Abstract
Coffee intake has been associated with a low risk of developing cancer, including prostate cancer, which is one of the most commonly diagnosed cancer in men. However, few studies have evaluated the chronic effects of caffeine, which is the most abundant methylxanthine in coffee, on prostate morphology and physiology. In the present study, we investigated the effects of chronic, low-dose caffeine intake on rat prostate morphology from puberty to adulthood. Five-week-old male Wistar rats were randomized into two experimental groups: caffeine-treated (20 ppm in drinking water, n = 12) and control (n = 12). The ventral and dorsolateral prostates were dissected, weighted and submitted to morphological, morphometrical and immunohistochemical analysis of cellular proliferation, apoptosis and androgen receptor (AR) tissue expression. The testosterone (T) and dihydrotestosterone (DHT) concentrations were measured in the plasma. Our results show that caffeine intake increased the concentrations of T and DHT, organ weight, epithelial cell proliferation and AR tissue expression in the ventral prostatic lobe. All the ventral prostates from the caffeine-treated animals presented various degrees of epithelial and stromal hyperplasia. Our results suggest that chronic caffeine intake from puberty increases androgenic signalling and cell proliferation in the rat prostate gland and can be related to the development of benign prostatic hyperplasia.
Keywords: androgen receptor, apoptosis, caffeine, cell proliferation, hyperplasia, prostate
Caffeine (1,3,7-trimethylxanthine) is a key component in many popular beverages including coffee, cocoa and tea. In addition, caffeine can be artificially synthesized and is included in foods and drinks such as energy drinks. Caffeine is probably the most frequently ingested neuroactive drug in the world (IARC 1991; Bode & Dong 2007). Studies have shown that caffeine induces a wide spectrum of cellular and pharmacologic responses in the central nervous system including motor activity stimulation (Fredholm et al. 1999) and antioxidant activity (Inkielewicz-Stepniak & Czarnowski 2010). Caffeine intake has been also associated with a reduced risk of developing type 2 diabetes (Huxley et al. 2009). Furthermore, current evidence suggests that coffee consumption is associated with reduced risks of liver, kidney and to a lesser extent, premenopausal breast and colorectal cancers, while it is unrelated to prostate, pancreas and ovarian cancers (Nkondjock 2009). Others studies have reported the role of coffee consumption in cancer prevention, including prostate cancer (Bettuzzi et al. 2006; Lu et al. 2006; Butt & Sultan 2011). However, coffee contains several bioactive molecules that exhibit distinct properties, such as chlorogenic acid (3-3,4-dihydroxycinnamoyl quinic acid), caffeic acid (3,4-dihydroconnamic acid), diterpenes (kahweol and cafestol) and hydroxyhydroquinone (1,2,4-trihydroxybenzene), which compromises the precise comprehension of its effects in the organism (Cavin et al. 2002; Butt & Sultan 2011).
It is well known that caffeine might act as an endocrine disruptor in the reproductive system owing to its effects on sex hormone levels, both in males and females (Pollard 1988; Hirose et al. 2007; Celec & Behuliak 2010). Experimental studies have shown that caffeine increases plasma testosterone levels via sympathetic stimulation of Leydig cells in the testis, but causes testicular atrophy and impaired spermatogenesis (Weinberger et al. 1978; Ezzat & Gohary 1994). Because caffeine intake disrupts circulating androgens, it would be expected to affect androgen-dependent sex accessory glands. Previous studies have associated chronic caffeine intake with the increased risk for clinical benign prostatic hyperplasia (BPH) (Signorello et al. 1999; Gass 2002; Yun & Doux 2006; Wilson et al. 2011). However, no previous experimental studies have evaluated the effects of long-term caffeine intake on the morphology of the prostate gland. Thus, this study was conducted to determine the effects of low-dose, long-term caffeine intake on the morphology, epithelial cell proliferation, apoptosis, androgen receptor (AR) protein expression and the incidence of prostatic diseases on the rat ventral and dorsal prostatic lobes (DP).
Materials and methods
Animals and treatment
Five-week-old male Wistar rats (n = 24) obtained from the central stock breeder of São Paulo State University – UNESP, Botucatu, SP/Brazil, were used in this study. The animals were maintained under controlled temperature and lighting conditions and received food and water ad libitum. The animals were randomized into two experimental groups (n = 12): a control group (CT) and a caffeine-treated group (CF) – three animals by cage. The treated group received caffeine (20 mg/l caffeine; Sigma™, Saint Louis, MO, USA) in the drinking water for 140 days, and the CT group received tap water only. The caffeine dose was chosen to simulate moderate human caffeine consumption (approximately 2–4 mg/kg/day) (Heckman et al. 2010). During the experiment, water and food intake and body weight were measured in the last day of each week.
After the treatment period, the animals were euthanized by CO2 inhalation followed by decapitation. The animals' weights were determined previous to euthanasia, and blood samples were collected from each animal. The ventral (VP) and dorsolateral (DLP) prostatic lobe pairs were excised and weighted. The right lobes were immersed in a fixative solution, and the left lobes were frozen for Western blotting analysis.
Ethical Approval
The experimental protocol followed the Ethical Principles in Animal Experimentation adopted by the Brazilian College of Animal Experimentation (COBEA) and has been approved by the Commission of Ethics in Animal Experimentation (CEEA) at the Institute of Biosciences at Botucatu (protocol no. 139/09).
Hormonal assay
The plasma was separated from the blood and stored at −4 °C. Plasma testosterone (T) and dihydrotestosterone (DHT) concentration were determined by chemiluminescence (VITROS ECi-Johnson™; Johnson Ultra-Sensitive Chemiluminescence™ analysis New Brunswick, NJ, USA.) in a renowned clinical analysis laboratory using specific reagents (Johnson™ and Johnson Orthoclinical™, New Brunswick).
Histopathological analysis
The VP and DLP were fixed by immersion in 4% paraformaldehyde/phosphate-buffered saline (PBS) solution for 24 h, washed in PBS for 24 h, dehydrated in a graded ethanol series and embedded in glycol methacrylate resin (Historesin® embedding kit; Leica™, Heidelberg, Germany) or cleared in xylene and embedded in Paraplast (Sigma-CO™, Saint Louis, MO, USA). Resin sections (3 μm) were stained with Haematoxylin–Eosin (HE) for morphological and morphometric analysis. Paraplast sections (5 μm) were stained with Picrosirius (Junqueira et al. 1979) for collagen (type I and type III collagen fibers) detection followed by stereological analysis or used to proceed to immunohistochemistry protocol. The histopathological classification of prostate lesions presented in the experimental animals was accomplished according to the Bar Harbor Classification System for the mouse prostate, developed by National Cancer Institute's Mouse Models of the Human Cancers Consortium, Prostate Steering Committee (Shappell et al. 2004).
Morphometric and stereological analysis
Morphometric analysis was performed using a Leica™ DMLB 80 microscope connected to a Leica™ DC300FX camera and Leica™ q-win software Version 3 for Windows™.
Epithelial height measurements were evaluated on 10 ventral and 10 dorsal lobes from both experimental groups. A total of 10 random microscopic fields (×40 magnification) of prostatic lobe sections from 10 different animals were acquired with 10 random interactive measurements per field (1000 measurements per experimental group).
To determine absolute collagen fibre volume, another 10 random microscopic fields (×20 magnification) of prostatic lobe sections from 10 different animals were acquired. The Sirius red–stained area was determined by automatic image detection of red colours, and the collagen volume fraction was calculated as a percentage of red-stained areas per total prostatic area. It has previously been determined that 1 mg of fresh rat ventral tissue has a volume of approximately 1 mm3 (DeKlerk & Coffey 1978). Therefore, the volume (or absolute volume) of collagen fibers was calculated by multiplying the collagen fiber volume fraction by the mean prostatic weight.
All the measurements were performed in the intermediate and distal regions of the prostate lobe ducts, which represent the major portions of the prostatic lobes (Nemeth & Lee 1996). The morphological criteria described in Shappell et al. (2004) were utilized for discerning lesions in the ventral and dorsal lobes.
Immunohistochemistry
Paraplast-embedded prostatic lobe samples were sectioned on to silanized glass slides. Antigen retrieval was performed by boiling the fixed samples in citrate buffer pH 6.0 in a pressure cooker for 35 min. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide in methanol for 10 min. The prostate sections were blocked with 3% bovine serum albumin (BSA) in PBS for 1 h at room temperature. These sections were then incubated overnight at 4 °C in a humidified chamber with mouse monoclonal anti-Ki-67 (Novocastra Laboratories™, Newcastle upon Tyne, UK) or polyclonal rabbit anti-AR (Santa Cruz Biotechnology™, CA, USA) diluted 1:100 in a 1% BSA/PBS solution. Excess primary antibody was removed with PBS washing, and the slides were incubated with a biotinylated goat antimouse IgG or anti-rabbit IgG antibody (Santa Cruz Biotechnology) diluted 1:100 in 1% BSA/PBS for 1 h at room temperature. After subsequent PBS washing, sections were incubated with an avidin–biotin–peroxidase solution (dilution 1:50) for 45 min (Elite ABC kit; Vector Laboratory, Burlingame, CA, USA). Chromogen colour was developed by incubation with 3.30-diaminobenzidine tetrahydrochloride (Sigma, MO, USA). Slides were then counterstained with haematoxylin.
Ki-67 detection and proliferation index
The proliferation index was determined by counting the number of Ki-67 positive epithelial cells in 10 microscopic fields (×40 magnification) visualized from 10 ventral and 10 dorsal lobe sections from both caffeine-treated and control animals. The results are expressed as a percentage of Ki-67 positive cells (number of labelled nuclei × 100/total cell number). Approximately 10,000 cells were counted per experimental group. Image acquisition and quantitative measurements were performed by an investigator who had no prior knowledge of animal identity or experimental group.
AR semi-quantitative analysis
Ten microscopic fields (×40 magnification) from histological sections of six different animals per group were analyzed and 10 AR positive nuclei were randomly selected by field (600 nuclei/group). A regular and constant square from the central part of each nucleus was selected and submitted to integrated optical densitometry analysis (IOD) using the image j software downloaded from the NIH website (http://rsb.info.nih.gov.ij/). The IOD methodological proceedings were based on Oliva et al. (2012) study and on physical principles of quantification performed Zhu et al. (2000).
In situ cell apoptosis detection by TUNEL assay
The apoptosis detection was based on a reaction of in situ Terminal Deoxynucleotidyl Transferase-Mediated Biotinylated UTP Nick End-Labelling (TUNEL). This was essentially performed using the FragELTM DNA (Calbiochem, La Jolla, CA, USA) according to the manufacturer's directions. Briefly, samples of the Paraplast-embedded tissue were collected in silanized glass slides. The slides were treated with Proteinase K (2 mg/ml) diluted at 1:100 in 10 mM Tris-HCl pH 8.0. After that the slides were washed in TBS, and endogenous peroxidase was blocked for 5 min in 3% H2O2 diluted in methanol. After washing in TBS, sections were treated with 1× TdT Equilibration Buffer at room temperature for 30 min and incubated with TdT Labelling Reaction Mix (57 µl Equilibration Buffer plus 3 µl of TdT Enzyme) for 1:30 h at 37 °C. The slides were rinsed in TBS and incubated with Blocking Buffer for 10 min at room temperature. Next, the sections were incubated with 50× Conjugated (diluted 1:50 in Blocking Buffer) for 30 min at room temperature. After three washings in TBS, the detection of TUNEL-positive cells was performed with DAB for 5 min. The specimens were counterstained with Haematoxylin and mounted in Permount. The epithelial cells were counted in 10 random microscopic fields (×40 magnification) from 10 different ventral and dorsal prostatic sections from both caffeine-treated and control animals. Approximately 10,000 cells were counted per experimental group. The TUNEL index was expressed as a percentage of total cells counted (number of TUNEL-positive cells × 100/total cell number). Image acquisition and quantitative measurements were performed by an investigator blind to both the animal identity and experimental group.
Western blotting analysis of PCNA and PAR-4
Frozen samples of ventral and dorsal adult rat prostatic lobes from control and caffeine groups were mechanically homogenized in 50 mM Tris-HCl buffer pH 7.5 plus 0.25% Triton-X 100 by Polytron for 30 s at 4 °C, centrifuged, and protein was extracted on supernatant and quantified as per Bradford methods (Bradford 1976). A protein sample (70 μg) was loaded into 10% SDS-PAGE under reducing conditions. After electrophoresis, the proteins were transblotted on to a nitrocellulose membrane (Sigma-CO™). The blot was blocked with 5% BSA in TBST (10 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.1% Tween-20) for 1 h. The blot was then incubated overnight at 4 °C with 3% BSA in TBST containing a 1:1000 dilution of monoclonal mouse anti-proliferation cell nuclear antigen (PCNA; Santa Cruz Biotechnology™) or polyclonal goat anti-PAR-4 (Prostate apoptosis response 4; Santa Cruz Biotechnology™) or polyclonal goat anti-beta-actin (Santa Cruz Biotechnology™) primary antibodies. The blot membranes were then washed five times for 20 minutes in TBST and incubated for 1 h at room temperature with a secondary peroxidase-conjugated antibody. After washing in TBS-T, antibody location was detected using chemiluminescence substrate kit (Pierce, ECL Western blotting substrate; Thermo Fischer Scientific TM, Rockford, IL, USA) according to the manufacturer's instructions. The substrates were removed from membranes, and ECL signals were captured by CCD camera (ImageQuant LAS 4000 mini®; GE Healthcare™ Waukesha, WI, USA). The IOD of the target proteins bands was measured using the image j software downloaded from the NIH website (http://rsb.info.nih.gov.ij/) to compare the protein levels. The PCNA and PAR-4 expression was normalized to the β-actin values, and the results were expressed as means ± standard deviation (SD).
Statistical analysis
Data are expressed as mean ± SD, and statistical significance (P < 0.05) was determined by Student's t-test. Statistical analyses were performed using the instat® software (version 3.0; GraphPad™, Inc., San Diego, CA, USA).
Results
Animals and prostatic lobes biometry
During the 140 days of experiment, no significant differences in water or food intake or body weight between control and caffeine-treated animals were observed (Table 1). However, the plasma T and DHT concentrations and the VP absolute and relative weights were significantly higher in the animals exposed to caffeine compared with the control animals (P < 0.01) (Table 1). Considering the mean daily intake of water by the caffeine-treated animals during the experiment, the mean estimated value of daily caffeine consumption was about 2.5 mg/kg of body weight (Table 1).
Table 1.
Caffeine effects on biometric parameters of the animals and prostatic lobes
| Experimental groups | ||
|---|---|---|
| Parameters | CT | CF |
| Body weight in the 1st week (g) | 128.31 ± 9.8 | 130.58 ± 10.2 |
| Body weight in the 10th week (g) | 356.75 ± 11.2 | 373.92 ± 17.8 |
| Body weight in the 20th week (g) | 487.95 ± 36.65 | 518.06 ± 49.99 |
| Testosterone (ng/ml) – 20th week | 1.34 ± 0.72 | 2.26 ± 0.44* |
| Dihydrotestosterone (ng/ml) – 20th week | 0.21 ± 0.03 | 0.33 ± 0.08* |
| Water intake in the 1st week (ml/day) | 28.9 ± 2.3 | 28.9 ± 2.8 |
| Water intake in the 10th week (ml/day) | 36.9 ± 4.3 | 38.7 ± 4.8 |
| Water intake in the 20th week (ml/day) | 39.38 ± 6.78 | 42.15 ± 6.57 |
| VP | ||
| Absolute weight (g) | 0.57 ± 0.09 | 0.71 ± 0.09* |
| Relative weight | 0.11 ± 0.01 | 0.14 ± 0.02* |
| Epithelium height (μm) | 26.06 ± 2.85 | 24.39 ± 3.95* |
| Focal epithelial hyperplasia | 0/12 | 5/12 |
| Diffuse epithelial and stromal hyperplasia | 0/12 | 3/12 |
| Collagen fibers volume (mm3) | 5.37 ± 1.85 | 6.06 ± 2.26 |
| DLP | ||
| Absolute weight (g) | 0.46 ± 0.10 | 0.50 ± 0.09 |
| Relative weight | 0.10 ± 0.02 | 0.10 ± 0.02 |
| DP | ||
| Epithelium height (μm) | 14.10 ± 2.73 | 12.67 ± 2.76 |
| Collagen fibers volume (mm3) | 4.94 ± 2.5 | 4.66 ± 0.77 |
CT, control group; CF, caffeine-treated group; VP, ventra prostatic lobe; DLP, dorsolateral prostatic lobe; DP, dorsal prostatic lobe.
Statistical analyses based on Student's t-test. Values represent mean ± SD.
P < 0.05 when compared with the CT group.
Histopathological and stereological analysis
The VPs from the CT animals presented regular glandular structures lined with tall, columnar and polarized epithelial cells surrounded by thin stroma (Figure 1a,b). In contrast, significant morphological changes were observed in the VPs from the CF animals (Figure 1–c–f) and about 67% of the animals presented glandular dysplasia classified as BPH (Table 1). Five VPs from the caffeine-treated animals had several acini with enlarged luminal spaces lined by simple squamous epithelial cells and reduced number of epithelial infoldings, resembling cystic hyperplasia (Figure 1c). Furthermore, these five VPs from the caffeine-treated animals showed glandular dysplasia classified as focal tufting and papilliferous epithelial hyperplasia dispersed throughout the distal regions of the acini, without stromal hyperplasia (Figure 1d). The other three VPs from the CF animals had large areas where there was combined and diffuse epithelial and stromal hyperplasia (Figure 1e,f). In these three VPs, the hyperplasic epithelium had a micropapillary pattern, and the cell nuclei exhibited prominent nucleoli, without signs of cellular atypia (Figure 1f). The DP did not exhibit significant morphological differences between the groups, and no dysplastic lesions were observed in this lobe from the caffeine-treated animals (Figure 1g,h). The morphometric analysis showed a slight reduction in epithelial cell height of the prostatic lobes from CF group, which was statistically significant only for the VP (Table 1).
Figure 1.
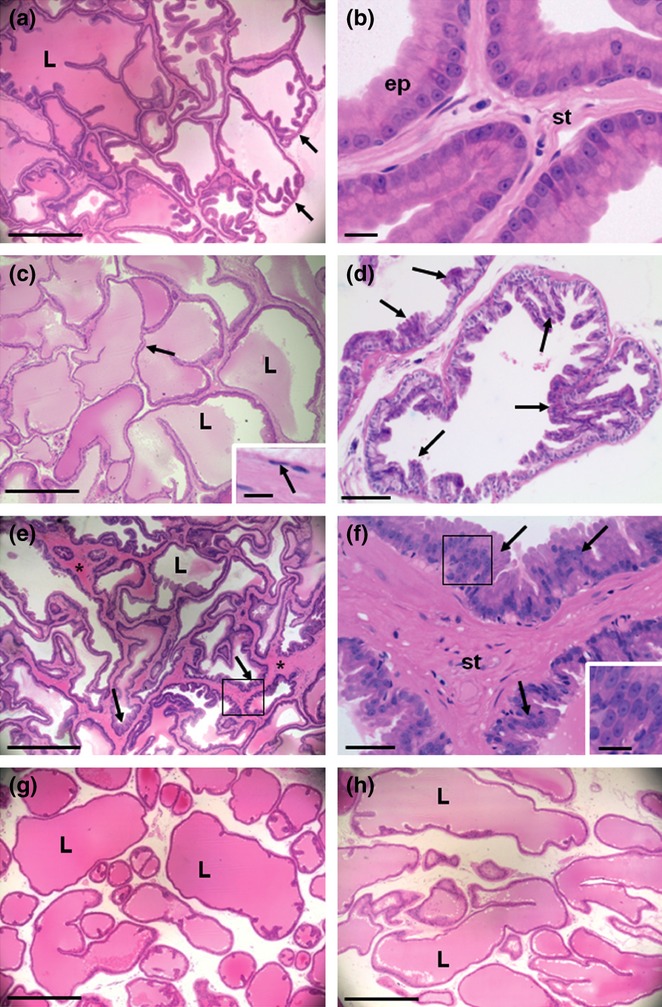
Morphological aspects of control (a, b, g) and caffeine-treated (c–f, h) ventral (VP) (a–f) and dorsal (DP) (g, h) prostatic lobes stained by haematoxylin–eosin. (a) VP from the control group showing the acini structures with large lumen (L) lined by regular tall columnar epithelium. The folds of the epithelium were concentrated in the distal edge of the acini (arrows). (b) Detail of the control VP showing regular and well-polarized epithelial cells (ep) and the thin stroma (st). (c) VP from caffeine-treated animals exhibiting enlarged luminal space (L) and reduced epithelium height and infoldings (arrows). Insert: detail from figure c showing simple squamous epithelium lining the acini (arrow). (d) VP from caffeine-treated animals presenting focal tufting and papilliferous epithelial hyperplasia (arrows), without stromal hyperplasia, at the distal region of the acini. (e) VP from caffeine-treated animals presenting more extensive and uniform process of combined and diffuse epithelial (arrows) and stromal (asterisks) hyperplasia. (f) Detail of figure e (square) showing stromal (st) enlargement and epithelial hyperplasia with micropapillary pattern (arrows). Insert: detail of figure f (square) showing micropapillary structures and nuclei with prominent nucleoli, without cellular atypia. (g) DP from control and (h) DP from caffeine-treated group. No significant morphological differences were observed in the DP between the groups. Scale bars: a, c, e, g and h = 500 μm; b and inserts = 10 μm; d = 100 μm; f = 50 μm.
Collagen fibers stained using picrosirius were located below the epithelium, around smooth muscle cells and in the interstitial space between the acini of the VP and DP from both experimental groups (Figure 2). The VP from CFs presented areas with similar distribution of the collagen fibers than CT group (Figure 2b), while other areas presented distended fibers associated with enlarged acini (Figure 2c) and increased amount of collagen fibers in the areas of stromal hyperplasia (Figure 2d). In the areas of the VP from the CF group where there was stromal hyperplasia the smooth muscle cell layer also appeared thickened (Figure 2d). Although three VP from CF showed diffuse stromal hyperplasia, quantitative analysis showed no significant differences in the collagen volume fraction in the VP and DP between CF and CT groups (Table 1).
Figure 2.
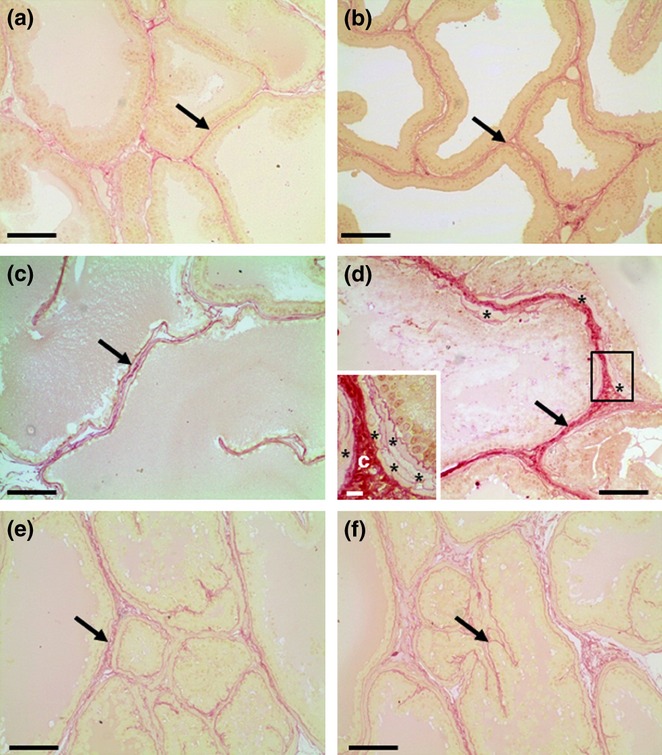
Picrosirius-stained sections of control (a, e) and caffeine-treated (b, c, d) prostatic lobes. Ventral (a–d) and dorsal (e–f) prostates. Collagen fibers (arrows) are observed around acini, mainly in the interstitial space. (b) Caffeine-treated prostate presenting area with similar morphology and collagen distribution (arrows) than control group. (c) Caffeine-treated prostate showing enlarged acini and regular collagen fibers (arrow) distribution in the reduced stroma. (d) Hyperplasic area of caffeine-treated prostate showing increased amount of collagen fibers (arrow) in the interstitial space and thickened layer of smooth muscle cells (asterisks). Insert: Detail of the figure d (square) showing thickened collagen fibers (c) and smooth muscle cells layer (asterisks). Scale bars: a–f = 50 μm; Insert = 10 μm.
Epithelial cell proliferation and apoptosis
To evaluate whether caffeine exposure interferes with epithelial cell proliferation, we performed immunohistochemistry to detect Ki-67 nuclear expression in the VP and DP sections (Figure 3). The number of positive nuclei was higher in the VPs from CF compared with the CT (Figure 3a,b), which was confirmed as statistically significant by quantitative analysis (Figure 5a).
Figure 3.
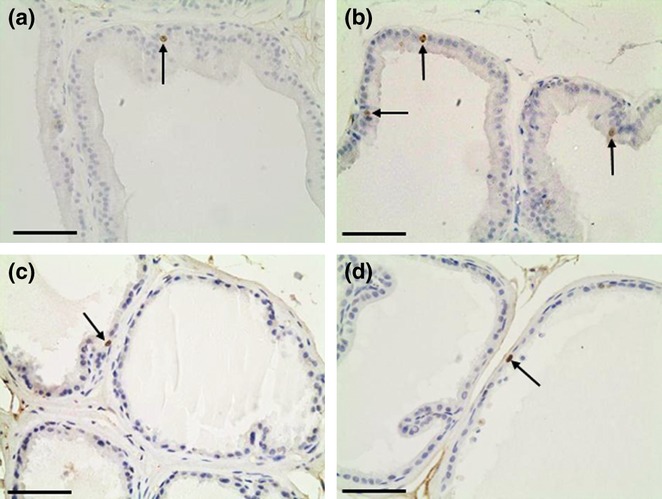
Representative sections of control (a, c) and caffeine-treated (b, d) prostatic lobes submitted to immunohistochemistry for Ki-67. Ventral (a, b) and dorsal (c, d) prostates. Note an increased number of Ki-67 positive nuclei (arrows) in caffeine-treated ventral prostates when compared with control. Scale bars: 30 μm.
Figure 5.
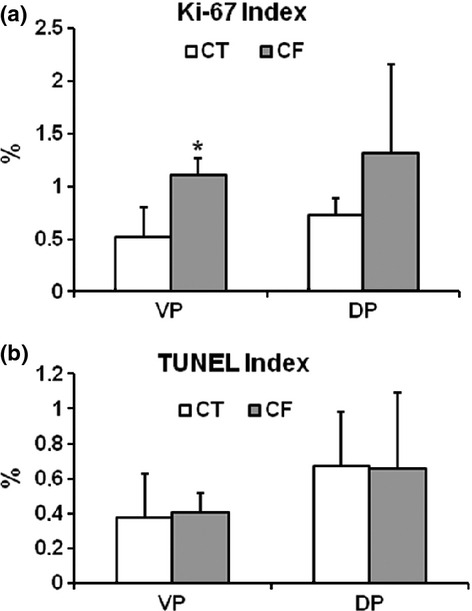
Bars graph of the proliferation and apoptotic indexes of the epithelial cells from control (CT) and caffeine-treated (CF) prostatic lobes. Ventral (VP) and dorsal (DP) prostatic lobes. (a) The proliferation index was significantly higher in the VP from the CF group. (b) No significant differences in the prostatic lobe epithelial cell apoptotic index were observed between the groups. Results are expressed as mean ± SD. *P < 0.05 when compared with the CT group.
The effect of caffeine treatment on epithelial cell apoptosis was also assessed by analyzing the frequency of positive nuclei by the TUNEL reaction (Figure 4). This analysis revealed no significant changes in the epithelial cell apoptotic index in both prostatic lobes from the caffeine-treated rats compared with the control rats (Figure 5b).
Figure 4.
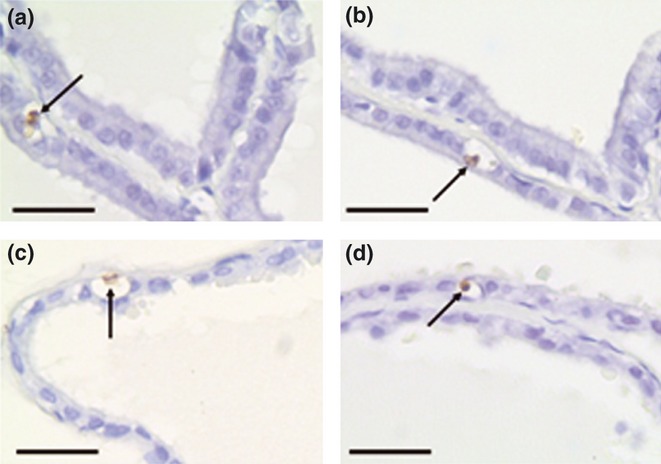
Representative sections of control (a, c) and caffeine-treated (b, d) prostatic lobes submitted to the Transferase-Mediated Biotinylated UTP Nick End-Labelling (TUNEL) reaction. Ventral (a, b) and dorsal (c, d) prostates. The arrows indicate typical positively stained apoptotic epithelial cells that were used to determine the TUNEL index. Scale bars: 10 μm.
Western blotting for PCNA and PAR-4
The Western blotting results confirmed the increased cellular proliferation observed in the VPs from the caffeine-treated animals (Figure 6). The Western blotting showed increased expression of PCNA in the VPs without changes in the DPs. The analysis of PAR-4 showed no significant difference between CT and CF groups in the expression of this protein (related to apoptosis) in both VP and DP.
Figure 6.
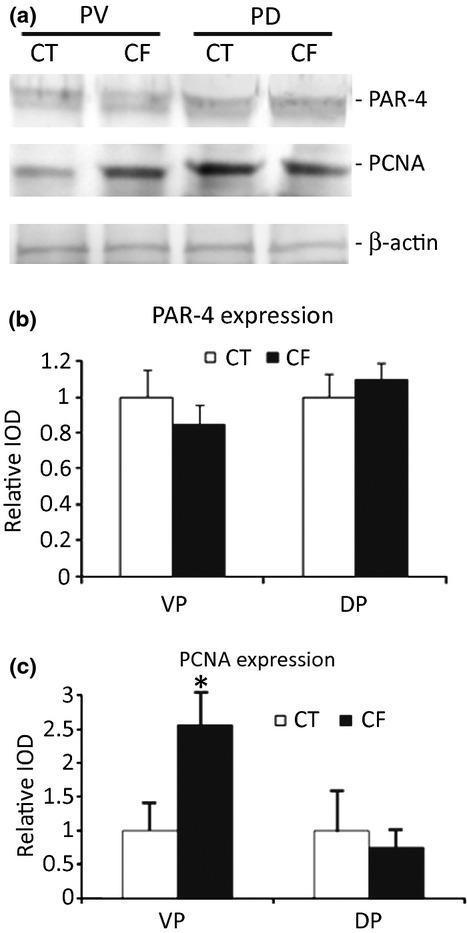
(a) Representative Western blotting for PAR-4, proliferation cell nuclear antigen (PCNA) and beta-actin expression in the rat ventral (VP) and dorsal (DP) prostatic lobes from the control (CT) and caffeine-treated animals (CF). (b) Densitometric analysis of the PAR-4 bands showing no differences between the groups in both prostatic lobes. (c) Densitometric analysis of the PCNA bands showing a significant higher expression only in the VP from the CF group. Results are expressed as mean ± SD. *Significantly different with P < 0.05.
AR immunostaining
Finally, we performed immunohistochemistry to evaluate the tissue expression of AR. Androgen receptor immunostaining was detected in the nuclei from columnar and basal epithelial cells of the VP and DP from both the control and caffeine-treated animals (Figure 7). However, the AR immunostaining was more intense in the nuclei of the VP epithelial cells from the CF and semi-quantitative IOD analysis confirmed this observation (Figure 7b,e). No statistically significant difference was found in the AR intensity from DP epithelial cell nuclei between CT and CF groups (Fig. 7e).
Figure 7.
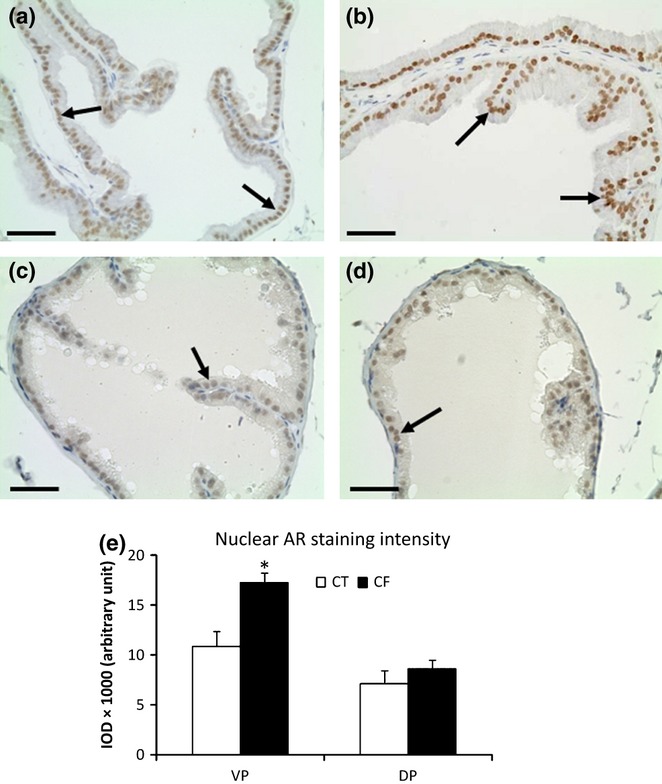
Immunohistochemistry for the androgen receptor (AR) in control (a, c) and caffeine-treated (b, d) prostates. Ventral (VP) (a, b) and dorsal (DP) (c, d) prostatic lobes. Note a uniform and higher intensity of the positive reaction in the nuclei (arrows) of the VPs from caffeine-treated group (CF) compared with the VPs from the control group. Scale bars: 30 μm. (e) Semi-quantitative analysis (IOD, integrated optical density) of the nuclear AR immunoreactivity of the epithelial cells from VP and DP in control (CT) and CF showing significant higher AR staining in the VP nuclei from CF group than CT group. Mean ± SD. Statistically significant differences: *P < 0.05.
Discussion
In the present study, we examined the effects of caffeine consumption on rat prostatic lobe growth, morphology and physiology. The findings demonstrated that long-term low-dose caffeine intake has induced deep alterations in the morphology and homoeostasis of the VP primarily. The VP from caffeine-treated animals showed increased absolute and relative weight and a higher incidence of glandular hyperplasia than CT. In our study, we observed glandular hyperplasia in eight of 12 VPs from the caffeine-treated rats, with different degree of severity and morphological patterns.
The increased incidence of prostatic hyperplasia in caffeine-treated animals observed in our study may be related to several different factors, one of which may be plasma androgen levels. Although the role of androgens as the causative factor for human BPH is debated, they undoubtedly play, at least, a permissive role (Bartsch et al. 2002; Ho & Habib 2011). In our study, we found that animals treated with caffeine developed increased plasmaic T and DHT levels, which is in agreement with previous work (Weinberger et al. 1978; Ezzat & Gohary (1994). Moreover, we found higher rates of epithelial cell proliferation in the VP of caffeine-treated rats than in control rats associated with unchanged apoptotic index, which is consistent with the development of epithelial hyperplasia. Normal prostate growth and maintenance are obtained by a tightly regulated balance between cell proliferation and cell death. Disruption of the molecular mechanisms that regulate these two processes may underline the abnormal growth of the gland leading to development of prostatic diseases (Kyprianou et al. 1996 Colombel et al. 1998; Xie et al. 2000). The results of epithelial cell proliferation associated with increased AR tissue staining observed in the VP strongly suggest that caffeine intake increases androgenic signalling in the VP epithelial cells, increasing their proliferative and secretory activities and inducing abnormal hyperplasic prostate growth.
On the other hand, it has also been suggested that elevated sympathetic stimulation by caffeine intake may cause BPH (Yun & Doux 2006). In agreement with this hypothesis, Huang et al. (2009) also have showed that overactivity of sympathetic nervous system by chronic stress induced hyperplasia in the ventral lobe of Wistar rats, while the dorsolateral lobe was almost unaffected. Hyperplasia was not observed after chemical sympathectomy had been performed during stress. Thus, a caffeine effect on sympathetic nervous system is another possible mechanism associated with development of prostatic hyperplasia observed in our study and future studies from our group should address this possible relationship.
Our group has previously demonstrated the major role of the stromal components and collagen fibers for the maintenance of prostate histoarchitecture and homoeostasis in several experimental conditions (Vilamaior et al. 2000; Delella et al. 2007; Felisbino et al. 2007; Justulin et al. 2008, 2010; Delella & Felisbino 2010; Lacorte et al. 2011). Caffeine has been associated with reduced collagen deposition and reduced fibrosis in the lung and liver (Feoktistov et al. 2009). This effect has been associated with an antagonist action of caffeine on adenosine receptors (Chan et al. 2006; Nakav et al. 2009). However, the inhibition of adenosine activity by caffeine is achieved by higher doses (25 mg/kg/day) than the dose used in our experiment (approximately 2.5 mg/kg/day). In our study, even though VP from CF group committed by stromal hyperplasia showed increased amounts of collagen fibers in the interstitial space of the gland, taking into account the number and random sampling of the fields from different areas and animals, the mean values of collagen volume fraction in the VP from CF group was not significantly different from the CT group.
In summary, our results suggest that chronic caffeine intake from puberty to adulthood increases androgenic signalling and cell proliferation in the prostate gland and can be related to the development of BPH. Future studies should address if caffeine co-exposure with others food, environmental and occupational carcinogens might be related to the onset of premalignant and malignant lesions in the prostate.
Acknowledgments
The authors thank Dr. Wagner José Fávaro, Dr. Renee Laufer Amorim and Ivan José Vechetti Junior for important contributions to the manuscript. This article is part of the dissertation presented by Carolina Sarobo to the Institute of Biosciences – Sao Paulo State University (UNESP) – to receive the degree of Master of Science.
Conflict of Interest
The authors declare that they have no conflit of interest.
Funding source
This research was funded by FAPESP (09/52747-7, 09/50850-5), CNPq, CAPESP and FUNDUNESP.
References
- Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia. World J. Urol. 2002;19:413–425. doi: 10.1007/s00345-002-0248-5. [DOI] [PubMed] [Google Scholar]
- Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention oh human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- Bode AM, Dong Z. The enigmatic effects of caffeine in cell cycle and cancer. Cancer Lett. 2007;247:26–39. doi: 10.1016/j.canlet.2006.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Butt MS, Sultan MT. Coffee and its consumption: benefits and risks. Crit. Rev. Food Sci. Nutr. 2011;51:363–373. doi: 10.1080/10408390903586412. [DOI] [PubMed] [Google Scholar]
- Cavin C, Holzhaeuser D, Scharf G, Constable A, Huber WW, Schilter B. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem. Toxicol. 2002;40:1155–1163. doi: 10.1016/s0278-6915(02)00029-7. [DOI] [PubMed] [Google Scholar]
- Celec P, Behuliak M. Behavioural and endocrine effects of chronic cola intake. J. Psychopharmacol. 2010;24:1569–1572. doi: 10.1177/0269881109105401. [DOI] [PubMed] [Google Scholar]
- Chan ES, Montesinos MC, Fernandez P, et al. Adenosine A2A receptors play a role in the pathogenesis of hepatic cirrhosis. Br. J. Pharmacol. 2006;148:1144–1155. doi: 10.1038/sj.bjp.0706812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombel M, Vacherot F, Diez SG, Fontaine E, Buttyan R, Chopin D. Zonal variation of apoptosis and proliferation in the normal prostate and in benign prostatic hyperplasia. Br. J. Urol. 1998;82:380–385. doi: 10.1046/j.1464-410x.1998.00752.x. [DOI] [PubMed] [Google Scholar]
- DeKlerk DP, Coffey DS. Quantitative determination of prostatic epihtleial and stromal hyperplasia by a new technique biomorphometrics. Invest. Urol. 1978;16:240–245. [PubMed] [Google Scholar]
- Delella FK, Felisbino SL. Doxazosin treatment alters stromal cell behavior and increases elastic system fibers deposition in rat prostate. Microsc. Res. Tech. 2010;73:1036–1044. doi: 10.1002/jemt.20828. [DOI] [PubMed] [Google Scholar]
- Delella FK, Justulin LA, Jr, Felisbino SL. Tissue inhibitor of metalloproteinase-2 (TIMP-2) location in the ventral, lateral, dorsal and anterior lobes of rat prostate by immunohistochemistry. Cell Biol. Int. 2007;31:229–234. doi: 10.1016/j.cellbi.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Ezzat AR, Gohary ZM. Hormonal and histological effects of chronic caffeine administration on the pituitary-gonadal and pituitary-adrenocortical axes in male rabbits. Funct. Dev. Morphol. 1994;4:45–50. [PubMed] [Google Scholar]
- Felisbino SL, Justulin LA, Jr, Carvalho HF, Taboga SR. Epithelial-stromal transition of MMP-7 immunolocalization in the rat ventral prostate following bilateral orchiectomy. Cell Biol. Int. 2007;31:1173–1178. doi: 10.1016/j.cellbi.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I, Cronstein BN. Adenosine receptors in wound healing, fibrosis and angiogenesis. Handb. Exp. Pharmacol. 2009;193:383–397. doi: 10.1007/978-3-540-89615-9_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Gass R. Benign prostatic hyperplasia: the opposite effects of alcohol and coffee intake. BJU Int. 2002;90:649–654. doi: 10.1046/j.1464-410x.2002.03001.x. [DOI] [PubMed] [Google Scholar]
- Heckman MA, Weil J, Gonzalez de Mejia E. Caffeine (1, 3, 7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010;75:77–87. doi: 10.1111/j.1750-3841.2010.01561.x. [DOI] [PubMed] [Google Scholar]
- Hirose K, Niwa Y, Wakai K, Matsuo K, Nakanishi T, Tajima K. Coffee consumption and the risk of endometrial cancer: evidence from a case-control study of female hormone-related cancers in Japan. Cancer Sci. 2007;98:411–415. doi: 10.1111/j.1349-7006.2007.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat. Rev. Urol. 2011;8:29–41. doi: 10.1038/nrurol.2010.207. [DOI] [PubMed] [Google Scholar]
- Huang S, Fang X, Meng Y, Chen Y, Zhang X, Zhao S. Sympathetic nervous system overactivity in the Wistar rat with proliferative lesions of ventral prostate induced by chronic stress. Urol. Int. 2009;83:230–235. doi: 10.1159/000230030. [DOI] [PubMed] [Google Scholar]
- Huxley R, Lee CM, Barzi F, et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch. Intern. Med. 2009;169:2053–2063. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- IARC – International Agency for Research on Cancer. Coffee, Tea, Mate, Methylxanthines and Methylglyoxal. Lyon: IARC; 1991. [monografia da internet], Volume 51. http://www.monographs.iarc.fr/ENG/Monographs/vol51/volume51.pdf. [Google Scholar]
- Inkielewicz-Stepniak I, Czarnowski W. Oxidative stress parameters in rats exposed to fluoride and caffeine. Food Chem. Toxicol. 2010;48:1607–1611. doi: 10.1016/j.fct.2010.03.033. [DOI] [PubMed] [Google Scholar]
- Junqueira LCU, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, specific method of collagen detection in tissue sections. Histochem. J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Justulin LA, Jr, Delella FK, Felisbino SL. Doxazosin reduces cell proliferation and increases collagen fibers in rat prostatic lobes. Cell Tissue Res. 2008;332:171–183. doi: 10.1007/s00441-007-0559-3. [DOI] [PubMed] [Google Scholar]
- Justulin LA, Jr, Della-Coleta HH, Taboga SR, Felisbino SL. Matrix metalloproteinase (MMP)-2 and MMP-9 activity and localization during ventral prostate atrophy and regrowth. Int. J. Androl. 2010;33:696–708. doi: 10.1111/j.1365-2605.2009.01016.x. [DOI] [PubMed] [Google Scholar]
- Kyprianou N, Tu H, Jacobs SC. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum. Pathol. 1996;27:668–675. doi: 10.1016/s0046-8177(96)90396-2. [DOI] [PubMed] [Google Scholar]
- Lacorte LM, Delella FK, Porto Amorim EM, et al. Early changes induced by short-term low-dose cadmium exposure in rat ventral and dorsolateral prostates. Microsc. Res. Tech. 2011;74:988–997. doi: 10.1002/jemt.20985. [DOI] [PubMed] [Google Scholar]
- Lu Y, Rosenfeld R, Bar-Joseph Z. Identifying cycling genes by combining sequence homology and expression Data. Bioinformatics. 2006;22:314–322. doi: 10.1093/bioinformatics/btl229. [DOI] [PubMed] [Google Scholar]
- Nakav S, Kachko L, Vorobiov M, et al. Blocking adenosine A2A receptor reduces peritoneal fibrosis in two independent experimental models. Nephrol. Dial. Transplant. 2009;24:2392–2399. doi: 10.1093/ndt/gfp041. [DOI] [PubMed] [Google Scholar]
- Nemeth JA, Lee C. Prostatic ductal system in rats: regional variation in stromal organization. Prostate. 1996;28:124–128. doi: 10.1002/(SICI)1097-0045(199602)28:2<124::AID-PROS8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Nkondjock A. Coffee consumption and the risk of cancer: an overview. Cancer Lett. 2009;277:121–125. doi: 10.1016/j.canlet.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Oliva SU, Scarano WR, Okada FK, Miraglia SM. Harmful effects of carbamazepine on the postnatal development of the rat ventral prostate. Reprod. Biol. Endocrinol. 2012;10:22–30. doi: 10.1186/1477-7827-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard I. Increases in plasma concentrations of steroids in the rat after the administration of caffeine: comparison with plasma disposition of caffeine. J. Endocrinol. 1988;119:275–280. doi: 10.1677/joe.0.1190275. [DOI] [PubMed] [Google Scholar]
- Shappell SB, Thomas GV, Roberts RL, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the mouse models of human cancer consortium prostate pathology committee. Cancer Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- Signorello LM, Tzonou A, Lagiou P, Samoli E, Zavitsanos X, Trichopoulos D. The epidemiology of benign prostatic hyperplasia: a study in Greece. BJU Int. 1999;84:286–291. doi: 10.1046/j.1464-410x.1999.00199.x. [DOI] [PubMed] [Google Scholar]
- Vilamaior PS, Felisbino SL, Taboga SR, Carvalho HF. Collagen fiber reorganization in the rat ventral prostate following androgen deprivation: a possible role for smooth muscle cells. Prostate. 2000;45:253–258. doi: 10.1002/1097-0045(20001101)45:3<253::aid-pros8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Weinberger MA, Friedman L, Farber TM, et al. Testicular atrophy and impaired spermatogenesis in rats fed high levels of the methylxanthines caffeine, theobromine, or theophylline. J. Environ. Pathol. Toxicol. 1978;1:669–688. [PubMed] [Google Scholar]
- Wilson KM, Kasperzyk JL, Rider JR, et al. Coffee consumption and prostate cancer risk and progression in the Health Professionals Follow-up Study. J. Natl Cancer Inst. 2011;103:1–9. doi: 10.1093/jnci/djr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Wong YC, Tsao SW. Correlation of increased apoptosis and proliferation with development of prostatic intraepithelial neoplasia (PIN) in ventral prostate of the noble rat. Prostate. 2000;44:31–39. doi: 10.1002/1097-0045(20000615)44:1<31::aid-pros5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Yun AJ, Doux JD. Opening the floodgates: benign prostatic hyperplasia may represent another disease in the compendium of ailments caused by the global sympathetic bias that emerges with aging. Med. Hypotheses. 2006;67:392–394. doi: 10.1016/j.mehy.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Zhu LJ, Hardy MP, Inigo IV, Huhtaniemi I, Bardin CW, Moo-Young AJ. Effects of androgen on androgen receptor expression in rat testicular and epididymal cells: a quantitative immunohistochemical study. Biol. Reprod. 2000;63:368–376. doi: 10.1095/biolreprod63.2.368. [DOI] [PubMed] [Google Scholar]


