Abstract
Background
Crimean Congo Hemorrhagic Fever (CCHF) is an acute febrile haemorrhagic disease. This study was conducted to ascertain the infection status amongst slaughterhouse workers in Iran’s north-eastern provinces (Razavi and northern and southern Khorasan), so that analysis of the results could help clarification of the epidemiology of this disease in the aforementioned provinces and eastern regions of Iran.
Methods:
In this cross-sectional study, conducted in 2004 and 2005, 108 slaughterhouse workers from 24 cities of the three previously mentioned provinces were randomly entered into the study. An IgG specific ELISA test was carried out on the participants’ serum samples.
Results:
Sixteen out of 108 (14.8%) participants under study were shown to have IgG against CCHF. The highest rate of infection was seen in Razavi Khorasan and southern Khorasan at 17.5% and 16.7%, respectively.
Conclusion:
The study showed a relatively high frequency of this disease amongst slaughterhouse workers in these provinces. Taking into account the small number of reported cases from these provinces, it would seem that more focus is required on primary diagnosis and on referral of suspected patients.
Keywords: Crimean Congo Hemorrhagic Fever, Slaughterhouse, ELISA, IgG, Iran
Introduction
Crimean Congo Hemorrhagic Fever (CCHF) is an acute febrile haemorrhagic disease. Although the causative virus of this disease is mostly present in animals, limited cases of the disease can occur in humans, with a mortality rate of 50% in certain instances. There are no specific clinical symptoms in animals, whereas in humans, various clinical symptoms may be observed, ranging from a flu-like syndrome to haemorrhage, and gastrointestinal and neurological conditions, etc (1).
The virus is transmitted to humans via tick bites or on contact with the blood or body tissues of infected livestock. Hospital acquired cases and transmission from human to human have also been reported. Multiple studies have demonstrated that most human infection in Iran has taken place amongst those having close contact with the blood, secretions, and tissues of infected livestock (1,2). The virus has prevailed in various countries stretching from the Far East (west of China) to the Middle East, and is also found in most areas of Africa, the southern parts of Russia and the Balkans in Europe (3).
The antibody against the virus can be discovered via serologic tests, specific ELISA, by IgM, and IgG titer. IgM can be isolated from approximately the sixth day until forty days later, while IgG can be isolated from around ten days after the virus has entered the body until five to six years later. In severe and lethal cases of the disease, where insufficient time exists for antibody production and manifestation of clinical symptoms, identification of the virus’s genome via RT-PCR is pertinent (2,3).
The history of the disease in Iran suggests that it has been presented since 1966 (4). In different studies conducted between 1966 and 1975, the disease has been reported in livestock in multiple regions in Iran (5–8). The first report of the clinical disease in humans goes back to 1999 in Shahrekord (9). Subsequently, the disease has been reported in different parts of the country. Most cases of the disease during this period were from Sistan and Baluchistan Province in the south-east of Iran and infected livestock imported from Iran’s eastern provinces can be surmised to be one of the main reasons for the disease’s prevalence in Iran (10). In addition, the majority of patients in Iran were of those who had close contact with the blood, secretions and body tissues of infected livestock (4).
Most reported cases of the disease in Iran are of butchers and slaughterhouse workers (4). In a 2002 report, distributed from Pakistan, it was revealed that most human infections were of people employed in the industry of keeping and slaughtering livestock (11).
Between the years 2001 to 2003, out of 448 livestock samples from northern Khorasan, Razavi Khorasan and southern Khorasan, referred to the Laboratory of Arboviruses and Viral Haemorrhagic Fevers of the Pasteur Institute of Iran, more than 50% of sheep and goats in these three provinces had a history of CCHF infection (12).
Asymptomatic infected cases among the ‘at risk’ population are not routinely discovered via routine surveillance systems. In addition, it would seem that most of the reported cases of the disease have been from Sistan and Baluchistan Province in the south-east of Iran, and the hypothesis that this infection has entered Iran from neighbouring countries in the east, is very strong. Taking all the aforementioned points into consideration, this study has been designed to assist in the understanding of the infection status among slaughterhouse workers of the north-eastern provinces of Iran (Razavi and northern and southern Khorasan).
Following the nosocomial outbreak of CCHF in 2011 in Birjand (South Khorasan) and in 2009 and 2012 in Mashad (13, 14), that lead to death of some of health care workers, as there were lots of non-expert opinions without adequate scientific support, the authors decided to contribute to further clarification of the epidemiology of this disease in eastern regions of Iran.
Materials and Methods
Study area
The region under study included provinces located in the north-east of Iran, i.e. Razavi and northern and southern Khorasan, each made up of 25, 7, and eight cities respectively. These three provinces border Turkmenistan in the north, Golestan, Semnan, Yazd and Kerman provinces in the west, Sistan and Baluchistan in the south, and Afghanistan in the east.
Sampling method
This cross-sectional study was conducted in 2004 and 2005. Twenty-four of the 40 cities in these provinces were randomly selected and entered into the study (Fig. 1). Samples were taken from workers of 27 slaughterhouses in these 24 cities (Mashhad, Torbat-e- Jam, Taibad, Khaf, Kashmar, Bardaskan, Chenaran, Ghuchan, Dargaz, Fariman, Sarakhas, Kalat, Neishabour, Torbat-e- Heydarie, Ferdous, Sabzevar, Gonabad (in Razavi Khorasan), Birjand, Ghaen, Nehbandan (in southern Khorasan), and Shirvan, Bojnord, Jajarm, Esfarayen (in northern Khorasan). The total number of workers in all the slaughterhouses was 635, of which 108 were randomly selected and entered into the study. From each city, one slaughterhouse was selected and from each slaughterhouse, four workers randomly selected for sampling. Due to the large number of slaughterhouses in Mashhad, four slaughterhouses and 16 workers were selected.
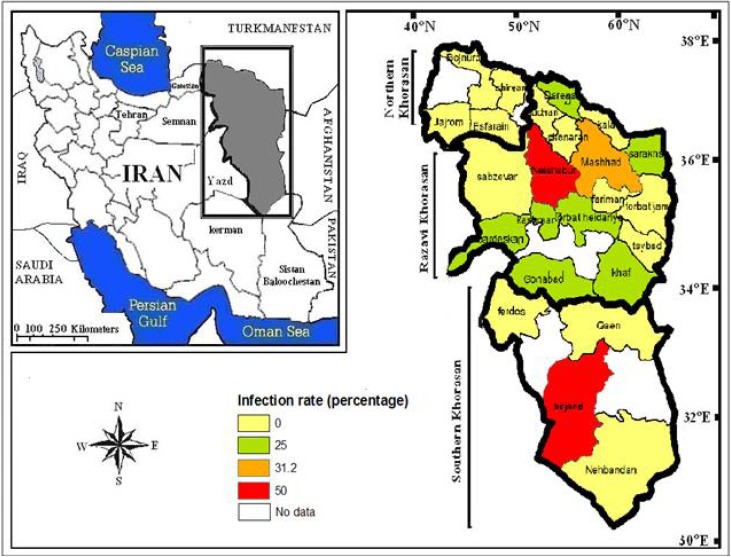
Fig. 1:
Infection rate of Crimean Congo Haemorrhagic Fever among Slaughterhouse Workers in North-Eastern Iran
Completing information and sampling
While the demographic questionnaires were being completed, a blood sample of 10ml was drawn from each of the participants. The blood samples were then centrifuged immediately after coagulation and the centrifuged samples were poured into a cryo tube; this was placed in a falcon tube for protection and then subsequently deposited in a vaccine carrier containing ice; it was then transferred to the Laboratory of Arboviruses and Viral Haemorrhagic Fevers (National Reference Laboratory) at the Pasteur Institute of Iran. In the laboratory, the specific IgG ELISA test was carried out on the dispatched serum samples.
Statistical analysis
Data were analyzed using SPSS 16; Chi square was applied for categorized data, while Kendall’s correlation was used to determine the relationship between ordinal and nominal variables.
Results
Totally 16 out of 108 (14.8%) workers of the slaughterhouses in Khorasan province (Razavi, northern, and southern Khorasan) tested positive for IgG against CCHF. The highest level of infection was seen in Razavi Khorasan with 17.5% (fourteen positive cases out of 80 samples), followed by southern Khorasan with 16.7% (two out of twelve samples). There were no positive cases among the 16 samples from northern Khorasan. Fifty percent of the dispatched samples from Neishabour (in Razavi Khorasan) and Birjand (in southern Khorasan) and one-third of the samples sent from Mashhad (in Razavi Khorasan) showed a history of the infection. IgG was observed in at least one out of four samples in ten out of 24 cities under study (41.6%). The most frequent history of the infection was observed in the cities of Razavi Khorasan (Fig. 1).
the infection was mostly seen in the age range of 20 to 39 years. The infection decreased with an increase in age (r=0.21). There was a significant relationship between the level of education and infection percentage; the infection percentage amongst workers decreased with an increase in level of education (P<0.001). There was no significant relationship between specifically the length of time working in a slaughterhouse and the percentage of infection amongst the workers. Although the infection rate in Razavi Khorasan (17.5%) and southern Khorasan (16.7%) was higher than in northern Khorasan (0%), such a difference was not statistically significant (Table 1).
Table 1:
Characteristics of subjects consenting to blood sampling (Seropositives are all ELISA IgG positive subjects)
| Variable | Category (yr) | Total No. (% Seropositive) | Correlation Coefficient | P-value |
|---|---|---|---|---|
| Age | 0–19 | 5 (0) | − 0.21 | 0.017a |
| 20–29 | 18 (16.7) | |||
| 30–39 | 37 (19) | |||
| 40–49 | 28 (14.3) | |||
| ≤50 | 20 (10) | |||
| Education | 0–5 | 62 (22.5) | − 0.19 | 0.04 a |
| 6–8 | 16 (12.5) | |||
| 9–12 | 19 (10) | |||
| 12≥ | 11 (0) | |||
| Work history | 0–5 | 15 (26.7) | − 0.1 | 0.201a |
| 6–10 | 19 (15.7) | |||
| 11–15 | 31 (12.9) | |||
| 16–20 | 21 (14.3) | |||
| 21≥ | 22 (9) | |||
| Province | North Khorasan | 16 (0) | - | 0.195b |
| Razavi Khorasan | 80 (17.5) | |||
| South khorasan | 12 (16.7) |
Kendall- tau correlation test
Chi-Square test
Discussion
In this study, the history of CCHF infection amongst butchers and slaughterhouse workers in the north-eastern provinces of Iran was 14.8%. This number is relatively high compared to the infection rate of 5% amongst the slaughterhouse workers in Isfahan province (15) which has the second highest rate of reported cases of this disease in Iran.
Various studies worldwide have identified butchers and slaughterhouse workers as a high-risk group for this disease. For instance, in Mauritania, half of the patients with this disease were butchers or slaughterhouse workers (16). In an epidemic in South Africa, 15 cases of CCHF were reported and all were workers in ostrich slaughterhouses (17).
Bearing in mind the common borders of the provinces of southern Khorasan and Razavi Khorasan with Afghanistan, and the likelihood of infected livestock being imported from this country, it was anticipated that we would be able to track the antibody in slaughterhouse workers in these two provinces. It is possible that the lower level of infection observed in northern Khorasan could be due to the geographical distance between this province and Afghanistan and fewer livestock imports to this province.
As seen in Table 1, education and increasing levels of awareness have been shown as effective in preventing CCHF among workers, and the percentage of positive samples has been reduced with an increase in educational level; this implies that appropriate education delivered to those in related careers, with respect to personal protection during the slaughtering of animals and when dealing with newly slaughtered animals, can decrease the infection rate amongst this population. A study in Turkey revealed that the risk of transmission of this disease could be decreased by providing more information to high-risk groups (18).
In this study, the highest percentage of infection was observed in the age range of 20 to 39. This age range is basically engaged more in the main activities and probability of contact with infected livestock due to slaughtering and contact with raw livestock products is more common.
Moreover, in this study, the infection rate decreased with an increase in age. In another study conducted in Senegal, it was revealed that the probability of a positive serum sample increased with an increase in age (19). A higher incidence of high risk behaviour and the failure to obey personal protection basics in younger butchers and slaughterhouse workers were likely to be important factors for the increased number of reports of infections, compared to older people.
As mentioned in the previous studies conducted on livestock in these three provinces, IgG is present in high levels in the livestock there (12); hence infection in groups in contact with livestock was anticipated and the results of this study additionally confirm these findings. Considering the low number of reported cases from these provinces(4, 12), it would seem that care is necessary in primary diagnosis and referral of suspected patients to the National Laboratory of Arboviruses and Viral Haemorrhagic Fevers of the Pasteur Institute of Iran, according to the national protocol of the surveillance system.
Bearing in mind that ticks and livestock reservoirs of this disease are present in different parts of Iran, such as the region under study, eradicating the disease appears to be extremely challenging and even impossible. However, by implementing pertinent plans, such as the population control of ticks and enhancing awareness levels of those groups in society that are particularly at risk, such as butchers and slaughterhouse workers, the disease could be controlled. It is strongly suggested that similar studies be conducted in other provinces of Iran in order to clarify the disease’s status amongst high-risk groups.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
We thank Ms S.KhakiFirooz and Ms F.S.RasiVaraee from Laboratory of Arboviruses and Viral Haemorrhagic Fevers (National Reference Laboratory), Pasteur Institute of Iran for their technical laboratory help in our project. The authors declare that there is no conflict of interests.
Reference
- 1.Chinikar S, Goya M, Shirzadi M, Ghiasi S, Mirahmadi R, Haeri A, et al. Surveillance and Laboratory Detection System of Crimean Congo Haemorrhagic Fever in Iran. Transbound Emerg Dis. 2008;55(56):200–204. doi: 10.1111/j.1865-1682.2008.01028.x. [DOI] [PubMed] [Google Scholar]
- 2.Izadihassan M, Salehi H, Chinikar S, Darvishi M, Jonaidi N, Ranjbar R, et al. A Geographical Distribution Survey on CCHF Positive Antibody Ovine’s of Isfahan Province in 1383–1384. Mil Med J. 2007;9(2):97–102. [Google Scholar]
- 3.Chinikar S, Ghiasi SM, Moradi M, Gooya M, Shirzadi MR, Zeinali M. Geographical Distribution and Survillance of crimean -congo Hemorrhagic Fever in Iran. Vector- Born and Zoonotic Disease. 2010;10:705–8. doi: 10.1089/vbz.2009.0247. [DOI] [PubMed] [Google Scholar]
- 4.Chinikar S. An overview of Crimean-Congo Hemorrhagic Fever in Iran. Iranian J Microbiol. 2009;1(1):7–12. [Google Scholar]
- 5.Ardoin A, Karimi Y. A focus of thrombocytopenic purpura in East Azerbaidjan province, Iran 1974–1975 (author’s transl) Médecine tropicale: revue du Corps de santé colonial. 1982;42(3):319–326. [PubMed] [Google Scholar]
- 6.Buckley SM. Cross plaque neutralization tests with cloned crimean hemorrhagic fever-congo (CHF-C) and Hazara viruses. Proc Soc Exp Biol Med. 1974;146(2):594–600. doi: 10.3181/00379727-146-38154. [DOI] [PubMed] [Google Scholar]
- 7.Burney M, Ghafoor A, Saleen M, Webb P, Casals J. Nosocomial Outbrak of Viral Hemorrhagic Fever Caused by Crimean Hemorrhagic Fever-Congo Virus in Pakistan, January 1976. Am J Trop Med Hyg. 1980;29(5):941. doi: 10.4269/ajtmh.1980.29.941. [DOI] [PubMed] [Google Scholar]
- 8.Saidi S, Casals J, Faghih M. Crimean hemorrhagic fever-Congo (CHF-C) virus antibodies in man, and in domestic and small mammals, in Iran. Am J Trop Med Hyg. 1975;24(2):353. doi: 10.4269/ajtmh.1975.24.353. [DOI] [PubMed] [Google Scholar]
- 9.Chinikar S, Mazaheri V. The seroepidemiological aspects of crimean congo hemorrhagic fever in three health workers: a report from Iran. Arch Iranian Med. 2002;5(4):255. [Google Scholar]
- 10.Chinikar S, Mazaheri V, Mirahmadi R, Nabeth P, Saron M, Salehi P, et al. A serological survey in suspected human patients of Crimean-Congo Hemorrhagic Fever in Iran by determination of IgM specific ELISA method during 2000–2004. Arch Iran Med. 2005;8(1):52–5. [Google Scholar]
- 11.Athar MN, Baqai HZ, Ahmad M, Khalid MA, Bashir N, AHMAD A, et al. Short report: crimean-congo hemorrhagic fever outbreak in Rawalpindi, Pakistan, February 2002. Am J Trop Med Hyg. 2003;69(3):284–87. [PubMed] [Google Scholar]
- 12.Bokaie S, Mostafavi E, Haghdoost A, Keyvanfar H, Gooya M, Meshkat M, et al. Crimean Congo Hemorrhagic Fever in Northeast of Iran. J Animal Vet Adv. 2008;7(3):354–361. [Google Scholar]
- 13.Ziayaei M, Azarkar G, Shayesteh M. Two cases of Crimean–Congo hemorrhagic fever and a suspected case in South Khorasan Province, 2011. Modern Care. 2011;8(3):174–178. [Google Scholar]
- 14.Naderi H, Sarvghad M, Bojdy A, Hadizadeh M, Sadeghi R, Sheybani F. Nosocomial outbreak of Crimean-Congo haemorrhagic fever. Epidemiology and Infection. 2011;139(06):862–866. doi: 10.1017/S0950268810002001. [DOI] [PubMed] [Google Scholar]
- 15.Karimi I, Rostami Jalilian M, Chinikar S, Ataei B, Kasaian N, Jalali N, et al. Seroepidemiological survey of crimean- congo hemorrhagic fever among slaughters and butchers in Isfahan. Journalof Isfahan Medical School. 2007;24(83):57–62. [Google Scholar]
- 16.Nabeth P, Cheikh DO, Lo B, Faye O, Vall I, Niang M, et al. Crimean-Congo hemorrhagic fever, Mauritania. Emerg Infec Dis. 2004;10(12):2143. doi: 10.3201/eid1012.040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capua I. Crimean-Congo haemorrhagic fever in ostriches: a public health risk for countries of the European Union? Avian Pathology. 1998;27(2):117–120. doi: 10.1080/03079459808419311. [DOI] [PubMed] [Google Scholar]
- 18.Ç lngrou N, Temel F, Altinta H. Public’s Knowledge, Opinions and Behaviors about Crimean-Congo Hemorrhagic Fever: An Example from Turkey. J Faculty Vet Med. 2010;16:S17–S22. [Google Scholar]
- 19.Chapman LE, Wilson ML, Hall DB, LeGuenno B, Dykstra EA, Ba K, et al. Risk factors for Crimean-Congo hemorrhagic fever in rural northern Senegal. J Infec Dis. 1991;164(4):686–692. doi: 10.1093/infdis/164.4.686. [DOI] [PubMed] [Google Scholar]