Abstract
Occupational neurotoxic diseases have become increasingly common in Taiwan due to industrialization. Over the past 40 years, Taiwan has transformed from an agricultural society to an industrial society. The most common neurotoxic diseases also changed from organophosphate poisoning to heavy metal intoxication, and then to organic solvent and semiconductor agent poisoning. The nervous system is particularly vulnerable to toxic agents because of its high metabolic rate. Neurological manifestations may be transient or permanent, and may range from cognitive dysfunction, cerebellar ataxia, Parkinsonism, sensorimotor neuropathy and autonomic dysfunction to neuromuscular junction disorders. This study attempts to provide a review of the major outbreaks of occupational neurotoxins from 1968 to 2012. A total of 16 occupational neurotoxins, including organophosphates, toxic gases, heavy metals, organic solvents, and other toxic chemicals, were reviewed. Peer-reviewed articles related to the electrophysiology, neuroimaging, treatment and long-term follow up of these neurotoxic diseases were also obtained. The heavy metals involved consisted of lead, manganese, organic tin, mercury, arsenic, and thallium. The organic solvents included n-hexane, toluene, mixed solvents and carbon disulfide. Toxic gases such as carbon monoxide, and hydrogen sulfide were also included, along with toxic chemicals including polychlorinated biphenyls, tetramethylammonium hydroxide, organophosphates, and dimethylamine borane. In addition we attempted to correlate these events to the timeline of industrial development in Taiwan. By researching this topic, the hope is that it may help other developing countries to improve industrial hygiene and promote occupational safety and health care during the process of industrialization.
Keywords: Occupational diseases, Neurotoxins, Manganese, Thallium, n-Hexane, Carbon disulfide, Dimethylamine borane
Introduction
Several episodes of neurological diseases due to occupational exposure have been reported during the industrialization of Taiwan. Most outbreaks of occupational neurotoxic disease developed prior to the 2000s. However, some toxins including thallium (Tl), dimethylamine borane (DMAB), and tetramethylammonium hydroxide (TMA) that are used in the electronic and biotechnology industries have been implicated in several recent outbreaks. Outbreaks of neurotoxic diseases can be related to the development of different kinds of industry, which is of concern to both the public and industries involved, but also requires governmental response. Reaction and prevention should include the provision of material safety data sheets from the industries, training and education programs for physicians and employees, improvements to industrial hygiene, and governmental modifications to the time-weighted average or threshold limited values (TLV) of each compound.
The aim of this review article is to detail the major outbreaks of occupational neurotoxic diseases from 1968 to 2012. The occupational neurotoxins involved include organophosphates, toxic gases, heavy metals, organic solvents, and other toxic chemicals peer-reviewed articles related to the electrophysiology, neuroimaging, treatment and long-term follow up of these neurotoxic diseases were also obtained. In addition to occupational neurotoxic diseases, two well-known environmental diseases, black foot disease and polychlorinated biphenyls (PCB) intoxication, which developed during the agricultural period and one case of heavy metal (Tl) poisoning due to homicide were also included.
The neurotoxic diseases studied involved heavy metals, organic solvents, and other toxic chemicals. The heavy metals consisted of lead (Pb), manganese (Mn), organic tin, mercury (Hg), Tl and arsenic (As) (Table 1). The organic solvents included n-hexane, toluene, and carbon disulfide (CS2). Toxic gas poisoning by hydrogen sulfide (H2S), and carbon monoxide (CO) was also reported. The toxic chemicals included PCB, TMA, organophosphates, and DMAB (Table 2). Both the peripheral nervous system (PNS) and central nervous system (CNS) are vulnerable to toxic agents because of their high metabolic rate. Some toxic agents may induce CNS impairment and some may induce PNS toxicity; most involve both of these reactions. The nervous system reactions reported in the Taiwanese population are summarized in Table 3. The major occupational neurotoxic diseases were also reviewed according to the corresponding industrial development stage of Taiwan at the time of outbreak.
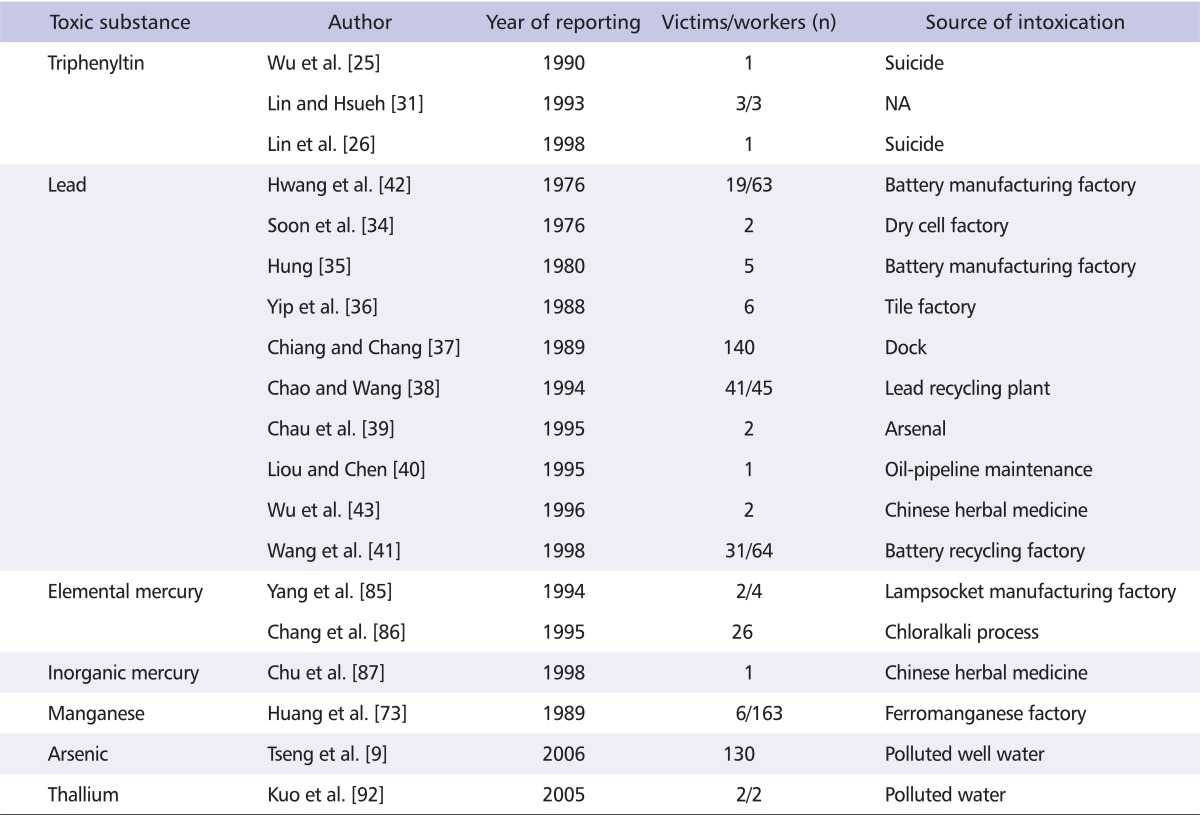
Table 1.
Major reported neurotoxicity of heavy metal poisoning in Taiwan
NA: not available.
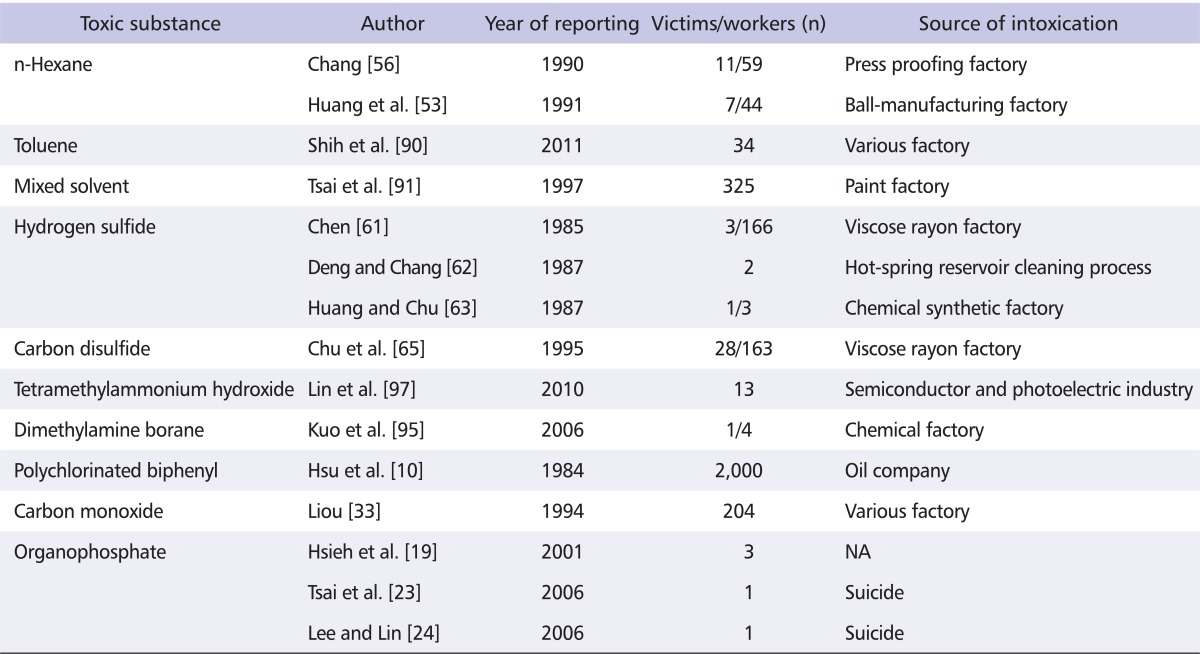
Table 2.
Major reported neurotoxicity of organic solvent, toxic chemicals and organophosphate in Taiwan
NA: not available.
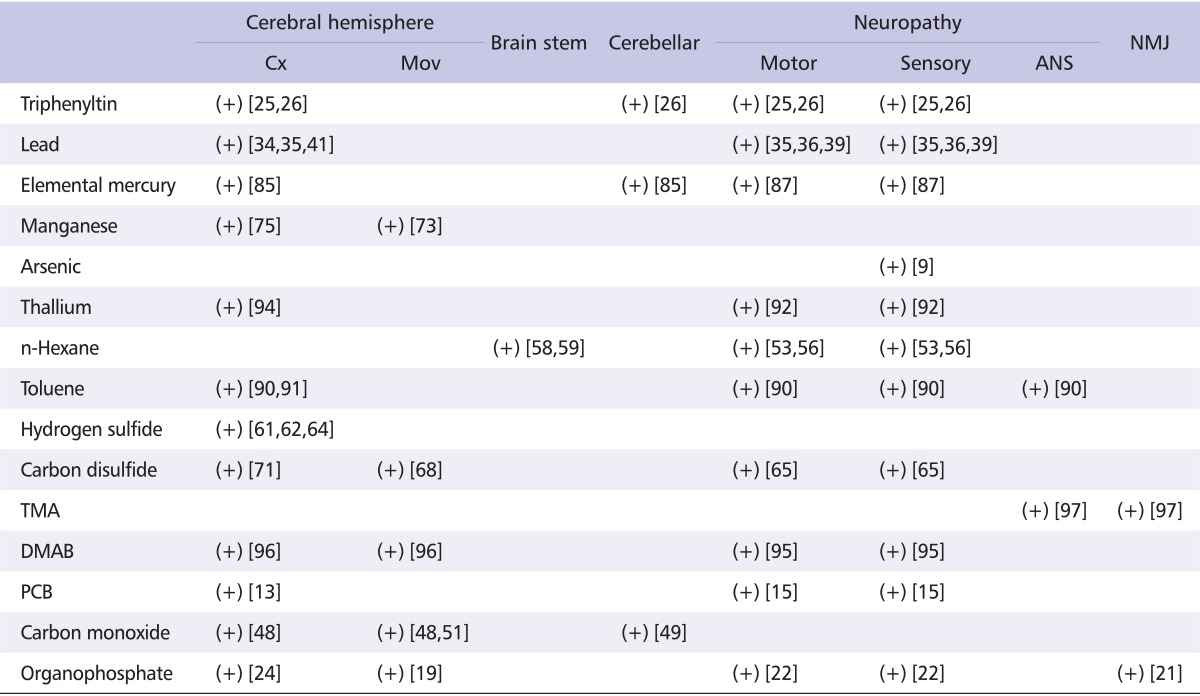
Table 3.
Reported involvements of nervous system in Taiwanese intoxicated patients
Cx: cortical function, Mov: movement disorder, ANS: autonomic nervous system, NMJ: neuromuscular junction, TMA: tetramethylammonium hydroxide, DMAB: dimethylamine borane, PCB: polychlorinated biphenyl.
(+) means 'reported'.
The Agricultural Period (Post 1950s)
Agriculture was the most important economic pillar of Taiwan during the period of Japanese occupation. After World War II and through the 1950s, agriculture was emphasized by the Taiwanese government, establishing the financial foundation on which subsequent industrialization could be built. Some chemical agents used as pesticides have become a continuous source of intoxication through over-exposure, misuse, or suicide attempts.
Arsenic
Arsenic is a well-known toxic agent that has been widely used in make-up, paints and pesticides. In Taiwan, a notorious endemic disease named "Black-foot disease" that occurred due to long-term drinking of arsenic-contaminated well water was reported during the 1950s [1]. The onset of black-foot disease was related to the duration of intake of well water containing a high concentration of arsenic [2]. Arsenic may also cause Bowen's disease, which may develop into squamous cell carcinoma if untreated, internal malignancies including bladder, lung and kidney cancers, and peripheral arterial diseases [3-5]. After disease onset, the average survival time is around 13.5 years [6]. Neurotoxic effects after an acute high concentration of arsenic exposure may develop within hours, but are more commonly noted 2-8 weeks after poisoning. Sensorimotor polyneuropathy resembling Guillain-Barre syndrome is the most common clinical manifestation in these patients [7]. In Thailand, arsenic poisoning was commonly found to develop from well water usage in mining areas [8]. In Taiwan, arsenic intoxication has usually developed after exposure to pesticides during the agricultural period. However one report revealed a slowing of nerve conduction velocity of the sural nerve in 130 junior high school students who drank arsenic-contaminated well water [9].
Polychlorinated biphenyls
PCBs have been produced since 1929. Some PCB compounds are structurally similar to dioxins and can exhibit dioxin-like toxicity. In 1968, an incident of "Yusho" disease occurred in Kyushu (southern Japan) after ingestion of rice bran oil contaminated with a heat-transfer medium containing PCB and polychlorinated dibenzofurans. In 1979, during the time of rice bran oil production, a related outbreak of PCB intoxication developed in central Taiwan, including the Taichung and Changhua areas, due to leakage from pipes which contained PCB [10]. This was the first major poisoning outbreak in Taiwan and involved around 2,000 people. The victims who ingested the contaminated rice bran oil developed chloracne, fatigue, arthralgia, headache and dizziness, which were collectively termed "Yucheng disease". In addition, menstrual irregularity and reproductive abnormalities were also noted [11]. Approximately half the victims experienced CNS disorder, including memory impairment, mental dullness, cognitive dysfunction, and learning difficulties [12,13]. Dose-dependent cognitive impairments in learning ability, attention, and visual memory were particularly noted in children born to women who had previously been exposed to PCB [13,14]. Furthermore, PNS symptoms such as weakness, paresthesia and pain in the distal extremities with a sensory predominant polyneuropathy were commonly noted [15,16].
Organophosphates
Organophosphates are widely used in farming as pesticides. Organophosphate poisoning was previously one of the leading toxic causes of suicide patients in Taiwan during the agricultural period, and has also been reported in pesticide production factory workers and those from a plastic bottle recycling plant [17-19]. However, organophosphate poisoning in Taiwan can clearly be traced back to the agricultural period [20]. Acute cholinergic crisis is the major presentation of organophosphate poisoning and may be lethal within minutes. Delayed toxic effects including sensorimotor polyneuropathy have also been reported [21,22]. In addition, intermediate syndrome, myelopathy, and transient extrapyramidal syndrome have also been noted in Taiwanese patients with organophosphate poisoning [19,21-24].
Triphenyltin acetate (TPTA)
TPTA is commonly used in plastic products manufacturing and farming due to its characteristic heat stability and bactericidal properties [25,26]. In Taiwan, it has been widely used to kill golden apple snails (Pomacea canaliculata), which have become a major pest to aquatic crops and have caused serious economical and agricultural damages since the 1980s [27,28]. However, TPTA is toxic to the CNS due to causing interstitial edema in white matter, and may cause pathological changes in cerebellar Purkinje's cells or limbic system structures [29,30]. In Taiwan, TPTA neurotoxicity has been noted in only three reports [25,26,31]. Suicidal attempt with molluscicidal agent was the main cause of intoxication in two of the five reported cases. Cognitive impairments including acalculia and disorientation without structural damage were noted in the early phase of poisoning, while ataxia was noted in one patient. Different to reports for Caucasian patients, delayed sensorimotor polyneuropathy was noted in a Taiwanese patient, which was reversible during the follow-up period [25,26]. Due to its toxicity, TPTA has been banned in agricultural use since 1999.
The Export-oriented Industrial Period (Post 1960s)
In the 1960s, Taiwan's electrical industry quickly prospered after successful manufacturing of black and white televisions. In 1965, the termination of aid from the United States forced local textile producers to begin investing in synthetic fibers and ready-made clothes. Since the 1970s, the petrochemical industry has developed rapidly, transforming oil into raw materials for plastics, textiles, rain equipment, bags and toys. During these years, the Taiwanese government also established export processing zones in Kaohsiung and Taichung. This policy helped light industry, including the textile and petrochemical industries, to grow rapidly. However, occupational diseases caused by neurotoxic agents commonly used in light industry, including Pb, n-Hexane, H2S and CS2, were observed in the following years. The use of poor-ventilated or confined space in these factories also made CO intoxication a common occupational disease during this period.
The first oil crisis developed due to the 1973 Arab-Israeli War. At this point the government started its "ten major construction projects" and built highways, airports, dockyards, or steel corporations, all of which promoted the advancement of heavy industry in Taiwan. Several episodes of intoxication due to heavy metals including Mn and Hg were notable in this period. However, the periods of light and heavy industry overlapped during these years.
Lead
The first reported case of Pb encephalopathy was that of a 4 year-old child in 1946, but the first epidemiological study of occupational Pb intoxication was reported in 1972 [32]. During the 1980s, Pb became the third most common cause of occupational disease in Taiwan [33]. Between 1975 and 1998, outbreaks occurred in battery manufacturing factories, ship-scrapping yards, cell recycling plants, weapons manufacturing factories oil-pipeline maintenance workers, in professional Buddha sculptors and painters, and in a tile factory [34-42]. Pb poisoning has also been related to the particular use of herbs in Chinese medicine [43,44]. Pb intoxication has been further associated with the use of Pb-containing gasoline and has therefore been found in bus drivers, police officers, gasoline station workers and freeway toll station workers due to polluted air exposure [32]. A decreased intelligence quotient was noted in a group of children who attended a kindergarden near to a battery recycling smelter in Taiwan [41]. Pb encephalopathy has been commonly noted in intoxicated children [35], while seizures, disorientation and personality change have been noted in adult patients [34]. Most intoxicated patients have shown distal limb weakness, especially in the upper extremities and wrist drop in more severe cases. In addition to anemia, and a pigment line is noted in the subgingival area [35,36,39]. However, Pb intoxication has been much reduced since the 2000s due to the successful performance of a Pb-surveillance program and government-led cessation of the use of Pb-containing gasoline [45].
Carbon monoxide
In the 1980s, CO intoxication was the second most common occupational disease in Taiwan [33]. This disease was frequently noted to occur due to improper use of gas hot water heaters, suicide by charcoal burning, or exposure in poor-ventilated spaces [46,47]. Acute CO intoxication due to accidental occupational exposure may induce anoxic or hypoxic encephalopathy with variable degrees of brain damages from confusion to deep coma. The common features include cognitive changes, apathy, sphincter incontinence, akinetic mutism, parkinsonism and dystonia [48]. Most patients have showed a prominent improvement, particularly in sphincter incontinence and akinetic mutism, although dystonia and cognitive impairment have persisted. Cerebellar involvement is rarely reported [49]. Brain magnetic resonance images (MRI) have revealed T2 high signal intensity lesions in the globus pallidus and subcortical white matter as an effect [48,50]. Using diffusion tensor imaging and dopamine transporter with Tc-99m tropane (99mTc-TRODAT) single photon emission computed tomography (SPECT) studies, it is suggested that the presence of pallidoreticular lesions may be related to extensive grey and white matter damage and may indicate a poorer cognitive state, while the Parkinsonian features may be associated with pallidal and presynaptic dopaminergic dysfunction [51]. Recovery from acute CO poisoning depends upon the CO concentration, duration of hypoxia and individual variation, while the prognosis of delayed CO encephalopathy is expected to be good.
n-Hexane
2,5-hexanedione, a common metabolite of n-hexane and methyl n-butyl ketone, is a potent neurotoxin [52]. Occupational exposure or recreational abuse of n-hexane may lead to neurotoxicity in both the PNS and CNS. In 1983, one patient visited Chang Gung Memorial Hospital because of distal numbness and weakness, and subsequently another six workers from the same press-proofing factory were found to have similar symptoms [53]. Marked prolonged distal latencies, decreased amplitudes of compound muscle and sensory nerve action potentials and slowing of motor and sensory nerve conduction velocities were noted indicating a demyelinating polyneuropathy [53]. Giant axons and demyelination in the paranodal areas were found in sural nerve biopsy [54]. Field studies exhibited a high concentration of n-hexane in the bulk samples and air samples. The event was reported to the Department of Labor in the Ministry of Internal Affairs of the Republic of China. Subsequently 59 workers from 16 press proofing factories were examined. Among these, 19 workers were found to have polyneuropathy and the air concentration of n-hexane ranged from 50 to 190 ppm. One patient who had been exposed to n-hexane 190 ppm for three months developed polyneuropathy. In 1988, a further outbreak of n-hexane poisoning developed in a ball-manufacturing factory. Poorly ventilated systems in the silk spinning and cement coating departments were the key reason, with n-hexane concentrations of 109 ppm and 86 ppm respectively [55,56]. A rapid deterioration of the clinical features was noted for 2-3 months and then a slow recovery developed during the following 1.5 year [57]. Besides this, subclinical CNS involvements were also noted for the brain stem and spinal cord in both outbreaks [58,59].
Hydrogen sulfide
H2S, a colorless gas, may result in convulsion, disturbed consciousness or sudden death after exposure [60]. Acute H2S intoxication has been reported in hot-spring reservoir cleansing workers and viscose rayon factory workers in Taiwan [61-63]. In 1985, three workers in a viscose rayon factory exposed to a high concentration of H2S developed convulsion and conscious disturbance. Two of the patients died and the other one had severe morbidity due to a lack of immediate treatment. In 1987, a chemical synthetic factory employee experienced status epilepticus after H2S poisoning. He improved rapidly under amyl nitrite treatment, and the sequalae of cognitive impairments completely recovered several years later [63,64].
Carbon disulfide
Acute high dose CS2 poisoning may cause cardiac arrest or acute psychosis, while a subacute moderate amount of CS2 intoxication may also induce encephalopathy with neurobehavioral abnormalities, Parkinsonism, intention tremor, and polyneuropathy. Low dose and chronic exposure to CS2 may lead to cardiovascular and cerebrovascular accidents. In 1994, a 45-year-old man working in a viscose rayon factory, reported glove and stocking like sensory impairment and distal weakness in the lower limbs for one year [65]. After a detailed survey in the same factory, nine further workers in the fiber-cutting department were found to suffer from the same disease (polyneuropathy) and 19 workers from various departments, mainly the spinning area, showed oligosymptoms of polyneuropathy. The fixed point air concentrations of CS2 were 450-900 mg/m3 in the cutting area and 45-300 mg/m3 in the spinning area. The estimated 8 hour-time weighted averages in the fiber cutting department were 120-201 mg/m3. Sural nerve biopsy showed vacuoles in the axon and then axonal degeneration and myelin degeneration developed. Large myelinated fibers were lost in the histogram of the sural nerve [66]. Chronic CS2 intoxication may induce Parkinsonian symptoms such as low body gait disturbance, rigidity, bradykinesia and loss of postural reflexes without resting tremor. Brain 99mTc-TRODAT SPECT in these patients showed a normal uptake of the radiotracer, indicating a normal presynaptic dopaminergic pathway, which differs from idiopathic Parkinson disease (PD) [67,68]. In addition, chronic CS2 exposure may also cause a small vessel disorder in the cerebral arterioles via a mechanism of an increase of low density cholesterol and triglycerides and a decrease of high density of cholesterol [69-71]. The data indicate that the clinical features may vary with variable concentrations of the toxic substance. After this outbreak, TLV of CS2 was changed from 60 mg/m3 to 30 mg/m3 by the government.
Manganese
Long-term exposure to high concentrations of Mn dust and fumes in welding or mining workers may cause an increased risk of manganism [72]. In 1985, six patients working in a ferromanganese alloy factory developed early onset Parkinsonism because of a six month delay in the installation of the ventilation system [73]. All patients had worked in the factory for more than two years and experienced masked face, rigidity, bradykinesia and loss of postural reflexes after an exposure to high concentration of Mn. The tremor was less prominent and not resting but postural; rigidity was more prominent in the lower limbs as well as more profound dystonia in the lower limbs, which is known as cock gait [74]. Micrographia and loss of voice were also noted. Besides this, impaired intelligence, defective manual dexterity, and visuoperceptive impairment were also recorded in workers with chronic exposure to Mn [75]. Using brain MRI, chronic Mn intoxication may show symmetrical high signal intensity lesions on T1-weighted MRI without T2-weighted signal abnormality in the globus pallidus [76,77]. The diagnosis of Mn-induced Parkinsonism was supported by a detailed survey in the worksite, which was found to have a high concentration of Mn (up to 28.8 mg per m3). Workers showed high Mn titers in the blood, urine, scalp hair and pubic hair. The brain positron emission tomography (PET) and 99mTc-TRODAT SPECT showed normal or only a minimal decrease in the putamen, suggesting the presynaptic dopaminergic terminals are not the main target of chronic manganese intoxication. This provided a useful, convenient and inexpensive tool for differentiation between chronic manganism and PD [78,79]. Postsynaptic D2 receptor PET scan with raclopride only showed a mild decrease in the putamen and caudate areas, indicating that these nuclei were also not the main targets of Mn poisoning [79]. The therapeutic effects with L-dopa were unsatisfactory [80]. A series of follow-up studies disclosed a rapid progression in the following 5-10 years [81,82]. However a long-term study revealed a plateau in the following 18 years [83]. An animal study in which monkeys were administered Mn showed that the most prominent pathological findings were severe gliosis in the globus pallidum [84]. When put together, the clinical course and the pathological findings indicate possible apoptosis in the globus pallidus neurons for 5-10 years [83].
Mercury: elemental and inorganic mercury
There has been no reported organic Hg poisoning in Taiwan, but elemental and inorganic Hg intoxications have been reported in 1994 and 1998 respectively [85-87]. Chronic elemental Hg intoxication occurred in lamp socket manufacturers who had worked in the industry for four years. They presented with gingivitis, erethism, action tremor, ataxia, dysarthria and visual field constriction similar to that found in organic Hg intoxication in Minamata disease. However, the clinical features were minor as compared with organic Hg poisoning. The time weighted average concentrations of air Hg were 0.709 to 0.945 mg/m3 in the plant. In the index patient, the Hg concentrations were 237 µg/L in blood and 610 µg/L in 24 hour urine. Patients could return to normal life after three months of medication with ethylenediaminetetraaetate and British anti-Lewisite [85,88]. In terms of inorganic Hg poisoning, one patient suffered from general weakness in 1997 after consuming herbal drugs named 'Huei Chen Sa', which contained a high concentration of inorganic Hg. The patient was initially misdiagnosed as having Guillain-Barre syndrome. After a careful history taking and in light of axonal degeneration in the sural nerve biopsy and a high concentration of Hg in the bulk samples, the diagnosis was confirmed [87].
The High-technology Oriented Period (Post 1980s)
The government established the first science park in Hsinchu and intensively supported the electronics industry during the 1980s. In 1995, Taiwan exported 4.66 million personal computers, 2.59 million notebook computers and 64-72% of the world's motherboards, keyboards and mice. In 2002, the government initiated the "Two Trillion and Twin Star" program, which planned to develop the digital media, liquid crystal display, and biotechnology industries. Some mixed solvent and toluene intoxication was still noted during this period. However, neurotoxic agents including Tl, DMAB, and TMA were of particular significance in this era.
Toluene and mixed solvents
Toluene is a widely used industrial solvent and a major ingredient of inhalant abuse products. Exposure to toluene or mixed solvent containing toluene has been studied in workers from paint manufacturing factories and semiconductor industries [89-91]. Neurobehavioral impairments or sympathetic and peripheral nerve dysfunction have been reported in workers with chronic exposure [90,91].
Thallium
Tl is currently used in the semiconductor and laser industries. In 2001, a middle aged couple suffered from severe painful sensation in their limbs after ingestion of water containing large amount of Tl. Initially they suffered angular stomatitis and diarrhea and then developed painful neuropathy with paresthesia especially in the lower extremities over the following day [92]. Skin hyperkeratosis was noted at one week and hair loss at two weeks. Complete alopecia developed in one month [93]. Tendon reflexes initially were normal and then hyporeflexia was found at two weeks. Motor weakness was more prominent in the lower limbs. Sural nerve biopsy revealed axonal degeneration with an involvement in the large and small fibers [92]. Skin biopsy showed perivascular mononuclear cell infiltration and cell debris and cutaneous nerve staining with protein gene product (PGP) 9.5 revealed loss of free nerve ending in the epidermis and dermis areas [93]. CNS involvements with confusion, disorientation, and hallucination in the acute stage and memory deficit in the chronic stage were also noted. The structural imaging studies failed to demonstrate extensive brain damage in the two reported cases [94]. However, 18F-fluorodeoxyglucose (FDG) PET showed an extent of brain involvement corresponding to clinical cognitive dysfunction in these two patients. Therefore PET functional neuroimages may better demenostrate the brain lesions than the structural neuroimages such as brain MRI.
Dimethylamine Borane
DMAB is a new synthetic agent used in the manufacturing of thin metal film, floppy discs, power transistors and high temperature printed circuit boards. The toxicity of DMAB was first reported in humans. In 2005, a 40-year-old man working in a semiconductor plant developed acute confusion and general weakness in four extremities following exposure to this new toxic chemical (DMAB), which was then unfamiliar to the public [95]. This was the first episode of human DMAB poisoning acute polyneuropathy with bilateral weakness in both legs and hyporefexia in both knee jerks and ankle jerks and sensory impairment was noted two weeks later [95]. Following exposure, the patient also developed mild Parkinsonism and cognitive deficits [96]. The polyneuropathy presented more motor symptoms, generally in the lower extremities. Sural nerve biopsy study revealed axonal degeneration and loss of free nerve ending was noted in the skin biopsy staining with PGP 9.5 [95].
Tetramethylammonium hydroxide
TMA is an etchant or developer used in the photoelectric or semiconductor industries [97]. TMA intoxication from dermal exposure may be fatal and is becoming a serious concern in Taiwan [98,99]. The structure of TMA ion is similar to the cationic portion of acetylcholine. TMA may stimulate the muscarinic or nicotinic autonomic ganglion and lead to depolarization blockade [97]. The neurologic manifestations of TMA intoxication include paralysis of respiratory muscles due to a ganglionic blocking effect. Cholinergic symptoms by accumulation of acetylcholine were first reported in Taiwanese patients in 2010 [99].
Conclusion
Over the past century, the most common occupational neurotoxic diseases in Taiwan have changed in relation to the nation's history of industrialization. The prevalence of occupational diseases decreased for multiple reasons, including an increasing knowledge of the toxic substances, awareness of toxic diseases in physicians and better public understanding of the risks, as well as improvements to the standard of occupational safety and health laws. In clinical presentation, occupation diseases are likely to have changed their appearance from acute intoxication to more chronic degenerative diseases. Correct diagnosis has therefore become more difficult. In addition, chronic degenerative diseases may occur due to long term, low dose exposure to the toxic agents, after which the cause-effect relationship may be vague. This will present a diagnostic challenge to general physicians in the future. Furthermore, occupational cancers are expected to become an important issue, which should be further emphasized.
Acknowledgments
The authors would like to express their thanks to Ms. YC Hsieh for typing the manuscript.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ch'i IC, Blackwell RQ. A controlled retrospective study of Blackfoot disease, an endemic peripheral gangrene disease in Taiwan. Am J Epidemiol. 1968;88:7–24. doi: 10.1093/oxfordjournals.aje.a120869. [DOI] [PubMed] [Google Scholar]
- 2.Tseng WP. Effects and dose-response relationships of skin cancer and blackfoot disease with arsenic. Environ Health Perspect. 1977;19:109–119. doi: 10.1289/ehp.7719109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CJ, Kuo TL, Wu MM. Arsenic and cancers. Lancet. 1988;1:414–415. doi: 10.1016/s0140-6736(88)91207-x. [DOI] [PubMed] [Google Scholar]
- 4.Tseng CH. An overview on peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Angiology. 2002;53:529–537. doi: 10.1177/000331970205300505. [DOI] [PubMed] [Google Scholar]
- 5.Chen YC, Guo YL, Su HJ, Hsueh YM, Smith TJ, Ryan LM, Lee MS, Chao SC, Lee JY, Christiani DC. Arsenic methylation and skin cancer risk in southwestern Taiwan. J Occup Environ Med. 2003;45:241–248. doi: 10.1097/01.jom.0000058336.05741.e8. [DOI] [PubMed] [Google Scholar]
- 6.Tseng WP. Blackfoot disease in Taiwan: a 30-year follow-up study. Angiology. 1989;40:547–558. doi: 10.1177/000331978904000606. [DOI] [PubMed] [Google Scholar]
- 7.Vahidnia A, van der Voet GB, de Wolff FA. Arsenic neurotoxicity--a review. Hum Exp Toxicol. 2007;26:823–832. doi: 10.1177/0960327107084539. [DOI] [PubMed] [Google Scholar]
- 8.Foy HM, Tarmapai S, Eamchan P, Metdilogkul O. Chronic arsenic poisoning from well water in a mining area in Thailand. Asia Pac J Public Health. 1992-1993;6:150–152. doi: 10.1177/101053959200600306. [DOI] [PubMed] [Google Scholar]
- 9.Tseng HP, Wang YH, Wu MM, The HW, Chiou HY, Chen CJ. Association between chronic exposure to arsenic and slow nerve conduction velocity among adolescents in Taiwan. J Health Popul Nutr. 2006;24:182–189. [PubMed] [Google Scholar]
- 10.Hsu ST, Ma CI, Hsu SK, Wu SS, Hsu NH, Yeh CC. Discovery and epidemiology of PCB poisoning in Taiwan. Prog Clin Biol Res. 1984;137:71–79. [PubMed] [Google Scholar]
- 11.Yu ML, Guo YL, Hsu CC, Rogan WJ. Menstruation and reproduction in women with polychlorinated biphenyl (PCB) poisoning: long-term follow-up interviews of the women from the Taiwan Yucheng cohort. Int J Epidemiol. 2000;29:672–677. doi: 10.1093/ije/29.4.672. [DOI] [PubMed] [Google Scholar]
- 12.Chen YJ, Hsu CC. Effects of prenatal exposure to PCBs on the neurological function of children: a neuropsychological and neurophysiological study. Dev Med Child Neurol. 1994;36:312–320. doi: 10.1111/j.1469-8749.1994.tb11851.x. [DOI] [PubMed] [Google Scholar]
- 13.Lai TJ, Guo YL, Yu ML, Ko HC, Hsu CC. Cognitive development in Yucheng children. Chemosphere. 1994;29:2405–2411. doi: 10.1016/0045-6535(94)90409-x. [DOI] [PubMed] [Google Scholar]
- 14.Lin KC, Huang PC, Yeh PS, Kuo JR, Ke DS. Comparing Mini-Mental State Examination and Attention and Digit Span in elderly exposed to polychlorinated biphenyls and polychlorinated dibenzofurans. Psychogeriatrics. 2010;10:191–197. doi: 10.1111/j.1479-8301.2010.00341.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen RC, Tang SY, Miyata H, Kashimoto T, Chang YC, Chang KJ, Tung TC. Polychlorinated biphenyl poisoning: correlation of sensory and motor nerve conduction, neurologic symptoms, and blood levels of polychlorinated biphenyls, quaterphenyls, and dibenzofurans. Environ Res. 1985;37:340–348. doi: 10.1016/0013-9351(85)90114-8. [DOI] [PubMed] [Google Scholar]
- 16.Chia LG, Chu FL. Neurological studies on polychlorinated biphenyl (PCB)-poisoned patients. Prog Clin Biol Res. 1984;137:117–126. [PubMed] [Google Scholar]
- 17.Wang CL, Chuang HY, Chang CY, Liu ST, Wu MT, Ho CK. An unusual case of organophosphate intoxication of a worker in a plastic bottle recycling plant: an important reminder. Environ Health Perspect. 2000;108:1103–1105. doi: 10.1289/ehp.001081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu YQ, Wang JD, Chen JS, Chung SC, Hwang SY. Occupational risk of decreased plasma cholinesterase among pesticide production workers in Taiwan. Am J Ind Med. 1989;16:659–666. doi: 10.1002/ajim.4700160605. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh BH, Deng JF, Ger J, Tsai WJ. Acetylcholinesterase inhibition and the extrapyramidal syndrome: a review of the neurotoxicity of organophosphate. Neurotoxicology. 2001;22:423–427. doi: 10.1016/s0161-813x(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 20.Lin TJ, Walter FG, Hung DZ, Tsai JL, Hu SC, Chang JS, Deng JF, Chase JS, Denninghoff K, Chan HM. Epidemiology of organophosphate pesticide poisoning in Taiwan. Clin Toxicol (Phila) 2008;46:794–801. doi: 10.1080/15563650801986695. [DOI] [PubMed] [Google Scholar]
- 21.Yang CC, Deng JF. Intermediate syndrome following organophosphate insecticide poisoning. J Chin Med Assoc. 2007;70:467–472. doi: 10.1016/S1726-4901(08)70043-1. [DOI] [PubMed] [Google Scholar]
- 22.Chuang CC, Lin TS, Tsai MC. Delayed neuropathy and myelopathy after organophosphate intoxication. N Engl J Med. 2002;347:1119–1121. doi: 10.1056/NEJM200210033471421. [DOI] [PubMed] [Google Scholar]
- 23.Tsai MH, Tsai NW, Chen SF, Tsai HH, Lu CH, Huang CR, Chang WN. Organophosphate intoxication-related coital-like involuntary movements: report of a case. Acta Neurol Taiwan. 2006;15:34–37. [PubMed] [Google Scholar]
- 24.Lee F, Lin JL. Intermediate syndrome after organophosphate intoxication in patient with end-stage renal disease. Ren Fail. 2006;28:197–200. doi: 10.1080/08860220500531294. [DOI] [PubMed] [Google Scholar]
- 25.Wu RM, Chang YC, Chiu HC. Acute triphenyltin intoxication: a case report. J Neurol Neurosurg Psychiatry. 1990;53:356–357. doi: 10.1136/jnnp.53.4.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin TJ, Hung DZ, Kao CH, Hu WH, Yang DY. Unique cerebral dysfunction following triphenyltin acetate poisoning. Hum Exp Toxicol. 1998;17:403–405. doi: 10.1177/096032719801700707. [DOI] [PubMed] [Google Scholar]
- 27.Liu WH, Chiu YW, Huang DJ, Liu MY, Lee CC, Liu LL. Imposex in the golden apple snail Pomacea canaliculata in Taiwan. Sci Total Environ. 2006;371:138–143. doi: 10.1016/j.scitotenv.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 28.Lo CC, Hsieh TT. Acute toxicity to the golden apple snail and estimated bioconcentration potential of triphenylphosphine oxide and series of related compounds. Bull Environ Contam Toxicol. 2000;65:104–111. doi: 10.1007/s0012800101. [DOI] [PubMed] [Google Scholar]
- 29.Besser R, Krämer G, Thümler R, Bohl J, Gutmann L, Hopf HC. Acute trimethyltin limbic-cerebellar syndrome. Neurology. 1987;37:945–950. doi: 10.1212/wnl.37.6.945. [DOI] [PubMed] [Google Scholar]
- 30.Colosio C, Tomasini M, Cairoli S, Foà V, Minoia C, Marinovich M, Galli CL. Occupational triphenyltin acetate poisoning: a case report. Br J Ind Med. 1991;48:136–139. doi: 10.1136/oem.48.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin JL, Hsueh S. Acute nephropathy of organotin compounds. Am J Nephrol. 1993;13:124–128. doi: 10.1159/000168601. [DOI] [PubMed] [Google Scholar]
- 32.Liou SH, Gu TL, Hsu SW, Wu DM, Chen LM. A review of occupational and environmental lead poisoning in Taiwan. J Inst Occup Saf Health. 1993;1:12–26. [Google Scholar]
- 33.Liou SH. Occupational disease profile in Taiwan, Republic of China. Ind Health. 1994;32:107–118. doi: 10.2486/indhealth.32.107. [DOI] [PubMed] [Google Scholar]
- 34.Soon WT, Tang KL, Chen RC, Hung TP, Wang CH. Lead encephalopathy in adults. Bull Chin Soc Neurol Psychiatry. 1976;2:16–20. [Google Scholar]
- 35.Hung IJ. Lead poisoning in two families. J Formos Med Assoc. 1980;79:740–748. [PubMed] [Google Scholar]
- 36.Yip PK, Chang YC, Wang JD, Tsasi SY, Chen JS. A small outbreak of lead neuropathy in a tile factory. J Formos Med Assoc. 1988;87:60–65. [PubMed] [Google Scholar]
- 37.Chiang HC, Chang PY. Lead intoxication in shipscrapping employees in Taiwan. Gaoxiong Yi Xue Ke Xue Za Zhi. 1989;5:284–290. [PubMed] [Google Scholar]
- 38.Chao KY, Wang JD. Increased lead absorption caused by working next to a lead recycling factory. Am J Ind Med. 1994;26:229–235. doi: 10.1002/ajim.4700260208. [DOI] [PubMed] [Google Scholar]
- 39.Chau TT, Chen WY, Hsiao TM, Liu HW. Chronic lead intoxication at an indoor firing range in Taiwan. J Toxicol Clin Toxicol. 1995;33:371–372. doi: 10.3109/15563659509028926. [DOI] [PubMed] [Google Scholar]
- 40.Liou SH, Chen LM. Lead poisoning in an oil-pipeline maintenance worker. Arch Environ Health. 1995;50:390. [PubMed] [Google Scholar]
- 41.Wang JD, Soong WT, Chao KY, Hwang YH, Jang CS. Occupational and environmental lead poisoning: case study of a battery recycling smelter in Taiwan. J Toxicol Sci. 1998;23(Suppl 2):241–245. doi: 10.2131/jts.23.supplementii_241. [DOI] [PubMed] [Google Scholar]
- 42.Hwang YF, Strickland GT, Chang NK, Beckner WM. Chronic industrial exposure to lead in 63 subjects. I. Clinical and erythrokinetic findings. Southeast Asian J Trop Med Public Health. 1976;7:559–568. [PubMed] [Google Scholar]
- 43.Wu TN, Yang KC, Wang CM, Lai JS, Ko KN, Chang PY, Liou SH. Lead poisoning caused by contaminated Cordyceps, a Chinese herbal medicine: two case reports. Sci Total Environ. 1996;182:193–195. doi: 10.1016/0048-9697(96)05054-1. [DOI] [PubMed] [Google Scholar]
- 44.Chi YW, Chen SL, Yang MH, Hwang RC, Chu ML. Heavy metals in traditional Chinese medicine: ba-pao-neu-hwangsan. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1993;34:181–190. Chinese. [PubMed] [Google Scholar]
- 45.Wu TN, Shen CY, Liou SH, Chao SL, Hsu CC, Lin FT, Ko KN, Chang PY. Reducing lead exposure by surveillance system: the Taiwan experience. Arch Environ Health. 1998;53:75–78. doi: 10.1080/00039899809605692. [DOI] [PubMed] [Google Scholar]
- 46.Cho CH, Chiu NC, Ho CS, Peng CC. Carbon monoxide poisoning in children. Pediatr Neonatol. 2008;49:121–125. doi: 10.1016/S1875-9572(08)60026-1. [DOI] [PubMed] [Google Scholar]
- 47.Pan YJ, Liao SC, Lee MB. Suicide by charcoal burning in Taiwan, 1995-2006. J Affect Disord. 2010;120:254–257. doi: 10.1016/j.jad.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Hsiao CL, Kuo HC, Huang CC. Delayed encephalopathy after carbon monoxide intoxication--long-term prognosis and correlation of clinical manifestations and neuroimages. Acta Neurol Taiwan. 2004;13:64–70. [PubMed] [Google Scholar]
- 49.Fan HC, Wang AC, Lo CP, Chang KP, Chen SJ. Damage of cerebellar white matter due to carbon monoxide poisoning: a case report. Am J Emerg Med. 2009;27:757.e5–757.e7. doi: 10.1016/j.ajem.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 50.Lin WC, Lu CH, Lee YC, Wang HC, Lui CC, Cheng YF, Chang HW, Shih YT, Lin CP. White matter damage in carbon monoxide intoxication assessed in vivo using diffusion tensor MR imaging. AJNR Am J Neuroradiol. 2009;30:1248–1255. doi: 10.3174/ajnr.A1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang CC, Chang WN, Lui CC, Huang SH, Lee CC, Chen C, Wang JJ. Clinical significance of the pallidoreticular pathway in patients with carbon monoxide intoxication. Brain. 2011;134:3632–3646. doi: 10.1093/brain/awr287. [DOI] [PubMed] [Google Scholar]
- 52.Wang JD, Chang YC, Kao KP, Huang CC, Lin CC, Yeh WY. An outbreak of N-hexane induced polyneuropathy among press proofing workers in Taipei. Am J Ind Med. 1986;10:111–118. doi: 10.1002/ajim.4700100202. [DOI] [PubMed] [Google Scholar]
- 53.Huang CC, Shih TS, Cheng SY, Chen SS, Tchen PH. n-Hexane polyneuropathy in a ball-manufacturing factory. J Occup Med. 1991;33:139–142. [PubMed] [Google Scholar]
- 54.Huang CC, Chen SS, Lu CS. Diagnostic value of sural nerve biopsy and electrophysiological studies in n-hexane intoxication. Gaoxiong Yi Xue Ke Xue Za Zhi. 1988;4:306–310. [PubMed] [Google Scholar]
- 55.Chang YC, Yip PK. N-hexane-induced electroneurographic changes and early detection of N-hexane intoxication. Taiwan Yi Xue Hui Za Zhi. 1987;86:194–200. [PubMed] [Google Scholar]
- 56.Chang YC. Patients with n-hexane induced polyneuropathy: a clinical follow up. Br J Ind Med. 1990;47:485–489. doi: 10.1136/oem.47.7.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang CC, Chu NS, Cheng SY, Shin TS. Biphasic recovery in n-hexane polyneuropathy. A clinical and electrophysiological study. Acta Neurol Scand. 1989;80:610–615. doi: 10.1111/j.1600-0404.1989.tb03936.x. [DOI] [PubMed] [Google Scholar]
- 58.Chang YC. Neurotoxic effects of n-hexane on the human central nervous system: evoked potential abnormalities in n-hexane polyneuropathy. J Neurol Neurosurg Psychiatry. 1987;50:269–274. doi: 10.1136/jnnp.50.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang CC, Chu NS. Evoked potentials in chronic n-hexane intoxication. Clin Electroencephalogr. 1989;20:162–168. doi: 10.1177/155005948902000309. [DOI] [PubMed] [Google Scholar]
- 60.Huang CC, Chu NS, Shih TS, Wu TN. Occupational neurotoxic diseases in Taiwan: a review of the outbreaks and clinical features. Changgeng Yi Xue Za Zhi. 1997;20:71–78. [PubMed] [Google Scholar]
- 61.Chen CH. Hydrogen sulfide intoxication. Bull Thorac Soc ROC. 1985;1:22–24. Chinese. [Google Scholar]
- 62.Deng JF, Chang SC. Hydrogen sulfide poisonings in hot-spring reservoir cleaning: two case reports. Am J Ind Med. 1987;11:447–451. doi: 10.1002/ajim.4700110407. [DOI] [PubMed] [Google Scholar]
- 63.Huang CC, Chu NS. A case of acute hydrogen sulfide (H2S) intoxication successfully treated with nitrites. Taiwan Yi Xue Hui Za Zhi. 1987;86:1018–1020. [PubMed] [Google Scholar]
- 64.Hua MS, Ku YW, Huang CC. Neuropsychological deficits in a case of H2S anoxic encephalopathy. Arch Clin Neuropsychol. 1992;7:63–76. [PubMed] [Google Scholar]
- 65.Chu CC, Huang CC, Chen RS, Shih TS. Polyneuropathy induced by carbon disulphide in viscose rayon workers. Occup Environ Med. 1995;52:404–407. doi: 10.1136/oem.52.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chu CC, Huang CC, Chu NS, Wu TN. Carbon disulfide induced polyneuropathy: sural nerve pathology, electrophysiology, and clinical correlation. Acta Neurol Scand. 1996;94:258–263. doi: 10.1111/j.1600-0404.1996.tb07062.x. [DOI] [PubMed] [Google Scholar]
- 67.Huang CC. Carbon disulfide neurotoxicity: Taiwan experience. Acta Neurol Taiwan. 2004;13:3–9. [PubMed] [Google Scholar]
- 68.Huang CC, Yen TC, Shih TS, Chang HY, Chu NS. Dopamine transporter binding study in differentiating carbon disulfide induced parkinsonism from idiopathic parkinsonism. Neurotoxicology. 2004;25:341–347. doi: 10.1016/S0161-813X(03)00147-5. [DOI] [PubMed] [Google Scholar]
- 69.Ku MC, Huang CC, Kuo HC, Yen TC, Chen CJ, Shih TS, Chang HY. Diffuse white matter lesions in carbon disulfide intoxication: microangiopathy or demyelination. Eur Neurol. 2003;50:220–224. doi: 10.1159/000073863. [DOI] [PubMed] [Google Scholar]
- 70.Luo JC, Chang HY, Chang SJ, Chou TC, Chen CJ, Shih TS, Huang CC. Elevated triglyceride and decreased high density lipoprotein level in carbon disulfide workers in Taiwan. J Occup Environ Med. 2003;45:73–78. doi: 10.1097/00043764-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 71.Chuang WL, Huang CC, Chen CJ, Hsieh YC, Kuo HC, Shih TS. Carbon disulfide encephalopathy: cerebral microangiopathy. Neurotoxicology. 2007;28:387–393. doi: 10.1016/j.neuro.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Wang JD, Huang CC, Hwang YH, Chiang JR, Lin JM, Chen JS. Manganese induced parkinsonism: an outbreak due to an unrepaired ventilation control system in a ferromanganese smelter. Br J Ind Med. 1989;46:856–859. doi: 10.1136/oem.46.12.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang CC, Chu NS, Lu CS, Wang JD, Tsai JL, Tzeng JL, Wolters EC, Calne DB. Chronic manganese intoxication. Arch Neurol. 1989;46:1104–1106. doi: 10.1001/archneur.1989.00520460090018. [DOI] [PubMed] [Google Scholar]
- 74.Huang CC, Chu NS, Lu CS, Calne DB. Cock gait in manganese intoxication. Mov Disord. 1997;12:807–808. doi: 10.1002/mds.870120532. [DOI] [PubMed] [Google Scholar]
- 75.Hua MS, Huang CC. Chronic occupational exposure to manganese and neurobehavioral function. J Clin Exp Neuropsychol. 1991;13:495–507. doi: 10.1080/01688639108401066. [DOI] [PubMed] [Google Scholar]
- 76.Cersosimo MG, Koller WC. The diagnosis of manganese-induced parkinsonism. Neurotoxicology. 2006;27:340–346. doi: 10.1016/j.neuro.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 77.Arjona A, Mata M, Bonet M. Diagnosis of chronic manganese intoxication by magnetic resonance imaging. N Engl J Med. 1997;336:964–965. doi: 10.1056/nejm199703273361319. [DOI] [PubMed] [Google Scholar]
- 78.Wolters EC, Huang CC, Clark C, Peppard RF, Okada J, Chu NS, Adam MJ, Ruth TJ, Li D, Calne DB. Positron emission tomography in manganese intoxication. Ann Neurol. 1989;26:647–651. doi: 10.1002/ana.410260510. [DOI] [PubMed] [Google Scholar]
- 79.Shinotoh H, Snow BJ, Chu NS, Huang CC, Lu CS, Lee C, Takahashi H, Calne DB. Presynaptic and postsynaptic striatal dopaminergic function in patients with manganese intoxication: a positron emission tomography study. Neurology. 1997;48:1053–1056. doi: 10.1212/wnl.48.4.1053. [DOI] [PubMed] [Google Scholar]
- 80.Lu CS, Huang CC, Chu NS, Calne DB. Levodopa failure in chronic manganism. Neurology. 1994;44:1600–1602. doi: 10.1212/wnl.44.9.1600. [DOI] [PubMed] [Google Scholar]
- 81.Huang CC, Lu CS, Chu NS, Hochberg F, Lilienfeld D, Olanow W, Calne DB. Progression after chronic manganese exposure. Neurology. 1993;43:1479–1483. doi: 10.1212/wnl.43.8.1479. [DOI] [PubMed] [Google Scholar]
- 82.Huang CC, Chu NS, Lu CS, Chen RS, Calne DB. Long-term progression in chronic manganism: ten years of follow-up. Neurology. 1998;50:698–700. doi: 10.1212/wnl.50.3.698. [DOI] [PubMed] [Google Scholar]
- 83.Huang CC, Chu NS, Lu CS, Chen RS, Schulzer M, Calne DB. The natural history of neurological manganism over 18 years. Parkinsonism Relat Disord. 2007;13:143–145. doi: 10.1016/j.parkreldis.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 84.Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- 85.Yang YJ, Huang CC, Shih TS, Yang SS. Chronic elemental mercury intoxication: clinical and field studies in lampsocket manufacturers. Occup Environ Med. 1994;51:267–270. doi: 10.1136/oem.51.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang YC, Yeh CY, Wang JD. Subclinical neurotoxicity of mercury vapor revealed by a multimodality evoked potential study of chloralkali workers. Am J Ind Med. 1995;27:271–279. doi: 10.1002/ajim.4700270211. [DOI] [PubMed] [Google Scholar]
- 87.Chu CC, Huang CC, Ryu SJ, Wu TN. Chronic inorganic mercury induced peripheral neuropathy. Acta Neurol Scand. 1998;98:461–465. doi: 10.1111/j.1600-0404.1998.tb07331.x. [DOI] [PubMed] [Google Scholar]
- 88.Hua MS, Huang CC, Yang YJ. Chronic elemental mercury intoxication: neuropsychological follow-up case study. Brain Inj. 1996;10:377–384. doi: 10.1080/026990596124386. [DOI] [PubMed] [Google Scholar]
- 89.Chiu KH, Wu BZ, Chang CC, Sree U, Lo JG. Distribution of volatile organic compounds over a semiconductor Industrial Park in Taiwan. Environ Sci Technol. 2005;39:973–983. doi: 10.1021/es049110m. [DOI] [PubMed] [Google Scholar]
- 90.Shih HT, Yu CL, Wu MT, Liu CS, Tsai CH, Hung DZ, Wu CS, Kuo HW. Subclinical abnormalities in workers with continuous low-level toluene exposure. Toxicol Ind Health. 2011;27:691–699. doi: 10.1177/0748233710395348. [DOI] [PubMed] [Google Scholar]
- 91.Tsai SY, Chen JD, Chao WY, Wang JD. Neurobehavioral effects of occupational exposure to low-level organic solvents among Taiwanese workers in paint factories. Environ Res. 1997;73:146–155. doi: 10.1006/enrs.1997.3704. [DOI] [PubMed] [Google Scholar]
- 92.Kuo HC, Huang CC, Tsai YT, Chu CC, Hsieh ST, Chu NS. Acute painful neuropathy in thallium poisoning. Neurology. 2005;65:302–304. doi: 10.1212/01.wnl.0000169021.26172.f8. [DOI] [PubMed] [Google Scholar]
- 93.Lu CI, Huang CC, Chang YC, Tsai YT, Kuo HC, Chuang YH, Shih TS. Short-term thallium intoxication: dermatological findings correlated with thallium concentration. Arch Dermatol. 2007;143:93–98. doi: 10.1001/archderm.143.1.93. [DOI] [PubMed] [Google Scholar]
- 94.Tsai YT, Huang CC, Kuo HC, Wang HM, Shen WS, Shih TS, Chu NS. Central nervous system effects in acute thallium poisoning. Neurotoxicology. 2006;27:291–295. doi: 10.1016/j.neuro.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 95.Kuo HC, Huang CC, Chu CC, Chu NS. Axonal polyneuropathy after acute dimethylamine borane intoxication. Arch Neurol. 2006;63:1009–1012. doi: 10.1001/archneur.63.7.1009. [DOI] [PubMed] [Google Scholar]
- 96.Liu CH, Wang HM, Lin KJ, Kuo HC, Weng YH, Shih TS, Huang CC. Long-term neurotoxic effects of dimethylamine borane intoxication. J Neurol Sci. 2012;319:147–151. doi: 10.1016/j.jns.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 97.Lin CC, Yang CC, Ger J, Deng JF, Hung DZ. Tetramethylammonium hydroxide poisoning. Clin Toxicol (Phila) 2010;48:213–217. doi: 10.3109/15563651003627777. [DOI] [PubMed] [Google Scholar]
- 98.Lee CH, Wang CL, Lin HF, Chai CY, Hong MY, Ho CK. Toxicity of tetramethylammonium hydroxide: review of two fatal cases of dermal exposure and development of an animal model. Toxicol Ind Health. 2011;27:497–503. doi: 10.1177/0748233710391990. [DOI] [PubMed] [Google Scholar]
- 99.Wu CL, Su SB, Chen JL, Lin HJ, Guo HR. Mortality from dermal exposure to tetramethylammonium hydroxide. J Occup Health. 2008;50:99–102. doi: 10.1539/joh.x7001. [DOI] [PubMed] [Google Scholar]