Abstract
Background
Gastrinomas are rare neuroendocrine tumours, and responsible for Zollinger–Ellison syndrome (ZES). Surgery is the only treatment that can cure gastrinomas. The success of surgical treatment of gastrinomas in a single centre was evaluated.
Methods
A retrospective review of all patients who underwent resection for a gastrinoma between 1992 and 2011 at a single institution was performed. Presentation, diagnostics, operative management and outcome were analysed.
Results
Eleven patients with a median age of 46 years were included. All patients had fasting hypergastrinaemia and a primary tumour was localized using imaging studies in all patients. A pylorus-preserving pancreaticoduodenectomy was performed in three patients: two patients underwent duodenectomy and one patient central pancreatectomy. The remaining five patients underwent enucleation. A primary tumour was removed in nine patients: five tumours were situated in the pancreas, three in the duodenum and one patient was considered to have a primary lymph node gastrinoma. The median follow-up was 3 years (range 1–15) after which 7 patients were disease-free and 3 patients had (suspected) metastatic disease. One patient died 13 years after initial surgery.
Conclusion
The success of surgical treatment of a gastrinoma in this series was 7/11 with a median follow-up of 3 years; comparable to recent published studies.
Introduction
Gastrinomas are rare neuroendocrine tumours with an estimated incidence of 0.5 to 4/million population/year.1 In 1955, Zollinger and Ellison described the symptoms of these gastrin-secreting tumours: the Zollinger–Ellison syndrome (ZES).2 Abdominal pain as a result of peptic ulcer disease or gastro-esophageal reflux disease is the most frequent complaint, followed by diarrhoea.3
In only 0.1–1% of patients with peptic ulcers, ZES is the underlying cause.1 The diagnosis of ZES should be suspected in patients with recurrent peptic ulcers, multiple ulcers or ulcers in unusual locations such as the distal duodenum and proximal jejunum. The diagnosis is established by measuring fasting gastrin levels after antisecretory therapy has been discontinued (proton pump inhibitors 1 week, histamine type-2 receptor antagonists 2 days).4 Other causes of hypergastrinaemia, such as achlorhydria, should be excluded. Additionally, a secretin-stimulation test should be performed.5
In about 75% of patients the tumours are sporadic. Twenty-five per cent of patients have gastrinomas as part of multiple endocrine neoplasia type-1 (MEN-1); a syndrome characterized by the combined occurrence of primary hyperparathyroidism, duodenopancreatic endocrine neoplasms and tumours of the anterior pituitary gland.6
The gastric hypersecretion of ZES can generally be treated effectively with antisecretory medication.7 However, surgery is the only known curative treatment for gastrinomas.5,8
The aim of this study was to present and evaluate a tertiary referral centre's 20-year experience in the surgical management of gastrinomas.
Patients and methods
Patients referred to a single institution for surgical assessment and treatment of gastrinomas were selected from a prospective database containing patients with pancreatic and periampullary tumours between January 1992 and December 2011. Additionally, histopathological diagnoses of gastrinomas were collected using the histopathology archive PALGA (‘Pathologisch Anatomisch Landelijk Geautomatiseerd Archief’; nationwide database for pathologic specimens). Only patients with pre-operative symptoms of ZES were included. Patients with neuroendocrine tumours immunohistochemical positive for gastrin but without evidence of ZES were excluded; these tumours should be considered non-functional NETs expressing gastrin.
Data concerning clinical presentation, time to diagnosis, pre-operative diagnostic techniques and type of surgery were studied; peri-operative mortality, morbidity and overall survival were calculated. Follow-up information was collected through medical records and telephone interviews with general practitioners.
The primary outcome measure of the study was success of surgical treatment (disease-free survival), defined as normalized serum gastrin levels and absent signs of recurrent or metastatic disease on imaging. Secondary outcomes measures were surgical morbidity and the post-operative use of drugs to control gastric hypersecretion.
Results
A total of 11 patients was indentified; 4 women and 7 men (Table 1). The median age was 46 years (range 73–69). Abdominal pain as a result of peptic ulcer disease was the most frequent complaint (9 of 11) follow by diarrhoea (5 of 11). Four patients developed complicated ulcer disease before the diagnosis of ZES was made. One patient presented with hypovolemic shock as a result of perforation of a duodenal ulcer.9 The median delay between onset of symptoms and the diagnosis of ZES was 8 years (range 1–17). One patient had ZES as part of MEN 1 syndrome.
Table 1.
Clinical characteristic of 11 patients undergoing surgical resection for a gastrinoma
| Patient | Gender | Age (years) | Symptoms | Time from onset disease to diagnosis (years) | Serum gastrin (ng/l) |
|---|---|---|---|---|---|
| 1 | M | 44 | Pain, diarrhoea | 15 | 2000 |
| 2 | F | 43 | Pain | 10 | 650 |
| 3 | F | 59 | Hypovolemic shock as a result of bleeding ulcer | 1 | 5750 |
| 4 | M | 43 | Pain | 17 | 610 |
| 5 | F | 44 | Pain, diarrhoea | 6 | 1650 |
| 6 | M | 59 | Pain | 5 | 165 |
| 7 | M | 69 | Diarrhoea | 8 | 600 |
| 8 | M | 37 | Pain, diarrhoea | 1 | 356 |
| 9a | M | 61 | Pain | 11 | 300 |
| 10 | M | 46 | Pain | 4 | 285 |
| 11 | F | 56 | Pain, diarrhoea | 10 | 1400 |
MEN 1 patient.
Pre-operative diagnostics
In all patients the diagnosis of ZES had been established by measuring fasting gastrin levels. The mean serum gastrin level was 1251 ng/l (range 165–5750, normal range 0–130). A secretin stimulation test was performed in two patients. Combined conventional imaging studies [computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound (US)] were able to successfully locate the gastrinoma in ten patients. Somatostatin receptor scintigraphy (SRS) was performed using [111In-DTPA-D-Phe1]-octreotide in all patients and was combined with single-photon emission computed tomography (SPECT)/CT with the exception of the first two patients. Imaging of the primary tumour and/or metastasis using SRS was successful in all patients (Fig. 1). Four patients had suspected lymph node metastases. One patient had a suspected neuroendocrine tumour metastasis in the right lung.
Figure 1.
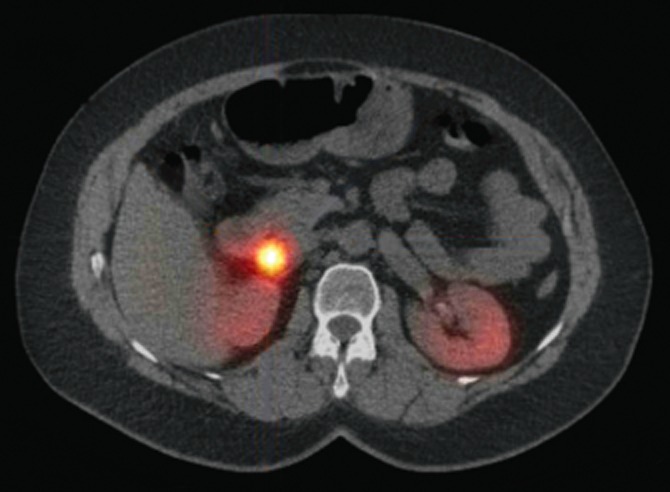
A transverse 111In-octreotide single-photon emission computed tomography (SPECT)/CT image showing a paraduodenal ‘hotspot’
Operative procedures
The decision on type of surgery was made pre- or peri-operatively based on tumour localization and size. Tumours located in the pancreas were enuclated if possible. A central or distal pancreatectomy was performed when a tumour, located in the neck, body or tail, was embedded deep in the pancreatic tissue. A pancreaticoduodenectomy was performed in patients with a large pancreatic head or duodenal tumour that could not be enucleated and in patients with multiple lymph nodes with a duodenal or pancreatic head tumour.
An intra-operative ultrasound (IOUS) was performed in five patients. Three patients underwent a pylorus-preserving pancreaticoduodenectomy (PPPD) (Table 2). In one patient an enucleation had been performed before a PPPD; only lymph node metastases were resected. Post-operative imaging located the primary tumour and a subsequent PPPD was performed. The post-operative course after PPPD was complicated in one of three patients; this patient had portal vein thrombosis for which anticoagulation therapy was subsequently administered for a course of 6 months. One patient underwent a central pancreatectomy. Two patients underwent a duodenectomy, of which one developed post-operative delayed gastric emptying requiring total parenteral nutrition. The remaining five patients underwent enucleation. In one of these five patients the post-operative course was complicated by the development of abdominal chyle leakage which was treated by percutaneous drainage and a medium-chain triglyceride diet.
Table 2.
Operative procedures and outcome in 11 patients with a gastrinoma
| Patient | Type of surgery | Localization of a gastrinoma | Lymph node metastases | Follow-up (years) | Outcome |
|---|---|---|---|---|---|
| 1 | Enucleation | Pancreas | - | 15 | Disease free |
| 2 | Enucleation | Duodenum | - | 13 | Disease free |
| 3 | Enucleation | Failed to localize primary | + | 5 | Metastatic disease Symptomatic treatment of ZES |
| 4 | Enucleation | Pancreas | - | 1 | Disease free |
| 5 | Enucleation | Lymph node primary | - | 1 | Disease free |
| 6 | Duodenectomy | Failed to localize | - | 2 | Symptomatic treatment of ZES |
| 7 | Duodenectomy | Duodenum | - | 1 | Disease free |
| 8 | Central pancreatectomy | Pancreas | - | 13 | Died of metastatic disease |
| 9a | PPPD | Pancreas | + | 3 | Suspected recurrence |
| 10 | PPPDb | Pancreas | + | 7 | Disease free |
| 11 | PPPD | Duodenum | + | 1 | Disease free |
MEN 1 patient.
Enucleation performed before PPPD.
PPPD, pylorus-preserving pancreaticoduodenectomy; ZES, Zollinger–Ellison syndrome.
Pathology
In 3 of the 11 patients the gastrinoma was located in the duodenum. Five patients had primary tumours located in the pancreas; three were situated in the pancreatic head and two in the body. Furthermore, pathological examination of the specimens showed lymph node involvement in five patients (median number of lymph nodes evaluated; 4, range 2–12). Of these patients, two had lymph node involvement (1 and 3 lymph nodes) without an identifiable primary tumour, in spite of extensive intra-operative exploration and imaging. One patient, to whom resection was proposed, underwent a duodenectomy elsewhere. The primary tumour could not be identified in the resected tissue. Of the successfully located gastrinomas, one was classified as a well-differentiated endocrine tumour of uncertain behaviour according to the WHO classification.10 The remaining tumours were well-differentiated endocrine carcinomas with low-grade malignant behaviour.
Follow-up and outcome
Follow-up consisted of yearly evaluation with biochemical studies (gastrin and chromogranin A). Imaging studies were not routinely performed.
After PPPD, two of the three patients remained disease-free during a follow-up of 1 and 7 years. The third patient, who underwent PPPD, had a gastrinoma as part of MEN-1 syndrome. During the 3-year follow-up, this patient has elevated fasting serum gastrin levels and imaging was not able to localize recurrent tumours or lymph node metastases. Symptomatic control has been achieved with proton pump inhibition.
After a duodenectomy, one of the two patients, with the suspected lesion in the lung, showed no biochemical signs of disease up to date (a follow-up period of 1 year). Further investigation of the lung has yet to be performed. In the other patient, who underwent duodenectomy elsewhere without removal of the gastrinoma, imaging studies repeatedly failed to localize the primary tumour. During 3 years of follow-up, symptoms of gastric hypersecretion were successfully treated with proton pump inhibitors.
Three of five patients treated with an enucleation are free of disease after a follow-up of 1, 13 and 15 years. Antisecretory therapy by means of proton pump inhibitors is being continued in two patients. One patient developed recurrent lymphatic metastatic disease after removal of lymph node metastases without an identifiable primary tumour. Because symptoms are relatively subtle and well controlled by proton pump inhibition, and the patient has severe comorbidity, no further treatment is being planned. In the fifth patient who underwent enucleation, only a single lymph node containing a gastrinoma was removed without an identifiable primary tumour (Fig. 2). The patient has normalized serum gastrin levels and no evidence of recurrent or metastatic disease after 1 year of follow-up. Therefore, this patient is considered to have a primary gastrinoma of a lymph node.11
Figure 2.
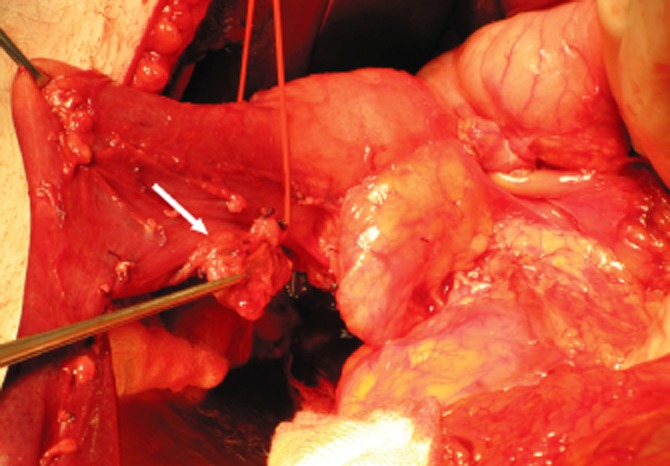
Intra-operative photograph of a patient with a lymph node containing a gastrinoma near the duodenal wall (arrow)
The patient who underwent a central pancreatectomy developed hepatic metastases after 5 years and a hemihepatectomy was subsequently performed. As a result of recurrent hepatic metastases, the patient was treated with multiple resections, radiofrequency ablations (RFA) and treatments with radiolabelled somatostatin analogues. The patient died of metastatic disease 13 years after initial surgery.
Discussion
In this study the results of surgical treatment of gastrinomas in a single centre are described. Only 11 patients were operated over a period of 19 years. The low incidence as well as the successful symptomatic treatment with antisecretory medication might be partially responsible for low referral numbers.12
The median delay between the onset of symptoms and the diagnosis of ZES in this study was 8 years (range 1–17). Previously published data show a typical delay in the diagnosis of 5 to 9 years.3 Patients are frequently being misdiagnosed before the diagnosis of ZES is properly established. Conventional imaging was able to localize the tumour in 10 out of 11 patients. However, numerous studies describe a negative result of conventional imaging in approximately one-third of patients with sporadic gastrinomas.1 Pre-operative somatostatin receptor scintigraphy (SRS) was positive in all patients. Correspondingly, data suggests SRS is more sensitive than conventional localization studies combined (CT, MRI and US) for localizing both primary tumours and metastases.13 The positive results of this study regarding imaging might be explained by selection bias; perhaps only patients with a localized tumour were referred for surgical treatment.
During exploration and IOUS, the primary tumour was successfully indentified in 9 out of 11 patients. The sensitivity of palpation and IOUS are 91% and 95%, respectively.1 Of the successfully localized tumours, seven were located in the so-called gastrinoma triangle, an anatomic triangle which includes the duodenum, the pancreatic head and the hepatoduodenal ligament (Fig. 3).14 Approximately 70–85% of all gastrinomas are located in the gastrinoma triangle.15,16 Duodenal tumours comprise 50–88% of sporadic gastrinomas and 70–100% of gastrinomas as part of MEN 1.15 The possible presence of lymph node primary gastrinomas in patients with ZES remains a controversial subject. However, numerous studies strongly support the notion that lymph node primary tumours exist and might account for approximately 10% of sporadic gastrinomas.11
Figure 3.
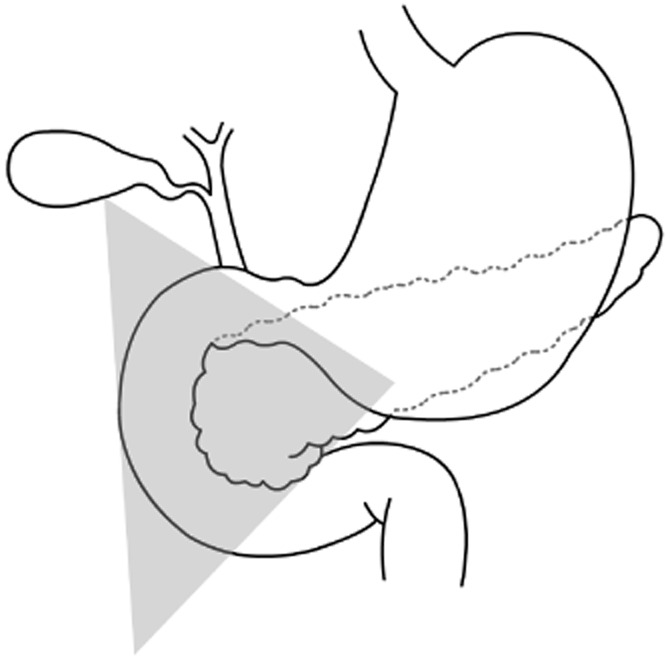
Gastrinoma triangle
In this study, five patients had lymph node involvement at the time of surgery, either as lymph node metastases or as a suspected lymph node primary gastrinoma. Even although most gastrinomas grow slowly, 50–70% have lymph node involvement at the time of diagnosis.1,17 Furthermore, 5–10% of duodenal gastrinomas and 20–25% of pancreatic gastrinomas are associated with liver metastases at diagnosis.5
After PPPD, two out of three patients are free of disease. The remaining patient, with MEN-1 syndrome, had persisting elevated serum gastrin levels but no identifiable tumour on imaging. Enucleation was successful in four of six patients; one patient underwent additional surgery and one patient only had multiple lymph nodes containing a gastrinoma removed without a primary tumour. A duodenectomy resulted in the successful removal of the gastrinoma in one of two patients. The patient that underwent a central pancreatectomy developed hepatic metastases and eventually died of metastatic disease. Within the limitations of this small series, the success of surgical treatment of a gastrinoma was 7/11 with a median follow-up of 3 years (range 1–15). Previously published studies describe success rates of 50–60% direct postoperatively and 25–30% 5–10 years after surgery.1,12
Surgical resection is the only chance of a complete cure of a gastrinoma.12 It is known to increase survival and reduce the rate of hepatic metastases.8 Routine surgical exploration should be performed in all patients with sporadic gastrinomas without evidence of diffuse hepatic metastases.5 If possible, pancreatic gastrinomas should be enucleated. If the pancreatic body and/or tail tumours can not be enucleated, a distal pancreatectomy is performed. A pancreaticoduodenectomy is typically reserved for patients with a large pancreatic head or duodenal tumours that cannot be enucleated or if the patient has multiple duodenal or pancreatic head tumours. A duodenotomy should be performed routinely to detect small duodenal gastrinomas, preferably combined with IOUS.18 A lymph node dissection should be carried out, even if no primary tumour is found.
Controversy remains regarding routine surgical exploration in patients with MEN-1/ZES. Disagreement not only exists concerning the indication for surgery but also the type of operation. Some authors recommend surgery when imaging studies identify tumours larger than 2–3 cm whereas others advocate a more aggressive treatment.17
The question whether a pancreaticoduodenectomy should be more frequently used in the management of ZES remains controversial.17 Several series report a better chance of a cure and increased survival, especially in patients with MEN 1 syndrome.17 Perhaps in the future a pancreaticoduodenectomy will be recommended in selected patients, especially as the mortality rate after pancreatic surgery in high-volume centres has decreased to less than 5%.19,20
In conclusion, this study describes a single-centre's experience of the surgical management of gastrinomas with a success rate of 7/11 during a median follow-up of 3 years (range 1–15). Even although controversies still exist in the management of patients with ZES, surgery offers the only possible complete cure and plays an important role in the treatment of gastrinomas. Given the complex management, every patient with a gastrinoma should be discussed in a multidisciplinary team consisting of gastroenterologists, endocrinologists, radiologists, nuclear medicine specialists and pancreatic- and endocrine surgeons.
Conflicts of interest
No conflict of interest, no disclaimers.
References
- 1.Fendrich V, Langer P, Waldmann J, Bartsch DK, Rothmund M. Management of sporadic and multiple endocrine neoplasia type 1 gastrinomas. Br J Surg. 2007;94:1331–1341. doi: 10.1002/bjs.5987. [DOI] [PubMed] [Google Scholar]
- 2.Zollinger RM, Ellison EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg. 1955;142:709–723. [PMC free article] [PubMed] [Google Scholar]
- 3.Roy PK, Venzon DJ, Shojamanesh H, Abou-Saif A, Peghini P, Doppman JL, et al. Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine (Baltimore) 2000;79:379–411. doi: 10.1097/00005792-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Dhillo WS, Jayasena CN, Lewis CJ, Martin NM, Tang KC, Meeran K, et al. Plasma gastrin measurement cannot be used to diagnose a gastrinoma in patients on either proton pump inhibitors or histamine type-2 receptor antagonists. Ann Clin Biochem. 2006;43:153–155. doi: 10.1258/000456306776021607. [DOI] [PubMed] [Google Scholar]
- 5.Jensen RT, Niederle B, Mitry E, Ramage JK, Steinmuller T, Lewington V, et al. Gastrinoma (duodenal and pancreatic) Neuroendocrinology. 2006;84:173–182. doi: 10.1159/000098009. [DOI] [PubMed] [Google Scholar]
- 6.Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, et al. Marx SJ: guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- 7.Metz DC, Pisegna JR, Fishbeyn VA, Benya RV, Jensen RT. Control of gastric acid hypersecretion in the management of patients with Zollinger-Ellison syndrome. World J Surg. 1993;17:468–480. doi: 10.1007/BF01655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norton JA, Fraker DL, Alexander HR, Gibril F, Liewehr DJ, Venzon DJ, et al. Surgery increases survival in patients with gastrinoma. Ann Surg. 2006;244:410–419. doi: 10.1097/01.sla.0000234802.44320.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van SS, van Soest EJ, Busch OR, van ES, Fockens P. A woman with shock as first sign of Zollinger-Ellison syndrome. Ned Tijdschr Geneeskd. 2007;151:478–483. [PubMed] [Google Scholar]
- 10.Hamilton SRAL. World Health Organization Classification of Tumours. 2000. Pathology and Genetics of Tumours of the Digestive System.
- 11.Norton JA, Alexander HR, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Possible primary lymph node gastrinoma: occurrence, natural history, and predictive factors: a prospective study. Ann Surg. 2003;237:650–657. doi: 10.1097/01.SLA.0000064375.51939.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norton JA, Fraker DL, Alexander HR, Venzon DJ, Doppman JL, Serrano J, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:635–644. doi: 10.1056/NEJM199908263410902. [DOI] [PubMed] [Google Scholar]
- 13.Gibril F, Reynolds JC, Doppman JL, Chen CC, Venzon DJ, Termanini B, et al. Somatostatin receptor scintigraphy: its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas. A prospective study. Ann Intern Med. 1996;125:26–34. doi: 10.7326/0003-4819-125-1-199607010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Stabile BE, Morrow DJ, Passaro E., Jr The gastrinoma triangle: operative implications. Am J Surg. 1984;147:25–31. doi: 10.1016/0002-9610(84)90029-1. [DOI] [PubMed] [Google Scholar]
- 15.Jensen RT. Zollinger-Ellison syndrome. In: Doherty GMSB, editor. Surgical Endocrinology: Clinical Syndromes. Philadelphia: Lipincott; 2001. pp. 291–344. [Google Scholar]
- 16.Yu F, Venzon DJ, Serrano J, Goebel SU, Doppman JL, Gibril F, et al. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J Clin Oncol. 1999;17:615–630. doi: 10.1200/JCO.1999.17.2.615. [DOI] [PubMed] [Google Scholar]
- 17.Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg. 2004;240:757–773. doi: 10.1097/01.sla.0000143252.02142.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norton JA, Alexander HR, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases, or survival in patients with Zollinger-Ellison syndrome? Ann Surg. 2004;239:617–625. doi: 10.1097/01.sla.0000124290.05524.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Heek NT, Kuhlmann KF, Scholten RJ, de Castro SM, Busch OR, van Gulik TM, et al. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–788. doi: 10.1097/01.sla.0000188462.00249.36. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Wilde RF, Besselink MG, van der Tweel I, de Hingh IH, van Eijck CH, Dejong CH, et al. Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg. 2012;99:404–410. doi: 10.1002/bjs.8664. [DOI] [PubMed] [Google Scholar]


