.367. [DOI] [PubMed] [Google Scholar]
- 44.Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: The atherosclerosis risk in communities study. Kidney Int. 2000;58:293–301. doi: 10.1046/j.1523-1755.2000.00165.x. [DOI] [PubMed] [Google Scholar]
- 45.Sasatomi Y, Tada M, Uesugi N, Hisano S, Takebayashi S. Obesity associated with hypertension or hyperlipidemia accelerates renal damage. Pathobiology. 2001;69:113–118. doi: 10.1159/000048764. [DOI] [PubMed] [Google Scholar]
- 46.Appel G. Lipid abnormalities in renal disease. Kidney Int. 1991;39:169–183. doi: 10.1038/ki.1991.22. [DOI] [PubMed] [Google Scholar]
- 47.Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, Morton RE, Febbraio M. Absence of cd36 protects against atherosclerosis in apoe knock-out mice with no additional protection provided by absence of scavenger receptor ai/ii. Cardiovasc Res. 2007:cvm093. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.