The synapsins are a family of neuronal phosphoproteins consisting of SYN1 at chrXp11.3, SYN2 at chr3p25, and SYN3 at chr22q12.3 with alternative splicing leading to as many as 10 isoforms. They are involved in synaptic transmission and plasticity, as well as various stages of neurodevelopment, including axon outgrowth and synapse formation (Cesca et al, 2010). All synapsins are highly concentrated at presynaptic nerve terminals of central neurons and associated with the cytoplasmic surface of synaptic vesicles, but SYN3 has markedly distinct developmental expression and subcellular distribution, suggesting divergent function (Pieribone et al, 2002). Therefore, not surprisingly, a role for synapsins in neuropsychiatry has been suggested and, indeed, several studies have indicated that genetic variants at these genes can be associated with epilepsy, autism, schizophrenia, and bipolar disorder (BD) (Cesca et al, 2010). Furthermore, mRNA- and protein-level postmortem brain studies have suggested dysregulation of these genes in both BD and major depressive disorder (MDD) (Cesca et al, 2010; Cruceanu et al, 2012). Thus, the study of mechanisms responsible for this dysregulation in mood disorders becomes pertinent.
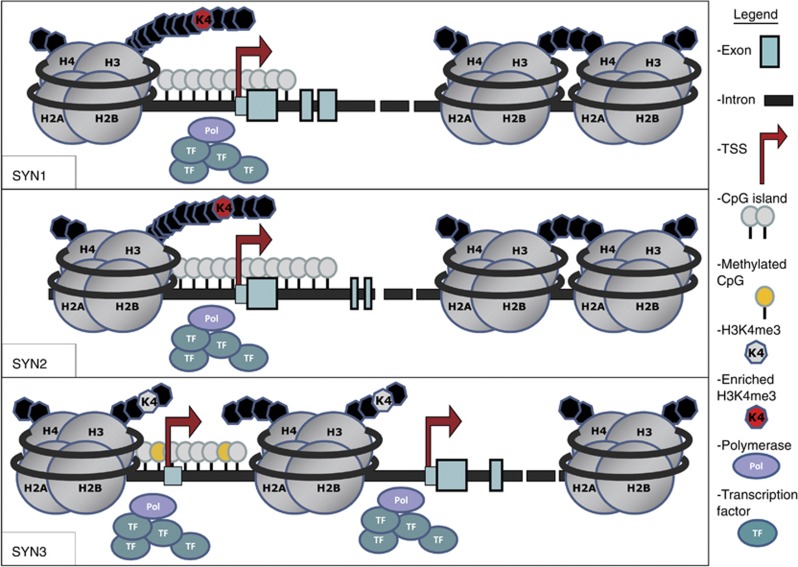
In the last few years, evidence has emerged suggesting that epigenetics play a role in neuropsychiatric disorders (Jiang et al, 2008), thus it is plausible that the dysregulation observed in synapsin expression could be attributed in part to epigenetic mechanisms. We found evidence that enrichment of H3K4me3—an epigenetic mark associated with increased transcription—at the promoters of SYN1 and SYN2, but not SYN3 is correlated with increased expression of these genes in the prefrontal cortex of patients with BD and MDD compared with controls (Cruceanu et al, 2012, see Figure 1). These findings are encouraging, but future research should better characterize these mechanisms by exploring the role of other chromatin epigenetic marks and brain-region specificity. In addition, the role of DNA methylation, an equally important epigenetic mechanism, should be investigated. In silico analyses have detected rich CpG islands at the proximal promoters of SYN1 (845 bp) and SYN2 (975 bp), as well as at a distal promoter of SYN3 (613 bp) (Miklem and Hillier, 2012, see Figure 1). To date there is no evidence in the literature of altered DNA methylation at synapsins in mood disorders, although one study of a single schizophrenia patient suggests potentially variably methylated sites in the distal CpG island of SYN3 (Murphy et al, 2008). Interestingly, the CpG islands at SYN1 and SYN2 are immediately preceded by regions of enriched H3K4me3 in mood disorders (Cruceanu et al, 2012, see Figure 1). The same is not true for SYN3, and considering this gene's distinct expression profile and potential implication throughout neurogenesis (Pieribone et al, 2002), perhaps different mechanisms regulate SYN3. The figure illustrates our current knowledge of the synapsin genes' structure, as well as the epigenetic mechanisms that have been identified in psychiatric disorders to date.
Figure 1.
Potential epigenetic mechanisms at the promoter regions of synapsin genes. Upper panel: the SYN1 gene (chrX:47 431 300–47 479 256). In silico analysis predicts a CpG island 845 bp in size at the 5′end of the gene (chrX:47 478 671–47 479 515) that spans from −259 bp upstream of the transcription start site (TSS) to +586 bp downstream. Evidence from chromatin immunoprecipitation assays for H3K4-trimethylation suggests that this epigenetic mark is enriched in mood disorders around roughly −200 bp to−350 bp upstream of the TSS. Middle panel: the SYN2 gene (chr3: 12 045 862–12 233 532). The first three coding exons are represented here. In silico analysis predicts a CpG island of 975 bp at the 5′end of the gene (chr3: 12 045 653–12 046 627) that spans from −208 bp upstream of the TSS to +767 bp downstream. Evidence from chromatin immunoprecipitation assays for H3K4-trimethylation suggests that this epigenetic mark is enriched in mood disorders around roughly −175 bp to−400 bp upstream of the TSS. Bottom panel: the SYN3 gene (chr22: 32 908 540–33 402 809). There is no predicted CpG island at the proximal promoter, but at a distal promoter upstream an alternative noncoding first exon, there is a CpG island 613 bp in size. Certain CpGs within this island have been suggested to be variably methylated in schizophrenia.
In conclusion, brain expression differences seen in synapsin genes in mood disorders may be explained in part by differences in H3K4me3. These results need additional and independent confirmation. Moreover, considering that promoter DNA methylation can modulate gene expression and lead to neuropsychiatric phenotypes, a study of DNA methylation patterns at the synapsin promoters is warranted. On the basis of growing evidence suggesting that epigenetic mechanisms may be involved in altered regulation of synapsins in mood disorders, it would be of interest to study these genes as potential therapeutic targets or biomarkers of treatment response. Evidence is starting to emerge pointing to epigenetic marks as potential biomarkers of treatment response. For instance, for brain-derived neurotrophic factor (BDNF), (Lopez et al, 2010) showed that promoter H3K27me3 levels could serve as a biomarker of response to citalopram in MDD, and (D'Addario et al, 2012) found distinct DNA methylation patterns at the BDNF promoter in BD patients depending on mood-stabilizer and antidepressant therapy. Although no such evidence has yet emerged for synapsins, a recent study showed that lithium, one of the most commonly prescribed drugs for BD, can modulate SYN2 expression in neuronal cell types (Cruceanu et al, 2012). Thus, an investigation of synapsin epigenetics in the brain compared with the periphery would be an interesting next step in elucidating their potential to serve as biomarkers for mood disorders or their treatment.
Acknowledgments
This work was supported by the Canadian Institute of Health Research (CIHR) grant MOP64410 to GT, MA, and GAR, and CIHR grant MOP119430 to GT.
Disclaimer
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no conflict of interest.
References
- Cesca F, Baldelli P, Valtorta F, Benfenati F. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol. 2010;91:313–348. doi: 10.1016/j.pneurobio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Cruceanu C, Alda M, Grof P, Rouleau GA, Turecki G. Synapsin II is involved in the molecular pathway of lithium treatment in bipolar disorder. PloS one. 2012;7:e32680. doi: 10.1371/journal.pone.0032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruceanu C, Alda M, Nagy C, Freemantle E, Rouleau GA, Turecki G. H3K4 tri-methylation in synapsin genes leads to different expression patterns in bipolar disorder and major depression. Int J Neuropsychopharmacol. 2012;9:1–11. doi: 10.1017/S1461145712000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Addario C, Dell'Osso B, Palazzo MC, Benatti B, Lietti L, Cattaneo E, et al. Selective DNA methylation of BDNF promoter in bipolar disorder: differences among patients with BDI and BDII. Neuropsychopharmacology. 2012;37:1647–1655. doi: 10.1038/npp.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Langley B, Lubin FD, Renthal W, Wood MA, Yasui DH, et al. Epigenetics in the nervous system. J Neurosci. 2008;28:11753–11759. doi: 10.1523/JNEUROSCI.3797-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JP, Mamdani F, Labonte B, Beaulieu MM, Yang JP, Berlim MT, et al. 2012Epigenetic regulation of BDNF expression according to antidepressant response Mol psychiatry;doi: 10.1038/mp.2012.38 [DOI] [PMC free article] [PubMed]
- Miklem G, Hillier L. UCSC CpG islands track (unpublished) based on Gardiner-Garden M. and Frommer M. CpG islands in vertebrate genomes. J Mol Biol. 2012;196:261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- Murphy BC, O'Reilly RL, Singh SM. DNA methylation and mRNA expression of SYN III, a candidate gene for schizophrenia. BMC Med Genet. 2008;9:115. doi: 10.1186/1471-2350-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Porton B, Rendon B, Feng J, Greengard P, Kao HT. Expression of synapsin III in nerve terminals and neurogenic regions of the adult brain. J Comp Neurol. 2002;454:105–114. doi: 10.1002/cne.10417. [DOI] [PubMed] [Google Scholar]