Abstract
Given the present challenges to attain effective treatment for β-amyloid (Aβ) toxicity in neurodegenerative disorders such as Alzheimer’s disease, development of novel cytoprotective pathways that can assist immune mediated therapies through the preservation of central nervous system microglia could offer significant promise. We show that the CCN4 protein, Wnt1 inducible signaling pathway protein 1 (WISP1), is initially up-regulated by Aβ and can modulate its endogenous expression for the protection of microglia during Aβ mediated apoptosis. WISP1 activates mTOR and phosphorylates p70S6K and 4EBP1 through the control of the regulatory mTOR component PRAS40. Loss of PRAS40 through gene reduction or inhibition by WISP1 is cytoprotective. WISP1 ultimately governs PRAS40 by sequestering PRAS40 intracellularly through post-translational phosphorylation and binding to protein 14-3-3. Our work identifies WISP1, mTOR signaling, and PRAS40 as targets for new strategies directed against Alzheimer’s disease and related disorders.
Keywords: Alzheimer’s disease, amyloid, CCN4, microglia, mTOR, PRAS40, WISP1
Introduction
The CCN family of proteins is composed of six secreted extracellular matrix associated proteins and each member contains four cysteine-rich modular domains that include insulin-like growth factor-binding domain, von Willebrand factor type C module, thrombospondin domain, and C-terminal cysteine knot-like domain (1). CCNs have a broad array of biological functions that include development of the skeletal system, vascular repair, extracellular matrix composition, and cellular proliferation and survival. In particular, Wnt1 inducible signaling pathway protein 1 (WISP1), a member of the CCN family termed CCN4, was initially shown to prevent p53 mediated apoptotic cell damage in renal fibroblasts (2). WISP1 is expressed during cell injury such as in the setting of cartilage and fracture repair (3, 4), cardiac ischemic injury (5), lung epithelial damage (6), and in primary neurons during exposure to oxidative stress (7, 8). Increased expression of WISP1 during cell injury may have a high correlation with enhanced cellular survival, since recent studies illustrate that WISP1 is protective against doxorubicin-induced cardiomyocyte death (9) and oxygen-glucose mediated neuronal injury (7, 8).
Given the potential of WISP1 to offer protection against neuronal injury, WISP1 may be a novel therapeutic target against neurodegenerative disorders such as Alzheimer’s disease. β-amyloid (Aβ) accumulation and toxicity in brain is considered to be a significant component for the onset and progression of Alzheimer’s disease (10-15). Current clinical strategies that target the immunomodulation and the removal of cortical Aβ in patients with Alzheimer’s disease remain without significant proven efficacy (16). However, additional gains may be acquired through the use of novel cytoprotective pathways such as WISP1 that may preserve central nervous system microglia survival to assist with immune mediated therapies to limit Aβ accumulation and sequester Aβ (17-22).
WISP1 fosters cellular survival through wingless mediated pathways (8, 9) and through pathways that involve phosphatidylinositol-3-kinase (PI 3-K) and protein kinase B (Akt) (2, 5, 7-9, 23). These pathways can converge upon mammalian target of rapamycin (mTOR) that has been shown to control inflammatory cell survival (22, 24, 25). In addition, mTOR relies upon activation of PI 3-K and Akt to block cell demise in the setting of toxic environments (22, 24, 26, 27). One component that can regulate the activity of mTOR is the proline rich Akt substrate 40 kDa (PRAS40). PRAS40 inhibits mTOR activity and its downstream signaling through the mTOR Complex 1 (mTORC1) to prevent the binding of p70S6K and the eukaryotic initiation factor 4E-binding protein 1 (4EBP1) to Raptor (28-30). PRAS40 activity is inhibited during post-translational phosphorylation (31) that has been shown to prevent cellular injury (32-34).
We therefore investigated whether WISP1 could preserve microglial cellular integrity during Aβ toxicity through cellular pathways that relied upon mTOR signaling and its regulatory component PRAS40. We show WISP1 can regulate its own expression and is necessary to prevent both early and late apoptotic injury in microglia through modulation of mTOR and its signaling pathways of p70S6K and 4EBP1. PRAS40 is vital to this cytoprotective pathway and is controlled by WISP1 through the post-translational phosphorylation of PRAS40 and the binding of PRAS40 to protein 14-3-3.
Materials and Methods
Microglial cell cultures
Per our prior protocols, the microglial cell line EOC 2 was obtained from American Type Culture Collection (ATTC, Manassas, VA.) (22, 35). Cells were maintained in Dulbecco’s modified Eagle medium (ATTC, Manassas, VA) and supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St Louis, MO), 50 μg/ml penicillin and streptomycin, and 20% media from the LADMAC cell line (ATCC, Manassas, VA) which contains colony stimulating factor-1 (CSF-1) secreted by the LADMAC cells. Cells were seeded onto 24-well plates or 35 mm culture dishes at a density of 1.5 × 106 cells per well or 4 × 106 cells per dish.
Experimental treatments
Per our prior protocols (21, 36, 37), β-amyloid (Aβ1-42) (American Peptide Co., Sunnyvale, CA) was dissolved in PBS at a concentration of 100 μM. To allow for Aβ aggregation, Aβ was incubated at 37°C for a 7 day period and then directly applied to microglial cell cultures per the experimental protocols. For treatments applied prior to Aβ, human recombinant WISP1 protein (R&D Systems, Minneapolis, MN) was applied 1 hour prior to Aβ administration and the treatment was continuous.
Assessment of cell survival
Microglial injury was determined by bright field microscopy using a 0.4% trypan blue dye exclusion method 24 hours following treatment with Aβ per our previous protocols (24, 37, 38). The mean survival was determined by counting eight randomly selected non-overlapping fields with each containing approximately 10-20 cells (viable + non-viable). Each experiment was replicated 3-6 times independently with different cultures.
Assessment of DNA fragmentation
Genomic DNA fragmentation was determined by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay (39-41). Briefly, microglial cells were fixed in 4% paraformaldehyde/0.2% picric acid/0.05% glutaraldehyde. The 3′-hydroxy ends of DNA were labeled with biotinylated dUTP using the enzyme terminal deoxytransferase (Promega, Madison, WI) followed by streptavidin-peroxidase and visualized with 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA).
Assessment of membrane phosphatidylserine (PS) membrane externalization
Per our prior protocols (42-44), externalization of membrane PS residues was determined by using Annexin V labeling. A 30 μg/ml stock solution of Annexin V conjugated to phycoerythrin (PE) (R&D Systems, Minneapolis, MN) was diluted to 3 μg/ml in warmed calcium containing binding buffer (10 mmol/L Hepes, pH 7.5, 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 1.8 mmol/L CaCl2). Plates were incubated with 500 μl of diluted Annexin V for 10 minutes. Images were acquired with “blinded” assessment with a Leitz DMIRB microscope (Leica, McHenry, IL) and a Fuji/Nikon Super CCD (6.1 megapixels) using transmitted light and fluorescent single excitation light at 490 nm and detected emission at 585 nm.
Gene reduction of WISP1 and PRAS40 with small interfering RNA (siRNA)
Microglia were plated into 35 mm dishes or 24-well plates. To silence WISP1 and PRAS40 gene expression, commercial reagents targeting WISP1 mRNA or PRAS40 mRNA (Santa Cruz, Santa Cruz, CA) were used. Transfection of siRNA duplexes was performed with LipofectamineTM RNAiMAX reagent according to the manufacturer guidelines (Life Technologies Corp, Carlsbad, CA). Experimental assays were performed 72 hours post-transfection. For each siRNA assay, scrambled siRNA was used as control.
Expression of WISP1, mTOR, p70S6K, 4EBP1, and PRAS40 with relevant phosphorylated moieties
Cells were homogenized and following protein determination, each sample (50 μg/lane) was then subjected to 7.5% (p-mTOR, mTOR, p-p70S6K, p70S6K) or 12.5% (WISP1, p-4EBP1, 4EBP1, PRAS40, p-PRAS40) SDS-polyacrylamide gel electrophoresis separation. After blocking for 1 hour at room temperature with 5% skim milk, the membranes were incubated overnight at 4 °C with a rabbit polyclonal antibody against WISP1 (1:200, Santa Cruz Biotechnologies, Santa Cruz, CA), a rabbit antibody against (p- = phosphorylated) p-mTOR (Ser 2448, 1: 1000), mTOR (1:1000), p-p70S6K (Thr389, 1:1000), p70S6K (1:1000), p-4EBP1 (Ser 65/Thr70, 1:1000), PRAS40 (1:1000), and p-PRAS40 (Thr246, 1:1000). All antibodies were obtained from Cell Signaling, Beverly, MA except for WISP1 as noted above. Following incubation, the membranes were incubated with a horseradish peroxidase (HRP) conjugated secondary antibody goat anti-rabbit IgG (goat anti-rabbit IgG, 1:5000, Thermo Scientific, Rockford, IL). The antibody-reactive bands were revealed by chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) and band density was performed using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
Immunoprecipitation of PRAS40 and 14-3-3
Cells lysates of total protein (200 μg) were incubated with primary antibody against protein 14-3-3 (1:100, Santa Cruz Biotech, Santa Cruz, CA) overnight at 4 °C. The complexes were collected with protein A/G-agarose beads, centrifuged, and then prepared for 14-3-3, PRAS40, and p-PRAS40 western analysis.
Statistical analysis
For each experiment, the mean and standard error were determined. Statistical differences between groups were assessed by means of analysis of variance (ANOVA) from 6 replicate experiments with the post-hoc Dunnett’s test. Statistical significance was considered at P<0.05.
Results
Exogenous WISP1 maintains endogenous WISP1 expression during Aβ exposure
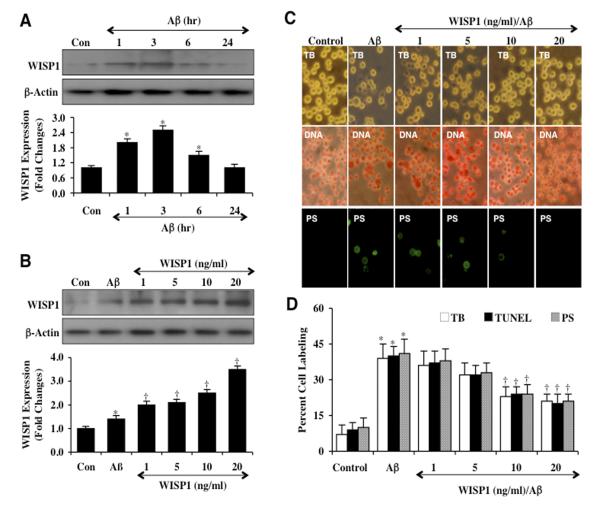
Cell protein extracts (50 μg/lane) were immunoblotted with anti-WISP1 antibody at 1, 3, 6, and 24 hours following Aβ (10 μM) administration. As shown in Figure 1A, WISP1 expression was mildly increased at 1, 3, and 6 hours following Aβ exposure, but returned to control untreated levels at the 24 hour time period. In the next study, WISP1 (1, 5, 10, and 20 ng/ml) was applied 1 hour prior to Aβ exposure, maintained for 6 hours and then removed through 3 media exchanges. Subsequent western analysis for endogenous WISP1 demonstrated significantly increased expression of WISP1 in a concentration dependent manner (Figure 1B), suggesting that application of exogenous WISP1 can promote endogenous WISP1 expression in microglia.
Figure 1. WISP1 increases endogenous expression and blocks apoptotic injury during Aβ exposure.
(A) Microglial cultures were exposed to Aβ at the concentration of 10 μM for the time period (hr=hour) as indicated and the expression of WISP1 was determined by western blot analysis (*P <0.01 vs. Control). Con = Control = untreated microglia. Each data point represents the mean and SEM from 3 experiments. (B) Exogenous WISP1 (1, 5, 10, and 20 ng/ml) was applied 1 hour prior to Aβ (10 μM) exposure, maintained for 6 hours and then removed through 3 media exchanges. Subsequent expression of WISP1 was determined by western blot analysis (*P<0.01 vs. untreated control; †P <0.01 vs. Aβ). Each data point represents the mean and SEM from 3 experiments. Con = control = untreated microglia. (C and D) WISP1 (1, 5, 10, and 20 ng/ml) was applied to cultures of microglia 1 hour prior to the administration of Aβ (10 μM) and cell survival, DNA fragmentation, and membrane PS exposure were determined 24 hours later through trypan blue dye exclusion (TB, for cell survival), TUNEL (DNA fragmentation), and annexin-V labeling (PS exposure) respectively. Representative images (C) and quantitative analysis (D) demonstrate that Aβ leads to a significant increase in trypan blue dye, TUNEL, and annexin-V labeling in microglia 24 hours after Aβ exposure when compared to untreated control cultures. In contrast, WISP1 (10 or 20 ng/ml) given 1 hour prior to Aβ exposure significantly reduced trypan blue dye, TUNEL, and annexin-V labeling (*P < 0.01 vs. Control; †P <0.01 vs. Aβ treated alone). Each data point represents the mean and SEM from 6 experiments.
WISP1 prevents microglial cell injury, DNA degradation, and phosphatidylserine externalization during Aβ exposure
Twenty-four hours following Aβ exposure, cell injury was determined by trypan blue dye exclusion, early apoptotic injury was assessed by membrane phosphatidylserine (PS) exposure (annexin V staining), and late apoptotic genomic DNA fragmentation was assessed by TUNEL (Figures 1C and 1D). Representative images and quantitative results demonstrate that Aβ exposure results in a significant increase in trypan blue staining, DNA fragmentation, and membrane PS exposure in microglia when compared to untreated control cultures. WISP1 (1, 5, 10, and 20 ng/ml) applied 1 hour prior to Aβ exposure significantly decreased trypan blue dye staining, DNA fragmentation, and membrane PS exposure in microglia 24 hours following Aβ administration for the WISP1 concentrations of 10 ng/ml and 20 ng/ml (Figure 1C). In Figure 1D, quantitative results illustrate that percent trypan blue staining, DNA fragmentation, and PS exposure were significantly increased to 39 ± 6%, 40 ± 4%, and 41 ± 6% respectively from 7 ± 4%, 9 ± 3%, and 10 ± 4% for untreated control cells. In contrast, WISP1 administration at the concentrations of 10 ng/ml and 20 ng/ml 1 hour prior to Aβ exposure significantly limited cell injury, DNA fragmentation, and membrane PS exposure.
Endogenous WISP1 is a necessary component for microglial protection against Aβ
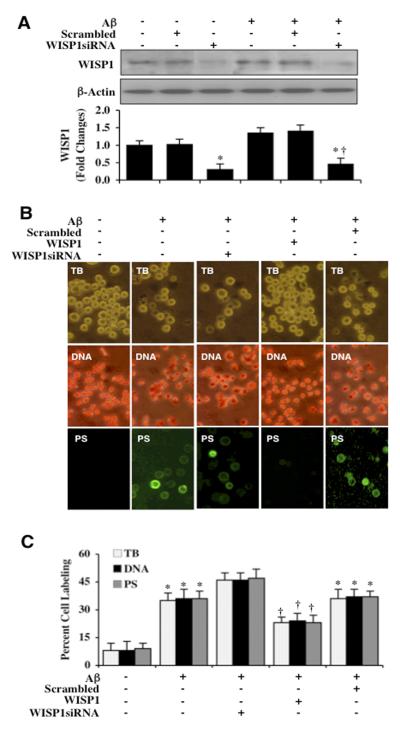
Transfection with WISP1 siRNA in either untreated microglia or microglia exposed to Aβ for 3 hours resulted in a significant reduction of WISP1 expression (Figure 2A). As a control, non-specific scrambled siRNA did not alter WISP1 protein expression in untreated control microglia or in microglia exposed to Aβ alone, demonstrating that WISP1 siRNA was specific to block protein expression of WISP1. In Figure 2B, representative images demonstrate that Aβ exposure leads to a significant increase in trypan blue staining, genomic DNA fragmentation, and PS membrane externalization in microglia 24 hours later. Gene reduction of WISP1 with siRNA further increased cell injury, genomic DNA fragmentation, and PS membrane externalization when compared with Aβ alone, illustrating that endogenous WISP1 provides a level of protection for microglia against Aβ toxicity (Figures 2B and 2C). As a control, non-specific scrambled siRNA did not alter survival, DNA fragmentation, or PS exposure when compared to Aβ treated cultures alone.
Figure 2. Endogenous WISP1 is a necessary for microglial cell survival against Aβ.
(A) Gene knockdown of WISP1 was performed with transfection of WISP1 siRNA prior to Aβ (10 μM) exposure in microglial cells. The expression of WISP1 was determined 3 hours following Aβ exposure by western blot analysis (*P <0.01 vs. Control; †P <0.01 vs. Aβ). Control = untreated microglia. Each data point represents the mean and SEM from 3 experiments. Transfection with scrambled siRNA did not change WISP1 expression following Aβ exposure when compared to Aβ exposure alone (*P < 0.01 vs. Control; †P <0.01 vs. Aβ treated alone). (B) Gene knockdown of WISP1 was performed with transfection of WISP1 siRNA prior to Aβ (10 μM) exposure in microglia cells and cell survival, DNA fragmentation, and membrane PS exposure were determined 24 hours later through trypan blue dye exclusion (TB), TUNEL (DNA), and annexin-V labeling (PS) respectively. Representative images demonstrate that Aβ results in a significant increase in trypan blue dye staining, DNA fragmentation, and membrane PS exposure in microglia 24 hours after Aβ exposure. WISP1 siRNA transfection further increased trypan blue dye staining, DNA fragmentation, and membrane PS exposure labeling following Aβ exposure, but not with statistical significance.. (C) Quantitative analysis demonstrates that transfection with WISP1 siRNA increased percent cell labeling of trypan blue dye staining, DNA fragmentation, and membrane PS exposure following Aβ exposure. Transfection with scrambled siRNA did not change the percent trypan blue dye staining, DNA fragmentation, and membrane PS exposure labeling following Aβ exposure when compared to Aβ exposure alone (*P < 0.01 vs. Control; †P <0.01 vs. Aβ treated alone). Each data point represents the mean and SEM from 3 experiments.
WISP1 promotes mTOR activation and phosphorylation of p70S6K and 4EBP1
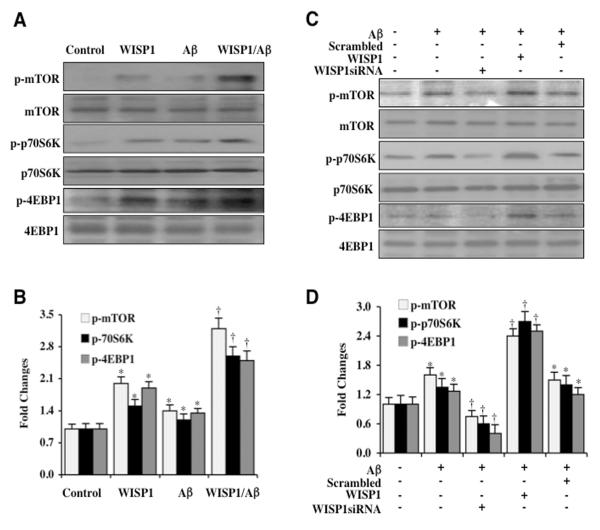
Since WISP1 cytoprotection in other systems has been tied to the pathways of PI 3-K and Akt1 (2, 5, 7-9, 23), we investigated whether WISP1 could alter mTOR signaling and the activity of its downstream targets p70S6K and 4EBP1. Phosphorylation sites of mTOR for its activation include serine2448 (31, 45). mTOR phosphorylates and activates p70S6K at threonine389 which serves as a marker of mTOR activity (46). 4EBP1 is phosphorylated by mTOR at serine65 and threonine70 (47). Phosphorylation of 4EBP1 results in the dissociation of 4EBP1 from eukaryotic translation initiation factor 4 epsilon (eIF4E) to promote the eukaryotic translation initiation factor 4 gamma (eIF4G) to begin mRNA translation (48, 49). We assessed the expression of phosphorylated mTOR (p-mTOR, Ser2448, active form) and phosphorylated forms of p-p70S6K and p-4EBP1 (p-p70S6K, Thr389; p-4EBP1, Ser65/Thr70) 3 hours following Aβ exposure. In Figures 3A and 3B, expression of p-mTOR, p-p70S6K, and p-4EBP1 was mildly increased during Aβ exposure alone. However, in the presence of WISP1 (10 ng/ml) alone or during WISP1 (10 ng/ml) with Aβ exposure, expression of p-mTOR, p-p70S6K, and p-4EBP1 were significantly increased. In addition, transfection of WISP1 siRNA significantly reduced phosphorylation of p-mTOR, p-p70S6K, and p-4EBP1 during Aβ exposure when compared to microglia treated with WISP1 ng/ml during Aβ exposure (Figures 3C and 3D), illustrating that WISP1 is necessary to phosphorylate and activate mTOR as well as phosphorylate p-p70S6K and p-4EBP1. As a control, non-specific scrambled siRNA did not alter mTOR, p70S6K, and 4EBP1 phosphorylation.
Figure 3. WISP1 activates mTOR and phosphorylates p70S6K and 4EBP1.
(A) WISP1 (10 ng/ml) was applied directly to microglial cultures and the expression of phosphorylated (p)-mTOR (Ser2448), p-p70S6K (Thr389), and p-4EBP1 (Ser65/Thr70) was determined at 3 hours later by western blot analysis. WISP1 significantly increased the expression of p-mTOR, p-p70S6K, and p-4EBP1 in microglia. Following WISP1 (10 ng/ml) application to microglia 1 hour prior to Aβ (10 μM) administration, the expression of p-mTOR, p-p70S6K, and p-4EBP1 was significantly increased when compared with Aβ cultures treated alone. (B) Quantitative results illustrate that WISP1 during Aβ exposure significantly increased the expression of p-mTOR, p-p70S6K, and p-4EBP1 in microglia (*P < 0.01 vs. Control; †P<0.01 vs. Aβ treated alone). (C) WISP1 siRNA was transfected into microglia and cell protein extracts (50 μg/lane) were immunoblotted with phosphorylated (p)-mTOR (Ser2448), p-p70S6K (Thr389), and p-4EBP1 (Ser 65/Thr70) antibodies at 3 hours following administration of Aβ (10 μM). Aβ resulted in a mild increase in the expression of p-mTOR, p-p70S6K, and p-4EBP1 that was further increased by WISP1 (10 ng/ml) administration 1 hour prior to Aβ exposure. WISP1 siRNA transfection significantly limited the expression of p-mTOR, p-p70S6K, and p-4EBP1 3 hours following Aβ exposure. (D) Quantitative results demonstrate that gene knockdown of WISP1 significantly limited the expression of p-mTOR, p-p70S6K, and p-4EBP1 3 hours following Aβ exposure (*P <0.01 vs. Control; †P<0.01 vs. Aβ treated alone). Scrambled siRNA transfection did not alter the expression of p-mTOR, p-p70S6K, and p-4EBP1 following Aβ exposure. Control=untreated microglia. In all cases, each data point represents the mean and SEM from 3 experiments.
Cellular reduction of PRAS40 protects against apoptotic DNA degradation and membrane PS exposure during Aβ
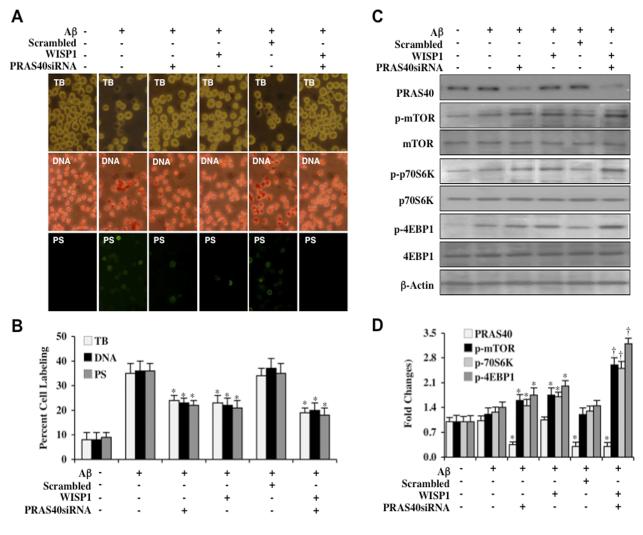
PRAS40 is a critical regulator of mTOR signaling that can associate with Raptor (29, 30) and prevent p70S6K and 4EBP1 binding to Raptor (28, 30). We therefore investigated whether loss of PRAS40 could affect microglial alter cell survival and apoptotic injury during Aβ exposure. Cell survival was assessed with trypan dye blue exclusion, DNA degradation with TUNEL, and PS externalization with annexin-V labeling 24 hours after Aβ exposure. In Figures 4A and 4B, gene reduction of PRAS40 during Aβ exposure significantly decreased trypan blue staining, TUNEL staining, and membrane PS externalization, demonstrating that loss of PRAS40 is protective during Aβ exposure. In addition, gene reduction of PRAS40 during WISP1 (10 ng/ml) treatment and Aβ exposure improved cell survival and reduced apoptotic injury to a greater extent than WISP1 alone (Figures 4A and 4B), suggesting that WISP1 relies upon inhibition of PRAS40 to prevent microglial cell injury during Aβ toxicity. As a control, non-specific scrambled siRNA did not alter survival or apoptotic injury when compared to WISP1 treatment and Aβ exposure alone.
Figure 4. PRAS40 controls apoptotic injury and the WISP1 phosphorylation of mTOR, p70S6K, and 4EBP1.
(A) PRAS40 siRNA was transfected into microglial cultures prior to administration of Aβ (10 μM) and cell injury was determined 24 hours following Aβ exposure through trypan blue dye exclusion (TB, for cell survival), TUNEL (DNA fragmentation), and annexin-V labeling (PS exposure) respectively. Representative images of trypan blue staining, TUNEL, and PS exposure following Aβ exposure in microglia show that PRAS40 siRNA transfection reduced trypan blue staining, DNA fragmentation, and PS exposure and tended to increase cytoprotection of WISP1 (10 ng/ml) without statistical significance. (B) The quantitative results of trypan blue dye exclusion, DNA fragmentation, and membrane PS exposure demonstrated that percent trypan blue staining, DNA fragmentation, and PS exposure was significantly decreased by PRAS40 siRNA transfection or WISP1 (10 ng/ml) administration prior to Aβ exposure (*P<0.01 vs. Aβ treated alone). Scrambled siRNA transfection did not alter trypan blue dye exclusion, DNA fragmentation, and membrane PS exposure following Aβ exposure when compared to Aβ exposure alone. Each data point represents the mean and SEM from 3 experiments. (C) Gene knockdown of PRAS40 was performed with transfection of PRAS40 siRNA prior to Aβ (10 μM) exposure in microglia and the expression of PRAS40, phosphorylated (p) p-mTOR (Ser2448), p-p70S6K (Thr389), and p-4EBP1 (Ser65/Thr70) was determined 3 hours following Aβ exposure. WISP1 (10 ng/ml) applied 1 hour prior to Aβ exposure significantly increased the expression of p-mTOR, p-p70S6K, and p-4EBP1. Transfection with PRAS40 siRNA significantly limited the expression of PRAS40 and significantly increased the expression of p-mTOR, p-p70S6K, and p-4EBP1 in microglia following a 3 hour period of Aβ exposure or in cells treated with WISP1 (10 ng/ml). Scrambled siRNA transfection did not alter the expression of PRAS40, p-mTOR, p-p70S6K, and p-4EBP1 following Aβ exposure. (D) Quantitative results of western blot band density in (C) illustrate that PRAS40 gene reduction leads to significantly increased expression of p-mTOR, p-p70S6K, and p-4EBP1 in microglia during Aβ exposure alone or during Aβ exposure with WISP1 (10 ng/ml) application (*P < 0.01 vs. Aβ treated alone; †P<0.01 vs. WISP1/Aβ).
PRAS40 modulates WISP1 phosphorylation of mTOR, p70S6K, and 4EBP1
We next examined the effects of PRAS40 gene reduction upon the expression of p-mTOR, p-p70S6K, and p-4EBP1 during Aβ exposure and also during WISP1 (10 ng/ml)with Aβ exposure. In Figures 4C and 4D, transfection with PRAS40 siRNA significantly reduced the expression of PRAS40 protein and increased the phosphorylation of p-mTOR, p-p70S6K, and p-4EBP1 during Aβ exposure. Reduction of PRAS40 with siRNA transfection also increased the expression of p-mTOR, p-p70S6K, and p-4EBP1 during WISP1 (10 ng/ml) administration with Aβ exposure, suggesting that WISP1 phosphorylation of mTOR, p70S6K, and 4EBP1 can be fostered by the inhibition or loss of PRAS40. As a control, scrambled siRNA did not alter the expression of p-mTOR, p-p70S6K, and p-4EBP1 during Aβ exposure.
WISP1 controls the phosphorylation and binding of PRAS40 to protein 14-3-3 during Aβ exposure
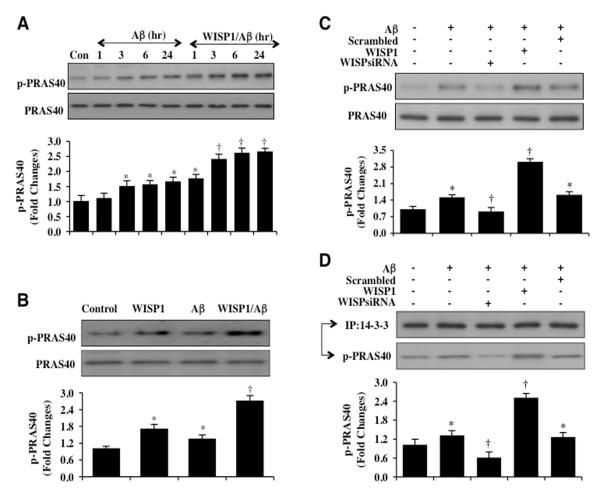
Phosphorylation of PRAS40 on threonine246 by Akt leads to its dissociation from the mTOR complex mTORC1 (28). As a result, phosphorylated PRAS40 binds to the docking protein 14-3-3 to inhibit PRAS40 and activate mTOR signaling (50, 51). Given that WISP1 can phosphorylate and activate Akt1 (2, 5, 7, 9), we examined whether WISP1 could lead to the phosphorylation of PRAS40 and promote the binding of PRAS40 to protein 14-3-3 in microglia. Western blot analysis for phosphorylated p-PRAS40 (Thr246) was performed at 1, 3, 6, and 24 hours following Aβ exposure. As shown in Figure 5A, phosphorylated p-PRAS40 expression was mildly increased at 3, 6, and 24 hours following Aβ exposure. In contrast, WISP1 (10 ng/ml) applied during Aβ exposure significantly increased phosphorylation of PRAS40 over a 24 hour course when compared to exposure to Aβ alone (Figure 5A). WISP1 (10 ng/ml) in cells not exposed to Aβ also significantly increased phosphorylation of PRAS40 within 3 hours (Figure 5B). In addition, gene reduction of WISP1 significantly limited the phosphorylation of PRAS40 during Aβ exposure alone and during Aβ exposure with WISP1 (10 ng/ml), illustrating that the presence of WISP1 was necessary for PRAS40 phosphorylation (Figure 5C). Non-specific scrambled siRNA did not alter PRAS40 phosphorylation illustrating the specificity for WISP1 in relation to PRAS40 phosphorylation.
Figure 5. WISP1 leads to the phosphorylation and binding of PRAS40 to protein 14-3-3 during Aβ exposure.
(A) Western blot was performed for phosphorylated-PRAS40 (p-PRAS40, Thr246) in microglia at 1, 3, 6, or 24 hours (hr) following Aβ (10 μM) exposure. Aβ resulted in a mild increase in the expression of p-PRAS40 after 3 hours following of Aβ (*P<0.01 vs. Control). Application of WISP1 (10 ng/ml) 1 hour prior to Aβ administration significantly increased the expression of p-PRAS40 at 3, 6, and 24 hours after Aβ exposure (†P<0.01 vs. Aβ at the corresponding time points). (B) Application of WISP1 (10 ng/ml) microglial cultures significantly increased the expression of p-PRAS40 3 hours later. WISP1 (10 ng/ml) treatment prior to Aβ (10 μM) administration also significantly increased the expression of p-PRAS40 when compared to Aβ treated alone 3 hours following Aβ exposure (*P < 0.01 vs. untreated Control; †P<0.01 vs. Aβ treated alone). (C) WISP1 siRNA was transfected into microglial cultures prior to the administration of Aβ (10 μM) and expression of p-PRAS40 was determined 3 hours following Aβ exposure by western blot analysis. Transfection of WISP1 siRNA reduced the expression of p-PRAS40 3 hours following Aβ exposure. Non-specific scrambled siRNA did not alter the expression of p-PRAS40 during Aβ exposure (*P < 0.01 vs. untreated Control; †P<0.01 vs. Aβ treated alone). In all cases, each data point represents the mean and SEM from three experiments. (D) WISP1 siRNA was transfected into microglial cultures prior to the administration of Aβ (10 μM) and cell extracts were immunoprecipitated by antibodies against protein 14-3-3 three hours later. Western blot analysis was performed to detect the expression p-PRAS40 and 14-3-3 in the precipitate. Application of WISP1 (10 ng/ml) increased the expression of p-PRAS40 in the precipitate. In contrast, WISP1 siRNA significantly reduced expression of p-PRAS40 in the precipitate following Aβ exposure (*P<0.01 vs. untreated control; †P< 0.01 vs. Aβ treated alone).
In regards to the binding of phosphorylated PRAS40 to protein 14-3-3, Aβ (10 μM) exposure for 3 hours mildly increased the expression of phosphorylated PRAS40 in the lysate that was immunoprecipited by antibody against 14-3-3 protein (Figure 5D). Yet, transfection with WISP1 siRNA in microglia significantly reduced the expression of phosphorylated PRAS40 in the precipitate following Aβ exposure, suggesting that loss of WISP1 prevents the binding of phosphorylated PRAS40 with protein 14-3-3 (Figure 5D). In addition, application of WISP1 (10 ng/ml) 1 hour prior to Aβ exposure significantly increased phosphorylated PRAS40 expression in the precipitate when compared to microglia exposed to Aβ alone (Figure 5D), further illustrating that WISP1 significantly increases the binding of phosphorylated PRAS40 to protein 14-3-3. Non-specific scrambled siRNA did not alter phosphorylated PRAS40 binding to protein 14-3-3 when compared to microglia exposed to Aβ alone.
Discussion
Control of mTOR signaling through cytoprotective pathways such as WISP1 (6-9, 52) may offer an exciting avenue to limit Aβ injury to microglial cells and preserve cognitive function (22, 53, 54). We show that WISP1 expression is endogenously present in central nervous system microglia and can be initially up-regulated in its expression following Aβ exposure, suggesting that WISP1 expression may be a cytoprotective reparative response similar to WISP1 up-regulation in other cells and systems of the body in response to environmental stress (5, 7, 8, 55, 56). Furthermore, exogenous WISP1 treatment in microglial cells can significantly increase the expression of endogenous WISP1 during Aβ exposure. A sustained cellular up-regulation of WISP1 may be critical to foster protection as well as repair to injured inflammatory cells. In cardiomyocytes (9) and primary neurons (8), exogenous WISP1 application has been shown to increase and sustain the expression of endogenous cellular WISP1 over time and that WISP1 may regulate its own expression through control of β-catenin phosphorylation (8, 55, 57) and nuclear trafficking (8).
Sufficient expression of WISP1 appears to be critical for the survival of microglial cells during Aβ exposure. In prior work, WISP1 has been shown to enhance survival of lung carcinoma cells during ultraviolet irradiation and etoposide treatment (2), prevent cardiomyocyte death during tumor necrosis factor exposure (56), and prevent neuronal apoptotic DNA degradation during oxidant stress (7, 8). We show that WISP1 can be necessary and sufficient to protect microglial cells from Aβ not only from the late stages of apoptotic injury with DNA degradation, but also from the early phase of apoptotic injury with membrane PS exposure. WISP application at concentrations of 10 ng/ml and 20 ng/ml similar to studies in other cellular systems prevented microglial demise during Aβ exposure (7-9, 56). Furthermore, we show that the loss of WISP1 with gene reduction of WISP1 resulted in significant cell injury, DNA degradation, and membrane PS exposure to even a greater extent than during exposure to Aβ alone. The ability of WISP1 to protect cells against both the early and late stages of apoptotic injury may be vital during reparative processes of the brain. Apoptotic exposure of membrane PS residues on injured cells can function as a “tag” for the eventual destruction of otherwise function cells such as neurons (58-60), microglia (21, 38, 61, 62), and vascular cells (41, 42, 63-65). By inhibiting membrane PS exposure as well as later genomic DNA degradation in microglia, WISP1 may be capable of blocking the demise of functional microglia to foster cytoprotection and potential repair of the brain during neurodegenerative disorders (17, 66, 67).
WISP1 also relies upon mTOR and its signaling pathways to foster cytoprotection in microglia. Previously, WISP1 has been shown to be dependent upon PI 3-K and Akt to offer cellular protection in neurons, cardiac cells, and renal fibroblasts (2, 5, 7-9, 23). The PI 3-K and Akt pathways are central pathways for cell proliferation and survival (41, 44, 68, 69) and for the control of metabolic pathways (70-73). mTOR has been shown to depend upon activation of the PI 3-K and Akt pathways to prevent cell injury (22, 24, 26, 27). We now show that WISP1 during Aβ exposure is necessary to phosphorylate mTOR, p70S6K and 4EBP1. Without WISP1, such as during gene reduction of WISP1, phosphorylation of mTOR, p70S6K and 4EBP1 is lost during Aβ exposure. These pathways can be vital for cell survival since loss of mTOR signaling prevents phosphorylation of both p70S6K and 4EBP1 and results in apoptosis (74). Although some studies that apply rapamycin to inhibit mTOR activity have observed cognitive improvement in mouse models of Alzheimer’s disease (75), rapamycin can alter a number of pathways that are not specific for mTOR through mTORC1 and mTORC2 (31, 76, 77), rapamycin may exacerbate amyloid toxicity (78), amyloid can be a detriment to cytoprotective mTOR signaling (53, 79), and in several scenarios mTOR signaling is necessary for protection against Aβ toxicity (22, 53, 54). In addition, activation of p70S6K by mTOR in astrocytes has been shown to be cytoprotective (80). In the absence of mTOR activity, 4EBP1 is hypophosphorylated and can bind to eIF4E that results in the translation of apoptotic promoting proteins (81).
Interestingly, we demonstrate that WISP1 governs mTOR signaling through PRAS40. PRAS40 can inhibit mTOR activity and the binding of p70S6K and 4EBP1 to Raptor (28-30). Furthermore, inhibition of PRAS40 has been shown to prevent cellular death during to toxic exposure such as oxidant stress. For example, inhibition and phosphorylation of PRAS40 reduces apoptotic cell death (32-34) and gene reduction of PRAS40 has been shown to prevent apoptosis against tumor necrosis factor and cyclohexamide (82). We show that during gene reduction of PRAS40, microglial cellular injury, genomic DNA degradation, and membrane PS exposure are significantly limited during Aβ exposure. In addition, gene reduction of PRAS40 during WISP1 administration and Aβ exposure further reduced cellular DNA degradation and membrane PS exposure, suggesting that WISP1 is employing PRAS40 inhibition to protect microglia against Aβ toxicity. These observations are further supported by our work that examined the ability of WISP1 to phosphorylate mTOR, p70S6K and 4EBP1 during gene reduction of PRAS40. Gene reduction of PRAS40 increased the phosphorylation of mTOR, p70S6K and 4EBP1 either during Aβ exposure alone or during treatment with WISP1, indicating that phosphorylation of mTOR, p70S6K, and 4EBP1 by WISP1 can be mediated through the inhibition or loss of PRAS40.
Ultimately, WISP1 fosters microglial cytoprotection by phosphorylating PRAS40 and promoting the binding of PRAS40 to protein 14-3-3 to inhibit its activity and increase mTOR activation. Phosphorylation of PRAS40 dissociates PRAS40 from mTORC1 (28) and allows PRAS40 to bind to protein 14-3-3 (50, 51). We show that WISP1 significantly increases phosphorylation of PRAS40 over a 24 hour course with or without Aβ exposure. In addition, gene reduction of WISP1 significantly limited the phosphorylation of PRAS40, demonstrating that WISP1 was necessary for PRAS40 phosphorylation. WISP1 also was necessary for the binding of phosphorylated PRAS40 to protein 14-3-3, since gene reduction of WISP1 prevented the binding of phosphorylated PRAS40 to protein 14-3-3.
Our studies highlight WISP1 and mTOR signaling as novel targets for neurodegenerative disease and Aβ toxicity that may be a result of inflammatory cell dysfunction. WISP1 is initially up-regulated by Aβ and can foster its own expression and block microglial early and late apoptotic demise during Aβ exposure through the phosphorylation of mTOR and its signaling pathways of p70S6K and 4EBP1. Ultimately, WISP1 controls mTOR signaling through PRAS40 through post-translational phosphorylation of PRAS40 to result in its binding to protein 14-3-3. Given the lack of efficacious treatments for neurodegenerative disorders, further understanding of the cellular pathways of the CCN family protein WISP1 could offer new promise for the development of novel therapeutic strategies for disease entities such as Alzheimer’s disease.
Acknowledgments
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association (National), Bugher Foundation Award, LEARN Foundation Award, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
Conflicts of interest: The authors have no conflicts of interest.
References
- 1.Yeger H, Perbal B. The CCN family of genes: a perspective on CCN biology and therapeutic potential. J Cell Commun Signal. 2007 Dec;1(3-4):159–64. doi: 10.1007/s12079-008-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002 Jan 1;16(1):46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.French DM, Kaul RJ, D’Souza AL, Crowley CW, Bao M, Frantz GD, et al. WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair. Am J Pathol. 2004 Sep;165(3):855–67. doi: 10.1016/S0002-9440(10)63348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macsai CE, Georgiou KR, Foster BK, Zannettino AC, Xian CJ. Microarray expression analysis of genes and pathways involved in growth plate cartilage injury responses and bony repair. Bone. 2012 May;50(5):1081–91. doi: 10.1016/j.bone.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Colston JT, de la Rosa SD, Koehler M, Gonzales K, Mestril R, Freeman GL, et al. Wnt-induced secreted protein-1 is a prohypertrophic and profibrotic growth factor. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1839–46. doi: 10.1152/ajpheart.00428.2007. [DOI] [PubMed] [Google Scholar]
- 6.Heise RL, Stober V, Cheluvaraju C, Hollingsworth JW, Garantziotis S. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J Biol Chem. 2011 May 20;286(20):17435–44. doi: 10.1074/jbc.M110.137273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S, Chong ZZ, Shang YC, Maiese K. Wnt1 inducible signaling pathway protein 1 (WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL. Curr Neurovasc Res. 2012 Feb;9(1):20–31. doi: 10.2174/156720212799297137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 (CCN4) autoregulates its expression and nuclear trafficking of beta-catenin during oxidant stress with limited effects upon neuronal autophagy. Curr Neurovasc Res. 2012 Apr 4;9(2):89–99. doi: 10.2174/156720212800410858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatesan B, Prabhu SD, Venkatachalam K, Mummidi S, Valente AJ, Clark RA, et al. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal. 2010 May;22(5):809–20. doi: 10.1016/j.cellsig.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajda M, Guzior N, Ignasik M, Malawska B. Multi-target-directed ligands in Alzheimer’s disease treatment. Curr Med Chem. 2011 Nov 1;18(32):4949–75. doi: 10.2174/092986711797535245. [DOI] [PubMed] [Google Scholar]
- 11.Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer’s disease. Brain Res Brain Res Rev. 2005 Jul;49(1):1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong YK, Lee S, Park SH, Lee JH, Han SY, Kim ST, et al. Inhibition of JNK/dFOXO pathway and caspases rescues neurological impairments in Drosophila Alzheimer’s disease model. Biochem Biophys Res Commun. 2012 Mar 2;419(1):49–53. doi: 10.1016/j.bbrc.2012.01.122. [DOI] [PubMed] [Google Scholar]
- 13.Maiese K, Chong ZZ, Shang YC, Wang S. Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom J Morphol Embryol. 2011;52(4):1173–85. [PMC free article] [PubMed] [Google Scholar]
- 14.Su SY, Cheng CY, Tsai TH, Hsiang CY, Ho TY, Hsieh CL. Paeonol attenuates HO-induced NF-kappaB-associated amyloid precursor protein expression. Am J Chin Med. 2010;38(6):1171–92. doi: 10.1142/S0192415X1000855X. [DOI] [PubMed] [Google Scholar]
- 15.Zeng KW, Wang XM, Ko H, Kwon HC, Cha JW, Yang HO. Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid beta-protein via the PI3K/Akt/Bad/Bcl(XL)-regulated mitochondrial apoptotic pathway. Eur J Pharmacol. 2011 Dec 15;672(1-3):45–55. doi: 10.1016/j.ejphar.2011.09.177. [DOI] [PubMed] [Google Scholar]
- 16.Delrieu J, Ousset PJ, Vellas B. Gantenerumab for the treatment of Alzheimer’s disease. Expert Opin Biol Ther. 2012 May 15; doi: 10.1517/14712598.2012.688022. [DOI] [PubMed] [Google Scholar]
- 17.Bach JP, Mengel D, Wahle T, Kautz A, Balzer-Geldsetzer M, Al-Abed Y, et al. The Role of CNI-1493 in the Function of Primary Microglia with Respect to Amyloid-beta. J Alzheimers Dis. 2011 Jan 1;26(1):69–80. doi: 10.3233/JAD-2011-110179. [DOI] [PubMed] [Google Scholar]
- 18.Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, et al. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat Neurosci. 2010 Mar 21; doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers J, Lue LF. Microglial chemotaxis, activation, and phagocytosis of amyloid beta-peptide as linked phenomena in Alzheimer’s disease. Neurochem Int. 2001 Nov-Dec;39(5-6):333–40. doi: 10.1016/s0197-0186(01)00040-7. [DOI] [PubMed] [Google Scholar]
- 20.Salminen A, Kaarniranta K. Siglec receptors and hiding plaques in Alzheimer’s disease. J Mol Med. 2009 Jul;87(7):697–701. doi: 10.1007/s00109-009-0472-1. [DOI] [PubMed] [Google Scholar]
- 21.Shang YC, Chong ZZ, Hou J, Maiese K. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009 Feb;6(1):20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012 Mar 3;4(3):187–201. doi: 10.18632/aging.100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy VS, Valente AJ, Delafontaine P, Chandrasekar B. Interleukin-18/WNT1-inducible signaling pathway protein-1 signaling mediates human saphenous vein smooth muscle cell proliferation. J Cell Physiol. 2011 Dec;226(12):3303–15. doi: 10.1002/jcp.22676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 Govern Pathways of mTOR, Apaf-1, and XIAP in Inflammatory Microglia. Curr Neurovasc Res. 2011 Oct 19;8(4):270–85. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sosunov AA, Wu X, McGovern RA, Coughlin DG, Mikell CB, Goodman RR, et al. The mTOR pathway is activated in glial cells in mesial temporal sclerosis. Epilepsia. 2012 Jun;53(Suppl 1):78–86. doi: 10.1111/j.1528-1167.2012.03478.x. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez G, Lal H, Fidalgo M, Guerrero A, Zalvide J, Force T, et al. A novel cardioprotective p38-MAPK/mTOR pathway. Exp Cell Res. 2011 Dec 10;317(20):2938–49. doi: 10.1016/j.yexcr.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magri L, Cambiaghi M, Cominelli M, Alfaro-Cervello C, Cursi M, Pala M, et al. Sustained activation of mTOR pathway in embryonic neural stem cells leads to development of tuberous sclerosis complex-associated lesions. Cell Stem Cell. 2011 Nov 4;9(5):447–62. doi: 10.1016/j.stem.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007 Mar 23;25(6):903–15. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Zhang Q, Wen Q, Zheng Y, Philip L, Jiang H, et al. Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal. 2012 Jan;24(1):17–24. doi: 10.1016/j.cellsig.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Harris TE, Roth RA, Lawrence JC., Jr PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem. 2007 Jul 6;282(27):20036–44. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- 31.Chong ZZ, Maiese K. Mammalian Target of Rapamycin Signaling in Diabetic Cardiovascular Disease. Cardiovasc Diabetol. 2012 Apr 30;11(1):45. doi: 10.1186/1475-2840-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das F, Dey N, Venkatesan B, Kasinath BS, Ghosh-Choudhury N, Choudhury GG. High glucose upregulation of early-onset Parkinson’s disease protein DJ-1 integrates the PRAS40/TORC1 axis to mesangial cell hypertrophy. Cell Signal. 2011 Aug;23(8):1311–9. doi: 10.1016/j.cellsig.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapoor V, Zaharieva MM, Das SN, Berger MR. Erufosine simultaneously induces apoptosis and autophagy by modulating the Akt-mTOR signaling pathway in oral squamous cell carcinoma. Cancer Lett. 2012 Jun 1;319(1):39–48. doi: 10.1016/j.canlet.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Xiao X, Zhang Y, Shi D, Chen W, Fu L, et al. Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J Pineal Res. 2012 Jan 3; doi: 10.1111/j.1600-079X.2012.00973.x. [DOI] [PubMed] [Google Scholar]
- 35.Shang YC, Chong ZZ, Hou J, Maiese K. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal. 2010 Sep;22(9):1317–29. doi: 10.1016/j.cellsig.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005 Dec;2(5):387–99. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007 Jun;19(6):1150–62. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res. 2006 Aug;3(3):187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002 Dec 3;106(23):2973–9. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 40.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003 Mar;138(6):1107–18. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010 May;7(2):95–112. doi: 10.2174/156720210791184899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO Relies upon Novel Signaling of Wnt1 that Requires Akt1, FoxO3a, GSK-3beta, and beta-Catenin to Foster Vascular Integrity During Experimental Diabetes. Curr Neurovasc Res. 2011 May 1;8(2):103–20. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c, and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J Cereb Blood Flow Metab. 2003 Mar;23(3):320–30. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- 44.Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003 Sep;64(3):557–69. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- 45.Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: hitting the bull’s-eye for neurological disorders. Oxid Med Cell Longev. 2010 Nov-Dec;3(6):374–91. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearson RB, Dennis PB, Han JW, Williamson NA, Kozma SC, Wettenhall RE, et al. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. Embo J. 1995 Nov 1;14(21):5279–87. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magagnin MG, van den Beucken T, Sergeant K, Lambin P, Koritzinsky M, Devreese B, et al. The mTOR target 4E-BP1 contributes to differential protein expression during normoxia and hypoxia through changes in mRNA translation efficiency. Proteomics. 2008 Mar;8(5):1019–28. doi: 10.1002/pmic.200700551. [DOI] [PubMed] [Google Scholar]
- 48.Gingras AC, Kennedy SG, O’Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998 Feb 15;12(4):502–13. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhandari BK, Feliers D, Duraisamy S, Stewart JL, Gingras AC, Abboud HE, et al. Insulin regulation of protein translation repressor 4E-BP1, an eIF4E-binding protein, in renal epithelial cells. Kidney Int. 2001 Mar;59(3):866–75. doi: 10.1046/j.1523-1755.2001.059003866.x. [DOI] [PubMed] [Google Scholar]
- 50.Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, et al. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem. 2003 Mar 21;278(12):10189–94. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- 51.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007 Mar;9(3):316–23. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 52.Kohara H, Tabata Y. Enhancement of ectopic osteoid formation following the dual release of bone morphogenetic protein 2 and Wnt1 inducible signaling pathway protein 1 from gelatin sponges. Biomaterials. 2011 Aug;32(24):5726–32. doi: 10.1016/j.biomaterials.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 53.Lafay-Chebassier C, Paccalin M, Page G, Barc-Pain S, Perault-Pochat MC, Gil R, et al. mTOR/p70S6k signalling alteration by Abeta exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer’s disease. J Neurochem. 2005 Jul;94(1):215–25. doi: 10.1111/j.1471-4159.2005.03187.x. [DOI] [PubMed] [Google Scholar]
- 54.Ma T, Hoeffer CA, Capetillo-Zarate E, Yu F, Wong H, Lin MT, et al. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer’s disease. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008 Oct 24;283(43):29196–205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venkatachalam K, Venkatesan B, Valente AJ, Melby PC, Nandish S, Reusch JE, et al. WISP1, a pro-mitogenic, pro-survival factor, mediates tumor necrosis factor-alpha (TNF-alpha)-stimulated cardiac fibroblast proliferation but inhibits TNF-alpha-induced cardiomyocyte death. J Biol Chem. 2009 May 22;284(21):14414–27. doi: 10.1074/jbc.M809757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marchand A, Atassi F, Gaaya A, Leprince P, Le Feuvre C, Soubrier F, et al. The Wnt/beta-catenin pathway is activated during advanced arterial aging in humans. Aging Cell. 2011 Apr;10(2):220–32. doi: 10.1111/j.1474-9726.2010.00661.x. [DOI] [PubMed] [Google Scholar]
- 58.Chong ZZ, Shang YC, Hou J, Maiese K. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid Med Cell Longev. 2010 Mar-Apr;3(2):153–65. doi: 10.4161/oxim.3.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schutters K, Reutelingsperger C. Phosphatidylserine targeting for diagnosis and treatment of human diseases. Apoptosis. 2010 Sep;15(9):1072–82. doi: 10.1007/s10495-010-0503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Witting A, Muller P, Herrmann A, Kettenmann H, Nolte C. Phagocytic clearance of apoptotic neurons by Microglia/Brain macrophages in vitro: involvement of lectin-, integrin-, and phosphatidylserine-mediated recognition. J Neurochem. 2000 Sep;75(3):1060–70. doi: 10.1046/j.1471-4159.2000.0751060.x. [DOI] [PubMed] [Google Scholar]
- 61.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007 Feb;19(2):263–72. [PMC free article] [PubMed] [Google Scholar]
- 62.Liu B, Wang K, Gao HM, Mandavilli B, Wang JY, Hong JS. Molecular consequences of activated microglia in the brain: overactivation induces apoptosis. J Neurochem. 2001 Apr;77(1):182–9. doi: 10.1046/j.1471-4159.2001.t01-1-00216.x. [DOI] [PubMed] [Google Scholar]
- 63.Chong ZZ, Kang JQ, Maiese K. AKT1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-xL and caspase 1, 3, and 9. Exp Cell Res. 2004 Jun 10;296(2):196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 64.Dombroski D, Balasubramanian K, Schroit AJ. Phosphatidylserine expression on cell surfaces promotes antibody-dependent aggregation and thrombosis in beta2-glycoprotein I-immune mice. J Autoimmun. 2000;14(3):221–9. doi: 10.1006/jaut.2000.0365. [DOI] [PubMed] [Google Scholar]
- 65.Popescu NI, Lupu C, Lupu F. Extracellular protein disulfide isomerase regulates coagulation on endothelial cells through modulation of phosphatidylserine exposure. Blood. 2010 Aug 12;116(6):993–1001. doi: 10.1182/blood-2009-10-249607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol. 2010 Mar;45(3):217–34. doi: 10.1016/j.exger.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pineda D, Ampurdanes C, Medina MG, Serratosa J, Tusell JM, Saura J, et al. Tissue plasminogen activator induces microglial inflammation via a noncatalytic molecular mechanism involving activation of mitogen-activated protein kinases and Akt signaling pathways and AnnexinA2 and Galectin-1 receptors. Glia. 2012 Apr;60(4):526–40. doi: 10.1002/glia.22284. [DOI] [PubMed] [Google Scholar]
- 68.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007 Nov;22(11):1251–67. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fokas E, Yoshimura M, Prevo R, Higgins G, Hackl W, Maira SM, et al. NVP-BEZ235 and NVP-BGT226, dual phosphatidylinositol 3-kinase/Mammalian target of rapamycin inhibitors, enhance tumor and endothelial cell radiosensitivity. Radiat Oncol. 2012 Mar 27;7(1):48. doi: 10.1186/1748-717X-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen JX, Tuo Q, Liao DF, Zeng H. Inhibition of Protein Tyrosine Phosphatase Improves Angiogenesis via Enhancing Ang-1/Tie-2 Signaling in Diabetes. Exp Diabetes Res. 2012;2012:836759. doi: 10.1155/2012/836759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deblon N, Bourgoin L, Veyrat-Durebex C, Peyrou M, Vinciguerra M, Caillon A, et al. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol. 2012 Apr;165(7):2325–40. doi: 10.1111/j.1476-5381.2011.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hou J, Chong ZZ, Shang YC, Maiese K. FoxO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol Cell Endocrinol. 2010 Mar 4;321(2):194–206. doi: 10.1016/j.mce.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14(9):3446–85. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hou G, Xue L, Lu Z, Fan T, Tian F, Xue Y. An activated mTOR/p70S6K signaling pathway in esophageal squamous cell carcinoma cell lines and inhibition of the pathway by rapamycin and siRNA against mTOR. Cancer Lett. 2007 Aug 18;253(2):236–48. doi: 10.1016/j.canlet.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 75.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ballou LM, Lin RZ. Rapamycin and mTOR kinase inhibitors. J Chem Biol. 2008 Nov;1(1-4):27–36. doi: 10.1007/s12154-008-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chong ZZ, Shang YC, Maiese K. Cardiovascular Disease and mTOR Signaling. Trends Cardiovasc Med. 2011 Jul;21(5):151–5. doi: 10.1016/j.tcm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lafay-Chebassier C, Perault-Pochat MC, Page G, Rioux Bilan A, Damjanac M, Pain S, et al. The immunosuppressant rapamycin exacerbates neurotoxicity of Abeta peptide. J Neurosci Res. 2006 Nov 1;84(6):1323–34. doi: 10.1002/jnr.21039. [DOI] [PubMed] [Google Scholar]
- 79.Chen TJ, Wang DC, Chen SS. Amyloid-beta interrupts the PI3K-Akt-mTOR signaling pathway that could be involved in brain-derived neurotrophic factor-induced Arc expression in rat cortical neurons. J Neurosci Res. 2009 Aug 1;87(10):2297–307. doi: 10.1002/jnr.22057. [DOI] [PubMed] [Google Scholar]
- 80.Pastor MD, Garcia-Yebenes I, Fradejas N, Perez-Ortiz JM, Mora-Lee S, Tranque P, et al. mTOR/S6 kinase pathway contributes to astrocyte survival during ischemia. J Biol Chem. 2009 Aug 14;284(33):22067–78. doi: 10.1074/jbc.M109.033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010 Aug;120(8):2805–16. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thedieck K, Polak P, Kim ML, Molle KD, Cohen A, Jeno P, et al. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS One. 2007;2(11):e1217. doi: 10.1371/journal.pone.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]