Abstract
Endoscopic submucosal dissection is a technically challenging but highly effective technique for the treatment of well selected early neoplasms in the digestive tract. Although it is frequently performed in East Asian countries, the Western world has not adopted this technique yet, probably due in part to the difficulty to learn it. Ex vivo and in vivo animal models are invaluable tools to overcome at least the beginning of the learning curve, although the initial step is the acquisition of basic knowledge about early diagnosis of neoplasias, and observing real procedures in expert centers. The practical issues, advantages, and disadvantages of the ex vivo and in vivo models are discussed.
Keywords: Endoscopic submucosal dissection, Animal models, Training
INTRODUCTION
Endoscopic submucosal dissection (ESD) appeared over one decade ago in Japan as a new and provocative method to achieve en bloc resection of intramucosal neoplasms in the digestive tract, almost without any size limit. Since then, this technique has evolved greatly, and it has shifted from the stomach to the esophagus and the colon.1-4 Geographically it expanded to Eastern Asia, and specially Korea has adopted faithfully this technique, with an increasing number of studies being published in endoscopy and gastroenterology journals from this country.5,6
The Western world has remained amazed but cautious about ESD: the lesions with an indication for ESD may not be as frequent in those countries, and ESD is one of the most complex endoscopic techniques, very operator-dependant, and with a potentially high perforation rate especially in the beginning of the learning curve.7 One of the most serious limitations to the application and spread of this technique outside Asia has been the lack of local experts.8 Strategies to overcome this limitation have come mostly from Western endoscopists who individually visited some Japanese expert centers, and observed ESD on real cases.9 However hands-on training on patients is generally speaking not possible in Japan. Therefore there is a clear need for well organized comprehensive strategies to get well trained in ESD. On the other hand, nowadays the high number of expert centers and endoscopists on ESD in Japan and Korea allows beginners to start training with patients, as supervision by experts can be provided. In this setting, the importance of training on simulators is evident. Considering that virtual simulators for ESD are not yet available, animal models are crucial to facilitate training at least in the first steps of ESD, and developing this fascinating technique outside Japan and Korea.
ANIMAL MODELS FOR TRAINING IN ESD: EX VIVO, IN VIVO
With the advent of new and more complex endoscopic methods, which are time consuming, and with a potential high complication rate, it is clearly not ethical to start practicing such techniques directly on human patients, unless supervision by real experts can be provided. Of course important ethical principles have to be respected also when training on animals. Large animals are usually selected for endoscopic training, and among them the pig is the most frequently used, because of the anatomical similarity with the human organs, the wide availability, and low cost.10 Therefore, in this review, pig models will be discussed unless specified otherwise.
The ideal setting for working with animal models (both ex vivo and in vivo models) is the experimental operation room with animal facilities, run by specialized veterinarians. Dedicated material (endoscopes, devices) should be used for working on animal models.
EX VIVO MODEL
The rationale for using ex vivo models is that in the beginning of the learning curve, the most important issue is having an initial exposure to the basic movements and maneuvers. It is known that ESD includes movements, mainly lateral cutting, which are not applied in other endoscopic techniques. Moreover, there are multiple different knives for ESD available, which have peculiarities in their use. Therefore it is considered that from the ethical standpoint, the beginner should have an initial exposure with several procedures performed on the isolated organ, before having to use a living animal. The use of the ex vivo model presents some more advantages over the in vivo model: the cost is negligible, a veterinarian and anesthesia would not be required.
Setting up the gastric/esophageal model
For this model, the stomach and at least a part of the esophagus are used.11,12
The gastric model should be the first one to use when training in ESD. There are different ways to set up this model, from the more rudimentary as were the initial ones, to the more sophisticated using manufactured plastic boxes with the different accessories required to attach and fix the organs. The isolated gastric model can be set up quite easily by anyone interested and motivated to practice ESD. The organs can be acquired in a slaughterhouse, usually with a special permission linked to experimental work. If possible, the model should be prepared with the fresh specimen. However for practicality it may be frozen and be taken care to have it at room temperature again for enough time (usually >12 hours) so that the organ recovers its softness as much as possible. However in our experience, pre-frozen organs frequently remain rigid and especially injecting in the submucosa becomes difficult.
Choosing the right size of the organs is important. Large pigs (>70 kg) provide large stomachs which allow for practicing multiple resections in different locations. The minimum size required for the pigs from which the stomach will be harvested is around 30 kg in our opinion. Organs from smaller animals have thinner walls with a higher risk of perforation, and the movements in the stomach are limited because of its reduced size. Using smaller stomachs would only have the potential benefit that increased skill is required, and therefore the endoscopists would face a higher step of difficulty.
A plastic box (approximately 45×30×20 cm) can be used to mount the isolated model in it; for that purpose, a hole 18 to 20 mm in diameter should be open in one of the short sides of the box, where a plastic tube (for instance an overtube) will be inserted and fixed.11 If small organs (coming from 12 to 20 kg pigs) are used, 14 mm tubes are more suitable; they can be obtained by cutting a 10 mL syringe, leaving only the cylinder. The esophagus will then be connected to it (Fig. 1). One or two plastic braces secure the esophagus tight to the tube, preventing the sliding of the esophagus and the leak of air with the movements of the endoscope.
Fig. 1.
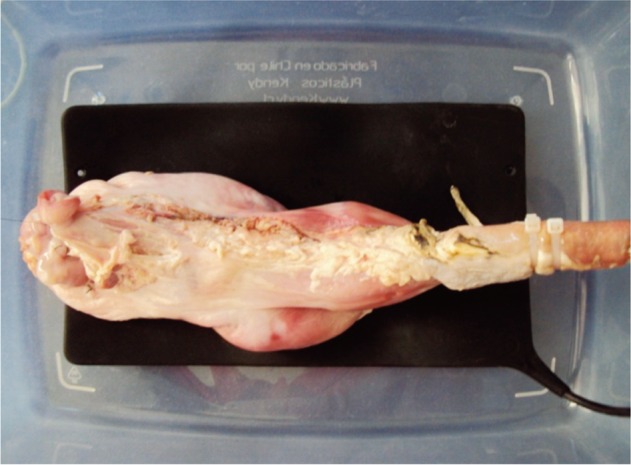
Ex vivo gastric porcine model.
The acquisition and preparation of the isolated organs can become the most tedious part of the story. Training sessions with ex vivo animal models are frequently performed outside working hours, and they can be tiring, it is advisable to have support for those procedures. Great support is usually obtained from veterinarians and other staff in experimental animal facilities, who collaborate to collect and prepare the organs. Cleaning the stomach is central to adequately perform the ESD procedures in the ex vivo model. Not only abundant mucus, but also large amounts of food can be found. In the authors' experience, there are two modalities for cleaning the isolated esophagus-stomach. One is introducing lukewarm water in the proximal end of the specimen (the esophagus), while closing the distal end (pylorus) with a forceps. A small amount of dishwashing liquid can be used too, and after vigorous shaking the liquid will be removed. This should be repeated until the effluent is clean. The second choice is making a 10 cm opening at the greater curvature with a scalpel; this is of considerable help to facilitate the washing, and even a brush can be used to gently eliminate the mucus. Then it can be dried with gauze (Fig. 2). An additional advantage is that markings can be created with tattooing of variable sizes and locations, as targets for ESD (Fig. 3). In this way the degree of difficulty can be established in an objective way. Usually the antrum is selected in the initial cases, followed by the gastric body in the greater curvature, then the lesser curvature, and finally subcardial locations will be selected. Finally, the defect is repaired with vicryl or prolene sutures, which provide water tightness, avoiding air-leakage during insufflation (Figs. 4, 5). As indicated above, for practical purposes, the organs may be stored in a freezer at -16℃. In that case, it is advisable to clean them beforehand.
Fig. 2.
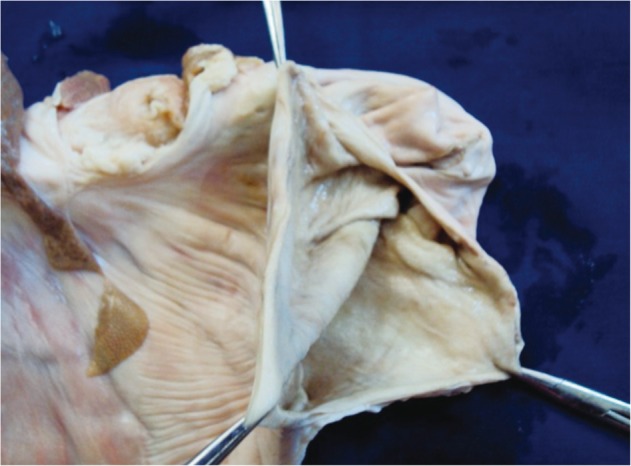
Open stomach after cleaned and dried.
Fig. 3.
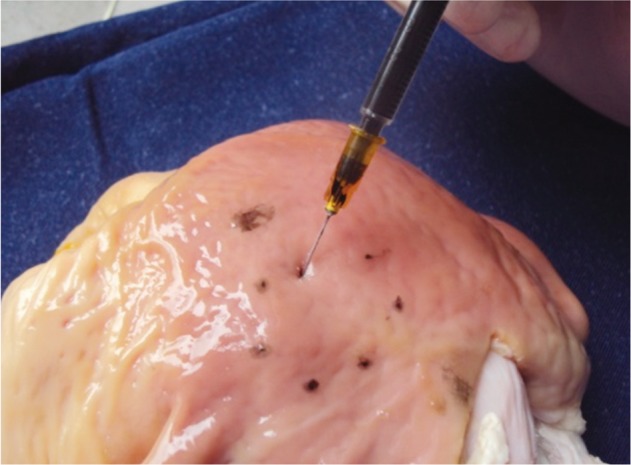
Tattooing with India ink to make measured markings in the ex vivo model.
Fig. 4.
Closure of the incision.
Fig. 5.
The tattooed area can be observed endoscopically.
A specific model for esophageal ESD has been described recently.13 A double overtube system was used to make the model more stable and to prevent the esophagus from sliding with the movements of the endoscope.
Setting up the colonic model
The feasibility and applicability of a simple porcine colonic model for training in colorectal ESD, which was adopted and modified from the model used in the National Cancer Center in Tokyo, was reported by Hon et al.14 from Hong Kong. In this model a plastic box is used similarly to the gastric model, and also a tube is inserted thorough which a laparoscopic port is used to simulate the anus. We have tried such method (Fig. 6).
Fig. 6.

Ex vivo colon model. In this case, not only the rectum, but a significant portion of the colon has also been left. The colon has been wrapped with aluminum foil in order to increase electrical conductivity (courtesy of Alfredo Zepeda, Equipos Médicos Zepeda, Chile).
The ex vivo porcine colonic model has the limitation that there is a large amount of fat tissue in the submucosa. It interferes with the progress of the dissection because the endoscopic lens becomes repeatedly stained by the fat, with the resulting vision impairment.
IN VIVO MODEL
As was previously indicated, it is important and advisable starting ESD in the living model as a previous step to the application of this technique in human cases, as this type of model offers several advantages.15,16 Using pigs over 30 kg is recommended, as the condition resembles more closely the situation of human ESD.
Preparation before ESD is required when working with the in vivo model.17 Fasting is absolutely a must, allowing only clear liquids 48 hours before the procedure, and complete fasting from at least 2 hours previous to the procedure. Any material which might be eaten by the pigs should be removed from the floor in the animal laboratory or the transportation (if pigs are going to be carried in the few days preceding the endoscopy). Unfortunately it is very frequent and discouraging finding large amounts of ingested material in the stomach when training ESD in the in vivo porcine model.
The in vivo pig model has proven to be adequate for endoscopic training in the upper digestive tract.9,11 Anatomy of the pig's stomach is quite similar to the human anatomy, with the main differences being that there is a gastric diverticulum near in cardia, and that the pylorus is strong and protruding (the so called Torus pyloricus).18 The most widely used type of pig is the Sus scrofa. The main advantage of the in vivo model as compared to the ex vivo is that the former is more realistic, with peristalsis, intraluminal secretions, and bleeding is a possible complication (although in our experience, bleeding during ESD in living pigs is less frequent and profuse than in humans). An additional advantage is the possibility to assess the post-treatment evolution of the animals in survival studies.
However, as described above, learning the basic movements and strategy of ESD can and should be learnt in ex vivo models first, and there is probably no justification to use living pigs initially, which are more expensive, require extensive preparation and technical support, and would not be ethically supportable. Moreover, pathological conditions can be prepared with the ex vivo model, where simulated polyps, bleeding, and other conditions can be produced. That is hardly possible with the in vivo model. In our experience it is adequate performing the initial 10 gastric ESDs in the ex vivo model, and then switching to the in vivo model.
Sedation must be conducted and controlled by a veterinarian, who must check the vital signs, and be watchful for signs of complications in survival studies. The veterinarians will be responsible for euthanasia of the animals. Two proposed strategies for sedation in porcine in vivo models: 1) both induction and maintenance with Ketamine and Midazolam, 2) premedication with ketamine intramuscular and maintenance with propofol and inhalatory anesthesia with isoflurane. Endovenous medication is administered with a line in the ear. Occasionally administration of butylscopolamine can be required to reduce gastrointestinal motility.
Of course, the in vivo model can be used not only for gastric, but also for esophageal and colonic ESD. One of the main limitations of the in vivo porcine model is the need to clean the colon, which is a difficult task. Preparation agents should be given orally for at least 2 days in advance of the examination, and additionally enemas can be administered after having anesthetized the animal. Additional limitations are the large amount of fat in the submucosal layer as indicated above, the reduced thickness of the colonic wall compared to the human colon, and the lack of abdominal fixations of the proximal colon. All of them can result in an increased technical difficulty and risk of perforation.19 Although all Japanese experts who participated in a survey agreed that the gastric porcine model should definitely be one initial step for Western endoscopists training in colonic ESD, approximately only half of them found the use of the ex vivo or in vivo colonic models important.15 The usefulness of the porcine colon model for training in colorectal ESD has not been clarified yet.
EVIDENCE ABOUT ANIMAL MODELS FOR TRAINING IN ESD
Currently, the use of animal models is an established recommendation when there is no local expertise in ESD, namely in most institutions in Western countries. When the initial descriptions of ESD outside Japan appeared, experts warned about the risks of self-taught ESD, and recommended using animal models.8 ESD training courses started being organized, where Japanese experts were invited to show their technique in animal models, and guide endoscopists in foreign countries in the initial training steps. Thirty supervised gastric ESDs in humans were considered the minimum number to achieve initial competence.8
The authors and their colleagues evaluated the usefulness of porcine ex vivo and in vivo models to overcome the initial learning curve.11 Thirty ESDs (nine esophageal and 21 gastric; eight ex vivo, 22 in vivo) were performed by one endoscopist. En bloc resection was achieved in 27 cases, and there was one perforation. The total resection time was significantly increased in the first half of the gastric cases, compared to the second half. Therefore, this study suggested that there is a learning curve in gastric ESD when using a porcine model. This result supports the importance of an initial training period in animal models before starting ESD in humans.
Another report also from Spain found that successful human gastric ESD is feasible after having trained with ex vivo and in vivo models which are not complex to prepare as described previously.12
In a study from Hong Kong, the results for 23 expert endoscopists who attended a hands-on course during an ESD training workshop were evaluated.20 There was a high incidence of complications, with perforations in 65% gastric and in 56% esophageal ESDs. Knives without any insulation were more prone to cause perforations. The majority of participants (96%) found the in vivo porcine model appropriate for simulation of esophageal and gastric ESD. This study is very interesting, and the results make evident the importance of simulation in animal models to avoid starting our learning curve on real human patients.
A European study evaluated also the usefulness of a 2-day training course in which 18 experienced endoscopists (39% with basic clinical experience in ESD) participated.21 Experts in ESD participated as supervisors, and the course included seminars and hands-on training with living pigs (17 to 25 kg). A mean 4.1 ESDs were performed by each endoscopist (84% gastric). The perforation rate was 22%, and the mortality rate because of perforations was 33% (8/24 piglets). These results once again suggest the usefulness of training on animal models to avoid the beginning of the learning curve in humans.
The results of training in an in vivo canine model have been reported.22 An endoscopist with previous training in an important endoscopy teaching center in Japan performed gastric ESDs in mongrel dogs that weighed 18 to 20 kg, either with a Hook knife or an IT-knife, successfully, and without any complications. The same group proved the feasibility of a similar canine model to train in circumferential esophageal ESD.23 Moreover, a learning curve was proven, as perforations occurred in the initial seven cases, whereas the last three cases were completed uneventfully.
An ex vivo model for esophageal ESD was used by three endoscopists with some previous experience in gastric ESD, and each of them could complete 10 esophageal ESDs.13 The learning curve when using this model was evident, as the operative time was reduced in the latter period and injuries to the proper muscle decreased as compared to the initial training period.
The results of a study in which one single endoscopist performed 10 ESDs in a colonic ex vivo model suggest that there is a learning curve when using this model; in this study in the two initial cases en bloc resection was not achieved, and perforations occurred.14 However the subsequent eight cases were completed successfully and without perforations.
Animal models are not only adequate for beginners in ESD who want to overcome the initial steps of the learning curve with them. The feasibility or effectiveness of ESD with new devices should ideally be tested in animal models. Table 1 shows studies performed with porcine models to test new methods for ESD.17,19,24-34
Table 1.
Feasibility or Comparative Studies of New Methods of Endoscopic Submucosal Dissection (ESD) Tested in Porcine Models (Ex Vivo and In Vivo)
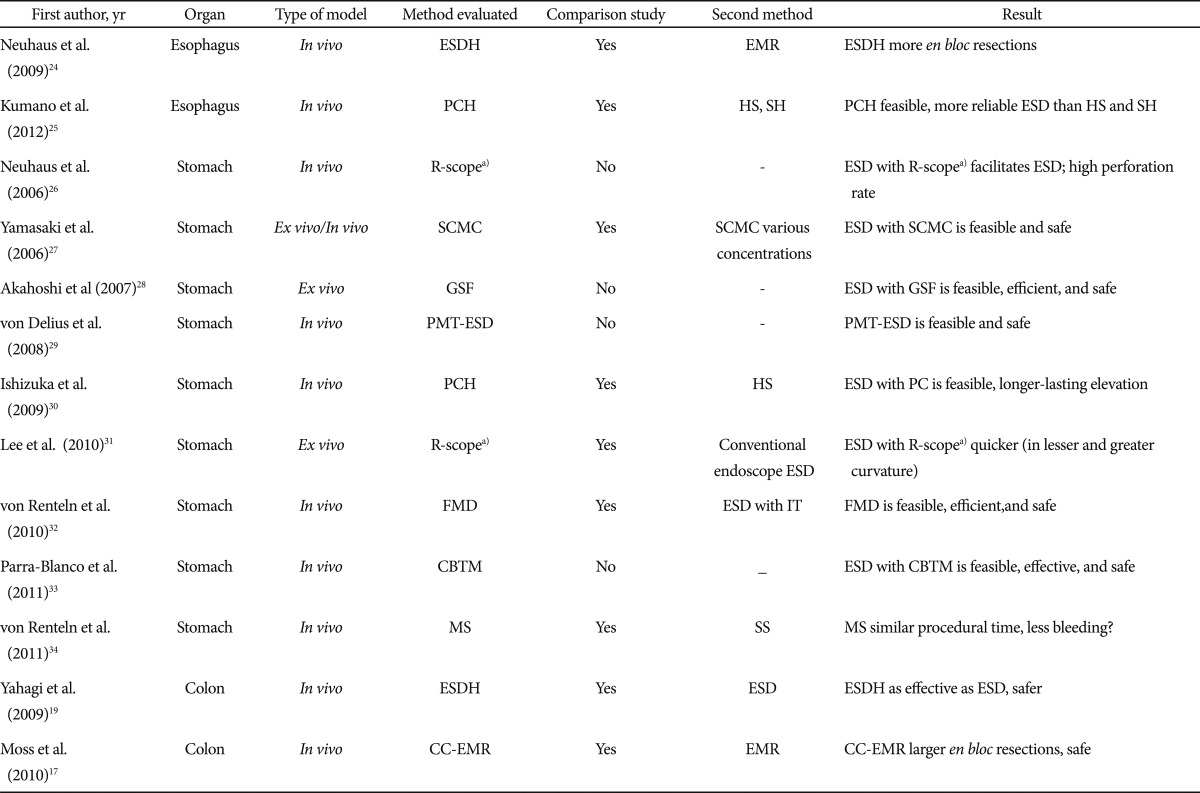
ESDH, ESD with Hybrid knife; EMR, endoscopic mucosal resection; PCH, photocrosslinkable chitosan hydrogel; HS, hypertonic saline; SH, sodium hyaluronate; SCMC, sodium carboxymethylcellulose; GSF, grasping type scissors forceps; PMT-ESD, PEG-minitrocar ESD; FMD, flexible Maryland dissector; IT, insulated-tip knife; CBTM, clip-band traction method; MS, Mesna solution; SS, saline solution; CC-EMR, circumferential cutting EMR.
a)R-scope, endoscope with two deflecting working channels.
PERSONAL EXPERIENCE IN ANIMAL MODELS FOR TRAINING IN ESD
The first author (A. Parra-Blanco) was trained in Japan for 4 years (1995 to 1999) in early detection and treatment of gastrointestinal neoplasms (Showa University Fujigaoka Hospital, National Cancer Center Hospital East, National Cancer Center Hospital, Tokyo Medical and Dental University). With that background, he returned to Japan in 2006 where he spent 1 month observing ESD cases (National Cancer Center Hospital, Jichi University, Cancer Institute Hospital). Moreover he performed seven ESD procedures in ex vivo pig models supervised by an expert. After 1 year training in ex vivo and in vivo pig models in his own institution in Spain,11 an ESD training course was organized in 2008 at University of La Laguna, together with the Research Animal Laboratory. Dr. Yutaka Saito was the expert, and 30 national experienced endoscopists received training. This was the first ESD training course in Spain and was among the few initial courses developed in Europe specifically for ESD training. The feedback obtained from the trainees was very positive. Although only three of the participating institutions have applied ESD in humans as of today, many of them have started or plan to set up their own ESD animal training programs. The first author has performed near 200 ESDs in pig model, and has performed around 40 human ESDs in the stomach, rectum and colon, some of them supervised by Japanese experts. We also proved the feasibility of a simple traction method (the clip-band method) for gastric ESD in a porcine model,33 which we have later on applied in human ESD successfully in 15 cases (unpublished results). The second author (N. Gonzalez) collaborated as an assistant in the animal ESD training program of the first author, then performed 30 gastric ESDs in an ex vivo model (Figs. 1-5) back in his institution in Uruguay, and has started performing human gastric ESD (five cases). The learning curve was evident in the period of animal training, and the human cases have gone uneventfully, with en bloc resection in each case and without perforations.
Therefore, in our own experience the animal models have been a useful method to learn ESD, where we have even been able to apply technical innovations to facilitate ESD, and have allowed us to apply ESD on our patients with confidence and good results.
CONCLUSIONS
ESD is one of the most challenging endoscopic techniques, which is still anecdotal in the Western countries. Probably one of the main reasons is that training is limited because of the absence of real experts. Ex vivo and in vivo models have been described for training in ESD from the esophagus to the rectum, and some of these reports come from Western countries, where with the aid of these models the authors have been able to start performing ESD in humans.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 2.Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3(7 Suppl 1):S67–S70. doi: 10.1016/s1542-3565(05)00291-0. [DOI] [PubMed] [Google Scholar]
- 3.Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video) Gastrointest Endosc. 2010;72:1217–1225. doi: 10.1016/j.gie.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Uraoka T, Kawahara Y, Kato J, Saito Y, Yamamoto K. Endoscopic submucosal dissection in the colorectum: present status and future prospects. Dig Endosc. 2009;21(Suppl 1):S13–S16. doi: 10.1111/j.1443-1661.2009.00863.x. [DOI] [PubMed] [Google Scholar]
- 5.Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1,000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–1235. doi: 10.1016/j.gie.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Lee EJ, Lee JB, Lee SH, et al. Endoscopic submucosal dissection for colorectal tumors-1,000 colorectal ESD cases: one specialized institute's experiences. Surg Endosc. doi: 10.1007/s00464-012-2403-4. Epub 2012 Jun 23. DOI: 10.1007/s00464-012-2403-4. [DOI] [PubMed] [Google Scholar]
- 7.Bergman JJ. How to justify endoscopic submucosal dissection in the Western world. Endoscopy. 2009;41:988–990. doi: 10.1055/s-0029-1215247. [DOI] [PubMed] [Google Scholar]
- 8.Gotoda T, Friedland S, Hamanaka H, Soetikno R. A learning curve for advanced endoscopic resection. Gastrointest Endosc. 2005;62:866–867. doi: 10.1016/j.gie.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 9.Kaltenbach T, Soetikno R, Kusano C, Gotoda T. Development of expertise in endoscopic mucosal resection and endoscopic submucosal dissection. Tech Gastrointest Endosc. 2011;13:100–104. [Google Scholar]
- 10.Ortiz-Fernández-Sordo J, Madrigal-Hoyos E, Waxman I. The role of live animal models for teaching endoscopy. Tech Gastrointest Endosc. 2011;13:113–118. [Google Scholar]
- 11.Parra-Blanco A, Arnau MR, Nicolás-Pérez D, et al. Endoscopic submucosal dissection training with pig models in a Western country. World J Gastroenterol. 2010;16:2895–2900. doi: 10.3748/wjg.v16.i23.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vázquez-Sequeiros E, de Miquel DB, Olcina JR, et al. Training model for teaching endoscopic submucosal dissection of gastric tumors. Rev Esp Enferm Dig. 2009;101:546–552. doi: 10.4321/s1130-01082009000800005. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka S, Morita Y, Fujita T, et al. Ex vivo pig training model for esophageal endoscopic submucosal dissection (ESD) for endoscopists with experience in gastric ESD. Surg Endosc. 2012;26:1579–1586. doi: 10.1007/s00464-011-2074-6. [DOI] [PubMed] [Google Scholar]
- 14.Hon SS, Ng SS, Lee JF, Li JC, Lo AW. In vitro porcine training model for colonic endoscopic submucosal dissection: an inexpensive and safe way to acquire a complex endoscopic technique. Surg Endosc. 2010;24:2439–2443. doi: 10.1007/s00464-010-0982-5. [DOI] [PubMed] [Google Scholar]
- 15.Parra-Blanco A, Saito Y, Yahagi N, et al. Recommendations about training for colorectal endoscopic submucosal dissection in the Western world: results of a survey to experts. Gastrointest Endosc. 2011;73(Suppl):AB419–AB420. [Google Scholar]
- 16.Deprez PH, Bergman JJ, Meisner S, et al. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy. 2010;42:853–858. doi: 10.1055/s-0030-1255563. [DOI] [PubMed] [Google Scholar]
- 17.Moss A, Bourke MJ, Tran K, et al. Lesion isolation by circumferential submucosal incision prior to endoscopic mucosal resection (CSI-EMR) substantially improves en bloc resection rates for 40-mm colonic lesions. Endoscopy. 2010;42:400–404. doi: 10.1055/s-0029-1243990. [DOI] [PubMed] [Google Scholar]
- 18.Freys SM, Heimbucher J, Fuchs KH. Teaching upper gastrointestinal endoscopy: the pig stomach. Endoscopy. 1995;27:73–76. doi: 10.1055/s-2007-1005637. [DOI] [PubMed] [Google Scholar]
- 19.Yahagi N, Neuhaus H, Schumacher B, et al. Comparison of standard endoscopic submucosal dissection (ESD) versus an optimized ESD technique for the colon: an animal study. Endoscopy. 2009;41:340–345. doi: 10.1055/s-0029-1214473. [DOI] [PubMed] [Google Scholar]
- 20.Teoh AY, Chiu PW, Wong SK, Sung JJ, Lau JY, Ng EK. Difficulties and outcomes in starting endoscopic submucosal dissection. Surg Endosc. 2010;24:1049–1054. doi: 10.1007/s00464-009-0724-8. [DOI] [PubMed] [Google Scholar]
- 21.Berr F, Ponchon T, Neureiter D, et al. Experimental endoscopic submucosal dissection training in a porcine model: learning experience of skilled Western endoscopists. Dig Endosc. 2011;23:281–289. doi: 10.1111/j.1443-1661.2011.01129.x. [DOI] [PubMed] [Google Scholar]
- 22.Tanimoto MA, Torres-Villalobos G, Fujita R, et al. Endoscopic submucosal dissection in dogs in a World Gastroenterology Organisation training center. World J Gastroenterol. 2010;16:1759–1764. doi: 10.3748/wjg.v16.i14.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanimoto MA, Torres-Villalobos G, Fujita R, et al. Learning curve in a Western training center of the circumferential en bloc esophageal endoscopic submucosal dissection in an in vivo animal model. Diagn Ther Endosc. 2011;2011:847831. doi: 10.1155/2011/847831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuhaus H, Wirths K, Schenk M, Enderle MD, Schumacher B. Randomized controlled study of EMR versus endoscopic submucosal dissection with a water-jet hybrid-knife of esophageal lesions in a porcine model. Gastrointest Endosc. 2009;70:112–120. doi: 10.1016/j.gie.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 25.Kumano I, Ishihara M, Nakamura S, et al. Endoscopic submucosal dissection for pig esophagus by using photocrosslinkable chitosan hydrogel as submucosal fluid cushion. Gastrointest Endosc. 2012;75:841–848. doi: 10.1016/j.gie.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 26.Neuhaus H, Costamagna G, Devière J, et al. Endoscopic submucosal dissection (ESD) of early neoplastic gastric lesions using a new double-channel endoscope (the "R-scope") Endoscopy. 2006;38:1016–1023. doi: 10.1055/s-2006-944830. [DOI] [PubMed] [Google Scholar]
- 27.Yamasaki M, Kume K, Yoshikawa I, Otsuki M. A novel method of endoscopic submucosal dissection with blunt abrasion by submucosal injection of sodium carboxymethylcellulose: an animal preliminary study. Gastrointest Endosc. 2006;64:958–965. doi: 10.1016/j.gie.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Akahoshi K, Akahane H, Murata A, Akiba H, Oya M. Endoscopic submucosal dissection using a novel grasping type scissors forceps. Endoscopy. 2007;39:1103–1105. doi: 10.1055/s-2007-966842. [DOI] [PubMed] [Google Scholar]
- 29.von Delius S, Karagianni A, von Weyhern CH, et al. Percutaneously assistennd endoscopic surgery using a new PEG-minitrocar for advanced endoscopic submucosal dissection (with videos) Gastrointest Endosc. 2008;68:365–369. doi: 10.1016/j.gie.2008.02.093. [DOI] [PubMed] [Google Scholar]
- 30.Ishizuka T, Ishihara M, Aiko S, et al. Experimental evaluation of photocrosslinkable chitosan hydrogel as injection solution for endoscopic resection. Endoscopy. 2009;41:25–28. doi: 10.1055/s-0028-1103483. [DOI] [PubMed] [Google Scholar]
- 31.Lee SH, Gromski MA, Derevianko A, et al. Efficacy of a prototype endoscope with two deflecting working channels for endoscopic submucosal dissection: a prospective, comparative, ex vivo study. Gastrointest Endosc. 2010;72:155–160. doi: 10.1016/j.gie.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Renteln D, Pohl H, Vassiliou MC, Walton MM, Rothstein RI. Endoscopic submucosal dissection by using a flexible Maryland dissector: a randomized, controlled, porcine study (with videos) Gastrointest Endosc. 2010;71:1056–1062. doi: 10.1016/j.gie.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 33.Parra-Blanco A, Nicolas D, Arnau MR, Gimeno-Garcia AZ, Rodrigo L, Quintero E. Gastric endoscopic submucosal dissection assisted by a new traction method: the clip-band technique. A feasibility study in a porcine model (with video) Gastrointest Endosc. 2011;74:1137–1141. doi: 10.1016/j.gie.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 34.von Renteln D, Dulai PS, Pohl H, Vassiliou MC, Rösch T, Rothstein RI. Endoscopic submucosal dissection with a flexible Maryland dissector: randomized comparison of mesna and saline solution for submucosal injection (with videos) Gastrointest Endosc. 2011;74:906–911. doi: 10.1016/j.gie.2011.05.030. [DOI] [PubMed] [Google Scholar]


