Abstract
Melioidosis is a tropical disease caused by ingestion, percutaneous inoculation or inhalation of the Gram-negative soil saprophyte Burkholderia pseudomallei. We developed a reproducible experimental murine model of pneumonic melioidosis induced by inhalation of aerosolized B. pseudomallei 1026b. In a series of experiments performed to bracket the lethal dose, we found that C57BL/6 mice were modestly more resistant than BALB/c mice (median lethal dose 334 CFU/lung vs 204 CFU/lung). We further characterized infection and pulmonary inflammation in C57BL/6 mice infected with a sublethal dose. We observed pulmonary replication and dissemination of bacteria to distant organs in the first days after infection, followed by bacterial containment by day 4 and no evidence of recrudescent infection for up to 2 months. We measured a robust host inflammatory response notable for a neutrophilic bronchoalveolar lavage fluid profile, elevated cytokines and chemokines in the lung and serum and scattered foci of neutrophilic infiltrates in the alveoli and in a perivascular distribution on histological analysis. We previously noted a similar pattern of inflammation in mice infected with aerosolized B. thailandensis. This report builds on the limited literature describing experimental murine pneumonic melioidosis induced by aerosol and characterizes pulmonary infection and resultant inflammation in C57BL/6 mice infected with aerosolized B. pseudomallei. This model has utility for the study of bacterial and host factors that contribute to the virulence of melioidosis.
Keywords: aerosol, animal model, Burkholderia pseudomallei, inhaled, melioidosis, mouse, pneumonia
The soil saprophyte Burkholderia pseudomallei is the causative agent of the tropical disease melioidosis (White 2003). Reflecting the environmental distribution of the organism, melioidosis is endemic in northern Australia and north-east Thailand. Mortality rates in these regions are 20% and over 40%, respectively. Burkholderia pseudomallei appears to infect the host by three potential routes: percutaneously, by inhalation or by ingestion (Limmathurotsakul & Peacock 2011). Clinically, the lung is involved in about 50% of melioidosis cases. Pulmonary melioidosis is particularly common during the rainy seasons, suggesting that the pathogen may be aerosolized and inhaled causing primary respiratory infection (Cheng et al. 2008). Lung involvement is associated with substantially greater lethality than non-pneumonic forms of melioidosis (Limmathurotsakul et al. 2005). Owing to the high mortality rate and severity of disease after inhalation and resultant biothreat concerns, B. pseudomallei is categorized as a select agent by the US government (http://www.selectagents.gov). There is therefore significant interest in developing a suitable animal model of pneumonic melioidosis.
A variety of mouse models of melioidosis have been developed using multiple routes of infection (Veljanov et al. 1996; Leakey et al. 1998; Hoppe et al. 1999; Santanirand et al. 1999; Gauthier et al. 2001; Liu et al. 2002; Jeddeloh et al. 2003; Barnes & Ketheesan 2005; Tan et al. 2008; Lever et al. 2009). Some of these studies have found C57BL/6 mice to be more resistant than BALB/c mice to intranasal, intravenous and intraperitoneal infection (Leakey et al. 1998; Hoppe et al. 1999; Liu et al. 2002; Barnes & Ketheesan 2005). An aerosol infection model of murine melioidosis was first described by Jeddeloh et al. (2003) but marked differences in susceptibility between C57BL/6 and BALB/c mice after inhalation of aerosolized B. pseudomallei strain 1026b were not observed. This finding contrasted with work by Tan et al. (2008), in which BALB/c mice infected by aerosol were noted to be at least 20-fold more susceptible to B. pseudomallei KHW than C57BL/6 mice. Lever et al. (2009) determined a median lethal dose of 5 CFU and described bacterial dissemination and histopathologic features of acute respiratory infection induced by aerosol infection of B. pseudomallei strain BRI in BALB/c mice. Given the discrepancies in the literature regarding differential mouse strain susceptibility to B. pseudomallei aerosol infection, here we report our investigations comparing aerosol infection of C57BL/6 and BALB/c mice with B. pseudomallei 1026b. C57BL/6 mouse strains are widely used and are a common background strain for creation of congenics. To date, description of pulmonary inflammation induced by inhalation of aerosolized B. pseudomallei in C57BL/6 mice is limited to cytokine release (Tan et al. 2008). Therefore, we further characterize infection and inflammatory changes in the lung of C57BL/6 mice in this aerosol model of pneumonic melioidosis.
Methods
Bacteria
Burkholderia pseudomallei 1026b, a clinical isolate obtained from a bacteraemic patient from Thailand (West et al. 2010), was grown in Luria-Bertani broth shaking in air at 37 °C, washed twice, resuspended in phosphate-buffered saline (PBS) containing 20% glycerol and frozen at −80 °C. Immediately before each aerosol infection experiment, the freezer stock was thawed and diluted in PBS to the desired concentration. Dilutions ranging from 10 to 100-fold yielded deposition doses of approximately 1 × 103–1 × 102 CFU/lung.
Animals and aerosol infection model
Specific pathogen–free BALB/c and C57BL/6 mice were obtained from Charles River Laboratories, Inc. (Wilmington, MA, USA) and Jackson Laboratories (Bar Harbor, ME, USA) and maintained in an approved animal facility. Mice were exposed to aerosolized bacteria or a sterile sham solution (PBS and glycerol) using a snout-only inhalation system (In-Tox Products, Moriarty, NM, USA). Aerosols were generated from a MiniHEART hi-flo nebulizer (Westmed, Tucson, AZ, USA) controlled by the Biaera AeroMP system (Biaera Technologies, Hagerstown, MD, USA). Nebulizer airflow was maintained at 8 l/min and dilution airflow over sterile water at 7 l/min for 10 min followed by 5 min purge with air. At this flow rate, the manufacturer reports generation of 2–3 μm particles by the nebulizer (http://www.westmedinc.com). Bacterial deposition in each experiment was determined from quantitative culture of lung tissue from four mice sacrificed immediately after infection. Animals were examined daily for illness or death, and when ill, abdominal surface temperatures were measured using a Ranger MX4P digital infrared thermometer (Raytek, Santa Cruz, CA, USA). Ill animals with temperatures <21.5 °C or a combination of ruffled fur, eye crusting, hunched posture and lack of resistance to handling were deemed terminal for study purposes and euthanized. At specific time points after infection, mice were sacrificed; the left lung, median hepatic lobe and spleen each were homogenized in 1 ml of sterile PBS. One hundred microliters each of homogenate and 10-fold serial dilutions were plated in duplicate on LB agar or Ashdown's medium. The lower limit of detection was approximately 5 CFU/organ. Colonies were counted after 2–4 days of incubation at 37 °C or up to a week incubating at room temperature. Animal studies were approved by the University of Washington Institutional Animal Care and Use Committee.
Bronchoalveolar lavage and lung histology
At serial time points after infection, mice were euthanized, the left pulmonary hilum tied off and a 20-gauge catheter inserted into the trachea. The right lung was lavaged with four 0.5 ml aliquots of 0.9% NaCl supplemented with 0.6 mM EDTA. The right lung was then inflated with 4% paraformaldehyde and immersed in the same fixative. Eukaryotic cell counts in bronchoalveolar lavage specimens were measured in a haemocytometer. Differentials were determined from examination of cytocentrifuge slides (Thermo Shandon, Pittsburgh, PA, USA) that were stained with a modified Wright-Giemsa technique (Diff-Quik; Siemens Healthcare Diagnostics Inc., Newark, DE, USA). Lung tissue was embedded in paraffin, sectioned and stained with haematoxylin and eosin. Tissue sections were examined by a veterinary pathologist.
Cytokine measurements
Left lung homogenates in PBS were diluted 1:1 in lysis buffer containing 2× protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany), incubated on ice for 30 min and then centrifuged at 1500 × g. Supernatants were collected and stored at −80 °C until assayed for cytokines. Whole blood was centrifuged, serum removed and stored at −80 °C until assayed. IFN-γ, IL-10, IL-12p70, IL-1β, IL-6, KC and TNF-α were measured in lung homogenates and serum using a 7-plex electrochemiluminescence detection assay (Meso Scale Discovery, Gaithersburg, MD, USA) and read on the MSD Sector Imager 2400.
Statistics
Combined data following a normal distribution are reported as mean ± SD. Comparisons between two groups of normally distributed data were made using Student's t-test. Survival analyses were performed with the log-rank test. All statistics were performed with Stata 11.2 (Stata Corp., College Station, TX, USA) or GraphPad Prism 4.0 (San Diego, CA, USA). A two-sided P-value ≤ 0.05 was considered significant.
Results
Survival
We first performed a series of experiments in which we aerosolized various dilutions of B. pseudomallei to C57BL/6 and BALB/c mice with deposition doses ranging from 56 to 1637 CFU/lung and monitored survival (Figure 1). All mice of each strain survived a deposition dose of 56 CFU/lung over a 2-week monitoring period. All C57BL/6 and the majority of BALB/c mice survived a deposition dose of 132 CFU/lung over a 5-day period when this experiment was censored. A deposition dose of 292 CFU/lung was lethal to the majority of BALB/c mice, but to none of the C57BL/6 mice that all remained well until the experiment was terminated at 61 days. All mice of both genetic backgrounds died in 2 days after deposition of 1637 CFU/lung. We performed two additional experiments to more precisely estimate the median lethal dose. All BALB/c mice infected with 1029 CFU/lung died within 3 days and all C57BL/6 mice infected with 375 CFU/lung died within 5 days. The results of all experiments are summarized in Table 1 and indicate that we successfully bracketed the narrow range encompassing the lethal dose. Using the method described by Reed and Muench (Reed & Muench 1938) but excluding the 132 CFU/lung experiment because of the short duration of observation, we estimated that the median lethal deposition dose was 204 CFU/lung for BALB/c mice and 334 CFU/lung for C57BL/6 mice. These results demonstrated that relatively low deposition doses of aerosolized B. pseudomallei 1026b are lethal to both strains and that BALB/c mice are only modestly more susceptible than C57BL/6 mice by this route of infection.
Figure 1.
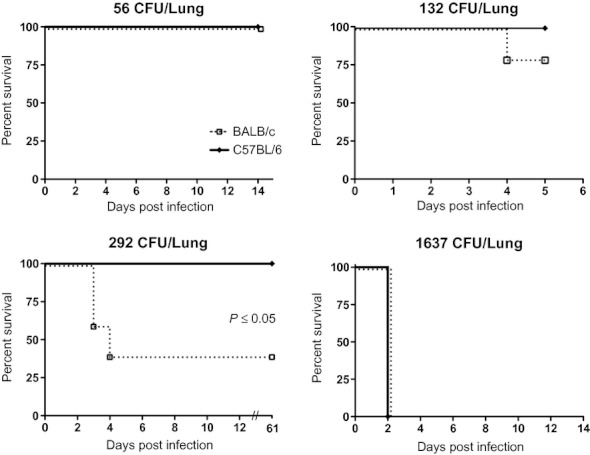
Survival of C57BL/6 and BALB/c mice following inhalation of Burkholderia pseudomallei. Burkholderia pseudomallei in a range of concentrations was deposited by aerosol in the lungs of C57BL/6 and BALB/c mice. The pulmonary deposition dose was quantified by culture of the left lung immediately following infection. Mice were monitored daily to determine survival. Differences between survival curves were calculated by the log-rank test. 56 CFU/lung: N = 4, 4; 132 CFU/lung: N = 5, 5; 292 CFU/lung: N = 5, 5; 1637 CFU/lung: N = 5, 5.
Table 1.
Mortality of mice following inhalation of Burkholderia pseudomallei
| Mouse strain | Deposition dose (CFU/lung) | Number of deaths/total* |
|---|---|---|
| BALB/c | 56 | 0/4 |
| 132 | 1/5 | |
| 292 | 4/5 | |
| 1029 | 4/4 | |
| 1637 | 5/5 | |
| C57BL/6 | 56 | 0/4 |
| 132 | 0/5 | |
| 292 | 0/5 | |
| 375 | 4/4 | |
| 1637 | 5/5 |
Observation periods for experiments with 100% mortality were ≤5 days. Observation periods for all other experiments were: 56 CFU/lung, 14 days; 132 CFU/lung, 5 days; 292 CFU/lung, 61 days.
Bacterial replication and dissemination
We next characterized patterns of bacterial replication and dissemination and host inflammatory responses in C57BL/6 mice infected by aerosol. We analysed these patterns in sublethal experimental infection to provide maximal information about a successful host response and the course of infection over time. We first quantified bacterial burdens in the lung, liver and spleen of C57BL/6 mice at serial time points after aerosol infection to assess the degree of bacterial replication and dissemination. One day following deposition of 56 CFU/lung, we observed clear evidence of replication (an increase in over two logs) in the lung but only scant CFUs in the liver and spleen (Figure 2). No bacteria were detectable in lung, liver or spleen 3 days after infection. An additional cohort of C57BL/6 mice infected with 56 CFU/lung was followed for 63 days and all mice remained clinically well. The monitoring period was ended at this time point, and on necropsy no bacteria were detected in lung, liver or spleen. We repeated this experiment in C57BL/6 mice inoculated with a higher deposition dose (292 CFU/lung), quantifying bacterial counts 1 and 4 days following infection (Figure 2). We again observed over two logs of replication in the lung 1 day following infection, and bacteria were detectable at low counts in the liver and spleen. Bacteria were still detectable but at lower levels in lung and liver (and in spleen in one mouse) on day 4. No bacteria were detected in lung, liver or spleen from an additional cohort of mice that were monitored for 61 days following infection and then euthanized and necropsied. Overall, the pattern of local replication and dissemination to distant organs at day 1 after infection followed by progressive bacterial clearance was comparable in both experiments. As a control experiment, we conducted a sham infection of age- and sex-matched mice and cultured lungs one and 4 days later. No bacteria were detected in sham-infected mice (data not shown). In two additional experiments, we quantified bacterial CFUs in lung and abdominal organs 1 and 2 days after deposition of 210 or 314 CFU/lung. We noted comparable replication and dissemination at day 1. At day 2, the number of CFUs was essentially unchanged in the lung but increased by about one log in the liver and spleen at day 2. Together, these findings indicated that while local bacterial replication and transient bacteraemia seeding distant organs occurred in the first few days after infection, by day 4 and beyond, infection was rapidly controlled by the host. There was no evidence of chronic infection in these experiments.
Figure 2.
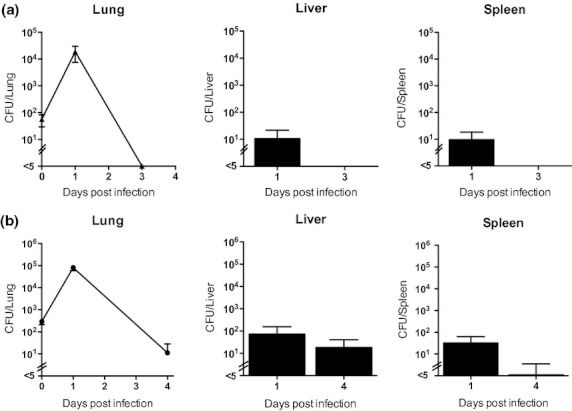
Burkholderia pseudomallei aerosol infection of C57BL/6 mice replicates in the lung and disseminates before infection is controlled by the host. C57BL/6 mice were infected by deposition of 56 (a) or 292 (b) CFU/lung B. pseudomallei. Lung, liver and spleen were quantitatively cultured at several time points following infection. The lower limit of detection was approximately 5 CFU/organ; <5 CFU/organ indicates no bacteria detected. 56 CFU/lung: N = 4 per time point; 292 CFU/lung: N = 5 per time point.
Alveolar space inflammation
To characterize the pulmonary inflammatory response in this model, we next examined cellular inflammation in the alveolar space in C57BL/6 mice following deposition of 292 CFU/lung and compared this to sham-infected mice (Figure 3). One day following infection, the total cell count was significantly increased in the bronchoalveolar lavage fluid of B. pseudomallei-infected mice in comparison with sham-infected mice, owing to a neutrophil predominance (mean total cell count in infected mice 0.40 × 106 cells/ml, 56% neutrophils vs 0.13 × 106 cells/ml, 0% neutrophils in sham-infected mice). Four days after infection, although the total cell count remained elevated, neutrophils in the alveolar space were replaced by mononuclear cells. Two subsequent experiments in which C57BL/6 mice were infected with similar deposition doses of B. pseudomallei and underwent bronchoalveolar lavage 1 and 2 days following infection confirmed a neutrophilic alveolitis at day 1 (mean total cell count 0.54 × 106 cells/ml, 61% neutrophils for mice infected with 314 CFU/lung) that was even more pronounced at day 2 (mean total cell count 1.68 × 106 cells/ml, 95% neutrophils) when pulmonary bacterial counts remained high. These data showed early recruitment of neutrophils to the alveolar compartment following inhalation of B. pseudomallei, followed by persistent inflammation as indicated by a subsequent increase in mononuclear cells.
Figure 3.
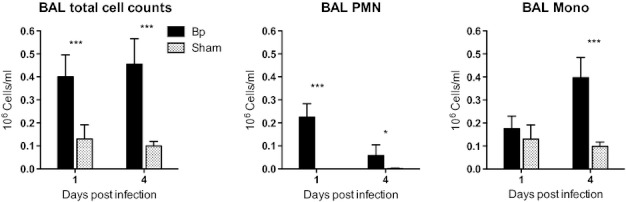
Burkholderia pseudomallei aerosol infection of C57BL/6 mice induces a neutrophil-predominant cellular response in the alveolar compartment. C57BL/6 mice were infected by deposition of 292 CFU/lung B. pseudomallei or were sham infected under similar conditions. Bronchoalveolar lavage was performed one and 4 days following infection. Cell and differential counts (polymorphonuclear vs mononuclear cells) were manually enumerated. N = 5 per time point. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 using the t-test.
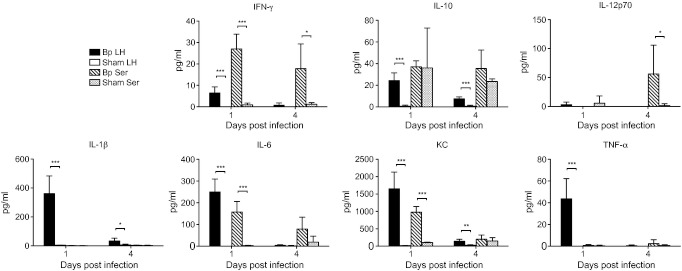
Lung and serum cytokine release
To assess whole lung and systemic inflammation after sublethal respiratory infection with B. pseudomallei, we quantified cytokine and chemokine levels in homogenized lung and in serum from the same mice infected with 292 CFU/lung (Figure 4). We observed an increase in IFN-γ, IL-10, IL-1β, IL-6, KC and TNF-α in the lung 1 day after infection with 292 CFU/lung B. pseudomallei compared to sham-infected mice. This inflammatory response substantially resolved by day 4. In serum, IFN-γ, IL-6 and KC were significantly increased in infected mice at day 1. Relative to lung, levels of serum mediators on day 1 tended to be lower, with the exception of IFN-γ, although absolute concentrations of IFN-γ were low overall. Levels of inflammatory mediators tended to stay elevated in the serum longer than in the lung, in particular for IFN-γ, IL-10, IL-12p70 and IL-6, reflecting a persistent systemic inflammatory response even as lung inflammation resolved.
Figure 4.
Pulmonary and systemic inflammation is induced by Burkholderia pseudomallei aerosol infection of C57BL/6 mice. C57BL/6 mice were infected by deposition of 292 CFU/lung B. pseudomallei or were sham-infected under similar conditions. Lung homogenates and serum cytokine/chemokine concentrations were measured in samples obtained one and 4 days following infection. N = 5 for each tissue sample per time point, except N = 3 for Bp serum at 4 days. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001 using the t-test.
Lung histology
We further characterized pulmonary inflammation following aerosol infection of C57BL/6 mice by performing histological examination of lungs 1 and 4 days after deposition of 292 CFU/lung B. pseudomallei (Figure 5). We noted widely scattered but defined and relatively compact foci of neutrophil-dense cellular infiltrates 1 day following infection. The orientation of these lesions was alveolar and perivascular rather than peribronchiolar. The lung parenchyma was otherwise completely preserved. Four days after infection, these foci had become substantially less dense and more dispersed and the underlying architecture of the affected alveolar spaces was again identifiable. At this stage, lesions were composed of cellular debris admixed with neutrophils and mononuclear cells. Additionally, smaller vessels within the vicinity of parenchymal lesions had mild perivascular accumulations of mostly mononuclear cells. There was no evidence of exudative bronchitis or bronchial necrosis. Comparable histological findings were observed in three confirmatory experiments in which mice were necropsied at 1 or 2 days following infection with similar or slightly lower deposition doses of B. pseudomallei.
Figure 5.
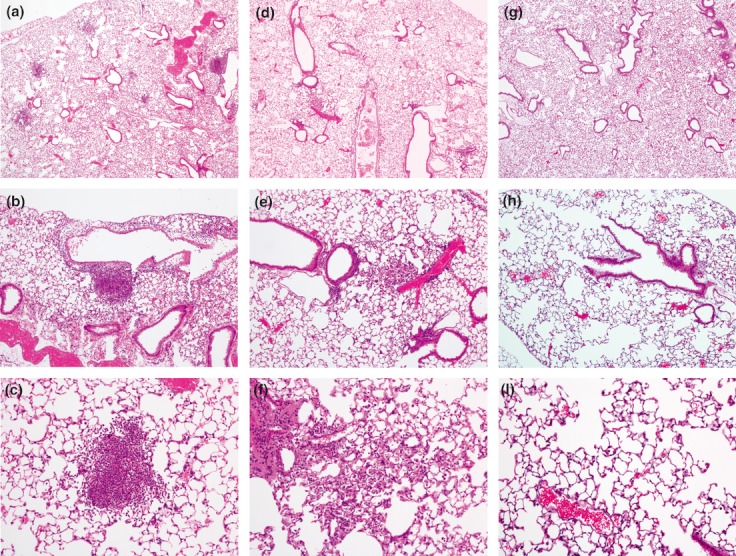
Burkholderia pseudomallei aerosol infection of C57BL/6 mice induces scattered, dense cellular infiltrates in the lung. C57BL/6 mice were infected by deposition of 292 CFU/lung B. pseudomallei or were sham-infected under similar conditions. Lungs harvested one (a,b,c) and four (d,e,f) days after infection or 1 day after sham infection (g,h,i) were fixed in 4% paraformaldehyde before sectioning and staining with haematoxylin and eosin.
Discussion
The purpose of this report is to describe our investigations comparing outcomes of BALB/c and C57BL/6 mice infected with aerosolized B. pseudomallei 1026b and to characterize more completely the course of infection and the pulmonary inflammatory response in C57BL/6 mice with pneumonic melioidosis. The main findings of our study are that (i) BALB/c mice are only modestly more susceptible than C57BL/6 mice in this reproducible airborne model of B. pseudomallei infection, (ii) C57BL/6 mice infected by aerosol with sublethal doses tolerate initial replication and dissemination beyond the pulmonary compartment before controlling and apparently eradicating infection and (iii) sublethal infection induces an inflammatory response in the lung characterized initially by neutrophil recruitment to the alveolar space, local and systemic cytokine release and histologically by scattered dense foci of pulmonary inflammation with otherwise normal lung parenchyma.
We have previously refined aerosol models of infection with a variety of pathogens, including B. thailandensis (Hawn et al. 2007; West et al. 2008a,b; Morris et al. 2009). In this work, we used similar methodology to develop a model of pneumonic murine melioidosis. Intranasal inoculation is a commonly used to generate respiratory infection in mice. While offering advantages in terms of practicality in many ways, this method does not mimic the clinical scenario of inhalation of airborne bacteria. Furthermore, intranasal B. pseudomallei infection may lead to neurological involvement via the olfactory nerve (Owen et al. 2009). Using strains of B. pseudomallei other than 1026b, it has been clearly shown that BALB/c mice are significantly more susceptible than C57BL/6 mice to B. pseudomallei infection by a variety of routes – intranasal, intravenous, intraperitoneal and subcutaneous (Leakey et al. 1998; Hoppe et al. 1999; Liu et al. 2002; Barnes & Ketheesan 2005). This has been attributed to greater – and deleterious – inflammatory responses in BALB/c mice compared to C57BL/6 mice (Ulett et al. 2000a,b;). However, Jeddeloh et al. (2003) did not demonstrate a significant difference in susceptibility when infecting mice with B. pseudomallei 1026b by the airborne route. While our median lethal dose determination must be interpreted in the context of some variation in duration of monitoring, group sizes and doses deposited, our results tend to confirm their findings of at most a modest difference in susceptibility and contrast with the results by Tan et al. (2008). Tan et al. aerosolized B. pseudomallei KHW to both strains of mice using a nose-only system and found a 200-fold increase in susceptibility for BALB/c mice compared with the C57BL/6 strain. These contrasting susceptibility observations may be attributable to differences in bacterial preparation and aerosolization method. Others have clearly demonstrated the dramatically varied virulence of different strains of B. pseudomallei to mice (Barnes & Ketheesan 2005); therefore, another likely explanation for this discrepancy between our findings and those of Tan et al. (2008) is the different bacterial strain used for the studies.
Our findings also suggest that C57BL/6 mice infected with sublethal doses of B. pseudomallei by the airborne route permit bacterial replication in the lungs and dissemination to distant organs before the infection is controlled. The time course of this infection is that bacteria continue to proliferate for at least 48 h following infection by the airborne route, but become contained by the host by 96 h. We did not observe any evidence of recrudescent infection in C57BL/6 mice either clinically or based on quantitative culture of selected organs at necropsy over 2 months following infection. These observations would indicate that C57BL/6 mice infected by the airborne route do not develop chronic infection within this time period and may completely clear bacteria. These findings contrast with those of Conejero et al. (2011) who developed a model of chronic infection using intranasally administered 100 CFU B. pseudomallei strain 576 to C57BL/6 mice and detected bacteria in lungs, blood or spleen of the majority of mice from 22 to 50 days postinfection. While potentially explicable by the different strains used, these discrepant observations may point to key differences in intranasal vs aerosol routes of infection.
Our experiments demonstrate a robust pulmonary inflammatory response in C57BL/6 mice infected with sublethal doses of B. pseudomallei. A neutrophilic alveolitis is identifiable at day 1 and is markedly increased by day 2, before bacterial burdens in the lung and distant organs have been contained. Pulmonary (and systemic) cytokine and chemokine levels are also elevated within 24 h following infection. The histological picture was characterized by scattered foci of alveolar and perivascular inflammation but preservation of most of the pulmonary architecture. Lever et al. (2009) also observed foci of alveolitis in their histological description of a high dose (20× median lethal dose) BALB/c model of inhaled B. pseudomallei. In contrast, however, we did not observe the same degree of lung inflammation or any exudative bronchitis. Our histological findings are very similar to what we have previously observed in a murine model of high dose, lethal, B. thailandensis aerosol infection (West et al. 2008a,b). In the absence of high-containment facilities, we have previously hypothesized that less virulent B. thailandensis serves as a useful surrogate for B. pseudomallei infection. The similarities between our prior work and the current model support this notion. Notably, the majority of our descriptions of host inflammation in the present report are based on analysis of data from mice infected with a deposition dose of B. pseudomallei that is <50 CFU below the median lethal dose. The median lethal dose is conceivably the threshold above which C57BL/6 mice infected by the airborne route fail to contain the disseminating bacteria. Based on the histological picture and as we have proposed in experimental B. thailandensis infection, we speculate that unchecked bacterial replication and resultant systemic inflammation rather than fulminant respiratory failure seem likely to be the proximate causes of death in this model.
In summary, this report expands our understanding of experimental melioidosis induced by inhalation of aerosolized bacteria, confirming the reproducibility and utility of this approach for melioidosis investigators. Furthermore, it fills a gap in the melioidosis literature by characterizing more completely the pulmonary inflammatory response to airborne B. pseudomallei infection in widely used C57BL/6 mice. The model is suitable for the study of bacterial and host factors in experimental melioidosis.
Acknowledgments
Tony Han and Loren Kinman provided technical assistance. This work was supported by grants from the National Institutes of Health, USA (HL094759 to TEW and AI057141 to SJS and TEW) and by a Parker B. Francis Fellowship in Pulmonary Research (to TEW).
Conflict of Interest
The authors declare no conflict of interest.
References
- Barnes JL, Ketheesan N. Route of infection in melioidosis. Emerg. Infect. Dis. 2005;11:638–639. doi: 10.3201/eid1104.041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AC, Jacups SP, Ward L, Currie BJ. Melioidosis and Aboriginal seasons in northern Australia. Trans. R. Soc. Trop. Med. Hyg. 2008;102(Suppl 1):S26–S29. doi: 10.1016/S0035-9203(08)70008-7. [DOI] [PubMed] [Google Scholar]
- Conejero L, Patel N, de Reynal M, et al. Low-dose exposure of C57BL/6 mice to Burkholderia pseudomallei mimics chronic human melioidosis. Am. J. Pathol. 2011;179:270–280. doi: 10.1016/j.ajpath.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier YP, Hagen RM, Brochier GS, et al. Study on the pathophysiology of experimental Burkholderia pseudomallei infection in mice. FEMS Immunol. Med. Microbiol. 2001;30:53–63. doi: 10.1111/j.1574-695X.2001.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Hawn TR, Berrington WR, Smith IA, et al. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J. Immunol. 2007;179:6981–6987. doi: 10.4049/jimmunol.179.10.6981. [DOI] [PubMed] [Google Scholar]
- Hoppe I, Brenneke B, Rohde M, et al. Characterization of a murine model of melioidosis: comparison of different strains of mice. Infect. Immun. 1999;67:2891–2900. doi: 10.1128/iai.67.6.2891-2900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeddeloh JA, Fritz DL, Waag DM, Hartings JM, Andrews GP. Biodefense-driven murine model of pneumonic melioidosis. Infect. Immun. 2003;71:584–587. doi: 10.1128/IAI.71.1.584-587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey AK, Ulett GC, Hirst RG. BALB/c and C57Bl/6 mice infected with virulent Burkholderia pseudomallei provide contrasting animal models for the acute and chronic forms of human melioidosis. Microb. Pathog. 1998;24:269–275. doi: 10.1006/mpat.1997.0179. [DOI] [PubMed] [Google Scholar]
- Lever MS, Nelson M, Stagg AJ, Beedham RJ, Simpson AJ. Experimental acute respiratory Burkholderia pseudomallei infection in BALB/c mice. Int. J. Exp. Pathol. 2009;90:16–25. doi: 10.1111/j.1365-2613.2008.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limmathurotsakul D, Peacock SJ. Melioidosis: a clinical overview. Br. Med. Bull. 2011;99:125–139. doi: 10.1093/bmb/ldr007. [DOI] [PubMed] [Google Scholar]
- Limmathurotsakul D, Wuthiekanun V, Chierakul W, et al. Role and significance of quantitative urine cultures in diagnosis of melioidosis. J. Clin. Microbiol. 2005;43:2274–2276. doi: 10.1128/JCM.43.5.2274-2276.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Koo GC, Yap EH, Chua KL, Gan YH. Model of differential susceptibility to mucosal Burkholderia pseudomallei infection. Infect. Immun. 2002;70:504–511. doi: 10.1128/IAI.70.2.504-511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AE, Liggitt HD, Hawn TR, Skerrett SJ. Role of Toll-like receptor 5 in the innate immune response to acute P. aeruginosa pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L1112–L1119. doi: 10.1152/ajplung.00155.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SJ, Batzloff M, Chehrehasa F, et al. Nasal-associated lymphoid tissue and olfactory epithelium as portals of entry for Burkholderia pseudomallei in murine melioidosis. J. Infect. Dis. 2009;199:1761–1770. doi: 10.1086/599210. [DOI] [PubMed] [Google Scholar]
- Reed L, Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Santanirand P, Harley VS, Dance DA, Drasar BS, Bancroft GJ. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei. Infect. Immun. 1999;67:3593–3600. doi: 10.1128/iai.67.7.3593-3600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GY, Liu Y, Sivalingam SP, et al. Burkholderia pseudomallei aerosol infection results in differential inflammatory responses in BALB/c and C57Bl/6 mice. J. Med. Microbiol. 2008;57:508–515. doi: 10.1099/jmm.0.47596-0. [DOI] [PubMed] [Google Scholar]
- Ulett GC, Ketheesan N, Hirst RG. Cytokine gene expression in innately susceptible BALB/c mice and relatively resistant C57BL/6 mice during infection with virulent Burkholderia pseudomallei. Infect. Immun. 2000a;68:2034–2042. doi: 10.1128/iai.68.4.2034-2042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulett GC, Ketheesan N, Hirst RG. Proinflammatory cytokine mRNA responses in experimental Burkholderia pseudomallei infection in mice. Acta Trop. 2000b;74:229–234. doi: 10.1016/s0001-706x(99)00075-3. [DOI] [PubMed] [Google Scholar]
- Veljanov D, Vesselinova A, Nikolova S, Najdenski H, Kussovski V, Markova N. Experimental melioidosis in inbred mouse strains. Zentralbl. Bakteriol. 1996;283:351–359. doi: 10.1016/s0934-8840(96)80071-5. [DOI] [PubMed] [Google Scholar]
- West TE, Frevert CW, Liggitt HD, Skerrett SJ. Inhalation of Burkholderia thailandensis results in lethal necrotizing pneumonia in mice: a surrogate model for pneumonic melioidosis. Trans. R. Soc. Trop. Med. Hyg. 2008a;102(Suppl 1):S119–S126. doi: 10.1016/S0035-9203(08)70028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West TE, Pelletier MR, Majure MC, Lembo A, Hajjar AM, Skerrett SJ. Inhalation of Francisella novicida Delta mglA causes replicative infection that elicits innate and adaptive responses but is not protective against invasive pneumonic tularemia. Microbes Infect. 2008b;10:773–780. doi: 10.1016/j.micinf.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West TE, Myers ND, Limmathurotsakul D, et al. Pathogenicity of high-dose enteral inoculation of Burkholderia pseudomallei to mice. Am. J. Trop. Med. Hyg. 2010;83:1066–1069. doi: 10.4269/ajtmh.2010.10-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ. Melioidosis. Lancet. 2003;361:1715–1722. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]