Abstract
In this study, age-related changes in the monoamine oxidases (MAO) were studied in the optic nerve (ON) of both young and aged male rats. The aim of the study was to assess the role of MAO in age-related changes in the rat ON and explain the mechanisms of neuroprotection mediated by MAO-B-specific inhibitors. Fifteen three month old and fifteen 26 month old Sprague–Dawley rats were used. The animals were killed by terminal anaesthesia. Staining of MAO, quantitative analysis of images, biochemical assays and statistical analysis of data were carried out. Samples of the ON were washed in water, fixed in Bowen fluid, dehydrated and embedded in Entellan. Histological sections were stained for MAO-enzymatic activities. The specificity of the reaction was evaluated by incubating control sections in a medium either without substrate or without dye. The quantitative analysis of images was carried out at the same magnification and the same lighting using a Zeiss photomicroscope. The histochemical findings were compared with the biochemical results. After enzymatic staining, MAO could be demonstrated in the ON fibres of both young and aged animals; however, MAO were increased in the nerve fibres of the elderly rats. These morphological findings were confirmed biochemically. The possibility that age-related changes in MAO levels may be attributed to impaired energy production mechanisms and/or represent the consequence of reduced energy needs is discussed.
Keywords: age-related change, deprenyl, glyceraldehyde-3-phosphate dehydrogenase, monoamine oxidase, optic nerve, oxidative stress
The monoamine oxidases (MAO) are intracellular enzymes linked to the mitochondrial membranes of nerve cells. MAO were first investigated thoroughly 40–59 years ago in the central nervous system (CNS; Studer et al. 1964; Blaschko 1974; Brus 1975; Saura et al. 1994a,b). MAO exist in three molecular forms: MAO-A, MAO-B and MAO-C. These forms differ both in specific substrates and inhibitor sensitivity. MAO-A is sensitive to clorgyline (Johnston 1968) and MAO-B is sensitive to deprenyl (Knoll & Magyar 1972), while MAO-C utilizes benzylamine as substrate and is sensitive to the inhibitor semi-carbazide (Cao Danh et al. 1984). MAO contribute to the regulation of neuronal activity by maintaining an unchanged level of catecholaminergic neurotransmitters.
Monoamine oxidases show a sharp age-dependent increase and play an important role in neurodegenerative diseases, as demonstrated by biochemical studies (Fowler et al. 1980; Hoffman et al. 1997; Moody et al. 1997; Thomas 2000).
In this study, we investigated the age-related changes in MAO-A and MAO-B in the rat optic nerve (ON), correlating morphological and biochemical data and performing a quantitative evaluation of the enzymatic staining.
Methods
Fifteen 3-month-old Sprague–Dawley rats and fifteen 26-month-old rats were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the Italian Health Ministry guidelines. The procedures have been approved by the Committee on Faculty Ethics. The rats were kept at a constant temperature (22 °C) with a 10–14 dark–light rhythm and free access to water and food. The maximum lifespan in our colony was 26/28 months. Each animal was weighed every day, and the weights were recorded. They were killed by means of terminal anaesthesia (50 mg/kg of endoperitoneal Nembutal).
The orbit cavity was opened, and the ON was identified, removed, weighed and washed in cold phosphate buffer solution (PBS). Samples of the ON were drawn with a scissor, weighed and fixed in Bowen fluid until they were required for analysis. Afterwards, they were embedded in Entellan (Merck, Darmstadt, Germany), sectioned at the microtome into 40–50 μ slices and treated for enzymatic staining of the MAO. Other samples of the ON were dissected under the operative microscope and homogenized in cold PBS 1:10 weight:volume and used for biochemical assay (proteins, enzymes). Staining of the MAO was performed according to the method described by Uchida and Koelle (1984):
incubation of the sections in 30% Na2S04 solution for 1 h at 37 °C;
incubation for 1 h at 37 °C in 0.05 M PBS (pH 7.6) containing sucrose (8%) either with or without 10–7 moles of clorgyline as MAO-A-specific inhibitor (Johnston 1968): or deprenyl as MAO-B-specific inhibitor (Knoll & Magyar 1972);
rinsing of the sections in PBS and staining with a solution containing phosphate buffer, nitroblue tetrazolium, sucrose (8%) and tryptamine (1 mM, used as a substrate and starter of the reaction) for 1–2 h at 37 °C.
The specificity of the reaction was evaluated by incubating control sections in a medium either without substrate (tryptamine) or without dye (nitroblue tetrazolium). In both instances, a negative result was expected. The reaction was considered to be positive when nitroblue tetrazolium precipitates were present. In such cases, the granules appear blue on the slides and black on B/W photographs.
The quantitative analysis of the images (QAI) was carried out at the same magnification and the same lighting as in the photographs, using a Zeiss II photomicroscope (Karl Zeiss, Jena, Germany). QAI makes it possible to obtain data for many morphological parameters (e.g. surface of a structure, specific parameters, number of cells, intensity of a reaction, white or black or grey intensity, etc.). The value 0 indicates a negative reaction and may be obtained, for example, by omitting the substrate in the incubation medium or the specific dye, but also by adding an excess of the specific reaction inhibitor. The value 100 indicates the maximum intensity of the reaction present in the samples with the strongest staining. The final values are expressed in Conventional Units (CU ± SEM) and are directly provided by the instruments (Handbook of Methods 1997).
To make the results as objective as possible, measurements were repeated three times by different researchers, using different instruments and with double-blind method. Samples were marked and identified only after completion of all the experimental procedures. Data were then analysed statistically to derive the significance index.
For the biochemical assay, samples were weighed and homogenized into an ice-cold buffer solution. Tissue protein concentrations were determined following the method described by Ledoux and Lamy (1986), using bovine serum albumin (BSA) as standard and Folin phenol as reagent.
Enzymatic assays
Subtypes of MAO were assayed on Zeiss Model PMQ II Spectrophotometer by the rate of change of concentrations of nicotinamide adenine dinucleotides (NAD) or nicotinamide adenine dinucleotides phosphate (NADP), as shown by extinction measurements at 340 μm at room temperature (19 °C). Results were recorded on a Varian model G-42A graphic recorder (Varian Instrument Group, Palo Alto, CA, USA). The values of the blank (endogenous values of extinction) were subtracted from each sample. The reaction mixture was buffered with 0.5 M potassium phosphate buffer (pH 7.4) until final volume of 2.7 ml in 1 cm quartz cuvette (Beisenherz 1995). The MAO-A was assessed with 5-hydroxytryptamine as substrate, while the subtype B of the same enzyme was assessed with benzylamine as substrate. Final values were expressed as nmol/mg protein, calculated on the basis of the extinction coefficient and of protein content (Owen et al. 1987).
The statistical analysis was carried out both for histochemical and for biochemical results, using basic statistical methods, that is, by calculating the arithmetic mean, the extreme values, the variance, the standard deviation, the standard error and the correlation coefficient as indicated by Serio (1986). Student's t-test was used to compare the mean values obtained in the young and elderly rats. Chi-square test was used to compare frequencies between the two groups. A P value <0.05 was considered statistically significant.
Results
Table 1 shows some data obtained in rats of different ages and MAO activity values in a transverse section of the ON of young and elderly rats. The microphotographs 1a and 1b must be compared to point out the difference between MAO activities in young (Figure 1a) and elderly (Figure 1b) rats. Increased MAO-enzymatic staining is apparent in the ON of the elderly rats compared with the young rats. Figures 1c and 1d are examples of MAO-A staining (i.e. using inhibitor deprenyl, which inhibits MAO-B and stains only MAO-A). In the elderly rats, an increase in enzymatic staining may be observed also in non-neuronal cells, such as glial cells, and in blood vessels and meningeal membranes.
Table 1.
Data summary for rats of different ages
| Transverse section of optic nerve (ON) | Young rats | Elderly rats | P |
|---|---|---|---|
| Age (months) | 3 | 26 | – |
| Numbers of animals | 15 | 15 | |
| Body weight (g ± SD) | 150 ± 35 | 360 ± 44 | <0.001 |
| Diameter of ON (μm ± SEM) | 221 ± 13 | 268 ± 9 | 0.006 |
| Ratio nerve fibres/meningeal membranes (%) | 74.6/25.4 | 52.2/47.8 | NS |
| Total number of nerve fibres ± SEM | 113,000 ± 894 | 67,300 ± 516 | <0.001 |
| Area of ON (μm2 ± SEM) | 30618.8 ± 12.1 | 28734.1 ± 10.6 | <0.001 |
| MAO activity | 76 ± 1.5 | 208 ± 4.2 | <0.001 |
Mean value ± standard deviation (SD) or standard error of the mean (SEM). Significant differences (P) were calculated comparing the data obtained in elderly and in young rats.
The values of MAOs activity are expressed in International Units (I.U.) ± SEM.
Figure 1.
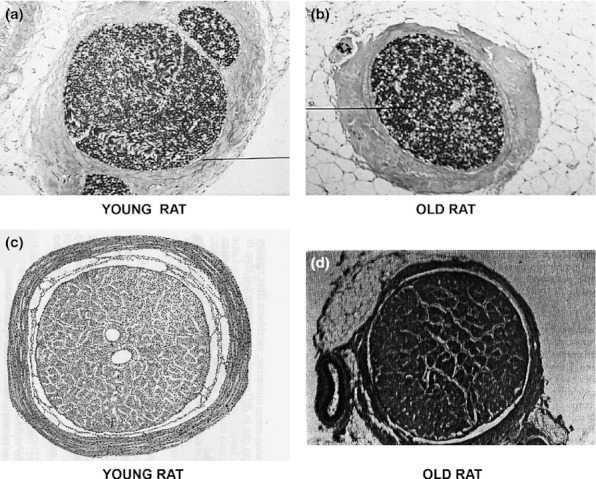
(a) Transversal section of a young rat optic nerve (ON). The black line indicates the connective sheath that envelops all the three fascicles of the ON (1, central; 2, nasal upper; and 3, temporal lower; magnification 100×). (b) Transversal section of an elderly rat ON. There is an increase in the enzymatic staining (total MAO-A-B-C) in the ON of the elderly rats compared with the young rats (a). The black line indicates atrophic ON fibres. The numbers and diameters of the nerve axons are strongly decreased, and the nerve fibres/meningeal membranes ratio is also decreased. The ON is surrounded by orbital adipose tissue (magnification 100×). (c) MAO activity in transversal section of the ON of a young rat. Centrally, cross sections of the ophthalmic artery and the central artery of the retina are observed. All the nerve structures (fascicles of nerve fibres, connective sheath and septa) are well preserved (magnification 250×). (d) MAO activity in a transversal section of the ON of an elderly rat. The ophthalmic artery and its branches, as well as the central artery of the retina, appear obstructed and strongly positive at the enzymatic staining. The connective septa are increased. All the structures of the ON are strongly positive for the MAO activity (magnification 250×).
Figures 2a and 2b compare samples of young (Figure 2a) and elderly (Figure 2b) rat ON stained using the specific MAO-A inhibitor clorgyline that thus provides an index of MAO-B enzymatic staining.
Figure 2.
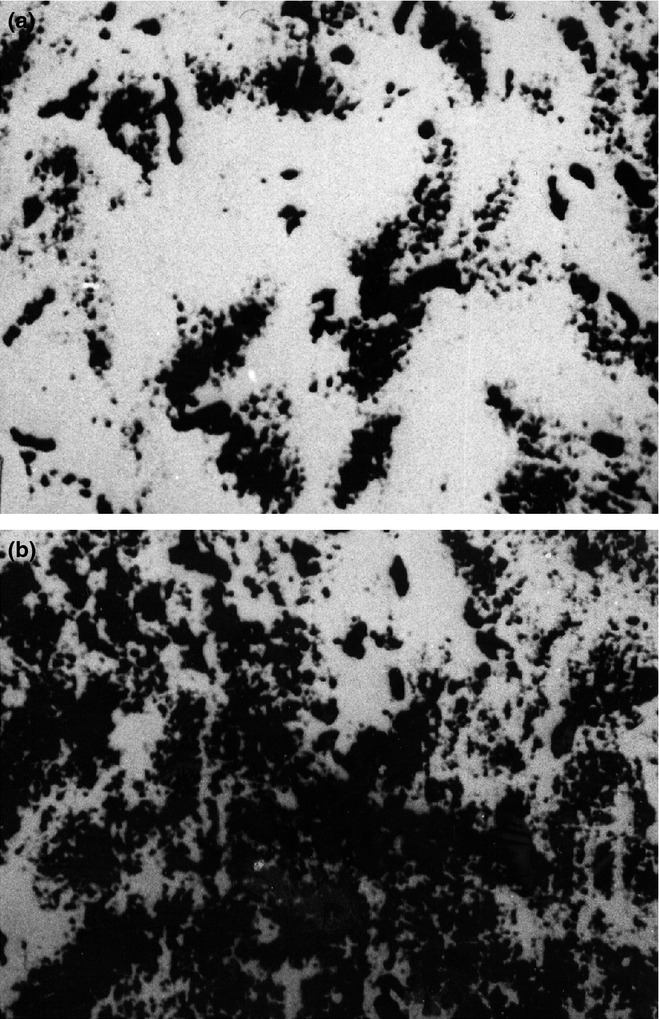
(a) Histochemical staining of MAO activity in the optic nerve (ON) of a young rat. A few nerve fibres stain positively for MAO. The whole sample appears black and therefore scarcely positive after the reaction (magnification 800×). (b) Histochemical staining of MAO activity in the ON of an elderly rat. An intense positive MAO reaction is apparent. The whole sample appears dark and therefore strongly positive after the reaction (magnification 800×).
This has to be seen against our background observation (unpublished) that in the heart, clorgyline inhibits MAO staining completely. Since the MAO-A inhibitor inhibits only 30% in the eye of staining, as demonstrated by QAI around 70% of MAO staining is not inhibited by clorgyline and is presumably associated with the presence of the other MAO.
Discussion
The of this study was to evaluate the involvement of MAO in the pathogenesis of neurodegenerative changes, particularly in the ON of experimental animals. Other researchers have already demonstrated a correlation between brain ageing, neuritic plaques and quantitative changes in the MAO (Fowler et al. 1980).
The MAO are localized mainly in the mitochondrial membranes and are able to oxidize biogenic amines (neurotransmitters) only when they are released by the presynaptic vesicles. This oxidation induces their catabolism and makes them lose the specific function of neurotransmitters (Blaschko 1974). Hyperactivity of MAO causes a reduction of biogenic amines and leads to an excessive production of oxygen free radicals (hydrogen peroxide) followed by oxidative stress and neurotoxicity.
The possible mechanisms of cell damage induced by free radicals include:
reactions with nucleic acids, nucleotides, polysaccharides, protein and non-protein thiols;
covalent bond with the components of membranes;
triggering of the lipid peroxidation reaction (Slater 1984).
The oxidative stress owing to the action of H2O2 is neurotoxic and may be inhibited by inhibitors of MAO-B as l-deprenyl and structurally related propargylamines (DRPs) as they can increase neuronal survival by reducing apoptosis (Tatton et al. 2003).
MAO inhibitors are able to facilitate the neuronal activity, to increase cerebral perfusion and to prevent neurodegeneration and ischaemic axonal damage. Therefore they can reduce neuronal death induced in vivo and in vitro by various kinds of damage in a number of different experimental neuronal models. These insults include ON or peripheral nerve crush or axotomy, hypoxia and/or ischaemia, trophic insufficiency, thiamine deficiency, nitric oxide (NO), ageing, glutathione depletion, etc. (Tatton et al. 2000; Rosenstiel et al. 2002).
Some authors state that l-deprenyl protects vascular endothelium from peptide amyloid-β toxicity, increases cerebral blood vessels and stimulates production of NO that reduces the oxidative stress in Alzheimer's Disease and related disorders (Thomas et al. 1999; Thomas 2000).
Carlile et al. (2000) showed that glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a glycolytic enzyme that plays a key role in energy metabolism, is a target for deprenyl and may contribute to the neuroprotection. In fact, GAPDH is also known to have several non-glycolytic cellular functions and has been implicated as an initiator signal of programmed cell death cascades. Therefore, it may be significant for a number of human disorders such as apoptosis, neurodegenerative diseases, prostate cancer and viral pathogenesis (Berry 2004). This was observed by the increased nuclear GAPDH in postmortem samples from patients with Parkinson's disease, Alzheimer's Disease, Huntington's Disease and glaucoma, among others. A number of small-molecule compounds, including CGP 3466 – a tricyclic deprenyl analogue showing anti-apoptotic activity as it interacts with GAPDH and prevents its nuclear accumulation – have now been identified (Berry 2004). In fact, CGP 3466 binds to GAPDH and, in addition, blocks growth factor deprivation-induced GAPDH nuclear translocation and apoptosis (Carlile et al. 2000). Therefore, MAO-B inhibitors (deprenyl, its metabolite desmethyl-deprenyl and CGP3466) can reduce apoptosis independent of MAO-B inhibition and have been found to bind to GAPDH (Carlile et al. 2000).
Furthermore, Kusner et al. (2004) reported nuclear accumulation of GAPDH in human retinal Müller cells undergoing apoptosis secondary to high glucose concentrations, effects that were prevented by treatment with deprenyl. The authors state that similar agents limit the mass movement of GAPDH into the nucleus and may be useful therapies for diabetic retinopathy.
Finally, Mazzola and Sirover (2005) provided evidence of structural changes in subcellular expression of the GAPDH protein associated with age-related neurodegenerative condition. These results suggest the possibility that subcellular interactions may mitigate oxidative stress–induced GAPDH modification in human ageing.
We have observed that in both young and elderly rats, the ON sheaths envelop the whole nerve and give off septa into the inner portion of the nerve, but in the elderly rats, there is an increase in thickness and area of the sheaths and septa. Moreover, in elderly rats ON, MAO activity is strongly increased. It is a possibility that age-related changes in MAO levels may be attributed to impaired energy production mechanisms; alternatively, these changes may follow reduced energy needs. In conclusion, therefore, we suggest that strong enzymatic staining correlates with ageing and with neurodegenerative changes.
Despite several research studies, the mechanisms of neuroprotection mediated by l-deprenyl remain poorly understood. Further studies will be needed to elucidate the causes of MAO increased values in the elderly rats and to better explain the mechanism of action of deprenyl.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the manuscript. Nebbioso M. and Pascarella A. contributed equally to this work.
References
- Beisenherz G. Methods in Enzymology. In: Colowick SP, Kaplan NO, editors. New York: Academic Press; 1995. p. 287. [Google Scholar]
- Berry M. Glyceraldehyde-3-phosphate dehydrogenase as a target for small-molecule disease-modifying therapies in human neurodegenerative disorders. J. Psychiatry Neurosci. 2004;29:337–345. [PMC free article] [PubMed] [Google Scholar]
- Blaschko H. The natural history of amine oxidase. Rev. Physiol. Biochem. Pharmacol. 1974;70:83–148. doi: 10.1007/BFb0034294. [DOI] [PubMed] [Google Scholar]
- Brus R. Activity of some enzymes which synthesize and metabolise catecholamines in the brain and peripheral organs in developing rats. Arch. Immunol. Ther. Exp. 1975;23:449–457. [PubMed] [Google Scholar]
- Cao Danh H, Strolin Benedetti M, Dostert P, Mousset A. Age-related changes in benzylamine oxidase activity in rat tissues. J. Pharm. Pharmacol. 1984;36:592–596. doi: 10.1111/j.2042-7158.1984.tb04904.x. [DOI] [PubMed] [Google Scholar]
- Carlile GW, Chalmers-Redman RM, Tatton NA, Pong A, Borden KE, Tatton WG. Reduced apoptosis after nerve growth factor and serum withdrawal: conversion of tetrameric glyceraldehyde-3-phosphate dehydrogenase to a dimer. Mol. Pharmacol. 2000;57:2–12. [PubMed] [Google Scholar]
- Fowler CJ, Wiberg A, Oreland L, Marcusson J, Winblad B. The effect of age on the activity and molecular properties of human brain monoamineoxidase. J. Neural. Transm. 1980;49:1–20. doi: 10.1007/BF01249185. [DOI] [PubMed] [Google Scholar]
- Hoffman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein and prevalence of dementia and Alzheimer disease in the Rotterdam study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- Johnston JP. Some observation upon a new inhibitor of monoamine oxidase in brain tissue. Biochem. Pharmacol. 1968;17:1285–1297. doi: 10.1016/0006-2952(68)90066-x. [DOI] [PubMed] [Google Scholar]
- Knoll J, Magyar K. Some puzzling pharmacological effects of monoamine oxidase inhibitors. Adv. Biochem. Psychopharmacol. 1972;5:393–408. [PubMed] [Google Scholar]
- Kusner LL, Sarthy VP, Mohr S. Nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase: a role in high glucose-induced apoptosis in retinal Muller cells. Invest. Ophthalmol. Vis. Sci. 2004;45:1553–1561. [PubMed] [Google Scholar]
- Ledoux M, Lamy F. Determination of proteins and sulfobetaine with the folin-phenol reagent. Anal. Biochem. 1986;157:28–31. doi: 10.1016/0003-2697(86)90191-0. [DOI] [PubMed] [Google Scholar]
- Mazzola JL, Sirover MA. Ageing of human glyceraldehyde-3-phosphate dehydrogenase is dependent on its subcellular localization. Biochim. Biophys. Acta. 2005;1722:168–174. doi: 10.1016/j.bbagen.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Microsystems imaging Solutions. Handbook of Methods. Cambridge, UK: Microsystems imaging Solutions; 1997. Quantimet 500 Leica. [Google Scholar]
- Moody DM, Brown WR, Challa VR, Ghazi-Birry HS, Reboussin DM. Cerebral microvascular alterations in ageing, leukariosis and Alzheimer's disease. Ann. N. Y. Acad. Sci. 1997;826:103–116. doi: 10.1111/j.1749-6632.1997.tb48464.x. [DOI] [PubMed] [Google Scholar]
- Owen F, Crow TJ, Frith CD, et al. Selective decreases in MAO-B activity in post-mortem brains from schizophrenic patients with type II syndrome. Br. J. Psychiatry. 1987;151:514–519. doi: 10.1192/bjp.151.4.514. [DOI] [PubMed] [Google Scholar]
- Rosenstiel P, Sievers J, Lucius R. Deprenyl fails to promote axonal regeneration of retinal ganglion cells in vitro and in vivo. Cell Tissue Res. 2002;308:167–175. doi: 10.1007/s00441-002-0551-x. [DOI] [PubMed] [Google Scholar]
- Saura J, Luque JM, Cesura AM, et al. Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme autoradiography. Neuroscience. 1994a;62:15–30. doi: 10.1016/0306-4522(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Saura J, Richards JG, Maly N. Differential age-related changes of MAO-A and MAO-B in mouse brain and peripheral organs. Neurobiol. Aging. 1994b;15:399–408. doi: 10.1016/0197-4580(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Serio A. Appunti delle lezioni di Statistica Sanitaria. Roma: Edizioni Kappa; 1986. [Google Scholar]
- Slater TF. Free-radical mechanisms in tissue injury. Biochem. J. 1984;222:1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer A, Baumgartner HR, Rever K. Histochemical evidence of monoamine oxidase activity in rats of different ages. Histochemie. 1964;79:43–47. doi: 10.1007/BF00304177. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Chalmers-Redman RM, Elstner M, et al. Glyceraldehyde-3-phosphate dehydrogenase in neurodegeneration and apoptosis signaling. J. Neural Transm. Suppl. 2000;60:77–100. doi: 10.1007/978-3-7091-6301-6_5. [DOI] [PubMed] [Google Scholar]
- Tatton W, Chalmers-Redman R, Tatton N. Neuroprotection by deprenyl and other propargylamines: glyceraldehyde-3-phosphate dehydrogenase rather than monoamine oxidase B. J. Neural. Transm. 2003;110:509–515. doi: 10.1007/s00702-002-0827-z. [DOI] [PubMed] [Google Scholar]
- Thomas T. Monoamine oxidase-B inhibitors in the treatment of Alzheimer's disease. Neurobiol. Aging. 2000;21:343–348. doi: 10.1016/s0197-4580(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Thomas T, McLendon C, Thomas G. L-Deprenyl protects vascular endothelium from amyloid-β toxicity and stimulates production of nitric oxide. In: K Igual, D.F Swaab, B Winblad, H. M Wisniewski., editors. Alzheimer Disease and Related Disorders. Chischestr: J Wiley & Sons Ltd; 1999. pp. 493–500. [Google Scholar]
- Uchida E, Koelle GB. Histochemical investigation of criteria for the distinction between monoamine oxidase A and B in various species. J. Histochem. Cytochem. 1984;32:667–673. doi: 10.1177/32.6.6202738. [DOI] [PubMed] [Google Scholar]


