Abstract
Objectives
The role of surgery in stage IV gallbladder (GB) cancer is not well established. This study analyses prognostic factors in patients with stage IV GB cancer following surgical resection with the aim of identifying a subgroup of patients who might benefit from surgical resection.
Methods
Clinicopathological details were analysed for 94 patients who were surgically treated for stage IV GB cancer at Seoul National University Hospital.
Results
Median survival was 8 months in patients with either stage IVa or IVb disease. Sixteen patients (17.0%) underwent resection with curative intent, which increased overall survival over that in patients undergoing palliative surgery (P < 0.001). No survival benefit was seen following surgery with curative intent in patients with stage IVa disease (P = 0.764). Surgery with curative intent resulted in a survival benefit in patients with stage IVb disease, patients with an isolated liver metastasis near the GB bed (median survival: 31 months vs. 9 months; P < 0.001) and patients with limited numbers of peritoneal implantations (median survival: 20 months vs. 6 months; P = 0.002). Preoperative serum carcinoembryonic antigen (CEA) (P = 0.018), surgery with curative intent (P = 0.045) and adjuvant chemotherapy (P = 0.002) were independent prognostic factors in patients with stage IV GB cancer.
Conclusions
Surgery in combination with systemic chemotherapy may be beneficial in carefully selected patients with stage IVb GB cancer.
Introduction
Gallbladder (GB) carcinoma is the most frequent of biliary tract cancers.1 Despite recent advances in diagnostic tools,2,3 a lack of symptoms or signs in its early stages delays the diagnosis of GB cancer until the disease is significantly advanced. In patients with early-stage GB cancer, surgical resection can yield favourable outcomes,4 but in patients with advanced disease the prognosis is usually poor.1,5,6
Patients with stage IV GB cancer are known to have a dismal prognosis,7 even after attempted curative resection.5,6,8,9 Advanced GB cancer with para-aortic lymph node metastasis or distant metastasis has been generally considered to represent a contraindication to surgery.6,10 Aggressive surgery that attempts to improve survival has been described, although no consensus regarding indications for radical surgery has been reached.8,11–13
The purpose of this study was to analyse factors for survival and prognosis in patients with stage IV GB cancer following surgical resection, with the aim of identifying a subset of patients who may benefit from surgical resection.
Materials and methods
This study was approved by the institutional review board at Seoul National University Hospital, which waived requirements for the provision of informed consent. Clinicopathological data and radiological images were prospectively collected in electronic medical record format. From January 1996 to January 2010, 421 patients diagnosed with GB cancer underwent surgery at Seoul National University Hospital. Gallbladder cancer was diagnosed using computed tomography (CT); when necessary, magnetic resonance imaging or positron emission tomography were selectively used. Hyperbilirubinaemia was defined as serum total bilirubin of >5 mg/dl. A serum carbohydrate antigen 19-9 (CA 19-9) level of >37 U/ml was considered to indicate elevated CA 19-9. Potential negative margin (R0) resections of both the primary tumour and any metastatic lesions were considered to represent operations with curative intent. At the beginning of the operation, the peritoneal cavity was explored to identify distant metastases. The locations of any distant metastases were specified. In patients with liver metastases, the sites of liver metastases were identified according to preoperative imaging and intraoperative findings. Liver metastases within 3 cm of the GB bed were considered to be ‘near’ the GB bed. In patients with peritoneal seeding, peritoneal implantation was identified during the operation and patients with fewer than three nodules of approximately 2–3 mm in size, without evidence of disseminated metastatic disease, were considered to have limited peritoneal implantation. Following surgery, a pathological diagnosis was established according to the seventh edition of the American Joint Committee on Cancer (AJCC) staging system.14 Patients were followed in the outpatient department using tumour markers and CT every 6 months. Recurrences were identified using radiological imaging and percutaneous biopsy if necessary.
Data were analysed using IBM spss Statistics Version 19.0 (IBM Corp., Somers, NY, USA). Continuous variables are presented as medians and ranges. Continuous parameters in each group were compared using the Mann–Whitney U-test; categorical parameters were compared using the chi-squared test or Fisher's exact test. Survival analysis was performed using the Kaplan–Meier method for univariate analysis and the Cox proportional hazard model for multivariate analysis. A two-sided P-value of ≤0.05 was accepted as indicating statistical significance and a two-sided P-value of 0.05–0.10 was accepted as indicating marginal statistical significance.
Results
Of the 421 patients treated for GB cancer during the study period, 94 (22.3%) patients who underwent surgery for stage IV GB cancer were included for further analysis. Clinical, operative and pathological variables are shown in Table 1. Seventy-one (75.5%) patients had died by the end of the study; the median follow-up in those still alive was 8.0 months (range: 1.0–101.0 months). Sixteen patients (17.0%) had stage IVa and 78 patients (83.0%) had stage IVb disease. Two patients had incidental GB cancer with peritoneal implantation, which was found during surgery for cholecystitis.
Table 1.
Demographic and surgical data for the 94 stage IV gallbladder cancer patients
| Characteristics | Values |
|---|---|
| Age, years, median (range) | 62.0 (43.0–82.0) |
| Sex (male : female) | 1 : 1.3 |
| Preoperative serum laboratory test | |
| Bilirubin, mg/dl, median (range) | 0.9 (0.3–30.6) |
| CEA, ng/ml, median (range) | 3.5 (0.7–3070.0) |
| CA 19-9, U/ml, median (range) | 31.0 (1.0–80 000.0) |
| Disease stage, AJCC 7th edition, n (%) | |
| IVa | 16 (17.0%) |
| T4N0 | 4 (4.3%) |
| T4N1 | 7 (7.4%) |
| T4Nx | 5 (5.3%) |
| IVb | 78 (83.0%) |
| Para-aortic lymph node metastasis | 22 (23.4%) |
| Liver metastasis | 20 (21.3%) |
| Peritoneal implantation | 36 (38.3%) |
| Operation, n (%) | |
| Resection with curative intent | 16 (17.0%) |
| Extended cholecystectomy | 6 (6.4%) |
| Hepatopancreatoduodenectomy | 4 (4.3%) |
| Extended right hemihepatectomy | 4 (4.3%) |
| Whipple's operation and extended cholecystectomy | 1 (1.1%) |
| Laparoscopic cholecystectomy with metastasectomy | 1 (1.1%) |
| Palliation | 78 (83.0%) |
| Resection margin status, n (%) | |
| R0 | 15 (16.0%) |
| R1 | 1 (1.0%) |
| R2 | 78 (83.0%) |
| Adjuvant chemotherapy, n (%) | 30 (31.9%) |
| Follow-up, months, median (range) | 8.0 (1–101) |
CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen 19-9; AJCC, American Joint Committee on Cancer.
Surgery with curative intent was performed in 16 patients (17.0%), in 15 of whom R0 resection was achieved. Palliative resections were performed in patients with combined cholecystitis or impending obstruction of the gastrointestinal tract. Surgical procedures in the 78 (83.0%) patients who underwent palliative resection included one hepatectomy (1.1%), four extended cholecystectomies (4.3%), 58 cholecystectomies (61.7%), eight bypass operations (8.5%) and seven open biopsies (7.4%). A comparison of outcomes in patients undergoing curative resection with those undergoing palliative resection showed no statistical difference in terms of age (mean age: 48.44 years vs. 47.31 years; P = 0.880), male : female ratio (1.7 vs. 1.2; P = 0.588), incidence of preoperative hyperbilirubinaemia (three of 16 patients vs. 14 of 78 patients; P = 0.940) or elevated CA 19-9 (eight of 15 patients vs. 40 of 68 patients; P = 0.697). The curative resection group included a lower percentage of patients with serum CEA of >5 ng/ml [two of 16 patients (12.5%) vs. 35 of 74 patients (47.3%); P = 0.010] and a higher proportion of patients treated with adjuvant chemotherapy (62.5% vs. 25.6%; P = 0.004). The curative resection rate was higher in stage IVa (n = 6, 37.5%) than in stage IVb (n = 10, 12.8%) GB cancer (P = 0.027).
The clinicopathological characteristics of the 16 patients who underwent surgery with curative intent are shown in Table 2.
Table 2.
Clinicopathological characteristics of 16 patients who underwent resection with curative intent
| Stage | Age, years/sex | Operation | T | N | M | Metastasis site | Survival, months | Final status | Chemotherapy | Radiation | Recurrence (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IVa | 80/M | HPD | 4 | 0 | 0 | 5 | Dead | − | − | Liver (4) | |
| 67/M | HPD and colectomy | 4 | 0 | 0 | 9 | Dead | − | − | Liver (7) | ||
| 66/F | ERH and colectomy | 4 | 0 | 0 | 7 | Dead | − | − | Seeding and regional LN (7) | ||
| 54/M | ERH, segmental duodenectomy | 4 | 0 | 0 | 16 | Dead | + | + | Seeding (14) | ||
| 53/F | HPD | 4 | 1 | 0 | 8 | Dead | − | − | Seeding (5) | ||
| 65/F | HPD | 4 | 1 | 0 | 8 | Dead | − | − | Liver (5) | ||
| IVb | 64/M | EC | 3 | 2 | 0 | Para-aortic LN | 34 | Dead | + | − | Regional LN (22) |
| 58/F | EC | 2 | 1 | 1 | S5 (1 lesion) | 30 | Alive | + | − | Regional LN (20) | |
| 44/F | ERH | 2 | 1 | 1 | S5/6 (2 lesions) | 22 | Dead | + | − | Liver (14) | |
| 74/F | EC | 3 | 1 | 1 | S5 (1 lesion) | 109 | Alive | − | − | No | |
| 55/F | ERH | 3 | 1 | 1 | S4 (2 lesions) | 39 | Alive | + | − | No | |
| 57/F | LC, seeding nodule excision | 2 | 0 | 1 | Seeding (abdominal wall, omentum, diaphragm) | 57 | Alive | − | − | No | |
| 67/F | EC | 2 | 1 | 1 | Seeding (mesentery) | 11 | Dead | + | + | Liver (6) | |
| 60/F | EC | 3 | 1 | 1 | Seeding (omentum) | 20 | Dead | + | − | Seeding (10) | |
| 73/M | EC | 4 | 0 | 1 | Seeding (abdominal wall) | 26 | Dead | + | − | Seeding (6) | |
| 59/M | HPD | 4 | 1 | 1 | Seeding (mesentery) | 11 | Alive | + | − | Para-aortic LN (11) | |
T, tumour; N, node; M, metastasis; M, male; F, female; LN, lymph node; ERH, extended right hemihepatectomy; HPD, hepatopancreatoduodenectomy; EC, extended cholecystectomy; LC, laparoscopic cholecystectomy.
Adjuvant chemotherapy was performed more often in patients who underwent curative resection than in those undergoing palliative surgery (10 of 16 patients vs. 20 of 78 patients; P = 0.004). Various chemotherapeutic agents were used: 11 patients received fluorouracil (5-FU)-based chemotherapy; seven received gemcitabine-based chemotherapy; six received titanium silicate (TS)-1 based chemotherapy, and four were treated with chemotherapy based on miscellaneous agents. The chemotherapeutic agents used in the other two patients were not identified.
Overall median survival in patients with stage IV GB cancer who underwent surgery was 8 months [95% confidence interval (CI) 7.2–8.8]. Median survival after surgery with curative intent (n = 16) was significantly higher than that in patients who underwent palliative surgery (n = 78) [median survival: 16.0 months (95% CI 6.2–25.8) vs. 8.0 months (95% CI 6.9–9.1); P < 0.001] (Fig. 1). Median survival rates in all patients with stage IVa (n = 16) and stage IVb (n = 78) disease were comparable [8.0 months (95% CI 6.5–9.5) vs. 8.0 months (95% CI 7.1–8.9); P = 0.400]. Of the patients who underwent surgery with curative intent (n = 16), those with stage IVb disease (n = 10) achieved better survival than those with stage IVa disease (n = 6) [median survival: 26.0 months (95% CI 13.6–38.4) vs. 8.0 months (95% CI 6.9–9.1); P < 0.001].
Figure 1.
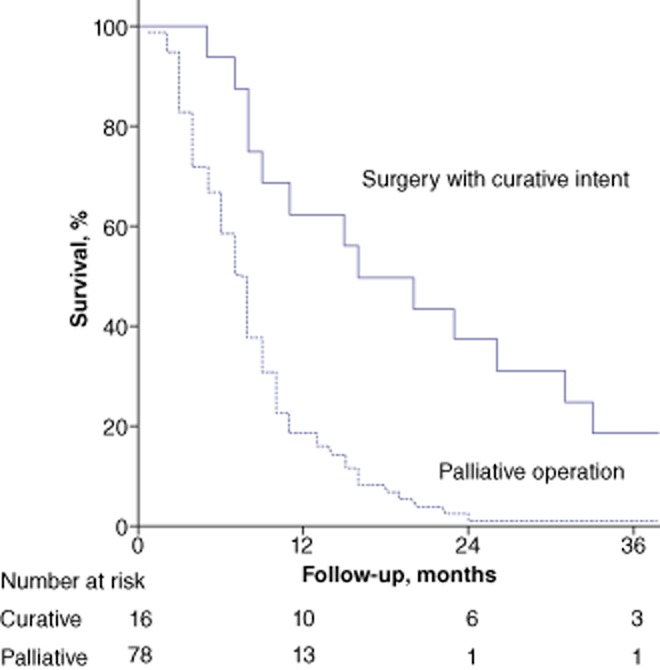
Overall survival in stage IV gallbladder cancer after surgery with curative intent (n = 16; median survival: 19 months) or palliative surgery (n = 78; median survival: 8 months) (P < 0.001)
In patients with stage IVa disease, no difference in survival was noted between those who underwent surgery with curative intent [n = 6; median survival: 8.0 months (95% CI 6.9–9.1)] and those who underwent surgical palliation [n = 10; median survival: 9.0 months (95% CI 6.2–11.8)] (P = 0.764). Of the 20 patients with liver metastases, four patients with one or two isolated liver metastases near the GB bed underwent curative resection. In patients with liver metastases, median survival in those who underwent surgery with curative intent (n = 4; 31.0 months) was higher than in those who underwent palliative surgery [n = 16; 9.0 months (95% CI 5.3–12.7)] (P < 0.001). Peritoneal implantation was observed in 36 patients at surgery. Five patients with limited numbers of seeding nodules underwent resection with curative intent that included the excision of seeding nodules. In patients with limited peritoneal implantation, median survival was higher in those who underwent surgery with curative intent [n = 5; 20.0 months (95% CI 9.3–30.7)] than in those who underwent palliative surgery [n = 31; 6.0 months (95% CI 3.3–8.7)] (P = 0.002).
Of the 16 patients who underwent surgery with curative intent, 13 developed recurrence (liver metastases, n = 5; peritoneal seeding, n = 5; metastatic lymphadenopathy, n = 3). In the 16 patients with stage IV GB cancer who underwent surgery with curative intent, median disease-free survival was 7 months (95% CI 6.7–7.3) and the rate of 1-year disease-free survival was 26.7%.
Univariate and subsequent multivariate analyses of potential important prognostic factors are shown in Table 3. Univariate analysis of potentially important factors in the 16 patients who underwent surgery with curative intent for stage IV GB cancer is shown in Table 4.
Table 3.
Prognostic factor analysis in 94 patients with stage IV gallbladder cancer
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| n | Median survival, months | P-value | HR | 95% CI | P-value | |
| Age, years, <60/≥60 | 39/55 | 8/8 | 0.172 | |||
| Sex, male/female | 41/53 | 8/8 | 0.525 | |||
| Bilirubin, maximum, mg/dl, ≤5/>5 | 77/17 | 8/9 | 0.801 | |||
| CEA, ng/ml, ≤5/>5 | 53/37 | 10/7 | <0.001 | 1.849 | 1.109–3.084 | 0.018 |
| CA 19-9, U/ml, ≤37/>37 | 35/48 | 8/8 | 0.803 | |||
| Stage, IVa/IVb | 16/78 | 8/8 | 0.400 | |||
| Distant metastases, +/− | 57/37 | 8/8 | 0.426 | |||
| Resection with curative intent, +/− | 16/78 | 16/8 | <0.001 | 2.042 | 1.016–4.101 | 0.045 |
| R0 resection, +/− | 15/79 | 16/8 | <0.001 | |||
| Adjuvant chemotherapy, +/− | 30/55 | 14/7 | <0.001 | 2.367 | 1.371–4.085 | 0.002 |
| Histological grade, G1/G2/G3/G4 | 15/42/25/1 | 8/9/8/33 | 0.462 | |||
The variables ‘Resection with curative intent’ and ‘R0 resection’ were linearly dependent covariates and their correlation efficient was 0.962 (P < 0.001). In consequence, R0 resection was removed from the multivariate analysis model.
HR, hazard ratio; 95% CI, 95% confidence interval; CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen 19-9.
Table 4.
Prognostic factors in 16 patients with stage IV gallbladder cancer who underwent surgery with curative intent
| Variables | n = 16 | Median survival, months | P-value |
|---|---|---|---|
| Age, years, <60/≥60 | 7/9 | 11/23 | 0.319 |
| Sex, male/female | 6/10 | 15/20 | 0.295 |
| Bilirubin, maximum, mg/dl, ≤5/>5 | 13/3 | 20/16 | 0.608 |
| CEA, ng/ml, ≤5/>5 | 14/2 | 20/8 | 0.158 |
| CA 19-9, U/ml, ≤37/>37 | 7/8 | 31/11 | 0.206 |
| Distant metastasis, +/− | 9/7 | 26/8 | 0.014 |
| Stage, IVa/IVb | 6/10 | 8/26 | <0.001 |
| Resection of hepatic metastasis near GB bed, +/− | 4/12 | 31/11 | 0.054 |
| Peritoneal implantation, +/− | 5/11 | 20/16 | 0.808 |
| Adjuvant chemotherapy, +/− | 10/6 | 23/8 | 0.091 |
| Histological grade, G1/G2/G3/G4 | 3/7/4/1 | 11/23/15/33 | 0.154 |
CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen 19-9; GB, gallbladder.
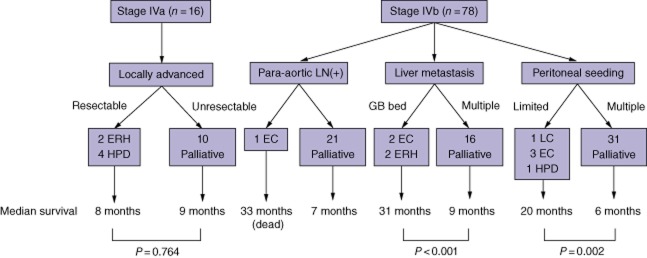
Figure 2 summarizes the treatment scheme for stage IV GB cancer in this study. In patients with liver metastases, adjuvant chemotherapy was given to three of the four patients treated with surgery with curative intent compared with five of the 15 patients treated palliatively (P = 0.262). In patients with peritoneal seeding, adjuvant chemotherapy was administered to all patients who underwent surgery with curative intent (n = 5) and nine of the 27 patients who underwent palliative surgery (P = 0.010).
Figure 2.
Treatment scheme in stage IV gallbladder (GB) cancer. Curative resection had limited prognostic value in stage IVa disease. In stage IVb disease, resection with curative intent was performed in patients with a solitary liver metastasis at the GB bed or a single peritoneal seeding nodule. In these selected cases, resection with curative intent resulted in a significant survival benefit. Statistical significance could not be calculated in patients with para-aortic lymph node metastasis because the curative resection group included only one such patient. LN, lymph node; ERH, extended right hepatectomy; HPD, hepatopancreatoduodenectomy; EC, extended cholecystectomy; LC, laparoscopic cholecystectomy with metastatectomy
Discussion
Gallbladder cancers are known to have a dismal prognosis unless the disease is treated in the early stages.1 Early GB cancers are usually found incidentally after cholecystectomy conducted as a result of a GB stone or cholecystitis, and curative resection surgery results in a favourable outcome.4 However, despite improvements in diagnostic modalities, the majority of GB cancers are not diagnosed until the disease has reached an advanced stage3 and the prognosis is apparently poor even after radical resection.5,6,13 Thus, although there are many challenges in improving survival outcomes in advanced GB cancer patients, some studies have reported positive results. Since the advantages of radical surgery in cases of liver invasion were reported,9 the use of extensive surgery in advanced GB cancer has gained support.5,6,8,12,15 Furthermore, there are reports of at least 22 patients who have survived for >5 years after aggressive surgery for stage IV GB cancer.13,16 The possibility of longterm survival after curative surgery in patients with para-aortic lymph node metastases or isolated liver metastases has also been suggested.17
However, the use of various staging systems for GB cancer, as defined by the AJCC,18 the International Union against Cancer [Union Internationale contre le Cancer (UICC)]19 and the Japanese Society of Biliary Surgery (JSBS),20 causes confusion in the interpretation of survival outcomes in advanced GB cancer. In particular, the JSBS staging system20 definitions of T3 and T4 disease differ from those of the AJCC18 and the UICC19 in terms of the depth of liver invasion. In addition, the tumour–node–metastasis (TNM) stage used to define stage IV varies across the systems. In consequence, in stage IV GB cancer defined according to the JSBS staging system, the curative resection and 5-year survival rates have been reported to be as high as 66% and 14%, respectively,12 whereas 5-year survival in patients staged according to the UICC system is reported to be 3–7%.5,6 Moreover, previous studies have dealt with limited numbers of patients in whom stage IV GB cancer with metastatic disease has been resected with curative intent. This study provides the largest cohort (n = 94) of patients with stage IV GB cancer, defined according to the seventh edition of the AJCC staging system,14 to have undergone surgery. The authors specifically compared survival in patients with locally advanced stage IV GB cancer (stage IVa) with that in patients with distant metastases (stage IVb disease).
In this study, overall median survival was 8 months in both stage IVa and stage IVb GB cancer. Surgery with curative intent was performed in 16 patients (17.0%), in whom outcomes were favourable compared with those in patients undergoing palliative surgery alone. The data in this study show that surgery with curative intent in locally advanced GB cancer with or without lymph node metastasis (stage IVa) did not improve survival rates. This finding confirms previous reports in patients with extensive nodal metastases or advanced T-stage disease which indicated that such patients are poor candidates for aggressive surgical intervention.15,21,22
In general, patients with advanced GB cancer with distant metastases (including para-aortic lymph node metastases) have not been thought to benefit from aggressive surgery.10,16 Although aggressive surgery for GB cancers with liver invasion has been reported to enhance survival outcomes,9,15 it has not been universally accepted as offering survival benefit.5,6,13 Moreover, longterm survival after surgical resection in patients with metastatic disease has rarely been reported.16 However, the current study shows that a carefully selected subset of patients with distant metastases (stage IVb disease) demonstrated a significant survival gain after surgery with curative intent. These included two longterm survivors, one of whom had a single S5 metastasis near the GB bed and underwent an extended cholecystectomy in which the extent of the liver resection included the hepatic metastatic nodule; this patient was alive and disease-free at 109 months postoperatively. The other patient, in whom three peritoneal seeding nodules were found at the abdominal wall, omentum and diaphragm, underwent cholecystectomy with seeding nodule excision and was alive and disease-free at 57 months postoperatively. Thus, distant metastases did not appear to be a negative prognostic factor in overall stage IV GB cancer in the current study. Additionally, in patients treated with curative intent, the presence of distant metastases was a positive prognostic factor. Given the prognostic outcome and the pattern of recurrence, these facts imply that the tumour burden in locally advanced disease is higher than that in patients with limited metastatic disease and therefore surgery with curative intent may be appropriate in selected patients with limited metastatic disease. This is in line with a recent report by Shimizu et al., who found that neither hepatic invasion nor liver metastases were negative prognostic factors in stage IV GB cancer.12 Nishio et al. also reported a superior survival outcome in patients with para-aortic lymph node metastases or isolated liver metastases compared with those with other distant metastases.17 Previous experiences in colorectal cancer23,24 and gastric cancer25,26 indicate that in GB cancer, resection with curative intent that includes complete metastatectomy may be beneficial in carefully selected patients with stage IV disease. Obviously, further prospective studies with larger study cohorts are needed.
Along with surgery, the provision of adjuvant chemotherapy was an independent prognostic factor in patients with stage IV GB cancer. Although the equivalent adjuvant chemotherapy was given to patients with liver metastases, whether or not they underwent curative resection, a significantly higher proportion of patients with peritoneal seeding in the curative resection group received adjuvant chemotherapy. Unfortunately, there is no established standard chemotherapy for patients with locally advanced GB cancer. A recent Phase III study reported improved survival outcomes after chemotherapy based on cisplatin in combination with gemcitabine in advanced biliary tract cancer.27 However, given the retrospective nature of the present study, it is difficult to evaluate treatment effects according to the chemotherapeutic agent. However, these results support those of studies that show the importance of using a multidiscipline treatment strategy that includes both surgery and systemic chemotherapy in advanced GB cancer.28
In summary, the present study found that overall median survival was 8 months in both stage IVa and stage IVb GB cancers. Resection with curative intent was performed in 17.0% of patients with stage IV GB cancer. Resection with curative intent increased overall survival compared with palliative surgery. The multivariate analysis indicated the independent prognostic factors in stage IV GB cancer were preoperative serum CEA levels, surgery with curative intent and adjuvant chemotherapy. After resection with curative intent, overall median survival was 8 months in stage IVa and 26 months in stage IVb disease. In patients with hepatic metastatic nodules near the GB bed or those with limited numbers of peritoneal implantations, resection with curative intent provided a significant survival benefit. Adjuvant chemotherapy made a marginally significant difference to survival after curative resection. Although this study was not a prospective comparative study, it affords the conclusions that surgery with systemic chemotherapy may be beneficial in carefully selected stage IVb GB cancer patients.
Conflicts of interest
The authors have no conflict of interest to declare.
References
- 1.Donohue JH, Stewart AK, Menck HR. The National Cancer Data Base report on carcinoma of the gallbladder, 1989–1995. Cancer. 1998;83:2618–2628. doi: 10.1002/(sici)1097-0142(19981215)83:12<2618::aid-cncr29>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 2.Chijiiwa K, Sumiyoshi K, Nakayama F. Impact of recent advances in hepatobiliary imaging techniques on the preoperative diagnosis of carcinoma of the gallbladder. World J Surg. 1991;15:322–327. doi: 10.1007/BF01658723. [DOI] [PubMed] [Google Scholar]
- 3.Paimela H, Karppinen A, Hockerstedt K, Perhoniemi V, Vaittinen E, Kivilaakso E. Poor prognosis of gallbladder cancer persists regardless of improved diagnostic methods. Incidence and results of surgery during 20 years in Helsinki. Ann Chir Gynaecol. 1997;86:13–17. [PubMed] [Google Scholar]
- 4.Lee SE, Jang JY, Lim CS, Kang MJ, Kim SW. Systematic review on the surgical treatment for T1 gallbladder cancer. World J Gastroenterol. 2011;17:174–180. doi: 10.3748/wjg.v17.i2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishikawa T, Horimi T, Shima Y, Okabayashi T, Nishioka Y, Hamada M, et al. Evaluation of aggressive surgical treatment for advanced carcinoma of the gallbladder. J Hepatobiliary Pancreat Surg. 2003;10:233–238. doi: 10.1007/s00534-003-0848-5. [DOI] [PubMed] [Google Scholar]
- 6.Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K. Extensive surgery for carcinoma of the gallbladder. Br J Surg. 2002;89:179–184. doi: 10.1046/j.0007-1323.2001.02001.x. [DOI] [PubMed] [Google Scholar]
- 7.Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg. 1994;219:275–280. doi: 10.1097/00000658-199403000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kai M, Chijiiwa K, Ohuchida J, Nagano M, Hiyoshi M, Kondo K. A curative resection improves the postoperative survival rate even in patients with advanced gallbladder carcinoma. J Gastrointest Surg. 2007;11:1025–1032. doi: 10.1007/s11605-007-0181-4. [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki M, Itoh H, Ambiru S, Shimizu H, Togawa A, Gohchi E, et al. Radical surgery for advanced gallbladder carcinoma. Br J Surg. 1996;83:478–481. doi: 10.1002/bjs.1800830413. [DOI] [PubMed] [Google Scholar]
- 10.Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Uesaka K. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg. 2000;87:418–422. doi: 10.1046/j.1365-2168.2000.01384.x. [DOI] [PubMed] [Google Scholar]
- 11.Sikora SS, Singh RK. Surgical strategies in patients with gallbladder cancer: nihilism to optimism. J Surg Oncol. 2006;93:670–681. doi: 10.1002/jso.20535. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu H, Kimura F, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, et al. Aggressive surgical approach for stage IV gallbladder carcinoma based on Japanese Society of Biliary Surgery classification. J Hepatobiliary Pancreat Surg. 2007;14:358–365. doi: 10.1007/s00534-006-1188-z. [DOI] [PubMed] [Google Scholar]
- 13.Chijiiwa K, Kai M, Nagano M, Hiyoshi M, Ohuchida J, Kondo K. Outcome of radical surgery for stage IV gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2007;14:345–350. doi: 10.1007/s00534-006-1186-1. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. New York: Springer; 2009. [Google Scholar]
- 15.Wakabayashi H, Ishimura K, Hashimoto N, Otani T, Kondo A, Maeta H. Analysis of prognostic factors after surgery for stage III and IV gallbladder cancer. Eur J Surg Oncol. 2004;30:842–846. doi: 10.1016/j.ejso.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Kondo S, Nimura Y, Kamiya J, Nagino M, Kanai M, Uesaka K, et al. Five-year survivors after aggressive surgery for stage IV gallbladder cancer. J Hepatobiliary Pancreat Surg. 2001;8:511–517. doi: 10.1007/s005340100018. [DOI] [PubMed] [Google Scholar]
- 17.Nishio H, Nagino M, Ebata T, Yokoyama Y, Igami T, Nimura Y. Aggressive surgery for stage IV gallbladder carcinoma; what are the contraindications? J Hepatobiliary Pancreat Surg. 2007;14:351–357. doi: 10.1007/s00534-006-1187-0. [DOI] [PubMed] [Google Scholar]
- 18.Greene FL American Joint Committee on Cancer. AJCC Cancer Staging Manual. New York: Springer-Verlag; 2002. [Google Scholar]
- 19.Sobin LH, Wittekind C. UICC: TNM Classification of Malignant Tumours. Hoboken: John Wiley & Sons; 2002. [Google Scholar]
- 20.Japanese Society of Biliary Surgery. Classification of Biliary Tract Carcinoma. 2nd English edn. Tokyo: Kanehara; 2004. [Google Scholar]
- 21.Bartlett DL, Fong Y, Fortner JG, Brennan MF, Blumgart LH. Longterm results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996;224:639–646. doi: 10.1097/00000658-199611000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benoist S, Panis Y, Fagniez PL. Longterm results after curative resection for carcinoma of the gallbladder. French University Association for Surgical Research. Am J Surg. 1998;175:118–122. doi: 10.1016/s0002-9610(97)00269-9. [DOI] [PubMed] [Google Scholar]
- 23.Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 24.Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg. 2007;84:324–338. doi: 10.1016/j.athoracsur.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 25.Koga R, Yamamoto J, Ohyama S, Saiura A, Seki M, Seto Y, et al. Liver resection for metastatic gastric cancer: experience with 42 patients including eight longterm survivors. Jpn J Clin Oncol. 2007;37:836–842. doi: 10.1093/jjco/hym113. [DOI] [PubMed] [Google Scholar]
- 26.Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008;19:1146–1153. doi: 10.1093/annonc/mdn026. [DOI] [PubMed] [Google Scholar]
- 27.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 28.Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30:1934–1940. doi: 10.1200/JCO.2011.40.5381. [DOI] [PubMed] [Google Scholar]