Abstract
Gastrointestinal (GI) stent has been developed for palliation of obstructive symptoms in various diseases causing obstruction of GI tract. Self-expanding metal stent (SEMS) has replaced old type of plastic stent, and endoscopic insertion of stent has replaced fluoroscopy-guided insertion. Nowadays, newly-designed SEMSs have been developed for prevention of complications such as stent migration and re-obstruction, and indications of stent recently have been widened into benign conditions as well as malignant obstruction. In this review, the types, method of insertion, indications and clinical outcomes of stent in the upper GI tract would be discussed.
Keywords: Self-expanding metal stent, Upper gastrointestinal tract, Obstruction
INTRODUCTION
Upper gastrointestinal (GI) obstruction is a preterminal event in patients with inoperable malignancies of the esophagus, stomach, duodenum and pancreas, and with direct invasion of other malignancies into the upper GI tract. Inadequate food intake results in malnutrition and impaired quality of life during a patient's remaining years. Surgical bypass has been the traditional palliative modality, but is limited by invasiveness, high complication rate, high cost, and longer hospital stay.
GI stent has been developed for palliation of obstructive symptoms in malignant obstruction of GI tract. Although plastic stent was initially used for malignant GI obstruction, it had many limitations such as the difficulty of insertion technique, high complication rate and poor compliance. In 1990s, self-expanding metal stent (SEMS) with convenient insertion technique, lower complication rate and better compliance has been developed and rapidly replaced the old plastic stent. Advanced technology nowadays enabled the development of new types of SEMSs designed to prevent common complications such as stent migration and re-obstruction, and to maintain longer patency. In addition, the indications for the upper GI stent have been widened to include benign conditions as well as malignant obstruction.
TYPES OF UPPER GI STENT
Upper GI stents can be categorized based on the existence of covered membrane, materials of the metal and accessories. Covered stents, including both fully-covered type and partially-covered type, are advantageous in lowering the rate of re-obstruction but is limited by higher rate of migration.1 Re-obstruction occurs usually by tumor in-growth in an uncovered stent, meaning that the embedded tumor tissue grows through the metal meshwork of the stent. Tumor over-growth can occur even with the covered stents by tumor growth at both ends of the stent, as well as tumor in-growth by membrane degradation over a long period of time. Stent migration is more common with the covered stents than with the uncovered stents because the tissue embedding through the metal meshwork of the uncovered types prevents the migration. Although late stent migration is not common even with the covered stents because of fibrotic reaction between the stent and tumor tissue, early stent migration can occur with the covered type before the stent is attached to the tumor tissue. Theoretically, newly-developed double-layered stent has the ability to prevent re-obstruction and migration simultaneously, but the efficacy should be verified by large-scale, prospective, randomized controlled trials.2,3
Materials of currently marketed SEMSs consist of nitinol or stainless steel. Nitinol, the most common material of SEMSs, is an alloy of nickel and titanium and has good shape-memory property and flexibility. Stainless steel is also used as a material for SEMSs, but is being substituted by nitinol due to limited flexibility and sharp ends of metal mesh which may injure the GI wall and lead to significant complications.4
In malignant obstruction of gastroesophageal (GE) junction, stent placement can cause GE reflux which may limit the quality of life of patients. Stent with one-way anti-reflux polyurethane or polyethylene valve has been developed to prevent GE reflux due to stent placement through GE junction. In some studies, reflux symptoms were improved with the stent with anti-reflux valve, whereas other reports could not demonstrate significant differences compared with the stent without anti-reflux valve.5-7 Moreover, the stent with anti-reflux valve, being a covered type, migrates frequently. To prevent migration of the covered stent with anti-reflux valve, the modified stent with a thread attached to the proximal end of the stent was developed, which is connected to the patient's earlobe until the stent is fixed to the esophageal wall. But this technique has the limitation of poor patient compliance.8 In malignant obstruction of cervical esophagus, a modified stent with shorter flanges and obtuse angles may be beneficial to alleviate foreign body sensation after the stent placement.9
Self-expanding plastic stent (SEPS) is composed of polyester and silicone and can be also used for malignant esophageal obstruction. Although the clinical results of SEPSs were not different from those of SEMSs in terms of symptom improvement, overall complication and survival, SEPSs are technically more difficult to apply and the stent migration rate is higher than SEMSs.10,11
Newly-developed stents are designed to complement the disadvantages of conventional SEMSs. Biodegradable stents, which have been developed for the treatment of benign stricture, are composed of poly-L-lactic acid monofilaments and are not required to be removed to prevent complications.12 Drug-eluting stents have been developed to prevent tumor in-growth by coating a stent with chemotherapeutic agents such as 5-fluorouracil or paclitaxel.13 Further long-term prospective data from controlled trials are awaited to confirm the efficacy of these newly-developed stents.
METHOD OF STENT INSERTION IN THE UPPER GI TRACT
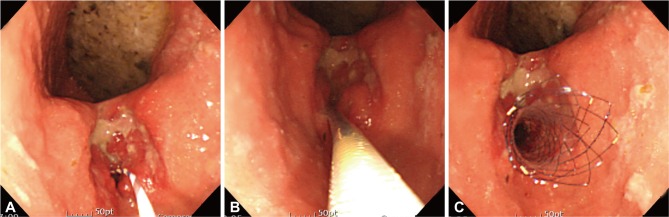
Most upper GI stents can be inserted into the obstructive lesions through the endoscopy. Firstly, a guide-wire is inserted through the obstructive lesion with or without fluoroscopic guidance. Adequate insertion of a guide-wire can be confirmed under fluoroscopy or when the passage of a guide-wire is without resistance under endoscopic guidance only. Secondly, stent is inserted through the guide-wire, and proximal end of the stent is placed at 2 cm proximal site of obstructive lesion to locate proximal flange adequately. After stent placement, the delivery device is withdrawn maintaining the stent in place, while assuring adequate expansion and location of the stent (Fig. 1). The length of stricture can be estimated by endoscopy, barium study or computed tomography (CT) prior to the stent insertion. If the length of stricture cannot be estimated before the insertion, stent with enough length should be inserted to prevent incomplete coverage of the stricture. Too much longer stent than the length of stricture, however, can lead to incomplete improvement of obstructive symptoms by food impaction.
Fig. 1.
Stent insertion. After insertion of guide-wire through the obstructive lesion with or without fluoroscopic guidance (A), stent is inserted through the guide-wire, and proximal end of the stent is placed at 2 cm proximal site of the obstructive lesion (B). Adequate expansion and location of the stent should be confirmed after withdrawing the delivery device (C).
After the stent was inserted, a simple radiography should be performed to confirm appropriate location and expansion of the stent. Stent migration can be detected by a series of follow-up radiography for several days after the stent insertion. Diet can be started gradually from water to regular meal in 24 to 48 hours after the insertion. Obstructive symptoms can recur with diet by stent migration, inappropriate location or expansion of stent, or multi-level obstruction, which can be confirmed by simple radiography, endoscopy, barium study, or CT.
INDICATIONS OF STENT INSERTION IN THE UPPER GI TRACT
Indications of stent insertion in the upper GI tract include malignant obstructions, benign strictures or fistulas of the esophagus, stomach or upper small bowel, which are inoperable diseases causing obstructive symptoms (Table 1). These malignant obstructions can be usually caused by esophageal cancer, stomach cancer, or periampullary cancer. Extrinsic tumor invasion or compression can also cause the upper GI obstruction by paraesophageal malignancies such as lung cancer or mediastinal mass; parapyloric or paraduodenal mass such as liver cancer, pancreatic cancer, or colon cancer; and small bowel obstruction by malignant peritoneal seeding. If obstructive symptoms are minimal, stent insertion is not beneficial because symptom improvement may not be evident in incomplete obstruction. Multi-level obstructions should also be excluded before stent insertion since single level stent insertion cannot solve the problem of the obstructive symptoms.
Table 1.
Indications for Stent Insertion in the Upper Gastrointestinal Tract

Although the Borrmann type-IV of advanced gastric cancer is not generally indicated for stent insertion because there is not focal obstructive portion but diffuse tumor infiltration along the gastric wall, stent may be beneficial for the localized scirrhous type in gastric outlet. Stent can be also indicated for malignant obstruction by a recurred tumor at anastomotic site after surgical resection and reconstruction for gastric cancer in cases of impossible curative resection.14-16
Although benign stricture caused by corrosive stricture, surgical anastomosis, recurrent peptic ulcer, or wide endoscopic resection is not generally indicated for stent insertion because of insufficient clinical results, stent insertion may be a feasible alternative to surgical bypass for intractable benign strictures with repetitive endoscopic dilatation or in inoperable cases.17 Retrievable covered stent should be inserted temporarily for benign stricture, and be removed in 4 to 8 weeks after insertion to prevent tissue embedment. Re-obstruction can be problematic after the retrieval of a stent in benign stricture, and biodegradable stent may be promising for it does not require reintervention to remove the stent.
In cases with malignant esophageal fistula or perforation by esophageal, lung, or mediastinal cancers, stent can be indicated in inoperable cases to prevent aspiration, dysphagia and infection as well as to seal off the fistula.18,19 SEPSs as well as SEMSs can be considered for the closure of fistula or perforation, although the quality of evidence for the use of stent in the management of such cases is very low.
WHICH TYPE OF STENT CAN BE INSERTED IN THE UPPER GI TRACT?
Malignant esophageal obstruction can be divided into the upper, the middle and the lower portions. For the upper obstruction, stent may be problematic with globus sensation, throat pain or incomplete expansion of proximal flange of the stent. Modified stent with shorter flange and obtuse angle may be beneficial to prevent foreign body sensation after the stent placement for cervical esophageal obstruction.9 For the middle obstruction, both covered and uncovered stents can be used for palliative purpose. Covered stents, despite the risk of migration, is good at lowering the rate of re-obstruction by the tumor growth than uncovered types. The risk of migration is lowered when there is a mass keeping the proximal flange of the stent in place. In the distal obstruction including GE junction, covered stents with anti-reflux valve will prevent reflux symptom after the stent placement. If there is no intrinsic mass in the esophageal obstruction by extrinsic tumor invasion or compression, uncovered stents are beneficial in terms of lowering the risk of migration and tumor growth. In cases with fistula or perforation, covered stents or SEPSs are effective in palliating symptoms and sealing off the defect on the esophageal wall.
For gastric outlet obstruction, both covered and uncovered stents can be used based on the relative risk of re-obstruction or migration. In cases with pyloric obstruction by antral mass formation, covered stents are effective for lowering the risk of tumor growth and migration, whereas uncovered stents are good for malignant obstruction by extrinsic tumor invasion or compression in periampullary cancer in which there is no anchoring mass for the proximal flange of the stent. Double-layered stents may be effective for the prevention of migration as well as re-obstruction, although large-scale prospective randomized studies are warranted to evaluate the efficacy.
CLINICAL OUTCOMES OF STENTS IN THE UPPER GI TRACT
Technical success is defined as successful insertion and adequate placement of a stent, and clinical success as adequate oral feeding with palliation of symptoms. The clinical success rates of stents in the upper GI tract are around 85% to 90%, usually lower than the technical success rates due to incomplete expansion of the stent, inadequate placement, kinking of the stent in bended stricture, early migration or multi-level obstruction. For cases with inadequate diet 72 hours after the stent insertion, the reasons of clinical failure should be confirmed by endoscopy, barium study, or CT.
The duration of stent patency is determined by underlying diseases, patients' performance, concomitant chemotherapy/radiotherapy, the location of the obstruction and the type of the stent. The median duration of stent patency in the upper GI tract are reported around 55 to 307 days in most studies and are not significantly different between covered and uncovered types or among products from various companies, except for the esophageal cancer. In pyloric obstruction, the reported patency rates were 71% at 4 weeks, 61% at 8 weeks, and 33% at 6 months, and in cardiac obstruction including esophageal cancer, the rates were 94% at 4 weeks, 78% at 3 months, and 67% at 6 months in previous studies.1,4,20,21 When comparing stents with surgical bypass in malignant upper GI obstruction, stent showed many advantages in terms of less invasiveness and complications, shorter hospital stay, and lower cost.22-25 Considering the median duration of patency of 6 months, stent may be beneficial for patients whose life expectancy is less than 6 months.
In benign strictures, retrievable SEMSs or SEPSs have showed significant limitations such as high migration and recurrent stricture rates, bleeding, fistula, and damage to the GI wall.26-29 Recurrent stricture is believed to be associated with the fibrosis resulting from mechanical injury due to the stent or the in-growth of the granulation tissue. Based on the previous studies, stent is not routinely recommended for benign stricture until we see a significant improvement in stent design. Although stents have showed good results in sealing off the defect of fistula or perforation in several case series, the quality of evidence regarding this indication is low.
In palliation of obstructive symptoms with recurrent unresectable malignant obstruction after surgical resection, stents have showed high clinical success rate, low morbidity, and low cost compared to surgical bypass, but these results are from single-arm observational studies with small sample size. Further prospective randomized controlled studies are mandatory to validate the effect of stents in recurrent malignant obstruction after surgical resection.14-16
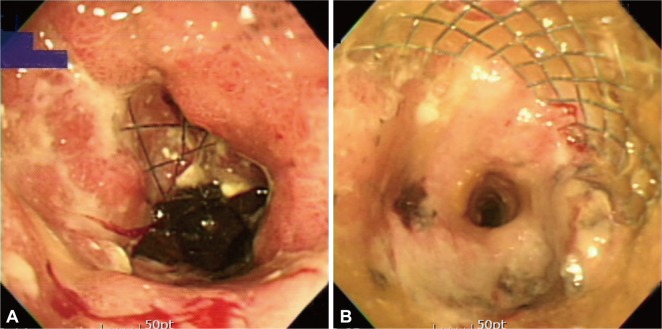
Re-obstruction and migration are the major complications of stents in the upper GI tract.1,20,29-33 Re-obstruction is developed by tumor over-growth, in-growth or food impaction (Fig. 2). The rate of re-obstruction has been reported to be 3% to 15% with covered stents and 10% to 42% with uncovered stents. It is unclear whether covered stents are superior to uncovered stents in terms of prevention of re-obstruction because tumor over-growth can occur at both ends of a stent and tumor in-growth can occur even in covered stents by membrane degradation in long-term follow-up.
Fig. 2.
Stent obstruction. Tumor over-growth occurs at both ends of a stent (A), and tumor in-growth occurs through the metal meshwork of stent (B).
Migration is more common in covered stents than in uncovered stents. The rate of migration has been reported to be 10% to 25% with covered stents, and 2% to 6% with uncovered stents. Although double-layered stents have been developed to overcome the drawbacks of covered and uncovered stents, the reported rates of re-obstruction and migration were both 9%, which were not significantly different from the conventional stents.8 It was reported that stent migration might be prevented by clipping of proximal end to GI wall in previous studies, but the efficacy should be confirmed by large-scaled prospective randomized trials.34,35
Other complications include pain, bleeding, aspiration, GE reflux, and stent dislodgement, which are usually temporary and manageable with symptomatic treatment.
CONCLUSIONS
Endoscopic stenting is currently the most common modality for palliation of symptoms in patients with unresectable upper GI cancer, and has replaced conventional surgical bypass. Endoscopic stenting is associated with less invasiveness and complications, shorter hospital stay, and lower cost. Although there have been problems of stent migration and re-obstruction, newly-developed stents are expected to overcome these limitations and to be extended to benign diseases as well as malignant obstruction.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Kim CG, Choi IJ, Lee JY, et al. Covered versus uncovered self-expandable metallic stents for palliation of malignant pyloric obstruction in gastric cancer patients: a randomized, prospective study. Gastrointest Endosc. 2010;72:25–32. doi: 10.1016/j.gie.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 2.Lee SM, Kang DH, Kim GH, Park WI, Kim HW, Park JH. Self-expanding metallic stents for gastric outlet obstruction resulting from stomach cancer: a preliminary study with a newly designed double-layered pyloric stent. Gastrointest Endosc. 2007;66:1206–1210. doi: 10.1016/j.gie.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Lee SH, Kang DH, Bae YM, et al. Treatment of gastric outlet obstruction by stomach cancer with using double-layered pyloric stent. Korean J Gastrointest Endosc. 2007;35:221–227. doi: 10.1016/j.gie.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 4.van Hooft JE, Uitdehaag MJ, Bruno MJ, et al. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointest Endosc. 2009;69:1059–1066. doi: 10.1016/j.gie.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Shim CS, Jung IS, Cheon YK, et al. Management of malignant stricture of the esophagogastric junction with a newly designed self-expanding metal stent with an antireflux mechanism. Endoscopy. 2005;37:335–339. doi: 10.1055/s-2005-861113. [DOI] [PubMed] [Google Scholar]
- 6.Homs MY, Wahab PJ, Kuipers EJ, et al. Esophageal stents with antireflux valve for tumors of the distal esophagus and gastric cardia: a randomized trial. Gastrointest Endosc. 2004;60:695–702. doi: 10.1016/s0016-5107(04)02047-4. [DOI] [PubMed] [Google Scholar]
- 7.Schoppmeyer K, Golsong J, Schiefke I, Mössner J, Caca K. Antireflux stents for palliation of malignant esophagocardial stenosis. Dis Esophagus. 2007;20:89–93. doi: 10.1111/j.1442-2050.2007.00646.x. [DOI] [PubMed] [Google Scholar]
- 8.Shim CS, Cho YD, Moon JH, et al. Fixation of a modified covered esophageal stent: its clinical usefulness for preventing stent migration. Endoscopy. 2001;33:843–848. doi: 10.1055/s-2001-17326. [DOI] [PubMed] [Google Scholar]
- 9.Shim CS, Jung IS, Bhandari S, et al. Management of malignant strictures of the cervical esophagus with a newly-designed self-expanding metal stent. Endoscopy. 2004;36:554–557. doi: 10.1055/s-2004-814555. [DOI] [PubMed] [Google Scholar]
- 10.Conio M, Repici A, Battaglia G, et al. A randomized prospective comparison of self-expandable plastic stents and partially covered self-expandable metal stents in the palliation of malignant esophageal dysphagia. Am J Gastroenterol. 2007;102:2667–2677. doi: 10.1111/j.1572-0241.2007.01565.x. [DOI] [PubMed] [Google Scholar]
- 11.Verschuur EM, Repici A, Kuipers EJ, Steyerberg EW, Siersema PD. New design esophageal stents for the palliation of dysphagia from esophageal or gastric cardia cancer: a randomized trial. Am J Gastroenterol. 2008;103:304–312. doi: 10.1111/j.1572-0241.2007.01542.x. [DOI] [PubMed] [Google Scholar]
- 12.Saito Y, Tanaka T, Andoh A, et al. Usefulness of biodegradable stents constructed of poly-l-lactic acid monofilaments in patients with benign esophageal stenosis. World J Gastroenterol. 2007;13:3977–3980. doi: 10.3748/wjg.v13.i29.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Q, Guo S, Wang Z. A type of esophageal stent coating composed of one 5-fluorouracil-containing EVA layer and one drug-free protective layer: in vitro release, permeation and mechanical properties. J Control Release. 2007;118:318–324. doi: 10.1016/j.jconrel.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Lee WH, Ko JH, Jung GS, Jeong KS, Kim KJ, Lee SH. Covered self-expandable metallic stent placement for a post-operative malignant anastomotic stricture secondary to recurrent gastric cancer. J Korean Radiol Soc. 2007;57:253–259. [Google Scholar]
- 15.Jo SJ, Yoon KY, Choi KH, et al. A comparative study of stenting versus surgical bypass in gastric outlet obstruction caused by gastric cancer. J Korean Gastric Cancer Assoc. 2007;7:82–87. [Google Scholar]
- 16.Cho YK, Kim SW, Nam KW, et al. Clinical outcomes of self-expandable metal stents in palliation of malignant anastomotic strictures caused by recurrent gastric cancer. World J Gastroenterol. 2009;15:3523–3527. doi: 10.3748/wjg.15.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song HY, Jung HY, Park SI, et al. Covered retrievable expandable nitinol stents in patients with benign esophageal strictures: initial experience. Radiology. 2000;217:551–557. doi: 10.1148/radiology.217.2.r00nv03551. [DOI] [PubMed] [Google Scholar]
- 18.Kozarek RA, Raltz S, Brugge WR, et al. Prospective multicenter trial of esophageal Z-stent placement for malignant dysphagia and tracheoesophageal fistula. Gastrointest Endosc. 1996;44:562–567. doi: 10.1016/s0016-5107(96)70009-3. [DOI] [PubMed] [Google Scholar]
- 19.Shin JH, Song HY, Ko GY, Lim JO, Yoon HK, Sung KB. Esophagorespiratory fistula: long-term results of palliative treatment with covered expandable metallic stents in 61 patients. Radiology. 2004;232:252–259. doi: 10.1148/radiol.2321030733. [DOI] [PubMed] [Google Scholar]
- 20.Im JP, Kang JM, Kim SG, Kim JS, Jung HC, Song IS. Clinical outcomes and patency of self-expanding metal stents in patients with malignant upper gastrointestinal obstruction. Dig Dis Sci. 2008;53:938–945. doi: 10.1007/s10620-007-9967-1. [DOI] [PubMed] [Google Scholar]
- 21.Kim TO, Kang DH, Kim GH, et al. Self-expandable metallic stents for palliation of patients with malignant gastric outlet obstruction caused by stomach cancer. World J Gastroenterol. 2007;13:916–920. doi: 10.3748/wjg.v13.i6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddiqui A, Spechler SJ, Huerta S. Surgical bypass versus endoscopic stenting for malignant gastroduodenal obstruction: a decision analysis. Dig Dis Sci. 2007;52:276–281. doi: 10.1007/s10620-006-9536-z. [DOI] [PubMed] [Google Scholar]
- 23.Hosono S, Ohtani H, Arimoto Y, Kanamiya Y. Endoscopic stenting versus surgical gastroenterostomy for palliation of malignant gastroduodenal obstruction: a meta-analysis. J Gastroenterol. 2007;42:283–290. doi: 10.1007/s00535-006-2003-y. [DOI] [PubMed] [Google Scholar]
- 24.Jeurnink SM, van Eijck CH, Steyerberg EW, Kuipers EJ, Siersema PD. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol. 2007;7:18. doi: 10.1186/1471-230X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeurnink SM, Steyerberg EW, van Hooft JE, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490–499. doi: 10.1016/j.gie.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 26.Dua KS, Vleggaar FP, Santharam R, Siersema PD. Removable self-expanding plastic esophageal stent as a continuous, non-permanent dilator in treating refractory benign esophageal strictures: a prospective two-center study. Am J Gastroenterol. 2008;103:2988–2994. doi: 10.1111/j.1572-0241.2008.02177.x. [DOI] [PubMed] [Google Scholar]
- 27.Sharma P, Kozarek R Practice Parameters Committee of American College of Gastroenterology. Role of esophageal stents in benign and malignant diseases. Am J Gastroenterol. 2010;105:258–273. doi: 10.1038/ajg.2009.684. [DOI] [PubMed] [Google Scholar]
- 28.Triester SL, Fleischer DE, Sharma VK. Failure of self-expanding plastic stents in treatment of refractory benign esophageal strictures. Endoscopy. 2006;38:533–537. doi: 10.1055/s-2006-925318. [DOI] [PubMed] [Google Scholar]
- 29.Kim GH, Kang DH, Lee DH, et al. Which types of stent, uncovered or covered, should be used in gastric outlet obstructions? Scand J Gastroenterol. 2004;39:1010–1014. doi: 10.1080/00365520410003146. [DOI] [PubMed] [Google Scholar]
- 30.Park HY, Kang DH, Eum JS, et al. Uncovered self-expandable metal stents (SEMS) for gastric outlet obstruction caused by stomach cancer. Korean J Gastrointest Endosc. 2008;36:57–63. [Google Scholar]
- 31.Kwon DS, Goh PG, Hwang SW, et al. The use of uncovered self-expandable metallic stents for palliation of gastric outlet obstruction caused by stomach cancer. Korean J Gastrointest Endosc. 2008;36:336–340. [Google Scholar]
- 32.Seo EH, Jung MK, Park MJ, et al. Covered expandable nitinol stents for malignant gastroduodenal obstructions. J Gastroenterol Hepatol. 2008;23(7 Pt 1):1056–1062. doi: 10.1111/j.1440-1746.2007.05260.x. [DOI] [PubMed] [Google Scholar]
- 33.Cho YK, Kim SW, Hur WH, et al. Clinical outcomes of self-expandable metal stent and prognostic factors for stent patency in gastric outlet obstruction caused by gastric cancer. Dig Dis Sci. 2010;55:668–674. doi: 10.1007/s10620-009-0787-3. [DOI] [PubMed] [Google Scholar]
- 34.Kim ID, Kang DH, Choi CW, et al. Prevention of covered enteral stent migration in patients with malignant gastric outlet obstruction: a pilot study of anchoring with endoscopic clips. Scand J Gastroenterol. 2010;45:100–105. doi: 10.3109/00365520903410554. [DOI] [PubMed] [Google Scholar]
- 35.Park SY, Park CH, Cho SB, et al. The usefulness of clip application in preventing migration of self-expandable metal stent in patients with malignant gastrointestinal obstruction. Korean J Gastroenterol. 2007;49:4–9. [PubMed] [Google Scholar]