Abstract
Muscle regeneration is a complex phenomenon, involving replacement of damaged fibers by new muscle fibers. During this process, there is a tendency to form scar tissue or fibrosis by deposition of collagen that could be detrimental to muscle function. New therapies that could regulate fibrosis and favor muscle regeneration would be important for physical therapy. Low-level laser therapy (LLLT) has been studied for clinical treatment of skeletal muscle injuries and disorders, even though the molecular and cellular mechanisms have not yet been clarified. The aim of this study was to evaluate the effects of LLLT on molecular markers involved in muscle fibrosis and regeneration after cryolesion of the tibialis anterior (TA) muscle in rats. Sixty Wistar rats were randomly divided into three groups: control, injured TA muscle without LLLT, injured TA muscle treated with LLLT. The injured region was irradiated daily for four consecutive days, starting immediately after the lesion using an AlGaAs laser (808 nm, 30 mW, 180 J/cm2; 3.8 W/cm2, 1.4 J). The animals were sacrificed on the fourth day after injury. LLLT significantly reduced the lesion percentage area in the injured muscle (p<0.05), increased mRNA levels of the transcription factors MyoD and myogenin (p<0.01) and the pro-angiogenic vascular endothelial growth factor (p<0.01). Moreover, LLLT decreased the expression of the profibrotic transforming growth factor TGF-β mRNA (p<0.01) and reduced type I collagen deposition (p<0.01). These results suggest that LLLT could be an effective therapeutic approach for promoting skeletal muscle regeneration while preventing tissue fibrosis after muscle injury.
Keywords: LLLT, Muscle cryolesion, Muscle regeneration, MRFs, Growth factors
Introduction
Skeletal muscle injuries are routinely found in rehabilitation centers. They often cause a significant reduction in the functional capacity of the patient, and are characterized by slow and incomplete recovery, leading to increased likelihood of scar tissue formation, re-injury, atrophy, contracture, and chronic disability [1, 2].
Muscle regeneration involves several highly organized molecular and cellular processes that ideally lead to structural and functional recovery of the injured muscle [3]. The initial phase of this process is characterized by an inflammatory response and necrosis of the injured tissue leading to activation and proliferation of adult satellite cells [1, 2, 4]. The satellite cells are myogenic precursor cells (stem cells) that are quiescent in normal muscle fibers, located between basal lamina and sarcolemma, and when activated, undergo cell cycle, divide, differentiate and fuse with muscle fibers to repair injured areas [5, 6].
Activated satellite cells increase their expression of myoD and myf5, which are basic helix-loop-helix transcription factors which belong to the myogenic regulatory factor family (MRFs) [7, 8]. MyoD is rapidly expressed within 12 h post-injury [9], and the peak of expression is at day 3 post-cryolesion in tibialis anterior (TA) muscle [10]. Subsequently, upregulation of the secondary MRFs like myogenin and MRF4 induces terminal differentiation of myoblasts into myocytes [4] in order to reestablish the structure and function of muscle tissue. Therefore, myoD and myogenin play an important role in the regeneration of skeletal muscle.
The development of new blood vessels (angiogenesis) plays an important role in successful muscle regeneration [11]. This process is regulated by vascular endothelial growth factor (VEGF) that exerts multiple effects on the vascular endothelium including stimulation of endothelial cell proliferation, rapid induction of microvascular permeability, promotion of endothelial cell survival, stimulation of endothelial cell adhesion and migration and subsequent connection between new vessels and the pre-existing circulation [12–14]. Hence, VEGF is considered an excellent biomarker for angiogenesis, and its expression is related to recovery of skeletal muscle.
Skeletal muscle regeneration is regulated by growth factors, such as insulin-like growth factor-1, basic fibroblast growth factor, hepatocyte growth factor, epidermal growth factor, and transforming growth factor-beta (TGF-β) [4, 6]. TGF-β is a multifunctional cytokine produced by fibroblasts, epithelial cells, and macrophages in the lesion site. It stimulates mesenchymal cell proliferation, collagen and fibronectin synthesis, and is important in causing fibrosis in several diseases [15]. Excessive production of TGF-β can be harmful in muscle repair, as it increases formation of fibrous non-functional scar tissue and can inhibit differentiation and fusion of myoblasts, impairing the regeneration of muscle tissue [15, 16]. Therapeutic approaches that have a dual action by reducing fibrous scar tissue formation while optimizing muscle repair would be helpful for rehabilitating muscle function after injury.
Low-level laser therapy (LLLT) is considered a safe and effective technique for the clinical treatment of a variety of diseases and injuries [17, 18]. Positive results have been reported with LLLT in several experimental models of skeletal muscle injury and repair [19–22]. This therapeutic modality was able to improve muscle repair by inducing a rapid growth of capillary vessels in the injured area [23, 24], a remarkable regeneration of muscle fibers [25–27] and parallel orientation of regenerating myofibers [28].
Although knowledge is steadily increasing concerning the biological and molecular mechanisms of LLLT, more studies are needed to determine which signaling pathways are triggered by LLLT in muscle and how the cellular effects are translated into improved skeletal muscle functions. This study therefore focused on exploring the effect of LLLT on skeletal muscle repair, evaluating its effect on the molecular markers involved in muscle regeneration and fibrosis, specifically measuring MyoD, myogenin, VEGF, and TGF-β at the transcriptional level as well type I collagen deposition.
Materials and methods
Experimental groups and freezing muscle injury (cryoinjury)
Adult male Wistar rats (Rattus norvegicus) weighing 300 g were used in this study. Good laboratory animal practice was observed according to the international standards for animal experimentation and following approval by our institution’s Animal Care and Ethics Committee.
The animals (n=60) were randomly divided into three groups (n=20 per group): control group—animals with no interventions (BC); injured TA muscle without treatment (IC); injured TA muscle submitted to laser irradiation treatment (IRI).
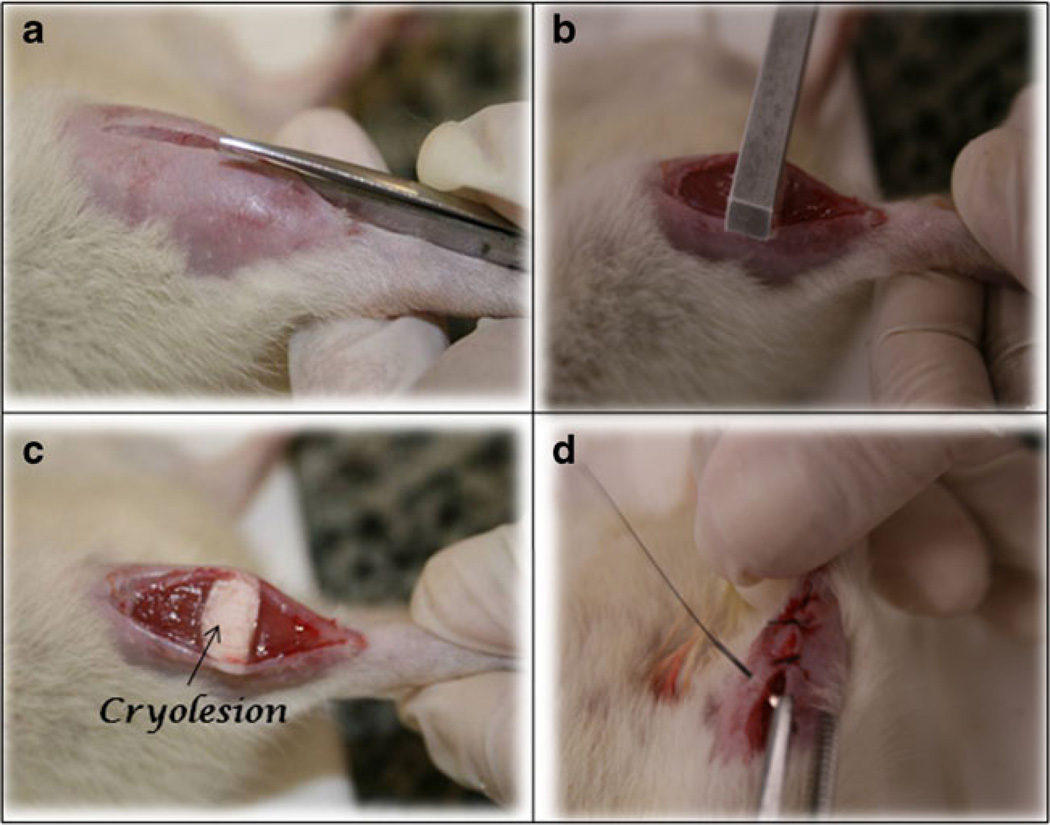
Surgical procedures (cryolesion) were performed based on those described by Miyabara et al. [29], under anesthesia with 1 ml/kg of 1 % ketamine HCl (Dopalen; Vetbrands; São Paulo; Brazil) and 2 % xylazine (Anasedan; Vetbrands; São Paulo; Brazil). After anesthesia, the skin around the right TA muscle was shaved and cleaned. Then, a transversal cut (about 1 cm) of the skin over the middle of the muscle was carried out, exposing the muscle. A rectangular iron bar (40×20 mm2), frozen in liquid nitrogen, was then kept for 10 s on the center of the muscle. The procedure was repeated twice consecutively, with a time interval of 30 s. Finally, the skin was sutured (Fig. 1). The right TA muscle was chosen because it is a superficial muscle, making the surgery easy. After surgery, the animals were housed in single plastic cages in a room with controlled environmental conditions and fed rat chow and water ad libitum.
Fig. 1.
Freezing right tibialis anterior muscle injury (cryoinjury) model. a Dissection and muscle exposition; b and c cryolesion procedure; d suture after surgical procedure
LLLT protocol
LLLT was performed using a gallium-aluminum-arsenide (GaAlAs) diode laser (PHOTON LASER II, DMC® equipamentos Ltda, SP, São Carlos, Brazil), with the following parameters: continuous radiation mode, 808 nm wavelength, 30 mW power output, 47 s irradiation time, 0.00785 cm2 spot area, dose 180 J/cm2, irradiance 3.8 W/cm2 and 1.4 J total energy per point. A LaserCheck power meter (Coherent, Santa Clara, CA) was used to determine the output of the equipment.
The skin having been shaved at the surgery site, the laser was applied over one point at in the middle of right TA muscle (lesion area). LLLT was performed daily and at the same time for four consecutive days, with the first application immediately after skin suturing. Laser was applied by contact technique, with the optical fiber kept perpendicular to the skin. The animals were handled gently, and LLLT did not produce any painful sensation or distress to the animals.
Muscle evaluation
Animals were weighed and euthanized with an anesthetic overdose, and the right TA muscles removed and weighed. Subsequently, ten animals from each group were used for morphometric analysis and the other ten animals for real-time polymerase chain reaction and immunoblotting analysis. For histological evaluation, the muscle fragment was immediately frozen in isopentane pre-cooled in liquid nitrogen, and then stored in a freezer at −80 °C (Forma Scientific, Marietta, OH). For further analysis, the muscle fragments from the injured site were pulverized in liquid nitrogen with a mortar and pestle. The proximal fragment was used for RNA extraction protocol. The distal samples were used for the other analyses and were homogenized in RIPA buffer containing 10 mM Tris–HCl (pH 7.5), 1 % Tergitol, 0.1 % SDS, 1 % sodium deoxycholate, 150 mM NaCl, and proteolytic enzyme inhibitors (Protease Inhibitor Cocktail, Sigma, St Louis, MO). After debris separation by centrifugation for 45 min at 14,000×g, the supernatants were collected and the protein concentration was determined using a BCA Protein Assay kit (Pierce, Rockford, IL). All samples were stored at −80 °C until analysis.
Injured muscle area
Serial muscle cross-sections for histology were obtained (one section of 10 µm in each 100 µm of tissue) using a cryostat microtome (Microm HE 505, Jena, Germany), across the middle of the TA muscle.
For morphometric evaluation by light microscopy (Axiolab, Carl Zeiss, Jena, Germany) tissue sections were stained using toluidine blue. One histological cross-section of each TA muscle located in the central region of muscle injury was chosen to measure the cross-sectional area of both injured and uninjured muscle, using software for morphometry (Axiovision 3.0.6 SP4, Carl Zeiss). Images were used to reconstruct the total muscle cross-section area, allowing the identification and measurement of both injured and uninjured areas. A double-blind procedure was used for both muscle cross-section image selection and injured and uninjured muscle area measurements.
Total RNA isolation and real-time polymerase chain reaction
Tissue fragments were homogenized in 1 mL TRIzol® reagent according to the manufacturer’s instructions (Invitrogen Life Science, Carlsbad, CA). RNA integrity was assessed by 260/268 nm ratio and by 1 % agarose gel electrophoresis stained with ethidium bromide.
Two nanograms of mRNA was used to carry out real-time PCR. The amplification was performed in a thermal cycler (Applied Biosystems StepOne™, Foster City, CA) at 50 °C for 10 min, 95 °C for 5 min and then 95 °C for 15 s followed by 60 °C for 30 s and 72 °C for 30 s for 40 cycles. Real-time PCR was performed in a 15 µl reaction mixture containing 7.5 µl 2× SYBR Green Reaction Mix (Invitrogen), 0.3 µl each primer, 0.3 µl Super Script III RT/Platinum Taq Mix (10 pmol/µl), 0.15 µl ROX Reference Dye and 5 µl sample in water. Quantification was performed by 2−ΔΔCT method, using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as housekeeping gene. This gene was chosen after genome analysis (http://medgen.ugent.be/~jvdesomp/genorm/) of five housekeeping genes. The following primers were used: MyoD forward: TAC GAC GCC GCC TAC TAC AGT G; reverse: GCA TCG CTT GAG GAT GTC TCC; Myogenin forward: AGG AAG TC TGT GTC TGT GGA CC; reverse: TGT ACT GGA TGG CAC TGC G; VEGF forward: TGA GAC CCT GGT GGA CAT CTT C; reverse: TCC TAT GTG CTG GCT TTG GTG; TGF-β1 forward: CCT ACA TTT GGA GCC TGG ACA C; reverse: CAC GAT CAT GTT GGA CAA CTG C; GAPDH forward: ATG ATT CTA CCC ACG GCA AG; reverse: CTG GAA GATGGT GAT GGG TT.
Immunoblotting
Protein expression was performed using SDS-polyacrylamide gel electrophoresis under reducing conditions. Tissue extracts (50 µg) were boiled in equal volumes of loading buffer (150 mM Tris–HCl—pH 6.8, 4 % SDS, 20 % glycerol, 15 % β-mercaptoethanol and 0.01 % bromophenol blue) and were subjected to electrophoresis in 9 % non-denaturing polyacrylamide gel. Following electrophoretic separation, proteins were transferred to Hybond-P membranes (Amersham Pharmacia Biotech, Buckinghamshire, UK). Membrane was blocked with 5 % non-fat dry milk in Tris-buffered saline and 0.5 % Tween 20 (TBST) for 1 h. Primary antibody (Ab) against the following was employed: Collagen I (goat polyclonal, 1:1000, Santa Cruz). Ab was diluted in TBST with 0.5 % bovine serum albumin and incubated in 4 °C overnight. After washing twice with TBST, secondary Ab horseradish peroxidase conjugate (Sigma Aldrich, St. Louis, MO) was applied at dilution 1:1,000 for 1 h. Blot was washed in TBST for 30 min, incubated in enhanced chemiluminesence reagents (Super signal detection kit, Pierce) and exposed and photographed using GBox Gel Document System (Syngene, Frederick, MD). The band intensity was quantified using Gene Tools software (Syngene).
Statistical analysis
Data are expressed as the mean ± standard error of the mean. Shapiro–Wilk’s and Levene’s test were applied to evaluate the normality and homogeneity of the results, respectively. Comparisons between experimental groups were performed by analysis of variance (one-way ANOVA), and the Tukey post-test used to compare individual groups. A P value <0.05 was considered significant. All analyses were performed using Sigma Stat Statistical Software (v.3.1).
Results
Tibialis anterior muscle weight and muscle injury area
There was no significant difference in the weight of the tibialis anterior muscle among the groups (p>0.05; Table 1). The total and percentual injured area in the LLLT-treated group were statistically lower than in the comparable areas in the non-treated injured muscles (IRI; p<0.05 vs IC).
Table 1.
Muscle weight, injury and uninjured cross-section area of TA muscle middle belly
| Groups | Muscle weight (g) | Total area mm2 | Injured area mm2 | Injured area (% total area) |
|---|---|---|---|---|
| BC | 0.46±0.05 | 50.04±4.7 | – | – |
| IC | 0.44±0.06 | 53.89±5.3 | 20.99±23 | 38.94 |
| IRI | 0.45±0.04 | 53.39±1.4 | 15.98±14* | 29.93* |
Data are means (±SD). Normal TA muscle (BC); TA muscle injured (IC); TA muscle injured submitted to infrared laser irradiation (IRI)
p<0.05: compared to injured area of IC group
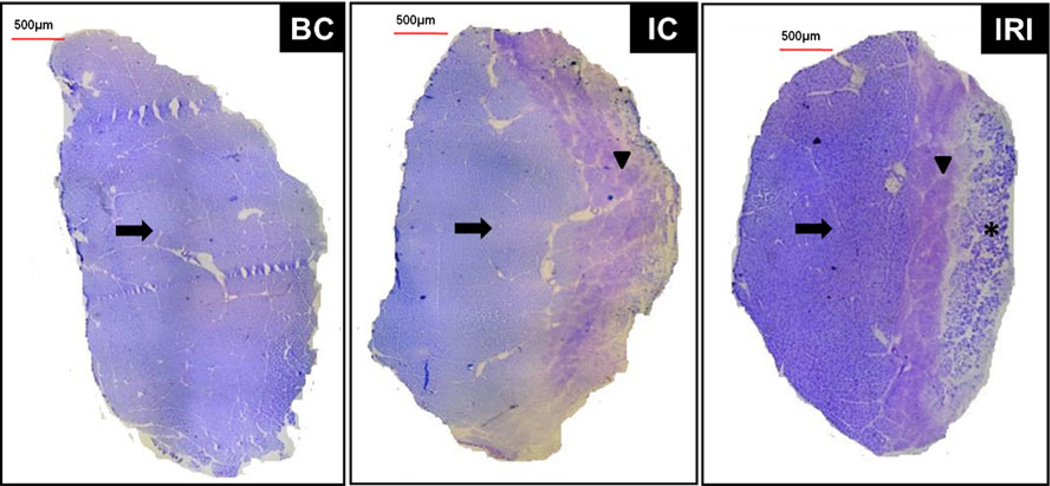
Representative images of the TA muscle sections are shown in Fig. 2. Injured groups show a homogeneous and well-defined lesion area, with fascicular disorganization, edema, tissue necrosis, and abundant extracellular matrix deposition (arrowheads). IRI group showed a repaired area (asterisk) on the surface of the tissue. The intact area was indicated by arrows.
Fig. 2.
Morphometric analysis of middle TA muscle cross-sections. Micrography of typical toluidine blue-stained muscle sections. Injured area (arrowhead), intact area (arrows) and reparative area (asterisk). Normal TA muscle—control (BC); injured TA muscle without LLLT (IC); injured TA muscle with LLLT (IRI)
Transcripts of the regeneration markers: MyoD, myogenin, and VEGF
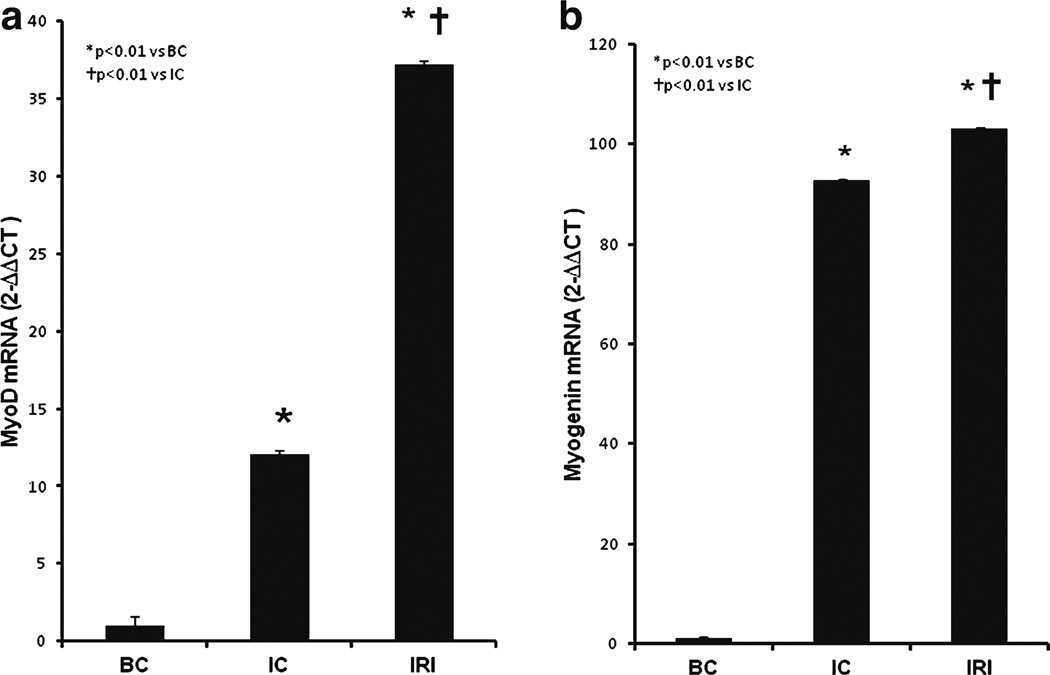
After production of the muscle lesion, the MyoD gene expression level was increased 13-fold and the myogenin gene expression level was increased 90-fold compared to uninjured controls (IC and IRI; p<0.01 vs BC; Fig. 3 A and B). MyoD mRNA was further increased more than twice by LLLT (IRI, 37.22±0.32 vs IC, 12.04±0.03) and myogenin mRNA also showed a small but significant increase compared to muscle lesion alone (IC, 92.74±0.2) after LLLT (IRI, 103.10±0.2). Both increases after LLLT were significantly higher than the non-LLLT group (IRI, p<0.01 vs IC).
Fig. 3.
Effect of LLLT on MyoD and myogenin gene expression. a MyoD mRNA and b myogenin mRNA
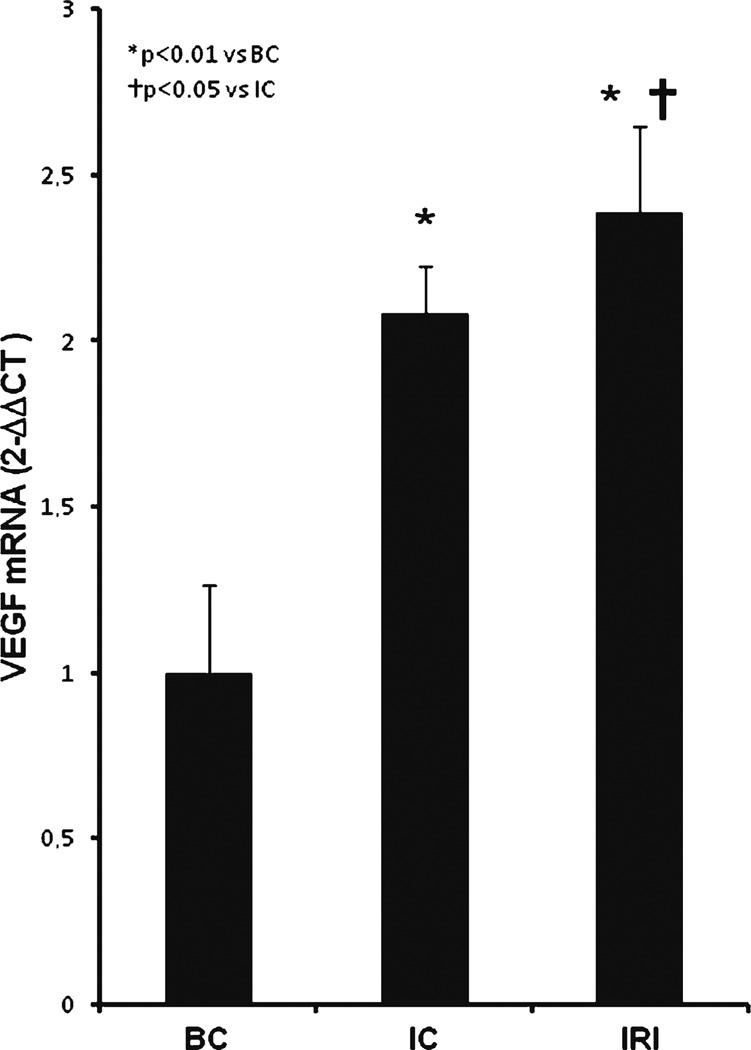
VEGF gene expression levels showed the same pattern observed in the myoD and myogenin mRNA quantification. VEGF levels were two times higher in injured muscle groups (IC, 2.08±0.1 and IRI, 2.38±0.2) compared to control group (BC, p<0.01; Fig. 4). LLLT further increased the expression of VEGF mRNA by a small but significant extent compared to non-LLLT group (IRI, p<0.05 vs IC).
Fig. 4.
Effect of LLLT on VEGF gene expression
Expression of scar markers: TGF-β and type I collagen
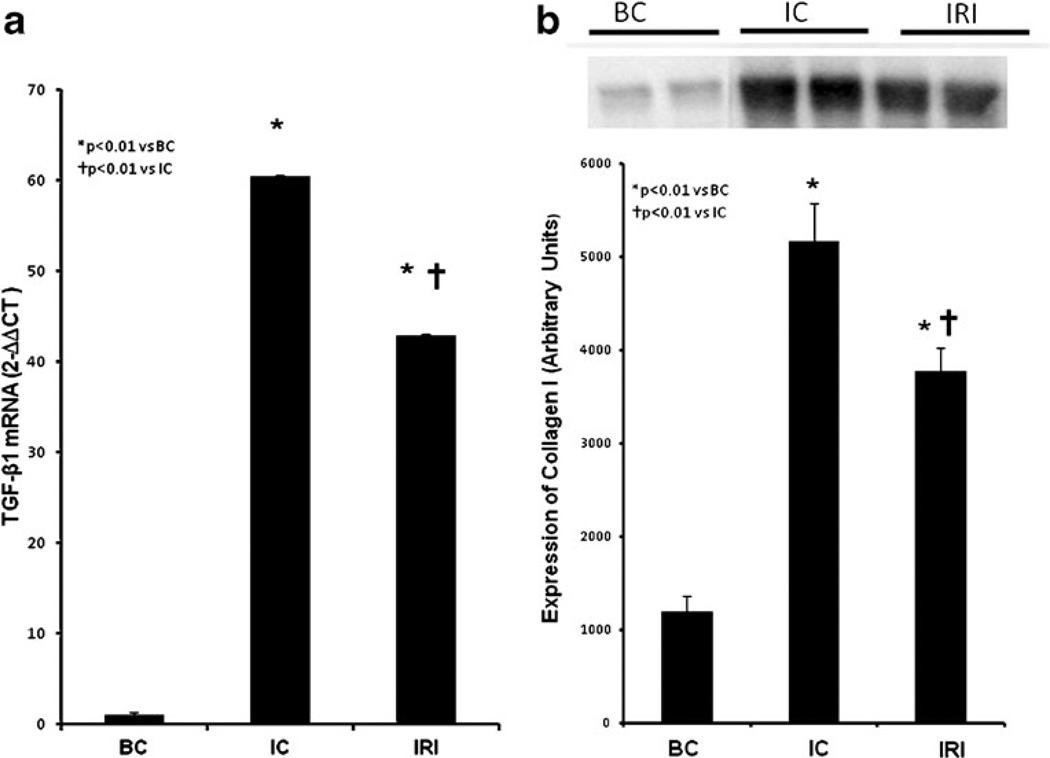
The cryolesion increased TGF-β gene transcription in the muscle homogenate 60-fold (IC, 60.39±0.15 and IRI, 42.92±0.12; p<0.01 vs BC; Fig. 5A). LLLT significantly reduced TGF-β gene expression by 25 % in injured muscle (IRI, p<0.01 vs IC).
Fig. 5.
Effect of LLLT on TGF-β gene expression and Type I collagen protein expression. a TGF-β mRNA and b Type I collagen I protein expression
Collagen type I production in the muscle tissue was fivefold-elevated after cryolesion (IC, 5080.2±339.0) when compared with non-injured group (p<0.01; Fig. 5B). LLLT reduced collagen I expression again by about 25 % in IRI group (3681.3±263.23; p<0.01 vs IC). There was a therefore a good correlation between reduced collagen production and reduced TGF-β expression.
Discussion
In spite of the fact that many studies of LLLT on muscle injury have demonstrated its beneficial effects [19–22, 30], little is known about how exactly LLLT is able to affect cellular systems involved in muscle repair and what are the molecular mechanisms involved in these processes. Herein, we have shown that LLLT improved skeletal muscle regeneration by reducing the injured area, increasing myoD, myogenin, and VEGF gene expression and, simultaneously, reducing TGF-β mRNA and type I collagen deposition in the injured tissue. Therefore, LLLT can increase muscle regeneration markers and reduce scar tissue formation which should favor tissue repair in muscle injuries.
The regeneration of skeletal muscle process is initiated by mechanical or chemical stimuli that lead to activation of quiescent satellite cells [31]. Even though the precise cause of the transition from the quiescent state to the activated state in satellite cells is unknown, some studies have proposed that disrupting the integrity of the sarcolemma and basal lamina causes mechanical stress that can induce satellite cell activation [32]. Many authors have proposed that the injury-stimulated release of substances such as cytokines, prostaglandins, nitric oxide could stimulate satellite cell activation [3, 5]. It is likely that there is a complex combination of these and other cellular events in which the use of LLLT might have an additional stimulating role [33, 34] on expression of transcription factors in the MRFs, such as myoD and myogenin. Some studies have shown that myoD and myogenin are traditionally considered markers of muscle growth and hypertrophy, as they can regulate the division of satellite cells and lead to incorporation of new myonuclei into mature muscle fibers. These transcription factors are expressed in the adult skeletal muscle in response to several stimuli, such as overcharge, denervation, and stretch, suggesting that myoD and myogenin have an important role in skeletal muscle regeneration [35]. In the present study, we observed that induction of cryolesion in the tibialis anterior muscle generated an increase in the transcription of myogenic factors, myoD and myogenin. However, there was an additional significant effect of LLLT in further increasing these markers.
LLLT has been reported to activate quiescent satellite cells and cause them to enter cell cycle and initiate proliferation [19, 34, 36]. Shefer et al. demonstrated that LLLT regulated proteins essential for the initial phase of protein synthesis in myoblasts (through MAPK/ERK) [34]. The same authors observed that the mean number of cells per fiber was tripled in the LLLT-irradiated group compared to control group [20]. Treatment with a GaAlAs laser (830 nm) was able to enlarge the muscle fiber diameter that had undergone atrophy [27]. Although there are still many questions to be clarified about the molecular mechanisms by which LLLT causes these effects, we suggest that in this model, the increase in myogenic factors favored satellite cell activation and muscle repair.
The increase in myoD and myogenin expression correlated with the results obtained in the morphometric analysis, where a reduction in the injured muscle area in the LLLT group was seen. This led us to infer that the regulation of transcription factors responsible for activation of satellite cells by LLLT contributed to new muscle fiber formation and to the reduction of the injured area.
Angiogenesis, stimulated by VEGF among other factors, is also essential for the formation of new muscle fibers after muscle damage. Angiogenesis is an important requirement for functional and morphological recovery of the injured muscle [11], as it restores injured blood vessels, promotes local blood flow, and restores a supply of oxygen and nutrients to the injured tissue.
Wagatsuma et al. showed that VEGF mRNA levels increased on the third day after cryolesion in murine gastrocnemius muscle [14]. Similarly in the present study, LLLT increased levels of this growth factor. In vivo studies in different regeneration models have highlighted the effect of LLLT in angiogenesis induction [37, 38]. It has been proposed that LLLT is able to increase the formation of new capillary vessels through the increased release of growth factors, such as VEGF [24, 38]. Our laboratory was able to confirm that LLLT induces angiogenesis through upregulating hypoxia-inducible factor (HIF-1α), increasing VEGF mRNA levels and increased formation of new blood vessels in rat skin (Cury et al., unpublished data). Increased expression of VEGF in this model could be considered an important contributor to muscle regeneration.
It is likely that recovery of the injured muscle function is dependent on an equilibrium between regeneration and fibrosis. This equilibrium is affected by the intensity of the acute inflammatory response, by satellite cells activation, and by expression of several growth factors and cytokines, especially TGF-β, present in the site of lesion [3]. Therefore, we decided to evaluate the possible effect of LLLT on TGF-β and collagen deposition in the site of lesion.
We found LLLT reduced TGF-β gene expression, lowered type I collagen deposition and could reduce fibrous tissue formation in rat skeletal muscle. TGF-β expression has a key role in the scar tissue formation during muscle repair. It regulates extracellular matrix component production and simultaneously blocks its degradation. It is also known that an increase in TGF-β production inhibits myoD expression and induces myogenic cells to differentiate instead to myofibroblasts able to produce type I collagen, thereby impairing muscle regeneration [15]. Li et al. observed that TGF-β stimulated C2C12 myoblasts to produce more TGF-β in an autocrine mode, suppressing muscle protein expression and enhancing fibrosis-related proteins [16].
Fibrous tissue formation in the skeletal muscle impairs regular muscle contraction, prompting pathological contractures and causing chronic muscle pain. In this scenario, there can be an intense deposition of collagen between the capillary vessels and the membrane of myofibrils that reduces nutrient support. TGF-β inactivation reduces fibrous tissue formation and improves the contractile properties of the repairing muscle [39]. Based on this information, our results suggest that LLLT had a positive effect, reducing TGF-β expression and, as a consequence, decreasing local collagen accumulation, preventing fibrous tissue formation and prompting skeletal muscle recovery.
Filliping et al. reported that an infrared laser (904 nm) reduced collagen deposition in rat tendons [40]. Rizzi et al. also detected reduced collagen expression after 904-nm laser treatment and suggested that NF-κB inactivation by LLLT could be the mechanism that regulated collagen production [41]. Mesquita-Ferrari et al. treated rat muscle with LLLT (660 nm) and found lower expression levels of TGF-β and TNF-α in the muscle fiber when the treatment ended, which supported fibrosis prevention and muscle contractility improvement [22]. Since TGF-β1 directly regulates collagen deposition, this growth factor is associated with complications arising in the muscle repair process, and therefore LLLT could be a good therapeutic approach for muscle repair.
In summary, our results indicate that near-infrared laser (808 nm) at the dose selected was able to improve skeletal muscle regeneration, increase myogenic regulatory factors (myoD and myogenin) and stimulate VEGF expression in an experimental animal model. Additionally, TGF-β1 and type I collagen mRNA were reduced in the lesion area, reducing skeletal muscle fibrosis. LLLT is inexpensive, non-invasive, and without reported side effects, and could therefore become a treatment of choice in patients with muscle injury. Nevertheless, more thorough investigations of the molecular mechanisms are needed to clarify how LLLT functions in muscle regeneration and fibrotic disorders in general.
Acknowledgments
We acknowledge CAPES, CNPQ and FAPESP for financial support. MR Hamblin was supported by NIH (grant R01AI050875). Emergency Medicine Division (LIM 51), Faculdade de Medicina da Universidade de São Paulo to provide technical support in biochemical and molecular biology analyses and NUPEN (Núcleo de Pesquisa e Ensino em Fototerapia nas Ciências da Saúde) for supporting and calibrating the laser equipment.
Footnotes
Disclosure of interests The authors indicate no potential conflict of interests.
Contributor Information
Lívia Assis, Laboratory of Electrothermophototherapy, Department of Physiotherapy, Federal University of São Carlos, São Carlos, SP, Brazil.
Ana Iochabel Soares Moretti, Email: aismoretti@yahoo.com.br, Emergency Medicine Division, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil; Post-Graduate Health Sciences Program, Instituto de Assistência, Médica ao Servidor Público Estadual—IAMSPE, São Paulo, SP, Brazil; Emergency Medicine Division, Faculdade de Medicina, Universidade de São Paulo, Av. Dr. Arnaldo, 455 sala 3189 01246-903, São Paulo, SP, Brazil.
Thalita Balsamo Abrahão, Laboratory of Vascular Biology, Department of Cardiopneumology, Heart Institute, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil.
Heraldo Possolo de Souza, Emergency Medicine Division, Faculdade de Medicina, Universidade de São Paulo, São Paulo, SP, Brazil.
Michael R Hamblin, Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA, USA; Department of Dermatology, Harvard Medical School, Boston, MA, USA; Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, USA.
Nivaldo Antonio Parizotto, Laboratory of Electrothermophototherapy, Department of Physiotherapy, Federal University of São Carlos, São Carlos, SP, Brazil.
References
- 1.Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002;84-A(5):822–832. [PubMed] [Google Scholar]
- 2.Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745–764. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- 3.Filippin LI, Cuevas MJ, Lima E, Marroni NP, Gonzalez-Gallego J, Xavier RM. Nitric oxide regulates the repair of injured skeletal muscle. Nitric Oxide. 2011;24(1):43–49. doi: 10.1016/j.niox.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 5.Ehrhardt J, Morgan J. Regenerative capacity of skeletal muscle. Curr Opin Neurol. 2005;18(5):548–553. doi: 10.1097/01.wco.0000177382.62156.82. [DOI] [PubMed] [Google Scholar]
- 6.Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol. 2007;19(6):628–633. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakuma K, Watanabe K, Sano M, Uramoto I, Sakamoto K, Totsuka T. The adaptive response of MyoD family proteins in over-loaded, regenerating and denervated rat muscles. Biochim Biophys Acta. 1999;1428(2–3):284–292. doi: 10.1016/s0304-4165(99)00086-0. [DOI] [PubMed] [Google Scholar]
- 8.Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20(13):1692–1708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- 9.Rantanen J, Hurme T, Lukka R, Heino J, Kalimo H. Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscle: evidence for two different populations of satellite cells. Lab Invest. 1995;72(3):341–347. [PubMed] [Google Scholar]
- 10.Warren GL, Hulderman T, Jensen N, McKinstry M, Mishra M, Luster MI, Simeonova PP. Physiological role of tumor necrosis factor alpha in traumatic muscle injury. FASEB J. 2002;16(12):1630–1632. doi: 10.1096/fj.02-0187fje. [DOI] [PubMed] [Google Scholar]
- 11.Deveci D, Marshall JM, Egginton S. Chronic hypoxia induces prolonged angiogenesis in skeletal muscles of rat. Exp Physiol. 2002;87(3):287–291. doi: 10.1113/eph8702377. [DOI] [PubMed] [Google Scholar]
- 12.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 13.Botusan IR, Sunkari VG, Savu O, Catrina AI, Grunler J, Lindberg S, Pereira T, Yla-Herttuala S, Poellinger L, Brismar K, Catrina SB. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA. 2008;105(49):19426–19431. doi: 10.1073/pnas.0805230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagatsuma A. Endogenous expression of angiogenesis-related factors in response to muscle injury. Mol Cell Biochem. 2007;298(1–2):151–159. doi: 10.1007/s11010-006-9361-x. [DOI] [PubMed] [Google Scholar]
- 15.Kollias HD, McDermott JC. Transforming growth factor-beta and myostatin signaling in skeletal muscle. J Appl Physiol. 2008;104(3):579–587. doi: 10.1152/japplphysiol.01091.2007. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164(3):1007–1019. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamblin MR. Introduction to experimental and clinical studies using low-level laser (light) therapy (LLLT) Lasers Surg Med. 2010;42(6):447–449. doi: 10.1002/lsm.20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demidova-Rice TN, Salomatina EV, Yaroslavsky AN, Herman IM, Hamblin MR. Low-level light stimulates excisional wound healing in mice. Lasers Surg Med. 2007;39(9):706–715. doi: 10.1002/lsm.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben-Dov N, Shefer G, Irintchev A, Wernig A, Oron U, Halevy O. Low-energy laser irradiation affects satellite cell proliferation and differentiation in vitro. Biochim Biophys Acta. 1999;1448(3):372–380. doi: 10.1016/s0167-4889(98)00147-5. [DOI] [PubMed] [Google Scholar]
- 20.Shefer G, Barash I, Oron U, Halevy O. Low-energy laser irradiation enhances de novo protein synthesis via its effects on translation-regulatory proteins in skeletal muscle myoblasts. Biochim Biophys Acta. 2003;1593(2–3):131–139. doi: 10.1016/s0167-4889(02)00350-6. [DOI] [PubMed] [Google Scholar]
- 21.Renno AC, Toma RL, Feitosa SM, Fernandes K, Bossini PS, de Oliveira P, Parizotto N, Ribeiro DA. Comparative effects of low-intensity pulsed ultrasound and low-level laser therapy on injured skeletal muscle. Photomed Laser Surg. 2011;29(1):5–10. doi: 10.1089/pho.2009.2715. [DOI] [PubMed] [Google Scholar]
- 22.Mesquita-Ferrari RA, Martins MD, Silva JA, Jr, da Silva TD, Piovesan RF, Pavesi VC, Bussadori SK, Fernandes KP. Effects of low-level laser therapy on expression of TNF-alpha and TGF-beta in skeletal muscle during the repair process. Lasers Med Sci. 2011;26(3):335–340. doi: 10.1007/s10103-010-0850-5. [DOI] [PubMed] [Google Scholar]
- 23.Iyomasa DM, Garavelo I, Iyomasa MM, Watanabe IS, Issa JP. Ultrastructural analysis of the low level laser therapy effects on the lesioned anterior tibial muscle in the gerbil. Micron. 2009;40(4):413–418. doi: 10.1016/j.micron.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Tuby H, Maltz L, Oron U. Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Lasers Surg Med. 2006;38(7):682–688. doi: 10.1002/lsm.20377. [DOI] [PubMed] [Google Scholar]
- 25.Amaral AC, Parizotto NA, Salvini TF. Dose-dependency of low-energy HeNe laser effect in regeneration of skeletal muscle in mice. Lasers Med Sci. 2001;16(1):44–51. doi: 10.1007/pl00011336. [DOI] [PubMed] [Google Scholar]
- 26.Silveira PC, Silva LA, Fraga DB, Freitas TP, Streck EL, Pinho R. Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. J Photochem Photobiol B. 2009;95(2):89–92. doi: 10.1016/j.jphotobiol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Nakano J, Kataoka H, Sakamoto J, Origuchi T, Okita M, Yoshimura T. Low-level laser irradiation promotes the recovery of atrophied gastrocnemius skeletal muscle in rats. Exp Physiol. 2009;94(9):1005–1015. doi: 10.1113/expphysiol.2009.047738. [DOI] [PubMed] [Google Scholar]
- 28.Cressoni MD, Dib Giusti HH, Casarotto RA, Anaruma CA. The effects of a 785-nm AlGaInP laser on the regeneration of rat anterior tibialis muscle after surgically-induced injury. Photomed Laser Surg. 2008 doi: 10.1089/pho.2007.2150. [DOI] [PubMed] [Google Scholar]
- 29.Miyabara EH, Martin JL, Griffin TM, Moriscot AS, Mestril R. Overexpression of inducible 70-kDa heat shock protein in mouse attenuates skeletal muscle damage induced by cryolesioning. Am J Physiol Cell Physiol. 2006;290(4):C1128–C1138. doi: 10.1152/ajpcell.00399.2005. [DOI] [PubMed] [Google Scholar]
- 30.Servetto N, Cremonezzi D, Simes JC, Moya M, Soriano F, Palma JA, Campana VR. Evaluation of inflammatory biomarkers associated with oxidative stress and histological assessment of low-level laser therapy in experimental myopathy. Lasers Surg Med. 2010;42(6):577–583. doi: 10.1002/lsm.20910. [DOI] [PubMed] [Google Scholar]
- 31.Holterman CE, Rudnicki MA. Molecular regulation of satellite cell function. Semin Cell Dev Biol. 2005;16(4–5):575–584. doi: 10.1016/j.semcdb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Hurme T, Kalimo H. Activation of myogenic precursor cells after muscle injury. Med Sci Sports Exerc. 1992;24(2):197–205. [PubMed] [Google Scholar]
- 33.Shefer G, Oron U, Irintchev A, Wernig A, Halevy O. Skeletal muscle cell activation by low-energy laser irradiation: a role for the MAPK/ERK pathway. J Cell Physiol. 2001;187(1):73–80. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1053>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Shefer G, Partridge TA, Heslop L, Gross JG, Oron U, Halevy O. Low-energy laser irradiation promotes the survival and cell cycle entry of skeletal muscle satellite cells. J Cell Sci. 2002;115(Pt 7):1461–1469. doi: 10.1242/jcs.115.7.1461. [DOI] [PubMed] [Google Scholar]
- 35.Gomes AR, Soares AG, Peviani S, Nascimento RB, Moriscot AS, Salvini TF. The effect of 30 min of passive stretch of the rat soleus muscle on the myogenic differentiation, myostatin, and atrogin-1 gene expressions. Arch Phys Med Rehabil. 2006;87(2):241–246. doi: 10.1016/j.apmr.2005.08.126. [DOI] [PubMed] [Google Scholar]
- 36.Oron U. Photoengineering of tissue repair in skeletal and cardiac muscles. Photomed Laser Surg. 2006;24(2):111–120. doi: 10.1089/pho.2006.24.111. [DOI] [PubMed] [Google Scholar]
- 37.Bibikova A, Belkin V, Oron U. Enhancement of angiogenesis in regenerating gastrocnemius muscle of the toad (Bufo viridis) by low-energy laser irradiation. Anat Embryol (Berl) 1994;190(6):597–602. doi: 10.1007/BF00190110. [DOI] [PubMed] [Google Scholar]
- 38.Kipshidze N, Nikolaychik V, Keelan MH, Shankar LR, Khanna A, Kornowski R, Leon M, Moses J. Low-power helium: neon laser irradiation enhances production of vascular endothelial growth factor and promotes growth of endothelial cells in vitro. Lasers Surg Med. 2001;28(4):355–364. doi: 10.1002/lsm.1062. [DOI] [PubMed] [Google Scholar]
- 39.Fukushima K, Badlani N, Usas A, Riano F, Fu F, Huard J. The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med. 2001;29(4):394–402. doi: 10.1177/03635465010290040201. [DOI] [PubMed] [Google Scholar]
- 40.Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, Marroni NP, Gonzalez-Gallego J. Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med. 2005;37(4):293–300. doi: 10.1002/lsm.20225. [DOI] [PubMed] [Google Scholar]
- 41.Rizzi CF, Mauriz JL, Freitas Correa DS, Moreira AJ, Zettler CG, Filippin LI, Marroni NP, Gonzalez-Gallego J. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg Med. 2006;38(7):704–713. doi: 10.1002/lsm.20371. [DOI] [PubMed] [Google Scholar]