Abstract
Objectives
This study evaluates the role of interventional radiology (IR) in the management of postoperative complications after pancreatoduodenectomy (PD).
Methods
A total of 120 consecutive patients were reviewed to identify IR procedures performed for early complications after PD.
Results
Findings showed that 24 patients (20.0%) required urgent radiological or surgical re-intervention for early complications, including 11 instances of post-pancreatectomy haemorrhage (PPH), six intra-abdominal abscesses, two bile leaks, one pancreatic fistula and one bowel ischaemia. Three of 24 complications were managed by surgery and 21 were managed by IR. Two of 11 PPHs involved intraluminal haemorrhage (ILH) and nine involved intra-abdominal haemorrhage (IAH). One ILH was managed conservatively and one required surgical intervention. In eight of nine patients with IAH, the bleeding site was identified on computed tomography angiography, and endovascular stenting or coil embolization were performed. No patient required a re-look laparotomy following IR for haemorrhage or intra-abdominal abscess. Overall, three of 120 patients required an urgent re-look laparotomy for early complications.
Conclusions
Rates of major morbidity after PD remain high. However, many significant complications (PPH, pancreatic fistula, intra-abdominal abscess) can be managed by IR, reducing the need for reoperation. Re-look surgery is still required in a small percentage (2.5%) of patients.
Introduction
Historically, pancreatoduodenectomy (PD) has been associated with high rates of mortality and morbidity in the early postoperative period.1,2 In recent years, refinements in surgical techniques and the evolution of high-volume specialist pancreatic centres have been associated with a significant drop in postoperative mortality from historically high levels of 25% to more acceptable rates of <5%.3,4
However, the substantial postoperative morbidity rates associated with proximal pancreatectomy have not decreased proportionately and most series have continued to report complication rates of 20–60%.3,5 When postoperative complications necessitate further surgery after PD, mortality following re-laparotomy is extremely high and lies in the range of 13–60%.6 However, considerable advances in radiological techniques and experience have increased the use of interventional radiology (IR) in specialist centres for the initial first-line management of postoperative complications, and this may reduce the need for re-look laparotomy for early serious postoperative complications.6,7 Interventional radiology has demonstrated an increasing role in the successful early management of post-pancreatectomy haemorrhage (PPH),8 postoperative intra-abdominal abscess, biloma and other collections.9
This study consists of a review of an institutional experience of the role of IR in the management of postoperative complications after PD, and an investigation into the impact of IR on the need for re-look or repeat surgical interventions.
Materials and methods
All patients who underwent PD from January 2002 to December 2011 at Ninewells Hospital, Dundee, were reviewed. Data were obtained from a prospectively maintained departmental hepatopancreatobiliary database.
All early and late postoperative complications were identified and reviewed. Early complications were defined as those that occurred within 90 days of the primary operation, or those that occurred prior to discharge from the index admission. In-hospital mortality was defined as death within 30 days of surgery or prior to discharge from the index hospital admission.
Reoperation within the first 90 days post-surgery and length of hospitalization were also recorded. Patients with complications were then reviewed to determine the use of IR procedures after surgery, including: (i) postoperative drainage of an intra-abdominal abscess; (ii) mesenteric angiography with or without coil embolization or endovascular stenting for PPH; (iii) radiologically guided peripheral insertion of central catheters for medium-term venous access and parenteral nutrition [in pancreatic fistula (PF) or delayed gastric emptying (DGE)], and (iv) percutaneous transhepatic cholangiography (PTC) (plus internal–external biliary drain) for bile leak or postoperative biliary stricture.
The surgical techniques used for PD in this patient cohort included classic PD and pylorus-preserving PD. The decision on whether to perform classic PD or pylorus-preserving PD was determined by the individual surgeon. In all patients, pancreaticoenteric reconstruction was achieved using end-to-side pancreatojejunostomy (with no pancreatogastrostomy) as previously described.10 Two abdominal drains were placed adjacent to the pancreatic anastomosis in all patients and drain fluid was routinely measured for amylase on day 5.
Definitions of complications
Post-pancreatectomy haemorrhage
Post-pancreatectomy haemorrhage was defined as postoperative bleeding from the surgical site accompanied by a drop in haemoglobin concentration of >3 g/dl with peripheral circulatory impairment requiring medical intervention. The definition of PPH was that proposed by the International Study Group on Pancreatic Surgery (ISGPS), in which early PPH is classified as PPH occurring at <24 h after the end of the index operation, and late PPH is classified as PPH that occurs at >24 h after surgery. The location of the bleeding is classed as indicating: (i) intraluminal haemorrhage (ILH); (ii) intra-abdominal (extraluminal) haemorrhage (IAH), or (iii) ILH and IAH combined. Bleeding may be either mild or severe. Three different grades of PPH (grades A, B and C) are defined according to time of onset, site of bleeding, severity and clinical impact.11
Pancreatic fistula
Pancreatic fistula was defined by an amylase concentration in the abdominal drain fluid exceeding three times that of serum amylase at any time after postoperative day (PoD) 3. Drain fluid was routinely tested for amylase and sent for culture on PoDs 5, 10 and 15 and every 5 days thereafter if appropriate. Clinical grading of PF as Grade A, B or C was performed according to the International Study Group on Pancreatic Fistula (ISGPF) definition.12
Delayed gastric emptying
Delayed gastric emptying was graded according to the ISGPS definition.13
Intra-abdominal abscess
An intra-abdominal abscess was defined according to culture-positive drain fluid obtained from a radiologically placed drain in the postoperative period.
Results
A total of 120 PDs were performed during the study period. Patient demographics and indications for surgery are summarized in Table 1. The indications for surgery were varied and included benign (n = 10) and malignant (n = 110) pathologies (Table 1). The median hospital stay was 28 days (range: 10–93 days). The overall postoperative mortality rate was 5.0% (n = 6/120), and the overall rate of postoperative morbidity that required further surgical or radiological intervention was 24.2% (n = 29/120).
Table 1.
Patient demographics, surgery and pathological diagnosis
| Demographics | |
| Age, years, median (range) | 67 (29–85) |
| Sex (male : female) | 70 : 50 |
| ASA Grades I and II, n | 114 |
| ASA Grade III, n | 6 |
| Intraoperative data | |
| Classic PD, n | 117 |
| Pylorus-preserving PD, n | 3 |
| Operative time, min, median (range) | 470 (300–840) |
| Blood loss, ml, median (range) | 1100 (300–5800) |
| Pathology, n | |
| Benign | 10 |
| Chronic pancreatitis | 7 |
| Autoimmune pancreatitis | 1 |
| Crohn's disease | 1 |
| Biliary papillomatosis | 1 |
| Malignant | 110 |
| Pancreatic ductal adenocarcinoma | 47 |
| Ampullary adenocarcinoma | 29 |
| Cholangiocarcinoma | 18 |
| Clear cell carcinoma | 2 |
| Neuroendocrine carcinoma | 5 |
| Tubulovillous adenoma | 5 |
| Renal cell metastasis | 2 |
| Acinar cell carcinoma | 1 |
| Pseudopapillary neoplasm | 1 |
ASA, American Society of Anesthesiologists; PD, pancreatoduodenectomy.
Early post-PD complications are summarized in Table 2. A total of 24 (20.0%) patients required early re-intervention postoperatively; 21 of these patients had radiological re-intervention and three underwent surgical re-intervention.
Table 2.
Early postoperative complications after pancreatoduodenectomy (n = 120)
| Early complications | |
| Pancreatic fistula, n (%) | 36 (32.5%) |
| Grade A, n | 23 |
| Grade B, n | 9 |
| Grade C, n | 4 |
| Post-pancreatectomy haemorrhage | 11 (9.1%) |
| Grade A, n | 1 |
| Grade B, n | 1 |
| Grade C, n | 9 |
| Delayed gastric emptying, n (%) | 8 (6.6%) |
| Bile leak, n (%) | 2 (1.6%) |
| Intra-abdominal collections, n (%) | 7 (5.8%) |
| Deep vein thrombosis, n (%) | 1 (0.8%) |
| Pulmonary embolism, n (%) | 1 (0.8%) |
| Acute renal failure, n (%) | 2 (1.6%) |
| Pneumonia, n (%) | 5 (4.1%) |
| Re-laparotomy, n (%) | 3 (2.5%) |
| In-hospital mortality, n (%) | 6 (5.0%) |
Five (4.2%) patients required late re-intervention (at >6 months) for biliary stricture at the bilioenteric anastomosis; in three cases this was caused by disease recurrence and in two by benign strictures. All five patients underwent PTC and brushings. Self-expanding metal stents were placed in the three patients with disease recurrence. In the two patients with benign biliary strictures, the anastomosis was surgically revised.
Interventional radiology
In total, 36 patients developed PF according to ISGPF guidelines (Table 2). Thirteen (10.8%) patients had Grade B or C PF. Five of these 13 patients subsequently developed an intra-abdominal abscess. In four of these patients, the PF-related abscesses were drained radiologically, but one patient required a re-look laparotomy and completion pancreatosplenectomy for uncontrolled sepsis and multi-organ dysfunction associated with a high-output PF (Grade C).
Eleven patients had PPH after surgery; PPH was classed as ILH in two cases and as IAH in nine. One patient with ILH was managed conservatively with an upper gastrointestinal endoscopy and blood transfusion, and one required a re-look laparotomy and surgical intervention to oversew significant bleeding from a stapled side-to-side gastrojejunostomy.
In eight of the nine patients with IAH, frank bleeding or blood-stained fluid was observed in the abdominal drain between PoD 3 and PoD 35 (at a median of PoD 23). One patient developed massive IAH at 9 days after discharge from hospital at 48 h after outpatient removal of an abdominal drain (PoD 30). This patient required urgent readmission for computed tomography (CT) angiography followed by mesenteric angiography and coil embolization of a small proximal branch of the superior mesenteric artery (SMA). Of these nine patients with IAH, four patients had an associated infected PF that preceded the onset of PPH.
The nine patients with IAH were classed as having late haemorrhage (>24 h) based on the ISGPS classification and all nine PPHs were considered to be Grade C. All nine patients underwent urgent CT angiography. The bleeding site was identified in eight patients, in whom mesenteric angiography with endovascular vessel stenting or coil embolization was carried out immediately (Table 3).
Table 3.
Outcome of endovascular intervention for post-pancreatectomy haemorrhage (PPH)
| Patient no. | Age, years | Sex | Primary pathology | Fistula | Day of PPH | Site of PPH | Coiling/stenting | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 67 | M | Ampullary adenocarcinoma | Yes | 25 | Pseudoaneurysm splenic artery | Coil | Survived |
| 2 | 58 | M | Ampullary adenocarcinoma | Yes | 30 | Pseudoaneurysm of jejunal branch of SMA | Coil | Survived |
| 3 | 71 | M | Neuroendocrine tumour | No | 7 | Pseudoaneurysm proximal branch of SMA | Coil | Survived |
| 4 | 77 | F | Ductal adenocarcinoma | No | 3 | Pseudoaneurysm CHA | Stent | Died |
| 5 | 75 | F | Cholangiocarcinoma | Yes | 17 | Pseudoaneurysm CHA | Stent | Died |
| 6 | 71 | F | Ductal adenocarcinoma | No | 23 | Pseudoaneurysm CHA | Stent | Died |
| 7 | 80 | M | Neuroendocrine tumour | Yes | 35 | GDA stump | Coil | Survived |
| 8 | 68 | M | Ductal adenocarcinoma | No | 5 | GDA stump | Coil | Survived |
M, male; F, female; SMA, superior mesenteric artery; CHA, common hepatic artery; GDA, gastroduodenal artery.
Endovascular stent grafting (n = 3) was used to control bleeding sites in the common hepatic artery (CHA) (Fig. 1a,b). Coil embolization (n = 5) was used to control bleeding originating from branches of the SMA, gastroduodenal artery (GDA) and splenic artery (Fig. 2a,b). One patient who underwent metal coil embolization to arrest bleeding from a pseudoaneurysm at the GDA stump required a second coil embolization to arrest flow because a CT scan performed 2 weeks after the initial embolization demonstrated persistent flow from the GDA stump into the pseudoaneurysm.
Figure 1.
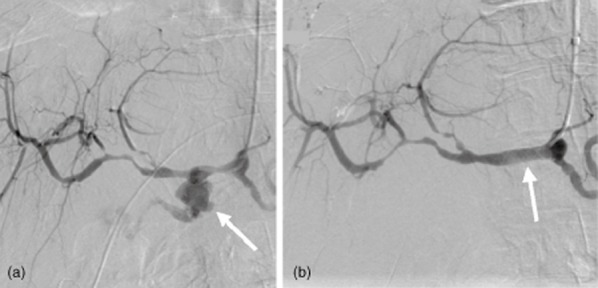
Catheter angiogram shows an intra-abdominal post-pancreatectomy haemorrhage. (a) A pseudoaneurysm with extravasation of contrast from the stump of the gastroduodenal artery (GDA) is indicated by the arrow. The tip of the catheter is in the common hepatic artery (CHA). (b) Imaging in the same patient after the deployment of a 6 × 20-mm stent-graft (arrow) in the CHA across the origin of the GDA. The stent-graft has excluded flow from the gastroduodenal stump
Figure 2.
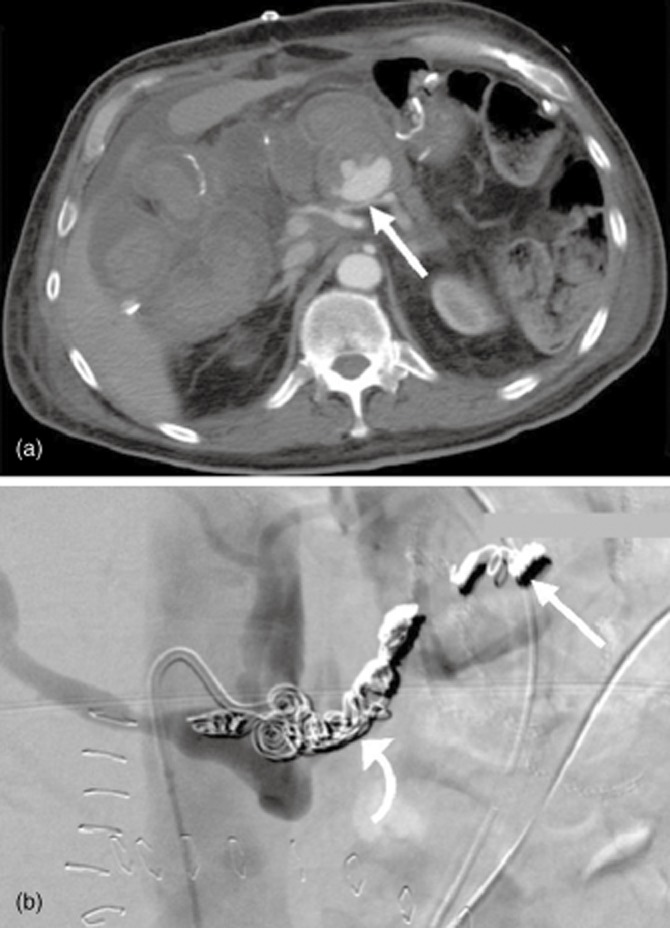
(a) Axial computed tomography demonstrates a pseudoaneurysm arising from the proximal splenic artery. The splenic artery is compressed by pressure from extravasated blood at the site of the pseudoaneurysm neck (arrow). (b) In the same patient, occlusive embolization coils are placed in the splenic artery both distal (‘back door’, straight arrow) and proximal (‘front door’, curved arrow) to the neck of the pseudoaneurysm. The tip of the catheter is in the coeliac trunk
No patient required re-look laparotomy for uncontrolled bleeding after IR and successful stenting or embolization. Overall, one of 11 patients required urgent surgery for management of PPH, eight of 11 instances of PPH were managed by radiological stenting or embolization, and two of 11 stable patients with PPH were managed conservatively. However, mortality associated with PPH remained high and three of the 11 patients in this series eventually succumbed to complications directly associated with PPH. The cause of death was acute kidney injury and renal failure in two patients, and an acute myocardial infarction in one patient. The site of haemorrhage in the three patients who died was the CHA. Sites of bleeding, types of intervention and outcomes for all patients who underwent mesenteric angiography and embolization or stenting are summarized in Table 3. Indications for all postoperative IR procedures are summarized in Table 4.
Table 4.
Indications for postoperative radiological intervention
| Interventional radiology | Patients, n | Indication |
|---|---|---|
| Early complications | ||
| Angiography and stenting or coil | 8 | Post-pancreatectomy haemorrhage |
| Insertion of percutaneous drain | 5 | Intra-abdominal abscess |
| Inferior vena cava filter | 1 | Deep vein thrombosis |
| Late complications | ||
| PTC and stent | 5 | Cancer recurrence (n = 3)/benign anastomotic stricture (n = 2) |
PTC, percutaneous transhepatic cholangiography.
Surgical intervention
Five of the 120 (4.2%) patients required surgical intervention. Three (2.5%) patients required surgery early in the index hospital admission for postoperative complications that included oversewing a staple-line for ILH (n = 1), completion pancreatectomy for PF (n = 1) and small bowel resection for acute small bowel volvulus (n = 1). Two patients required surgical intervention in the form of hepaticojejunostomy in the medium term for late benign biliary strictures at the bilioenteric anastomosis.
Three (2.5%) patients required urgent re-look surgery for serious early postoperative complications after PD, and a further 21 (17.5%) patients required rapid access to diagnostic and interventional radiology for the management of early complications.
Discussion
This study has shown that the majority of procedure-related complications after PD can be managed by IR so that surgical re-intervention is required in only a small percentage (2.5%) of patients. In the present study, rapid access to specialist IR proved to be successful for the urgent first-line management of postoperative complications in most patients, and reduced the need for urgent re-look laparotomy that is historically associated with high rates of morbidity and mortality.6 The beneficial role of IR is increasingly recognized in the management of postoperative complications after colorectal surgery,14 oesophagogastric surgery15 and bariatric surgery.16 However, the potential benefits to the patient are even more significant in pancreatic surgery, which carries an inherent risk for significant postoperative morbidity in 20–60% of patients.
Interventional radiology is increasingly recognized as having a significant role in the management of complications that arise after pancreatic surgery7,17 and should be considered an integral component of specialist pancreatic surgery centres. In the present study, the urgent 24-h availability of IR proved invaluable in the early postoperative management of serious intra-abdominal complications, such as intra-abdominal abscess and PPH. Image-guided percutaneous drainage is a well-documented standard of care for post-surgical causes of intra-abdominal abscess.7 In one institutional review, intra-abdominal abscess was identified as the most common indication for IR after PD and a small proportion of treated patients required a second radiological re-intervention with IR for the further drainage of peripancreatic collections.7 Overall, 86% of patients with intra-abdominal abscesses were managed successfully with IR without the need for reoperation.7 This is similar to findings in the present study, in which four of five patients with peripancreatic collections were successfully treated with IR and percutaneous drainage. It is well established that concurrent intra-abdominal sepsis associated with PF increases the risk for the formation of pseudoaneurysms and PPH and therefore a high index of suspicion for PPH is warranted in the presence of an infected PF, which should be managed aggressively with the radiological drainage of all associated peripancreatic collections and appropriate antibiotic therapy.18,19
Standop et al.6 reviewed the indications for re-look surgery after PD and identified early postoperative extraluminal bleeding (PPH) and intra-abdominal abscess as the two main indications for surgical re-intervention, with associated patient mortality of 13%. In the present series, PPH and intra-abdominal abscess were successfully managed with IR in >90% of patients. However, one patient with severe ILH and one patient with an intra-abdominal abscess required index admission re-look laparotomy; these patients required over-sewing of a stapled gastroenterostomy and completion pancreatectomy, respectively. Similar observations were noted in another study that assessed the role of IR after PD. Sohn et al.7 presented a series of 1062 patients, 12% (n = 129) of whom required postoperative IR. Nineteen of these 129 patients required surgical re-intervention after IR for uncontrolled PPH or further surgical drainage of an intra-abdominal abscess.7
It is well established that PPH is associated with significant morbidity and mortality, especially when further surgery is required to control the bleeding.19,20 However, IR has been shown to rapidly control bleeding in the majority of patients with both early and late PPH and appears to be associated with a significant reduction in PPH-associated mortality.20 The data from the present study show that IR can be extremely helpful in establishing rapid control of IAH, even in the presence of PF: in eight of nine patients IAH was successfully controlled by endovascular techniques, with no subsequent re-bleeding, emergency angiographic re-intervention or salvage laparotomy. However, one patient with PPH did require a second elective endovascular coiling of the GDA stump because a follow-up CT scan showed minor extravasation of contrast at 2 weeks after the initial embolization of the GDA stump and pseudoaneurysm.
In summary, major pancreatic resectional surgery is associated with lower rates of mortality in specialist pancreatic centres, but PD continues to be associated with considerable morbidity. It is increasingly recognized that IR has a significant role to play in the immediate management of early procedure-related postoperative complications (PPH, PF), and that early re-look surgery is required in a small but significant percentage of patients.
Conflicts of interest
None declared.
References
- 1.Brunschwig A. One-stage pancreaticoduodenectomy. Surg Gynecol Obstet. 1943;77:581–584. [Google Scholar]
- 2.Shapiro TM. Adenocarcinoma of the pancreas: a statistical analysis of bypass vs. Whipple resection in good risk patients. Ann Surg. 1975;182:715–721. doi: 10.1097/00000658-197512000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braga M, Capretti G, Pecorelli N, Balzano G, Doglioni C, Ariotti R, et al. A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg. 2011;254:702–707. doi: 10.1097/SLA.0b013e31823598fb. [DOI] [PubMed] [Google Scholar]
- 4.Pecorelli N, Balzano G, Capretti G, Zerbi A, Di Carlo V, Braga M. Effect of surgeon volume on outcome following pancreaticoduodenectomy in a high-volume hospital. J Gastrointest Surg. 2012;16:518–523. doi: 10.1007/s11605-011-1777-2. [DOI] [PubMed] [Google Scholar]
- 5.Balzano G, Zerbi A, Capretti G, Rocchetti S, Capitanio V, Di Carlo V. Effect of hospital volume on outcome of pancreaticoduodenectomy in Italy. Br J Surg. 2008;95:357–362. doi: 10.1002/bjs.5982. [DOI] [PubMed] [Google Scholar]
- 6.Standop J, Glowka T, Schmitz V, Schäfer N, Overhaus M, Hirner A, et al. Operative re-intervention following pancreatic head resection: indications and outcome. J Gastrointest Surg. 2009;13:1503–1509. doi: 10.1007/s11605-009-0905-8. [DOI] [PubMed] [Google Scholar]
- 7.Sohn TA, Yeo CJ, Cameron JL, Geschwind JF, Mitchell SE, Venbrux AC, et al. Pancreaticoduodenectomy: role of interventional radiologists in managing patients and complications. J Gastrointest Surg. 2003;7:209–219. doi: 10.1016/s1091-255x(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 8.Sanjay P, Fawzi A, Fulke JL, Kulli C, Tait IS, Zealley IA, et al. Late post-pancreatectomy haemorrhage. Risk factors and modern management. JOP. 2010;11:220–225. [PubMed] [Google Scholar]
- 9.Bruno O, Brancatelli G, Sauvanet A, Vullierme MP, Barrau V, Vilgrain V. Utility of CT in the diagnosis of pancreatic fistula after pancreaticoduodenectomy in patients with soft pancreas. AJR Am J Roentgenol. 2009;193:175–180. doi: 10.2214/AJR.08.1800. [DOI] [PubMed] [Google Scholar]
- 10.Tait IS. Whipple's resection – proximal pancreaticoduodenectomy (PD) J R Coll Surg Edinb. 2002;47:528–540. [PubMed] [Google Scholar]
- 11.Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Post-pancreatectomy haemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. International Study Group on Pancreatic Fistula definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Llaguna OH, Calvo BF, Stitzenberg KB, Deal AM, Burke CT, Dixon RG, et al. Utilization of interventional radiology in the postoperative management of patients after surgery for locally advanced and recurrent rectal cancer. Am Surg. 2011;77:1086–1090. [PubMed] [Google Scholar]
- 15.Arellano RS, Gervais DA, Mueller PR. Computed tomography-guided drainage of mediastinal abscesses: clinical experience with 23 patients. J Vasc Interv Radiol. 2011;22:673–677. doi: 10.1016/j.jvir.2011.01.427. [DOI] [PubMed] [Google Scholar]
- 16.Serra C, Baltasar A, Andreo L, Pérez N, Bou R, Bengochea M, et al. Treatment of gastric leaks with coated self-expanding stents after sleeve gastrectomy. Obes Surg. 2007;17:866–872. doi: 10.1007/s11695-007-9161-8. [DOI] [PubMed] [Google Scholar]
- 17.Baker TA, Aaron JM, Borge M, Pierce K, Shoup M, Aranha GV. Role of interventional radiology in the management of complications after pancreaticoduodenectomy. Am J Surg. 2008;195:386–390. doi: 10.1016/j.amjsurg.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Sanjay P, Fawzi A, Kulli C, Polignano FM, Tait IS. Impact of methicillin-resistant Staphylococcus aureus (MRSA) infection on patient outcome after pancreatoduodenectomy (PD) – a cause for concern? Pancreas. 2010;39:1211–1214. doi: 10.1097/MPA.0b013e3181e00cad. [DOI] [PubMed] [Google Scholar]
- 19.Grützmann R, Rückert F, Hippe-Davies N, Distler M, Saeger HD. Evaluation of the International Study Group of Pancreatic Surgery definition of post-pancreatectomy haemorrhage in a high-volume centre. Surgery. 2012;151:612–620. doi: 10.1016/j.surg.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 20.Yekebas EF, Wolfram L, Cataldegirmen G, Habermann CR, Bogoevski D, Koenig AM, et al. Post-pancreatectomy haemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007;246:269–280. doi: 10.1097/01.sla.0000262953.77735.db. [DOI] [PMC free article] [PubMed] [Google Scholar]


