Abstract
The hip joint is one of the major structures in the human body and the resultant force acting through the hip joint is 300% of body weight. Therefore, weight bearing, as a cause of ischaemia, may contribute to the development of non-traumatic osteonecrosis of the femoral head (ONFH). However, it remains unclear whether weight bearing is related to the development of non-traumatic ONFH. Therefore the aim of this study was to clarify the role of weight bearing in the development of non-traumatic ONFH. Non-weight-bearing (NWB) rats were tail suspended to prevent any weight coming to bear on the hindlimbs from day 1 to the time of sacrifice. The weight-bearing (WB) group rats were also housed individually, although without tail suspension. All rats were injected with lipopolysaccharide and methylprednisolone to promote the development of non-traumatic ONFH. All animals were sacrificed three weeks after the final methylprednisolone injection. Histopathological analysis was performed. Osteonecrosis of the femoral head was observed not only in the NWB but also in the WB rats; however, no osteonecrosis of the humeral head was observed in either group. We confirmed that non-traumatic ONFH developed in NWB rats, suggesting that weight bearing does not contribute to the development of non-traumatic ONFH in rats.
Keywords: animal model, femoral head osteonecrosis, pathogenesis, steroid, weight bearing
Non-traumatic osteonecrosis is classified as corticosteroid-related, alcohol-related, or idiopathic (Mont et al. 2006). Most cases of non-traumatic osteonecrosis are observed in the femoral head (Lafforgue 2002). Non-traumatic osteonecrosis of the femoral head (ONFH) is believed to be a multifactorial disease. Several factors have been invoked in the pathogenesis abnormalies of lipid metabolism (Miyanishi et al. 1999), thrombosis (Glueck et al. 2003), oxidative stress (Ichiseki et al. 2006), genetic polymorphisms (Glueck et al. 2008; Levasseur 2008) and direct or indirect disruption of vascular supply (Mankin 1992; Jones & Hungerford 2004). However, the pathogenesis of non-traumatic ONFH remains unclear.
The hip joint supports the weight of the upper body and decreases impulsion loading from the lower body to the upper body. Experimental studies have found that the resultant force acting through the hip joint is around 300% of total body weight during normal walking (Bergmann et al. 1989). An experimental study using spontaneously hypertensive rats demonstrated that weight bearing and disruption of the retinacular vessels that feed the femoral head caused ischaemic damage in the bone, resulting in ONFH of Perthes' disease (Mihara & Hirano 1998). Therefore, weight bearing, as a cause of ischaemia, may contribute to the development of non-traumatic ONFH.
We reported that lipopolysaccharide (LPS)-treated rats developed ONFH after the administration of corticosteroids (Okazaki et al. 2009). However, in this rat model, we could not exclude the possibility of weight bearing contributing to the development of ONFH.
On the other hand, a hindlimb unloading rat model was developed at the National Aeronautics and Space Administration (NASA) Ames Research Centre to study the consequences of skeletal unloading, as occurs during space flight, by removing weight-bearing (WB) loads from the hindquarters (Morey-Holton & Globus 1998).
Therefore, we speculated whether or not weight bearing might lead to the development of non-traumatic ONFH in the rat model. In this study, we examined the incidence of non-traumatic ONFH between WB and non-weight-bearing (NWB) rats to clarify the role of weight bearing in the development of non-traumatic ONFH.
Materials and methods
Animals
Male Wistar rats (10 weeks old) were obtained from Sankyo Labo Service, Co. Ltd. (Sapporo, Japan). Each animal was housed in an individual cage in a temperature- and humidity-controlled room with unlimited food and water and a 12-h light/dark cycle. All animals (n = 22) were given 0.3-mg/kg LPS (from Escherichia coli serotype 055: B5; Sigma, St. Louis, MO, USA) intravenously on days 1 and 2. On days 3, 4 and 5, the animals were given 20-mg/kg methylprednisolone intramuscularly to promote development of non-traumatic ONFH as described previously (Okazaki et al. 2009). All the injections were administered at 7:00 p.m.
Hindlimb unloading was accomplished as described by Wronski and Morey-Holton (Morey-Holton & Globus 1998) with some modifications (Okazaki et al., unpublished observation). We used minimal restraints to avoid stressing threat during the hindlimb unloading procedure. Briefly, the tail was cleaned with alcohol and allowed to dry. The tail was then varnished with instant adhesive (ARON ALPHA®; Toagosei Co., Ltd., Tokyo, Japan), and a strip of traction tape (Skin-trac, 3 × 40 in.; Zimmer, Warsaw, IN, USA) was then attached to the tail beginning at the base of the tail above the hairline. To facilitate free movement about the cage, the cast was attached to a swivel anchored to the cage that allowed a 360° range of movement. The animals were then suspended in a head-down tilted position. Over time, the suspension angle was readjusted as the body size increased to prevent any weight coming to bear on the hindlimbs. In NWB rats (n = 11), the tail was suspended from day 1 to sacrifice. All animals were sacrificed 3 weeks after the last methylprednisolone injection. The femur and humerus were harvested from each rat and fixed with 10% formalin-0.1 M phosphate buffer, pH 7.4.
Histopathology
Bone samples were decalcified with Kalkitox™ (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and then neutralized with sodium sulphate buffer. The tissues were processed for routine haematoxylin and eosin staining to assess the general architecture and osteonecrosis of the femoral and humeral heads. We defined histopathological osteonecrosis as the diffuse presence of empty lacunae or pyknotic osteocyte nuclei in the bone trabeculae, accompanied by surrounding bone marrow cell necrosis (Figure 1) (Ichiseki et al. 2006; Okazaki et al. 2009). Three pathologists individually assessed the histological findings for the femoral and humeral heads.
Figure 1.
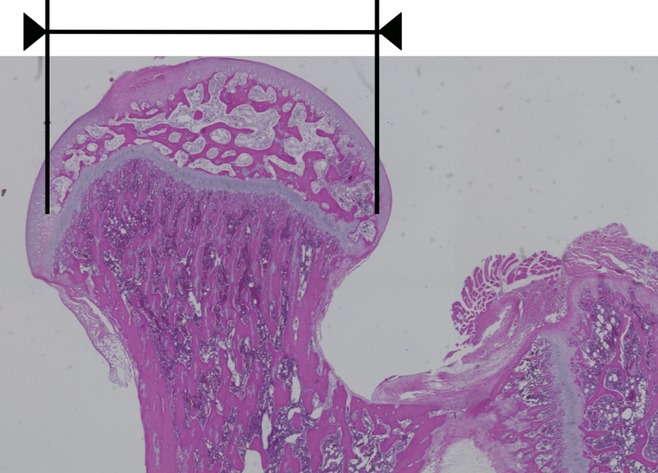
Histopathological appearance of proximal femoral epiphysis of weight-bearing (WB) rat with haematoxylin and eosin staining. Osteonecrosis was located in most area of proximal femoral epiphysis: femoral head.
Statistical analysis
Data were compared between WB and NWB groups using the chi-square test. A P-value of <0.05 was considered to be significant.
Ethical approval statement
All experiments adhered to the guidelines of the Ministry of Sports, Culture, Science and Technology of Japan and followed protocols approved by the Animal Ethics Committee of the Sapporo Medical University (#09-008).
Results
Femoral head osteonecrosis was observed in seven of 11 (63.6%) WB rats and six of 11 (54.5%) NWB rats, with no significant intergroup difference observed (P = 0.66). Figure 2 shows the histopathological appearance of the femoral head after haematoxylin and eosin staining in typical specimens from the WB (a, b) and NWB (c, d) groups. In the WB group, empty lacunae were seen at the necrotic bone trabeculae, and the number of hematopoietic cells was decreased in the medullary space at most areas of the femoral head (Figure 2a,b). In the NWB group, empty lacunae were also seen at the necrotic bone trabeculae. In addition, the number of haematopoietic cells was again decreased, and necrotic cell debris was accumulated in the medullary space at most areas of the femoral head (Figure 2c,d), as observed in the WB group. On the other hand, no humeral head osteonecrosis was observed in any of the WB or NWB rats.
Figure 2.
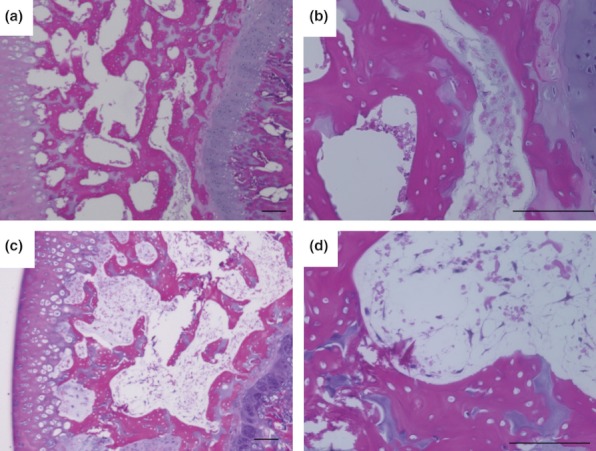
Histological appearance of femoral head osteonecrosis. Haematoxylin and eosin staining of the femur. Typical specimens of weight-bearing (WB) (a, b) and non-weight-bearing (NWB) (c, d) rats. Both WB and NWB group shows diffuse presence of empty lacunae in the bone trabeculae, accompanied by surrounding bone marrow cell necrosis at most areas of femoral head. Scale bar = 100μm.
Figure 3 shows the histopathological appearance of the humeral head in typical specimens from the WB (a, b) and NWB (c, d) groups. In the WB group, normal trabeculae, as well as haematopoietic and fat cells, were observed (Figure 3a,b). Normal trabeculae were also observed in the NWB group rats. In comparison with the WB group, a large number of haematopoietic cells, but few fat cells were observed in the NWB group rats (Figure 3c,d).
Figure 3.
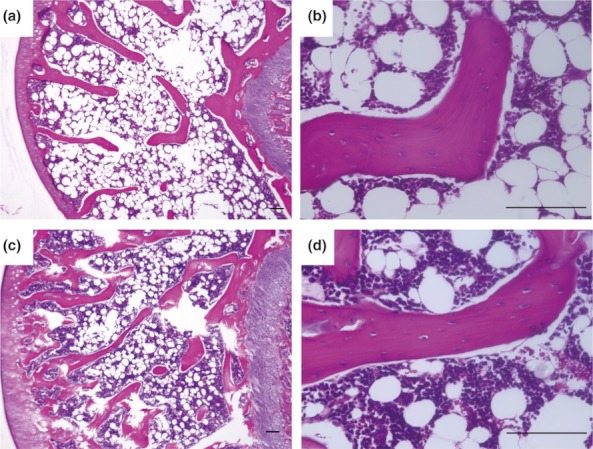
Histological appearance of the humeral head. Haematoxylin and eosin staining of the humerus. Typical specimens of weight-bearing (WB) (a, b) and non-weight-bearing (NWB) (c, d) rats. Both WB and NWB group shows no pathological change. Scale bar = 100μm.
Discussion
Osteonecrosis of the femoral head often develops after corticosteroid therapy for inflammatory diseases, such as autoimmune diseases (Fukushima et al. 2010). On the other hand, it has been reported that mega-dose corticosteroid therapy for trauma, such as spinal cord injury, does not induce ONFH (Wing et al. 1998). Therefore, we developed a non-traumatic ONFH animal model in which ONFH was encouraged by the administration of LPS (a ligand of toll-like receptor 4, and implicated in inducing inflammatory status) and methylpredonisolone treatment in the rats. In this animal model, however, rats were allowed to walk and move independently in individual cages, with the hip joints supporting the loading from the lower body. We could not exclude the possible influence of weight bearing on the hip joints on the induction of ONFH. Therefore, we employed a hindlimb unloading procedure, in which the rat tail was fitted on to a suspension system with the rats in a head-down tilted position to prevent weight bearing on the hindlimbs. In this system, the rats were allowed to move freely in individual cages. We observed that NWB group rats developed ONFH at a comparable rate as did WB group rats. Taken together, the results of the present study confirm that weight bearing does not contribute to the development of non-traumatic ONFH in rats.
To clarify the pathogenesis of ONFH, several quadruped animal models of osteonecrosis, including both traumatic and non-traumatic forms, have been reported (Yamamoto et al. 1995; Norman et al. 1998; Ichiseki et al. 2006; Okazaki et al. 2009). However, the quadruped animals in these models fail to progress to end stage mechanical collapse similar to that in bipedal animals, such as humans (Conzemius et al. 2002). Xu et al. (2010) reported that this is caused by a difference in weight bearing through the hip joint between quadruped and bipedal animals. This report suggests that weight bearing may contribute to eventual collapse. However, our results showed that weight bearing did not contribute to the initial development of ONFH in NWB rats. Therefore, it is speculated that the rat model may help us to elucidate the pathogenesis of ONFH and to devise a prevention strategy for the condition, but may not be useful for the observation of the mechanism of femoral head collapse.
In conclusion, the present study revealed that weight bearing is not related to the development of non-traumatic ONFH in the rat model, although the aetiology, pathogenesis and prognosis of non-traumatic ONFH remain unclear.
Acknowledgments
This work was supported in part by the Grants-in-Aid for Scientific Research (B) (H.M., 20390196) and for Young Scientists (B) (S.O., 22791390) of the Japanese Society for the Promotion of Science.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Bergmann G, Rohlmann A, Graichen F. In vivo measurement of hip joint stress. 1. Physical therapy. Z. Orthop. Ihre Grenzgeb. 1989;127:672–679. doi: 10.1055/s-2008-1040311. [DOI] [PubMed] [Google Scholar]
- Conzemius MG, Brown TD, Zhang Y, Robinson RA. A new animal model of femoral head osteonecrosis: one that progresses to human-like mechanical failure. J. Orthop. Res. 2002;20:303–309. doi: 10.1016/S0736-0266(01)00108-5. [DOI] [PubMed] [Google Scholar]
- Fukushima W, Fujioka M, Kubo T, Tamakoshi A, Nagai M, Hirota Y. Nationwide epidemiologic survey of idiopathic osteonecrosis of the femoral head. Clin. Orthop. Relat. Res. 2010;468:2715–2724. doi: 10.1007/s11999-010-1292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glueck CJ, Freiberg RA, Wang P. Role of thrombosis in osteonecrosis. Curr. Hematol. Rep. 2003;2:417–422. [PubMed] [Google Scholar]
- Glueck CJ, Freiberg RA, Boppana S, Wang P. Thrombophilia, hypofibrinolysis, the eNOS T-786C polymorphism, and multifocal osteonecrosis. J. Bone Joint Surg. Am. 2008;90:2220–2229. doi: 10.2106/JBJS.G.00616. [DOI] [PubMed] [Google Scholar]
- Ichiseki T, Ueda Y, Katsuda S, Kitamura K, Kaneuji A, Matsumoto T. Oxidative stress by glutathione depletion induces osteonecrosis in rats. Rheumatology. 2006;45:287–290. doi: 10.1093/rheumatology/kei149. [DOI] [PubMed] [Google Scholar]
- Jones LC, Hungerford DS. Osteonecrosis: etiology, diagnosis, and treatment. Curr. Opin. Rheumatol. 2004;16:443–449. doi: 10.1097/01.moo.0000127829.34643.fd. [DOI] [PubMed] [Google Scholar]
- Lafforgue P. Osteonecrosis of the femoral head. Rev. Prat. 2002;52:616–620. [PubMed] [Google Scholar]
- Levasseur R. Mechanisms of osteonecrosis. Joint Bone Spine. 2008;75:639–642. doi: 10.1016/j.jbspin.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis) N. Engl. J. Med. 1992;326:1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- Mihara K, Hirano T. Standing is a causative factor in osteonecrosis of the femoral head in growing rats. J. Pediatr. Orthop. 1998;18:665–669. doi: 10.1097/00004694-199809000-00022. [DOI] [PubMed] [Google Scholar]
- Miyanishi K, Yamamoto T, Irisa T, Noguchi Y, Sugioka Y, Iwamoto Y. Increased level of apolipoprotein B/apolipoprotein A1 ratio as a potential risk for osteonecrosis. Ann. Rheum. Dis. 1999;58:514–516. doi: 10.1136/ard.58.8.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J. Bone Joint Surg. Am. 2006;88:1117–1132. doi: 10.2106/JBJS.E.01041. [DOI] [PubMed] [Google Scholar]
- Morey-Holton ER, Globus RK. Hindlimb unloading of growing rats: a model for predicting skeletal changes during space flight. Bone. 1998;22:83S–88S. doi: 10.1016/s8756-3282(98)00019-2. [DOI] [PubMed] [Google Scholar]
- Norman D, Reis D, Zinman C, Misselevich I, Boss JH. Vascular deprivation-induced necrosis of the femoral head of the rat. An experimental model of avascular osteonecrosis in the skeletally immature individual or Legg-Perthes disease. Int. J. Exp. Pathol. 1998;79:173–181. doi: 10.1046/j.1365-2613.1998.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki S, Nishitani Y, Nagoya S, Kaya M, Yamashita T, Matsumoto H. Femoral head osteonecrosis can be caused by disruption of the systemic immune response via the toll-like receptor 4 signalling pathway. Rheumatology. 2009;48:227–232. doi: 10.1093/rheumatology/ken462. [DOI] [PubMed] [Google Scholar]
- Wing PC, Nance P, Connell DG, Gagnon F. Risk of avascular necrosis following short term megadose methylprednisolone treatment. Spinal Cord. 1998;36:633–636. doi: 10.1038/sj.sc.3100647. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang X, Toney CB, Seamon J, Cui Q. Blood supply to the chicken femoral head. Comp. Med. 2010;60:295–299. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Hirano K, Tsutsui H, Sugioka Y, Sueishi K. Corticosteroid enhances the experimental induction of osteonecrosis in rabbits with Shwartzman reaction. Clin. Orthop. Relat. Res. 1995;316:235–243. [PubMed] [Google Scholar]


