Abstract
In utero irradiation (IR) and postnatal hyperthermia (HT) exposure cause infertility by decreasing spermatogenic colony growth and the number of sperm in rats. Four groups were used: (i) Control group, (ii) HT group (rats exposed to hyperthermia on the 10th postnatal day), (iii) IR group (rats exposed to IR on the 17th gestational day) and (iv) IR + HT group. Three and six months after the procedures testes were examined by light and electron microscopy. Some degenerated tubules in the HT group, many vacuoles in spermatogenic cells and degenerated tight junctions in the IR group, atrophic tubules and severe degeneration of tight junctions in the IR + HT group were observed. ZO-1 and occludin immunoreactivity were decreased and disorganized in the HT and IR groups and absent in the IR + HT group. The increase in the number of apoptotic cells was accompanied by a time-dependent decrease in haploid, diploid and tetraploid cells in all groups. Degenerative findings were severe after 6 months in all groups. The double-hit model may represent a Sertoli cell only model of infertility due to a decrease in spermatogenic cell and alterated blood-testis barrier proteins in rat.
Keywords: apoptosis, blood-testis barrier, hyperthermia, irradiation, rat, testis
Spermatogenesis is a long, complex and finely tuned process. During this process, the developing sperm cell is sensitive to endogenous and exogenous factors. Exposure to reproductive cytotoxic agents may damage somatic testicular cells or germ cells at different stages of differentiation, leading to a temporary or permanent impairment of fertility (Take et al. 2009). Many studies have shown that irradiation (IR) and hyperthermia (HT) lead to infertility due to decrease in spermatogenic colony growth, decrease in the number of sperm and in the weight of the testis in rats (Moore & Chase 1923; Brinkworth & Handelsman 2000). Moreover, short-term exposure of the testis to heat (Lue et al. 1999; Xu et al. 2003) and radiation (Allan et al. 1988; Hasegawa et al. 1997) causes apoptosis-related loss of germ cells in the adult rat.
It is evident that significant permanent tissue hypoplasia can result following ionizing IR exposure in late foetal development. Exposure to ionizing IR in early development causes infertility by affecting the gonocytes (Jensh & Brent 1988). Ionized radiation triggers the apoptosis of the spermatogenic cells (Allan et al. 1988; Hasegawa et al. 1997). Depending on the dose and duration of ionizing radiation, spermatogenic cell damage can be irreversible (Brinkworth & Handelsman 2000).
Another environmental factor causing infertility in males is high-temperature. Experimental studies have shown that HT leads to spermatogenic cell death depending on its duration and amplitude (Collins & Lacy 1969).
All germ cells are associated with adjacent spermatogenic cells and with Sertoli cells. This dynamic relation is mediated by tight, gap and anchoring junctions (Russell 1993). Tight junctions between Sertoli cells and epididymal epithelia are important for the formation and functioning of the blood-testis and blood-epididymal barriers. Sertoli cell tight junctions in the seminiferous epithelium are close to the basement membrane. These junctions at the basal domain of Sertoli cells break down to allow the passage of preleptotene and leptotene spermatocytes across the blood-testis barrier, while new tight junction fibrils are formed under the migrating preleptotene and leptotene spermatocytes (Pelletier 2001). During this process, tight junctions have a coordinating role in the movement of early spermatocytes to the adluminal compartment of the seminiferous epithelium.
Epithelial tight junctions are multimolecular membrane specializations comprising multiple integral membrane proteins such as zonula occludens-1 (ZO-1) and occludin. ZO-1, a 225-kDa protein, is an integral membrane protein found in the most superficial region of the tight junctions. Occludin, a 65-kDa protein, was the first tight junction–associated integral membrane protein identified in different types of epithelia (Furuse et al. 1993), including the rat testis and epididymis (Moroi et al. 1998). ZO-1 is necessary for the localization of occludin in tight junction. The main roles of tight junctions are to act as a physical barrier to prevent solutes and water from passing freely through the intercellular spaces and to create and maintain cell polarity (Tsukita & Furuse 1999). In the testis, tight junctions between Sertoli cells form part of the blood-testis barrier, which is important for the maintenance of a special physiological milieu for spermatogenesis and for the protection of germ cells from the immune system (Russell & Peterson 1985).
Gonocytes are radiosensitive between 15–19 gestational days, and gonocytes proliferate and differentiate to the type A spermatogonia at postnatal first week (Moreno, 2001). The effects of ionizing radiation are reversible at 6 months after exposure in adults (Brinkworth & Handelsman 2000). Moreover, hyperthermia causes apoptosis in spermatogenic cells over long term (Xu et al. 2003).
In this study, we aimed to show the ultimate stem cell death and blood-testes barrier damage due to the synergistic effect of in utero ionizing radiation and postnatal hyperthermia using histopathological and ultrastructural analysis, TUNEL assay for cell death, flow cytometry for DNA ploidy ratio, ZO-1 and occludin immunohistochemistry at 3 and 6 months in rat testes.
Materials and methods
Animals and experimental design
Thirteen pregnant Wistar albino rats and their litters were studied. Rats were controlled daily for the presence of sperm cells by vaginal swab, and the day on which insemination was detected was defined as embryonic day 0 (E0). Birth occurred on E21 or E22, and this date was designated as postnatal day 0 (P0). Rat pups remained with their mothers until they were weaned at P21. All animals were maintained on a 12-h light/dark cycle with food and water available ad libitum and an average room temperature of 21 ± 3 °C.
Ethical Approval
The experimental study was approved by The Animal Care and Ethical Committee for Experimental Animals in Marmara University (23.11.2006-40.2005. Mar).
The experimental design of this study was as follows: (i) IR group (n = 14), in which the rats were only exposed to 100 cGy radiation on E17; (ii) HT group (n = 14), in which the rats were only exposed to hyperthermia on day P10; (iii) IR plus HT group (n = 14), in which the rats were exposed to 100 cGy irradiation on E17, followed by subjection to hyperthermia of the litter on day P10; (iv) Sham-anesthesized control group (n = 7), in which neither irradiation nor hyperthermia was applied to age-matched control rats. Only the anaesthesia was applied to this group.
Exposure to irradiation and hyperthermia
In the irradiation experiments, the pregnant rats were anesthetized with 0.2 ml of ketamine (100 mg/kg) and xylazine hydrochloride (20 mg/kg) intramuscularly and placed in the irradiator device. In the IR group, the abdomen of the rat was exposed to 100cGy radiation of a single fraction using a cobalt 60 teletherapy system with the SSD (Source to skin distance) technique on E17. The duration of the IR session was 35 s.
In the hyperthermia experiments, the litters of rats which were exposed to IR in utero and another group of litters without exposure to IR were subjected to HT by heating them with a heating machine on day P10. The duration of the HT sessions was 10 ± 5 min until the rectal temperature reached 40 ± 1 °C.
Light microscopic preparation and histopathological scoring
Three or 6 months after IR, HT or both applications, rats were deeply anesthetized with 50 mg/kg ketamine and 12 mg/kg xylazine hydrochloride and then perfused through the aorta with a solution of 10% formaldehyde. Then, testes were removed, left in the same fixative at 4 °C for 4 h and then washed in tap water for 2 h. Thereafter, the testes were dehydratated with subsequent 70%, 90%, 96% and 100% ethanol and cleared with toluene. After overnight incubation of paraffin in a 60 °C incubator, testes were embedded and blocked in paraffin at room temperature. Sections were cut at 5 μm from these blocks. Every fifth section was collected. Five sections were randomly selected from the series and stained with haematoxylin–eosin (H&E) for microscopic examination and measurement of the testicular areas. Ten testicular areas from serial cross-sections of mid-testicular region for each animal were photographed and calculated by the NIH Image Analysis image j (National Institutes of Health, Bethesda, MD, USA) program.
Stained sections from all the animals were analysed by a blinded observer. Five similar areas were examined at ×200 magnification. Histopathological scoring was evaluated by the modification of Hess's data and organized as normal, regressive, degenerative or atrophic tubules (Hess et al. 1988). Normal tubules are tubules with normal morphology of spermatogenesis and intercellular junctions. Regressive tubules are seminiferous tubules with one or more abnormalities: whereas intercellular tight junctions are normal, there is loose cellular organization and cellular degeneration (pyknotic nucleus, granular eosinophilic cytoplasm and karyolysis). Degenerative tubules show an irregular arrangement of germ and Sertoli cells with loss of intercellular tight junctions. Atrophic tubules show either Sertoli cells or a few germ cells and Sertoli cells with loss of tight junctions. Interstitial connective tissue was evaluated as showing a mild, moderate or severe increase according to the thickening of connective tissue structures between the seminiferous tubules.
Transmission electron microscopic (TEM) preparation
Approximately 3 mm3 tissue blocks from the left testis were fixed by immersion into 2.5% glutaraldehyde in PBS (0.1 M, pH 7.2), postfixed in 1% osmium tetroxide in PBS (0.1 M, pH 7.2), dehydrated in a graded serie of ethanol and embedded in Epon 812 resin (Fluka, Sigma-Aldrich Chemica, Steinheim, Switzerland) polymerized at 60 °C. The epon blocks were sectioned with glass knives on a Leica Ultracut R microtome (Wien, Austria). Ultrathin sections were cut at 60 nm and were collected on 200-mesh naked copper grids and stained with uranyl acetate and lead citrate. The ultrathin sections were investigated using a JEOL 1200 EXII transmission electron microscope (Tokyo, Japan) at 80 kV accelerating voltage and photographed with a side mounted digital camera (Morada Soft Imaging System, Olympus, USA).
ZO-1 and Occludin Immunohistochemistry
Paraffin sections cut at 5 μm thickness were collected into positively charged slides, incubated in 56 °C overnight, deparaffinized with toluene for 30 min, rehydrated with an ethanol series and washed with distilled water. Slices were incubated with protease (1 mg/ml; Sigma-Aldrich) at 37 °C for 10 min and washed with phosphate-buffered saline (PBS). Sections were delineated with a Dako pen (Dako, Glostrup, Denmark) and incubated in a solution of 3% H2O2 for 10 min to inhibit endogenous peroxidase activity. After washing with PBS (phosphate buffer solution), sections were incubated with blocking solution (Scy Tek Ultra Tek HRP, anti-polivalent, UHP 125 (ScyTek Laboratories Inc., Logan, UT, USA)) for 15 min. Then, the sections were washed with PBS and incubated with primary anti-ZO-1 antibody developed in rabbit (1:100; Zymed Laborotories S. San Francisco, CA, USA) for 6 h and primary anti-occludin, an antibody developed in rabbit (1:150; Zymed Laboratories), for one night at +4 °C. Next, the sections were incubated with biotinylated IgG and then with streptavidin-peroxidase conjugate (Histostain-Plus Bulk kits; Zymed laboratories). The sections were washed with PBS, incubated with 3-Amino-9-ethylcarbazole (AEC) for 15 min to visualize immunostaining and finally counterstained with Mayer's haematoxylin (Zymed Laboratories). Control samples were processed in the same manner except that the primary antibody was omitted. An observer blinded to experimental groups evaluated the staining intensity semiquantitatively. Staining intensity was graded as none (−), least (+/−), weak (+), dense (++) and intense (+++) respectively.
TUNEL method
The TUNEL method was used in accordance with the manufacturer's manual (Apoptag Plus Peroxidase in situ Apoptosis kit, Chemicon International, S7101, Temecula, CA, USA). The procedure was as follows: every fifth section (a total of five sections from each animal) was incubated with proteinase K for 5 min, washed with distilled water and incubated with 3% hydrogen peroxide in PBS for 5 min. The sections were then washed with PBS, put in the equilibrium buffer for 30 min and incubated in recombinant terminal transferase TdT enzyme at 37 °C for 1 h. The sections were agitated in washing buffer for 15 s, washed in PBS, put into anti-digoxigenin conjugate for 30 min and then washed with PBS. After incubation with peroxidase for 6 min, they were washed with distilled water, stained with Mayer's haematoxylin, and after the dehydration procedure, they were covered with Entellan (Merck, Darmstadt, Germany). Every fifth section was collected, and in each section, TUNEL-positive cells in five similar areas were counted at ×400 magnification. An eye-piece graticule (0.0785 mm2) was used to define the counting area, and TUNEL-positive cell density was expressed as number of cells per unit area. All light microscopic sections were observed and photographed with the digital camera (Olympus C-5060, Tokyo, Japan) of a photomicroscope (Olympus BX51, Tokyo, Japan).
Flow cytometry
For measuring the DNA ploidy content of the cells, the paraffin embedded tissues were deparaffinized with toluene and rehydrated with an ethanol series. After incubation with proteinase K in PBS (1:10) at 37 °C for 30 min, the sections were washed with PBS, filtered and centrifuged at 240 g for 15 min. For DNA staining, propidium iodide solution was added to the pellet and incubated for 15 min at 4 °C. The specimens were examined by a Fac Scan device (FAC Scan; Becton Dickinson, NJ, USA).
Statistical analysis
Data were analysed using one-way analysis of variance (anova). Differences between groups were determined with Tukey's multiple comparisons test, and the data were expressed as mean ± standard error of the mean (SEM). Significance of differences was taken at the level of P < 0.05.
Results
Measurement of testis weight and area
The testicular weight was 1.52 ± 0.1 g, and the area of testes was 3.83 ± 0.3 mm2 in control group. There were no significant changes in weight and cross-sectional area in the HT group after 3 (weight: 1.52 ± 0.08; area: 3.61 ± 0.2) and 6 (weight: 1.51 ± 0.1; area: 3.62 ± 0.15) months compared with the control group. However, the weight (Figure 1a) and cross-sectional area (Figure 1b) of the testis were decreased in the IR group (weight: 1.21 ± 0.03; area: 1.9 ± 0.1, P < 0.001 at 3 months, weight: 0.98 ± 0.02; area: 1.72 ± 0.02, P < 0.001 at 6 months) and in the IR + HT groups (weight: 0.92 ± 0.03; area: 1.61 ± 0.02, P < 0.001 in 3 months, weight: 0.93 ± 0.01; area: 1.55 ± 0.04, P < 0.001 in 6 months) compared with control groups (Figure 1).
Figure 1.

The graph of (a) testis weight (g) and (b) testis cross-sectional area (mm2) in control, HT (hyperthermia), IR (irradiation) and IR + HT (irradiation + hyperthermia) groups at 3 and 6 months (MNTH). Differences between groups are significant at the level of **P < 0.01, ***P < 0.001 compared with control group, +++P < 0.001 compared with HT groups.
Histological observations
Normal testis morphology with regular seminiferous tubules and spermatogenic cells were observed in the control group (Figure 2a).
Figure 2.

(a) Normal testis morphology in control group; (b) Degenerated seminiferous tubules (d) and irregular cell borders ( , insert) in hyperthermia (HT) group at 3 months; (c) Degenerated seminiferous tubules (d) and luminal cell deposits (+), dilatation of intercellular tight junctions (→, insert) in HT group at 6 months; (d) Some atrophic seminiferous tubules (a), vacuolized spermatogenic cells (>) and increase in interstitial tissue (*, insert) in irradiation (IR) group at 3 months; (e) Regressive (r), degenerated (d) and atrophic (a) seminiferous tubules and increase in interstitial tissue (*) in IR group at 6 months; (f) several atrophic tubules (a) and an increase in interstitial tissue (*) (insert) in IR + HT group at 3 months; (g) several atrophic tubules (a) in IR + HT group at 6 months. Staining: H&E, Scale Bars: 50 μm.
, insert) in hyperthermia (HT) group at 3 months; (c) Degenerated seminiferous tubules (d) and luminal cell deposits (+), dilatation of intercellular tight junctions (→, insert) in HT group at 6 months; (d) Some atrophic seminiferous tubules (a), vacuolized spermatogenic cells (>) and increase in interstitial tissue (*, insert) in irradiation (IR) group at 3 months; (e) Regressive (r), degenerated (d) and atrophic (a) seminiferous tubules and increase in interstitial tissue (*) in IR group at 6 months; (f) several atrophic tubules (a) and an increase in interstitial tissue (*) (insert) in IR + HT group at 3 months; (g) several atrophic tubules (a) in IR + HT group at 6 months. Staining: H&E, Scale Bars: 50 μm.
Although in the HT group after 3 months most of the seminiferous tubules were of normal morphology, there were some degenerated tubules with dilatations in the intercellular spaces, vacuole formation in spermatogenic cells, and a decrease in the spermatogenic germ cells. Cell debris was present in the lumen (Figure 2b). After 6 months, the number of degenerative tubules increased, and atrophic tubules were present (Figure 2c).
In the IR group after 3 months, the number of atrophic and degenerative seminiferous tubule increased parallel to a decrease in spermatogenic germ cells and severe thickening of the interstitial tissue. Vacuole formation was observed in proportionally undamaged spermatogenic cells (Figure 2d). In the IR group after 6 months, atrophy and degeneration of seminiferous tubules with a decrease in spermatogenic germ cells and an increase in interstitial tissue were more severe than in the same IR group after 3 months (Figure 2e).
In the IR + HT group after 3 (Figure 2f) and 6 (Figure 2g) months, there were no normal cell structures. Severe degeneration of seminiferous tubules with depletion of spermatogenic germ cells and increase in interstitial tissue were observed.
Histopathological scoring results
Histopathological scoring results for seminiferous tubules in all experimental groups are shown in Figure 6a. Normal morphologic seminiferous tubules in all stages were observed in the control group. In the HT group, regressive seminiferous tubule numbers were higher than in the control group (P < 0.001) after 3 months with further progressive increment at 6 months. In the IR group after 3 months, the number of normal tubules decreased (P < 0.001), whereas the number of regressive, degenerative and atrophic tubules increased. After 6 months, the number of atrophic tubules was significantly increased (P < 0.001). After IR + HT application, the number of normal tubules was almost zero, and the number of atrophic tubules was statistically increased (P < 0.001) at three and 6 months (Figure 6a).
Figure 6.
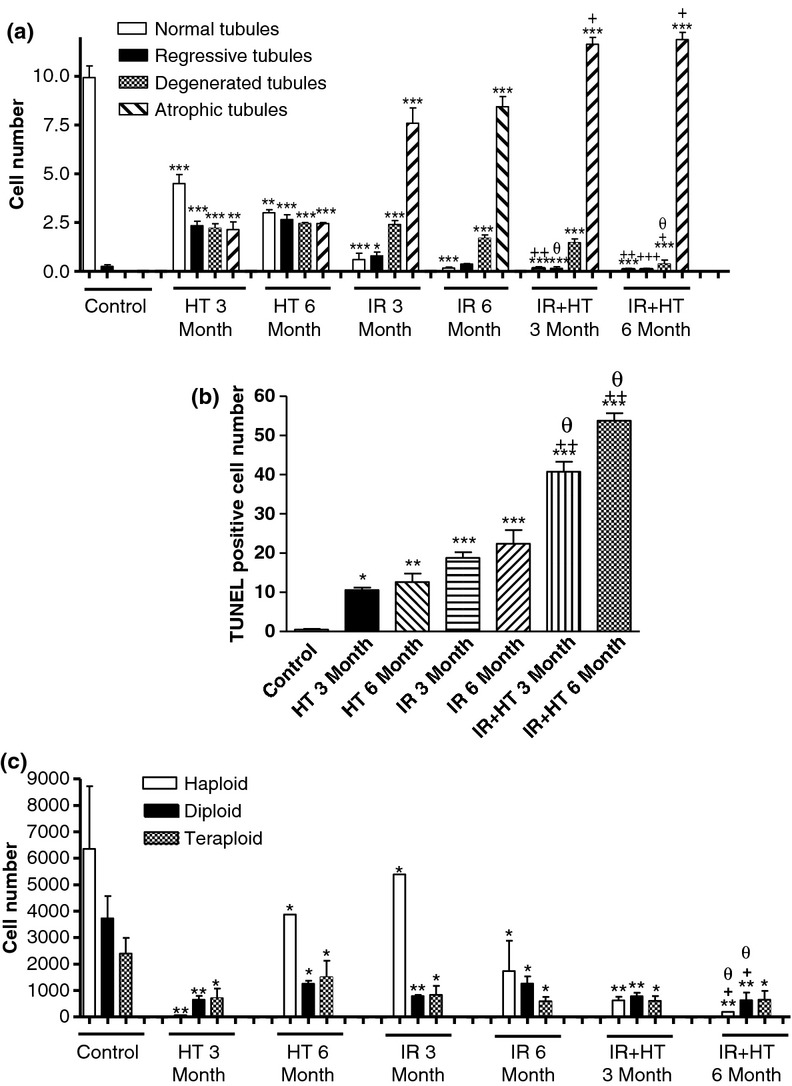
The number of (a) normal, regressive, degenerated and atrophic seminiferous tubules, (b) TUNEL-positive cells per 0.0785 mm2 and (c) haploid, diploid, and tetraploid cells in control and hyperthermia (HT), irradiation (IR) and IR + HT groups. *; P < 0.05, **; P < 0.01, ***; P < 0.001 compared with the control group. +; P < 0.05, ++; P < 0.01 compared with HT groups. θ; P < 0.05 compared with IR groups.
Electron microscopic analysis
In the control groups, normal testis ultrastructure and basal membrane thickness were observed. There were regular Sertoli-to-Sertoli and Sertoli-to-germ cell junctions (Figure 3).
Figure 3.

(a) Regular ultrastructure of the tight junction regions (→), spermatogenic cells, Sertoli cells and spermatozoa in control group; (b) Vacuole (→) around the tight junctions, and lipid droplets (*) in hyperthermia (HT) group at 3 months; (c) Large vacuoles (*) in the cytoplasm and around the tight junctions (→) in HT group at 6 months; (d) Huge vacuoles (*) and irregular tight junctions (→) in irradiation (IR) group at 3 months; (e) Fingerlike projections in the basal lamina ( ), huge vacuoles (*) and irregular tight junctions (→, insert) in IR group at 6 months; (f) Finger-like projections in the basal lamina (
), huge vacuoles (*) and irregular tight junctions (→, insert) in IR group at 6 months; (f) Finger-like projections in the basal lamina ( ), large vacuoles (*) and irregular tight junctions (→) and degenerated nuclei (n) in IR + HT group at 3 months; (g) Finger-like projections in the basal lamina (
), large vacuoles (*) and irregular tight junctions (→) and degenerated nuclei (n) in IR + HT group at 3 months; (g) Finger-like projections in the basal lamina ( ), large vacuoles (*) and irregular tight junctions (→) and nucleus (n) in IR + HT group at 6 months.
), large vacuoles (*) and irregular tight junctions (→) and nucleus (n) in IR + HT group at 6 months.
In the HT groups, the spermatogonia and Sertoli cells located on the basal membrane showed normal morphology in most regions 3 (Figure 3b) and 6 (Figure 3c) months after application. However, vacuoles were observed in the cytoplasm and around the tight junctions in Sertoli cells in some seminiferous tubules. Further, spermatozoa with regular morphology as well as spermatozoa with head defects were observed in the lumen in both HT groups.
There were thickenings of the basal lamina and fingerlike extensions towards the cytoplasm in the IR group after 3 (Figure 3d) and 6 months (Figure 3e). Moreover, there were many enlarged vacuoles in the cytoplasm of Sertoli cells, degenerated germ cells, depletion of spermatogenic cells, many apoptotic cells, spermatozoa with head defects and dilatations in the intercellular space near the basal lamina.
In the IR + HT groups, there was severe thickening of the basal lamina and fingerlike extensions towards the cytoplasm of the Sertoli cells, as well as degenerated nuclei of spermatogonia, severe enlarged vacuoles in the cytoplasm of Sertoli cells and spermatogonia, dilatation and separations of tight junctions and foamy cell residues in the lumen at 3 (Figure 3f) and 6 months (Figure 3g). The germinal epithelium was reduced to one or two cell thickness, and there were no spermatozoa in the lumen.
Immunohistochemical localization of ZO-1 and occludin
Reddish-coloured ZO-1 immunoreactivity (ir) was observed in the peripheral cytoplasm of Sertoli cells and in the basally located spermatogenic cells in all of the seminiferous tubules in the control group (Figure 4a). Occludin immunostaining was concentrated and localized in a linear fashion at the basolateral site of the adjacent Sertoli cells in the control group as shown in Figure 4c.
Figure 4.

Negative control of ZO-1 and occludin immunoreactivity in control group (b, d) ZO-1 (a) and occludin (c) immunoreactivity (→) in control group, hyperthermia (HT) group at 3 months; (e, g) and HT group at 6 months (f, h); decrease in ZO-1 (i) and occludin (k) immunoreactivity (→) in irradiation (IR) group at 3 months and IR group at 6 months (j, l) and no ZO-1 (m) and occludin (o) immunoreactivity (→) in IR + HT group at 3 months; IR + HT group at 6 months (n, f). Scale Bars: 50 μm.
ZO-1 and occludin-ir were decreased at 3 (Figure 4e,g) and 6 (Figure 3f,h) months after HT compared with the control group. There was a significant decrease and irregular immunolocalization of ZO-1 and occludin at 3 (Figure 4i,k) and 6 (Figure 4j,l) months after IR application, and no ZO-1 ir or occludin-ir cells in the seminiferous tubules at 3 (Figure 4m,o) or 6 (Figure 4n,p) months after IR + HT application. The intensity of ZO-1 and occludin immunostaining is summarized in Table 1.
Table 1.
The intensity of zonula occludens-1 (ZO-1) and occludin immunoreactivity (ir) in control, HT (hyperthermia), IR (irradiation) and IR + HT (irradiation + hyperthermia) groups at 3 and 6 months (M)
| Control | HT 3 M | HT 6 M | IR 3 M | IR 6 M | IR + HT 3 M | IR + HT 6 M | |
|---|---|---|---|---|---|---|---|
| ZO-1 ir | +++ | ++ | + | +/− | – | – | – |
| Occludin-ir | +++ | ++ | + | +/− | – | – | – |
+++, intense; ++, dense; +, weak; +/−, least; –, none.
Quantitative analysis of the TUNEL assay
Brown-coloured TUNEL-positive cells mostly spermatogonia and primary spermatocytes in seminiferous tubules were observed in all groups (Figure 5). The total number of TUNEL-positive cells throughout the testis was significantly increased at 3 (P < 0.05) and 6 (P < 0.01) months after HT application, and also at 3 (P < 0.001) and 6 (P < 0.001) months after IR application, compared with the control group. The total number of TUNEL-positive cells throughout the testis increased significantly at 3 (P < 0.001) and 6 (P < 0.001) months after IR + HT application, compared with the controls, the HT and IR groups, and it was further increased in the HT, IR and IR + HT application groups after 6 months compared with the 3 monthly groups (Figure 6b).
Figure 5.
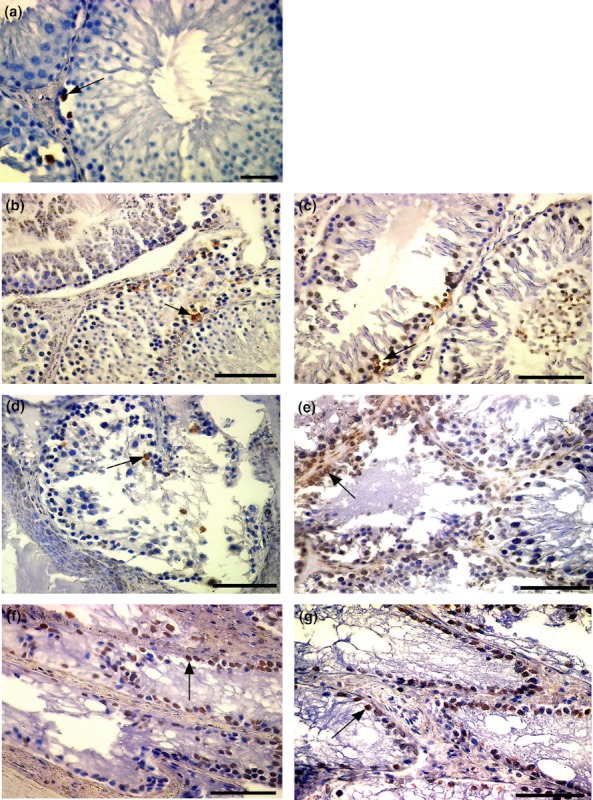
TUNEL-positive spermatogenic cells (→) in seminiferous tubules in; (a) control group; (b) hyperthermia (HT) group at 3 months; (c) HT group at 6 months; increase in the number of TUNEL-positive spermatogonium (→) in (d) irradiation (IR) group at 3 months; (e) IR group at 6 months; (f) IR + HT group at 3 months; (g) IR + HT group at 6 months. Scale Bars: a, b, c, d; 50 μm.
Flow cytometric analysis
In the HT group, the number of haploid (3 month: P < 0.01, 6 month: P < 0.05), diploid (3 month: P < 0.01, 6 month: P < 0.05) and tetraploid cells (P < 0.05) was decreased at 3 and 6 months compared with the control group. In the IR group, the number of haploid (P < 0.05), diploid (3 month: P < 0.01, 6 month: P < 0.05) and tetraploid cells (P < 0.05) was decreased at 3 and 6 months compared with control group. In the IR + HT group, the number of haploid (P < 0.01), diploid (P < 0.01) and tetraploid cells (P < 0.05) was significantly decreased at 3 and 6 months after application compared with control, as well as the HT and IR groups (Figure 6c).
Discussion
Our study shows that exposure to in utero irradiation followed by hyperthermia on postnatal day 10 causes more degeneration in the testis than does irradiation or hyperthermia alone. Degenerative findings were a decrease in testicular weight and area, a reduction in the number of haploid, diploid and tetraploid cells, an increase in the number of atrophic seminiferous tubules and TUNEL-positive cells, thickening of the basal membrane, and a disorganization in the distribution of ZO-1 and occludin protein.
Radiation is one of the initial and most studied agents in male infertility. The anti-spermatogenic effects of radiation depend on dose and repetition. It has been shown that the application of a single exposure of irradiation leads to irreversible damage in human spermatogenesis. With irradiation of 100cGy, the azoospermia was shown to be reversible 6 months later (Rowley et al. 1974; Brinkworth & Handelsman 2000). In another study, rats exposed to in utero X-ray irradiation of 1.5 Gy on gestational day 18 were examined 90 days after birth. Radiation exposure during the embryonic period inhibited testicular development decreased spermatogenesis significantly and increased the infertility rate of the rats (Jensh & Brent 1988).
In the present study, we observed that irradiation of 100cGy on embryonic day 18 leads to severe damage to spermatogenic cells after 3 and 6 months resulting in azoospermia; many seminiferous tubules consisted merely of an aligned row of Sertoli cells. After 6 months, testicular and spermatogenic damage were more severe. This form of exposure to radiation in the embryonic period was irreversible.
Twelve hours after a single exposure of 0.5 to 5 Gy irradiation of adult rats, nuclear chromatin condensation and margination, necrosis and morphological manifestations of apoptosis such as nuclear disintegration, and an increase in TUNEL-positive cells were observed in the spermatogenic cells (Hasegawa et al. 1997). Liu et al. (2007) applied X-ray for 10 min to 8–10-week-old male rats and reported a high number of atrophic seminiferous tubules in the high-dosage X-ray group and a dosage-dependent increase in the number of TUNEL-positive cells.
We observed that TUNEL-positive cells increased in number, mainly amongst spermatogonia and primary spermatocytes, in both IR and IR + HT groups. Furthermore, the irreversibility of the damage may result from the cell death occurring at the level of spermatogonia functioning as stem cells. Moreover, cellular debris and no spermatogonia in the lumen and atrophic tubules can cause azospermia by cell death in spermatogonia and primary spermatocyte.
Hacker et al. (1981) showed alterations in the amount of DNA of the spermatogenic series in animals exposed to radiation. Rats having been exposed to 0.1 and 1.5 Gy radiation were studied by flow cytometry at 2–35 days. Although tetraploid and S phase cells decreased, an increasing amount of DNA was observed in time. They also reported a decrease in the number of diploid cells, showing a high incidence of spermatogonial cell death (Hacker et al. 1980). In our study, parallel to the studies above, we observed that haploid, diploid and tetraploid DNA content decreased within 3 months and further at 6 months, although the greatest decrease in the haploid cells in both observation periods ocurred of the IR + HT group. A considerable decrease in spermatogonia leads to a decrease in haploid cells in the long term.
Infertility after irradiation is caused by spermatogonia undergoing apoptosis instead of differentiation (Boekelheide et al. 2005). A possible mechanism explaining this finding could be that radiation has been shown to increase the reactive oxygen species (ROS) in the testis (Kanter et al. 2010; Kim et al. 2012). This increase in ROS induces apoptosis in testicular tissue (Turner & Lysiak 2008). According to these studies, we believe that the increase in apoptosis in the testis in the irradiation group might be related to the increase of ROS in long-term especially in HT groups.
Miraglia and Hayashi showed that 90 days after application of 45 °C heat for 45 min to 35-day-old rats, testicular weight was lowered because the parenchymal volume had decreased. They also reported damage to the spermatogenic cells and multinuclear bodies of the spermatids (Miraglia & Hayashi 1993).
We observed that the high heat (40–41 °C) applied at postnatal day 10 caused no statistical difference in testicular weight and area compared with the control groups after 3 and 6 months. However, we observed histopathological changes as vacuoles in some spermatogenic cells of seminiferous tubules in HT groups after 3 months. Besides, only one row of cells remained in a few seminiferous tubules in HT groups after 6 months. In addition, we observed that in the IR + HT groups there was a significant decrease in the weight and area of the testes after 3 and 6 months, and only one row of serial Sertoli cells remained in almost all of the seminiferous tubules in testes. The weight and area of testes were decreased due to the increament of the number of atrophic tubules in IR and IR + HT groups.
Khan and Brown showed that programmed cell death was higher after 15 h if the body temperatures were kept at 41.3 ± 0.8 °C for 1 h with the application of HT on postnatal day 7. The heat sensitive cells were the spermatogonia, primary spermatocytes and round spermatids, whereas the elongated spermatids and Sertoli cells were not heat sensitive (Khan & Brown 2002). In another study, it had been observed that the weight of the testes was decreased and the number of TUNEL-positive cells, mostly seen among the spermatocytes, was increased after 4, 15, 28, 38 and 68 h after the body temperatures had been increased to 43 °C in 10–11-week-old rats (Rockett et al. 2001). Gasinska and Hill (1990) showed that high temperatures cause degeneration of neighbouring cells by denaturation of cytoplasmic bridges. In another study, apoptosis was observed in spermatogenic cells of locally heated rats. The temperature of the testes was raised to 43 °C and the testes studied after 12 h, 1, 3, 6, 10, 50 and 80 days. The high-temperature application caused a decrease in haploid cells and an increase in TUNEL-positive cells. As a result, this study showed that local testicular heating causes spermatogenic cell apoptosis. The most sensitive cells were the spermatocytes, and apoptosis of spermatogonia was increased over long-term exposure (Xu et al. 2003). De Vita et al. (1990) showed that with a body temperature rise to 38–40 °C for 20–60 min, the weight of mice testes had decreased after 14 and 28 days, the immature spermatids hereby being more sensitive to heat than the spermatogonia.
We observed increased number of TUNEL-positive cells in seminiferous tubules especially in spermatogonia and spermatocytes in HT, IR and IR + HT groups. Moreover, the most TUNEL-positive cell number was observed in IR + HT groups at 6 months. HT has an additive effect on triggering of cell death after irradiation.
Many in vivo models have examined the blood-testis barrier to study junctional dynamics in the testes. Morales et al. examined that the massive apoptosis found in the zygotene–pachytene spermatocytes between days 14 and 20 coincides with an open blood-testis barrier. This absence of the blood-testis barrier could be one of the factors causing massive apoptosis of zygotene-pachytene spermatocytes, at least within the time span analysed; the zygotene-pachytene spermatocytes are left exposed in an open environment instead of being isolated in the adluminal compartment to which they are destined (Morales et al. 2007). In our study, we detected ZO-1 and occludin protein by immunohistochemistry to show damage of blood-testis barrier. Damage of the tight junctional proteins of the blood-testis barrier with ageing and experimental cryptorchidism has been examined by pathology before. Damage occurred in the tight junctions of Sertoli and Leydig cells and in proliferative and differentiated spermatogenic cells in cryptorchid dogs, mice, rats and human. Furthermore, cryptorchidism led to damage of the blood-testis barrier due to functional damage of internal tight junctions with fewer tight junction fibrils and abnormal spermatogenesis in the seminiferous epithelium. Moreover, it was determined that the basal membrane was thicker. Lui et al. (2003) showed that damage in this kind of experimental model was related to occludin, ZO-1, E-cadherin, protease and protease inhibitors. Ribeiro and David-Ferreira (1996) demonstrated that 3 months after irradiation in the prenatal period, tight junctional proteins changed in relation to the degeneration of stem cells in the rat testis. Expression of occludin in Sertoli cells is controlled by an array of molecules including proteases, protease inhibitors, cytokines, cAMP and intracellular Ca2+ (Mruk & Cheng 2004). In normal seminiferous epithelium, occludin forms a continuous belt of strong immunohistochemical staining at the level of the blood-testis barrier. This distribution coincides with the expression of occludin reported in the dog (Gye 2004), wild rabbit (Yoon et al. 2008) and rat (Altay et al. 2008; Contuk et al. 2011) testis. In the present study, there was a constant decrease in ZO-1 and occludin immunoreactivitiy, which was parallel to spermatogenic damage in the IR and HT groups, whereas no immunoreactivitiy was observed in IR + HT groups. We also observed that tight junctional components were damaged in the IR and IR + HT groups with formation of large vacuoles in the cytoplasm and damaging of basal membrane at level of the tight junctions. There was a decrease and even absence in immunoreactivity, and the highly damaged tight junctional parts might have been either the result of an inhibition of synthesis or of a change of interaction between the tight junctional proteins under the influence of HT and/or IR.
Increased production of glycosaminoglycans and proteoglycans in basal membrane has been also considered a defence reaction against the damaging activity of free radicals. Sertoli cell functions, such as differentiation, cell growth and migration, are dependent on the substratum on which cells are attached (Dym 1994). Damage and thickening of the basal membrane had been demonstrated in the seminiferous epithelium in rat testis after gamma radiation application (Topcu-Tarladacalisir et al. 2009). Basal membrane thickening (Siu & Cheng 2004; Contuk et al. 2011) and disorganized occludin distribution (Contuk et al. 2011) had also been shown in vasectomized rats.
In the present study, the ultrastructure of the seminiferous epithelium and the basal membrane in the rat testis were also examined by transmission electron microscopy. Electron microscopic results were in line with the immunohistochemical and histopathological results. Ultrastructural examination revealed minimal degeneration in the 3-month hyperthermia group, whereas the degeneration progressed by 6 months and in both the 3 and the 6 months IR groups. Degenerative findings like vacuole formation in the spermatogonial cells, dilatation of tight junction, foamy cell residues in the lumen of seminiferous tubules, thickening of the basal membrane and fingerlike extensions towards the cytoplasm were severe in the IR + HT group. The disruption of the barrier may be related to the dislocation of ZO-1 and occludin from the basolateral region of the cytoplasm.
Conclusion
Thus, in conclusion, in this study we have shown that IR + HT application led to a series of morphologic degenerations including apoptosis of spermatogenic cells, reduction in haploid, diploid and tetraploid cells and alterations in the distribution of ZO-1 and occludin in the blood-testis barrier and thickening of the basal membrane. The disruption of the blood-testis barrier might be related to the dislocation of ZO-1 and occludin from the basolateral region of Sertoli cells, after which the germ cell damage becomes irreversible. We assume that this double-hit model can serve as a powerful experimental tool to study irradiation and hyperthermia toxicity in spermatogonial stem cell and Sertoli cell functions in immature human testis. The double-hit model can be introduced as an experimental Sertoli cell only infertility model important for identifying this type of irreversible infertility and highlights a possible target for male infertility treatment in the future.
Acknowledgments
The authors thank Dr. Ray Guillery and Dr. Susanne Oner for their contributions to our manuscript.
Funding
This study was supported by Marmara Scientific Research Project Committee (SAG-DKR 290506-0067, SAG-DKR-290506-0084).
Conflict of interest
None.
References
- Allan DJ, Gobé GC, Harmon BV. Sertoli cell death by apoptosis in the immature rat testis following x-irradiation. Scanning Microsc. 1988;2:503–512. [PubMed] [Google Scholar]
- Altay B, Turna B, Oktem G, Aktug H, Semerci B, Bilir A. Immunohistochemical expression of connexin 43 and occludin in the rat testis after epididymal and vassal ligation. Fertil. Steril. 2008;90:141–147. doi: 10.1016/j.fertnstert.2007.05.065. [DOI] [PubMed] [Google Scholar]
- Boekelheide K, Schoenfeld HA, Hall SJ, et al. Gonadotropin- releasing hormone antogonist (cetrorelix) therapy fails to protect nonhuman primates (Macaca arctoids) from radiation induced spermatogenic failure. J. Androl. 2005;26:222–234. doi: 10.1002/j.1939-4640.2005.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Brinkworth MH, Handelsman DJ. Environmental influences on male reproductive health. In: Nieschlag E, Behre HM, editors. Andrology Male Reproductive Health and Dysfunction. 2nd edn. Germany: Springer press; 2000. pp. 257–258. [Google Scholar]
- Collins P, Lacy D. Studies on the structure and function of the mammalian testis. II. cytological and histochemical observations on the testis of the rat after a single exposure to heat applied for different lengths of time. Proc. R. Soc. Lond. B Biol. Sci. 1969;172:17–38. doi: 10.1098/rspb.1969.0009. [DOI] [PubMed] [Google Scholar]
- Contuk G, Orun O, Demiralp-Ekşioğlu E, Ercan F. Morphological alterations and distribution of occludin in rat testes after bilateral vasectomy. Acta Histochem. 2011;114:244–251. doi: 10.1016/j.acthis.2011.06.006. [DOI] [PubMed] [Google Scholar]
- De Vita R, Calugi A, Chiarantano C, Forte D, Mauro F, Uccelli R. Effects of heat on mouse spermatogenesis monitored by flow cytometry. Int. J. Hyperthermia. 1990;6:543–551. doi: 10.3109/02656739009140950. [DOI] [PubMed] [Google Scholar]
- Dym M. Basement membrane regulation of Sertoli cells. Endocr. Rev. 1994;15:102–115. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasinska A, Hill S. The effect of hyperthermia on the Mouse testis. Neoplasma. 1990;37:357–366. [PubMed] [Google Scholar]
- Gye MC. Expression of occludin in canine testis and epididymis. Reprod. Dom. Anim. 2004;39:43–47. doi: 10.1046/j.1439-0531.2003.00474.x. [DOI] [PubMed] [Google Scholar]
- Hacker U, Schumann J, Göhde W. Effects of acute gamma-irradiation on spermatogenesis as revealed by flow cytometry. Acta Radiol. Oncol. 1980;19:361–368. doi: 10.3109/02841868009131321. [DOI] [PubMed] [Google Scholar]
- Hacker U, Schumann J, Göhde W, Müller K. Mammalian spermatogenesis as a biologic dosimeter for radiation. Acta Radiol. Oncol. 1981;20:279–282. doi: 10.3109/02841868109130207. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Wilson G, Russell LD, Meistrich ML. Radiation-induced cell death in the mouse testis: relationship to apoptosis. Radiat. Res. 1997;147:457–467. Erratum in: Radiat Res 1998 Mar; 149: 316. [PubMed] [Google Scholar]
- Hess RA, Linder RE, Strader LF, Perreault SD. Acute effects adn long-term sequelae of 1,3-dinitrobenzene on male reproduction in the rat II. Quantitative and Qualitative Histopathology of the Testis. J. Androl. 1988;9:327–342. doi: 10.1002/j.1939-4640.1988.tb01059.x. [DOI] [PubMed] [Google Scholar]
- Jensh RP, Brent RL. Effects of prenatal X-irradiation on postnatal teticular development and function in the Wistar rat: develoment/teratology/behavior/radiation. Teratology. 1988;38:443–449. doi: 10.1002/tera.1420380507. [DOI] [PubMed] [Google Scholar]
- Kanter M, Topcu-Tarladacalisir Y, Parlar S. Antiapoptotic effect of L-carnitine on testicular irradiation in rats. J. Mol. Histol. 2010;41:121–128. doi: 10.1007/s10735-010-9267-5. [DOI] [PubMed] [Google Scholar]
- Khan RV, Brown IR. The effect of hyperthermia on the induction of cell death in brain, testis, thymus of the adult and developing rat. Cell Stres Chaperones. 2002;7:73–90. doi: 10.1379/1466-1268(2002)007<0073:teohot>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Heo K, Yi JM, et al. Genistein mitigates radiation-induced testicular injury. Phytother. Res. 2012;26:1119–1125. doi: 10.1002/ptr.3689. [DOI] [PubMed] [Google Scholar]
- Liu FH, Yang DZ, Wang YF, et al. Making of the animal model with sterilized testes. Zhonghua Nan Ke Xue. 2007;13:125–129. [PubMed] [Google Scholar]
- Lue YH, Hikim AP, Swerdloff RS, et al. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone on stege specificity. Endocrinology. 1999;140:1709–1717. doi: 10.1210/endo.140.4.6629. [DOI] [PubMed] [Google Scholar]
- Lui W, Dolores M, Lee WM, Cheng CY. Sertoli cell tight junction dynamics: their regulation during spermatogenesis. Biol. Reprod. 2003;68:1087–1097. doi: 10.1095/biolreprod.102.010371. [DOI] [PubMed] [Google Scholar]
- Miraglia SM, Hayashi H. Histomorphometry of immature rat testis after heating. J. Morphol. 1993;217:65–74. doi: 10.1002/jmor.1052170106. [DOI] [PubMed] [Google Scholar]
- Moore CR, Chase HD. Heat application and testicular degeneration. Anat. Rec. 1923;26:344–403. [Google Scholar]
- Morales A, Mohamed F, Cavicchia JC. Apoptosis and blood-testis barrier during the first spermatogenic wave in the pubertal rat. Anat. Rec. (Hoboken) 2007;290:206–214. doi: 10.1002/ar.20417. [DOI] [PubMed] [Google Scholar]
- Moreno SG, Dutrillaux B, Coffigny H. High sensitivity of rat foetal germ cells to low dose-rate irradiation. Int. J. Radiat. Biol. 2001;77:529–538. doi: 10.1080/09553000010030211. [DOI] [PubMed] [Google Scholar]
- Moroi S, Saitou M, Fujimoto K, et al. Occludin is concentrated at tight junctions of mouse/rat but not human/guinea pig Sertoli cells in testes. Am. J. Physiol. 1998;274:1708–1717. doi: 10.1152/ajpcell.1998.274.6.C1708. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Pelletier RM. The tight junctions in the testis, epididymis, and vas deferens. In: Cereijido M, Anderson J, editors. Tight Junctions. 2nd edn. Boca Raton, FL: CRC Press; 2001. pp. 599–628. [Google Scholar]
- Ribeiro AF, David-Ferreira JF. The inter-sertoli cell tight junctions in germ cell-free seminiferous tubules from prenatally irradiated rats: a freeze-fracture study. Cell Biol. Int. 1996;20:513–522. doi: 10.1006/cbir.1996.0066. [DOI] [PubMed] [Google Scholar]
- Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, Dix DJ. Effect of hyperthermia on spermatogenesis, apoptosis, gene expression and fertility in adult mal emice. Biol. Reprod. 2001;65:229–239. doi: 10.1095/biolreprod65.1.229. [DOI] [PubMed] [Google Scholar]
- Rowley MJ, Leach DR, Warner GA, Heller CG. Effect of graded doses of ionizing radiation on the human testis. Radiat. Res. 1974;59:665–678. [PubMed] [Google Scholar]
- Russell LD. Morphological and functional evidence for Sertoli germ cell relationships. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater, FL: Cache River Press; 1993. pp. 365–390. [Google Scholar]
- Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int. Rev. Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- iu MK, Cheng CY. Extracellular matrix: recent advances on its role in junction dynamics in the seminiferous epithelium during spermatogenesis. Biol. Reprod. 2004;71:375–391. doi: 10.1095/biolreprod.104.028225. [DOI] [PubMed] [Google Scholar]
- Take G, Erdogan D, Helvacioglu F, et al. Effect of melatonin and time of administration on irradiation-induced damage to rat testes. Braz. J. Med. Biol. Res. 2009;42:621–628. doi: 10.1590/s0100-879x2009000700006. [DOI] [PubMed] [Google Scholar]
- Topcu-Tarladacalisir Y, Kanter M, Uzal MC. Role of L-carnitine in the prevention of seminiferous tubules damage induced by gamma radiation: a light and electron microscopic study. Arch. Toxicol. 2009;83:735–746. doi: 10.1007/s00204-008-0382-y. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- Turner TT, Lysiak JJ. Oxidative stress: a common factor in testicular dysfunction. J. Androl. 2008;29:488–498. doi: 10.2164/jandrol.108.005132. [DOI] [PubMed] [Google Scholar]
- Xu D, Qin DN, Wang Y, Zhuang CX, Li WQ. Effect of local testicular heating on spermatogenic cell apoptosis in rat. Zhonghua Nan Ke Xue. 2003;9:170–174. [PubMed] [Google Scholar]
- Yoon SI, Park CJ, Nah WH, Gye MC. Expression of occludin in testis and epididymis of wild rabbits, Lepus sinensis coreanus. Reprod. Dom. Anim. 2008;10:1–6. doi: 10.1111/j.1439-0531.2008.01064.x. [DOI] [PubMed] [Google Scholar]


