Abstract
Background
Engineered zinc-finger nucleases (ZFN) represented an innovative method for the genome manipulation in vertebrates. ZFN introduced targeted DNA double strand breaks (DSB) and initiated non-homologous end joining (NHEJ) after pronuclear or cytoplasmatic microinjection into zygotes. Resulting frame shift mutations led to functional gene ablations in zebra fish, mice, pigs and also in laboratory rats. Therefore, we targeted the rat Rag1 gene essential for the V(D)J recombination within the immunoglobulin production process and for the differentiation of mature B and T lymphocytes to generate an immunodeficient rat model in the LEW/Ztm strain.
Results
After microinjection of Rag1 specific ZFN mRNAs in 623 zygotes of inbred LEW/Ztm rats 59 offspring were born from which one carried a 4 bp deletion. This frame shift mutation led to a premature stop codon and a subsequently truncated Rag1 protein confirmed by the loss of the full-length protein in Western Blot analysis. Truncation of the Rag1 protein was characterized by the complete depletion of mature B cells. The remaining T cell population contained mature CD4+/CD3+/TCRαβ+ as well as CD8+/CD3+/TCRαβ+ positive lymphocytes accompanied by a compensatory increase of natural killer cells in the peripheral blood. Reduction of T cell development in Rag1 mutant rats was associated with a hypoplastic thymus that lacked follicular structures. Histological evaluation also revealed the near-complete absence of lymphocytes in spleen and lymph nodes in the immunodeficient Rag1 mutant rat.
Conclusion
The Rag1 mutant rat will serve as an important model for transplantation studies. Furthermore, it may be used as a model for reconstitution experiments related to the immune system, particularly with respect to different populations of human lymphocytes, natural killer cells and autoimmune phenomena.
Keywords: Rag1, Zinc-finger nucleases, Rat, Lymphocytes, Natural killer cells, Hypoplastic thymus
Background
Several modern techniques facilitate the genetic manipulation of the rat genome in vivo. Aside from the pronuclear injection of recombinant DNA constructs, the integration of lentiviral vectors into the rat genome after injection into the perivitelline space and transposon-mediated insertions have enabled studies in transgenic rats [1-5]. Random integration of Sleeping Beauty and PiggyBac transposons caused gene inactivation at the integration sites [6,7]. Moreover, the cultivation of rat embryonic stem cells does allow gene targeting and functional deletion through homologous recombination (HR) in vitro[8-11]. However, due to the technical complexity of these procedures only the p53 gene and the Par-2 gene have been inactivated through HR in ES cells to date [12,13]. Alternative strategies for the functional inactivation of genes use the highly efficient Zinc-finger nuclease (ZFN) [14] and transcription activator-like effector nucleases (TALEN) technologies [15], both of which offer a rapid approach in gene targeting. Zinc-finger nucleases are hybrid proteins consisting of polymeric zinc-finger proteins fused to the endonuclease domain of the restriction enzyme FokI. A pair of zinc-finger nucleases binds to two contiguous target sequences in each DNA strand separated by a 6 bp cleavage site. Subsequent dimerization of the FokI domains causes DSB and initiate endogenous repair processes. Inaccurate repairs by NHEJ in response to DNA damage introduce deletions or insertions in the spacer region. The resulting frame shift leads to a premature stop codon and the translation of a truncated, inactive protein [16,17]. Therefore, ZFN have been used for the manipulation of human and rodent cells in vitro[18-20] as well as for gene targeting or for homology directed repair in zebra fish, mouse, rat and pig embryos in vivo[21-25]. In the rat, genes such as immunoglobulin heavy chain 6 (Igh-6), immunoglobulin heavy chain (gamma polypeptide) (JH locus), transgenic eGFP, renin (Ren), interleukin 2 receptor gamma (Il2rg), ATP-binding cassette, sub-family B (MDR/TAP), member 1A (Mdr1a) and Rab38 have been successfully inactivated by ZFN mediated gene disruption in single cell embryos [26-30].
Here we report about generating a Rag1 knockout by ZFN in LEW rats, with the intention of establishing an immunodeficient rat model on a defined genetic background.
Rag1 as well as Rag2 are highly conserved genes located in close vicinity in head to tail arrangement on chromosome 11p13 in humans, on chromosome 2qE2 in the mouse, and at chromosome 3q31 in the rat. In humans, Rag1 mutations lead to severe combined immunodeficiency (SCID) due to B and T cell loss (T-B-SCID) or Omenn’s Syndrome (T+B-SCID) with a shortened life expectancy reviewed in [31-34]. A similar phenotype was described after gene targeting of Rag1 in the mouse with an arrest of B and T precursor cells in an early developmental stage, no serum IgG and an elevated number of natural killer cells (NKC) [35-38].
The generated Rag1 mutant rat on a defined genetic background can provide a useful tool for studies of the immune system, in transplantation studies, and in autoimmune diseases.
Results
Embryo manipulation and Rag1 gene targeting
A pair of zinc-finger nucleases consisting of five zinc-finger modules recognizing 15 bp at both sides of a 6 bp cut side located close to the 5’ end of Exon 2, were used to induce DSBs and NHEJ in the Rag1 gene (Figure 1A). The injection of the Rag1 specific ZFNs into 623 zygotes harvested from 35 hyperovulated donors resulted in 444 morphologically intact embryos transferred to 19 pseudopregnant (LEW x WKY) F1 females. From the 59 pups born, 49 survived the weaning period.
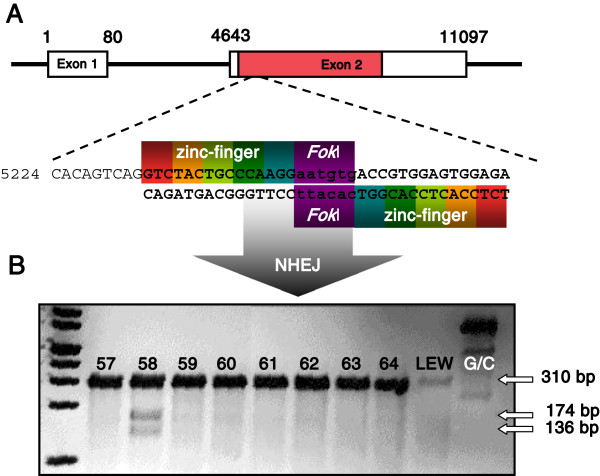
Figure 1.
Introduction of a frame shift mutation. A The 11097 base pairs of genomic Rag1 are separated in two exons. Of these only Exon 2 contains the complete coding sequence (red bar). Rag1 specific zinc-finger nucleases recognizing 18 bp upstream and downstream of the 6 bp cutting site between position 5233 and 5267 introduced DSBs through the FokI endonuclease domain and initiated NHEJ. BCel-I endonuclease digest of the 310 bp heteroduplex DNA derived from the hybridization of the DNA from LEW wild type rats and founder 58 into 174 bp and 136 bp fragments proofed the mutagenesis of one Rag1 allele in founder 58. G/C: heteroduplex control DNA; LEW: LEW/Ztm wild type DNA.
Surveyor mutation detection assay was performed to identify offspring carrying a Rag1 mutation by cutting of heteroduplex DNA derived from hybridization of wild type LEW/Ztm DNA with founder DNA through the Cel-I endonuclease [39,40]. Analysis of the offspring revealed partial cleavage of the 310 bp hybrid DNA into 174 bp and 136 bp fragments in one female (denominated “founder 58”), giving a 1.7% rate of transgenesis of the Rag1 gene (Figure 1B).
Rag1 frame shift mutation
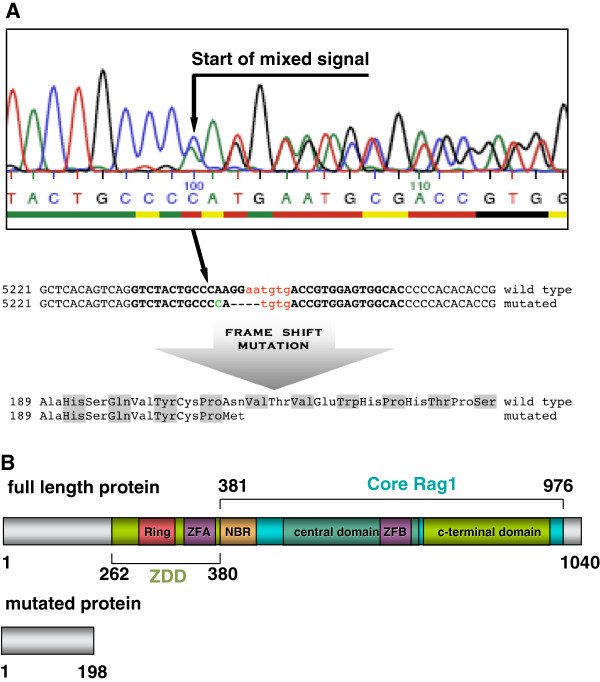
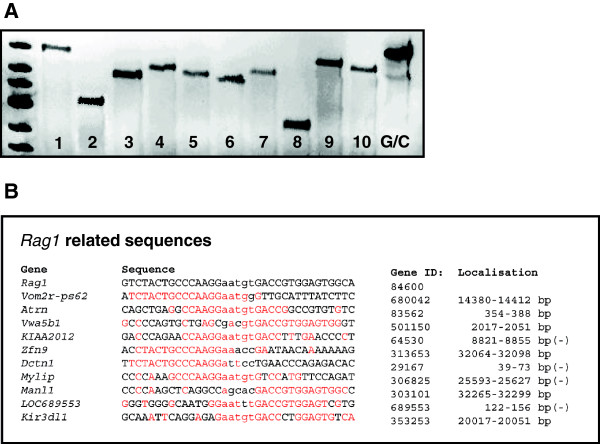
Sequencing of exon 2 of the Rag1 gene from “founder 58” verified an A to C base exchange at position 5244 combined with a 4 bp deletion at position 5246 – 5249 while mutations were absent on the second allele (Figure 2A). The 4 bp deletion in the Rag1 gene of “founder 58” caused a frame shift mutation and generated a premature stop codon at position 667 of the Rag1 mRNA, thereby encoding a truncated 198 aa protein. This shortened protein was homologous to the N-terminal part of wild type Rag1 but lost the zinc-binding dimerization domain (ZDD) and the c-terminal Core domain of the 1040 aa full length protein [38] (Figure 2B). No off-target mutations were seen in the 10 loci with the highest sequence homology to the zinc-finger binding and cut site in the Rag1 gene by the SURVEYOR mutation detection assay in “founder 58” (Figure 3).
Figure 2.
Analysis of the mutant Rag1 allele. A Heterozygosity at the genomic Rag1 locus of founder 58 was confirmed by sequencing. The start of the mixed signal indicated the Rag1 mutation identified as A to C exchange (green upper case) plus 4 bp deletion at the ZFN cut site (red lower case) and the ZFN binding regions (black upper case). The introduced frame shift caused a premature stop codon leading to a truncation of the Rag1 protein to a 198 aa N-terminal residue. B Full length Rag1 protein consists the functional important zinc-binding dimerization domain (ZDD) containing the RING finger (RING) and the zinc finger A (ZFA) as well as the c-terminal core domain with the nonamer-binding region (NBR) and the zinc finger B (ZFB). Mutated Rag1 lost all domains essential for Rag1 function in V(D)J recombination.
Figure 3.
Off-target mutation analysis. A Cel-I digest of heteroduplex DNA revealed no additional off-target mutations at the 10 loci with highest homology to Rag1; 1: Vom2r-ps62; 2: Atrn; 3: Vwa5b1; 4: KIAA2012; 5: Zfn9; 6: Dctn1; 7: Mylip; 8: Manl1; 9: LOC689553; 10: Kir3d1l; G/C: control heteroduplex DNA. B Genes, gene IDs, sequence homologies and localization of Rag1 related sequences to exclude off-target mutations; upper case: ZFN binding sites; lower case: ZFN cut site; homolog base pairs in red; (-): Gene located on the minus strand.
Germ line transmission and genotyping
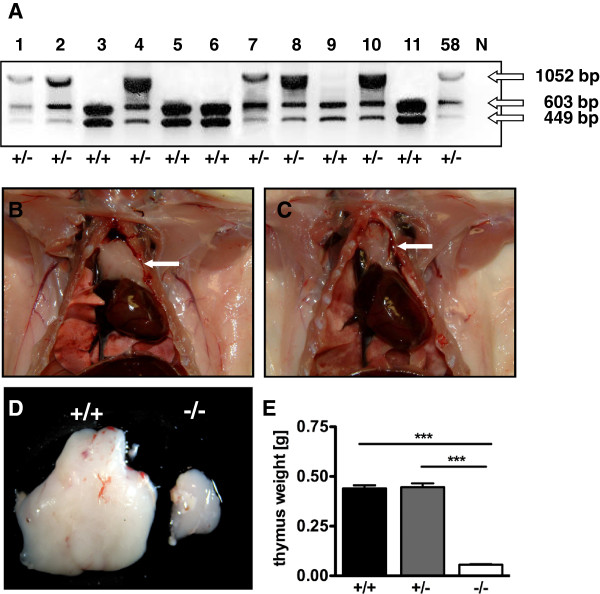
Subsequently, the heterozygous “founder 58” was mated to a wild type LEW male to evaluate germ line transmission and to establish the novel Rag1 deficient LEW/Ztm-Rag1em1Ztm coisogenic strain. To distinguish between wild type and heterozygous pups, we used the loss of the recognition site of the restriction endonuclease StyI caused by the 4 bp deletion in Exon 2 of Rag1. Therefore, genotyping was performed by PCR amplification of a 1052 bp sequence of Rag1. While the PCR products derived from wild type allele were cleaved into 603 bp and 449 bp fragments by StyI, mutated Rag1 sections remained uncut. Six out of 11 offspring were heterozygous, demonstrating germ line competence and confirming the heterozygosity of “founder 58” (Figure 4A). Offspring carrying the mutation was used for further breeding. Heterozygous LEW/Ztm-Rag1em1Ztm rats showed normal reproduction with litter sizes ranging from 6 to 12. The mutation was inherited in the expected Mendelian ratio.
Figure 4.
Genotyping and macroscopical examination. A Genotyping of the offspring derived from founder 58 using PCR amplification and StyI-HF digest. Half of the 1052 bp PCR products from heterozygous animal are digested into 603 bp and 449 bp fragments, while the Rag1 PCR product from wild type offspring was digested completely; N: negative control. 58: PCR product from founder 58 as positive control. B Normal thymus of wild type animal of the strain LEW-Rag1em1/Ztm-RaglemlZtm. C Hypoplastic thymus in a Rag1 mutant rat. D Comparison of the thymus sizes of wild type and Rag1 mutant rats. E Comparison of thymus weigths, *** : P < 0.0001.
Macroscopical and histological evaluation
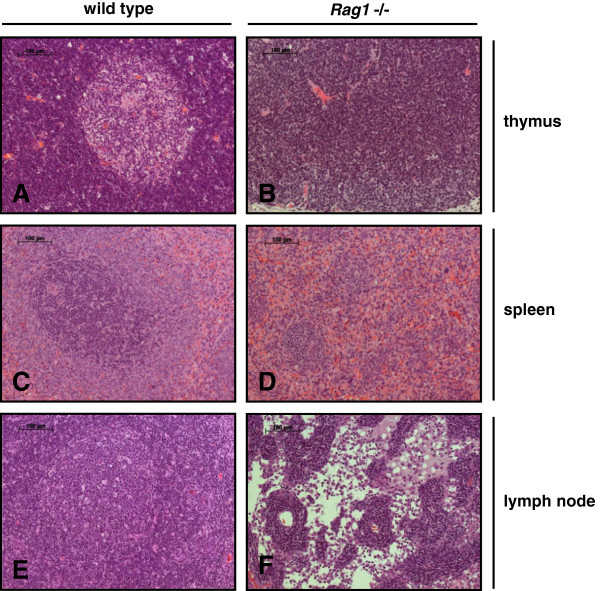
Phenotypical characterization revealed no ontogenetic disorders despite of underdeveloped thymi of the Rag1 mutant rats (Figure 4B and C). The mean thymus weight was significantly reduced from 0.44 g (SD ±0,04) in wild type and 0.45 g (SD ±0,05) in heterozygous animals to 0.06 g (SD ±0,01) in homozygous Rag1 mutant rats (Figure 4D and E). The reduced size of the thymus of Rag1 mutant rats was associated with a complete loss of the follicular structure showing the absence of T lymphocytes (Figure 5B). Histological evaluation of the spleen demonstrated the differentiation in red and white pulp containing B cells in the lymphatic follicles and T lymphocytes in the periarteriolar lymphoid sheaths (PALS) in wild type animals (Figure 5C). Besides the central artery the follicles of the white pulp are reduced and the PALS were lost in rats with partial Rag1 inactivation (Figure 5D). In wild type lymph nodes the cortex was characterized through trabecular structures separating follicles where B cell differentiation occurred in the germinal centers (Figure 5E). The lymph nodes of homozygous LEW/Ztm-Rag1em1Ztm rats appeared devoid of T and B lymphocytes and follicular structures, in which physiological B cell differentiation would occur, were absent (Figure 5F). No further macroscopic differences in organ size and structures were found in homozygous LEW/Ztm-Rag1em1Ztm rats, and careful histological evaluation revealed no malformation of organs outside of the lymphatic system.
Figure 5.
Histological evaluation of lymphatic organs of wild type and Rag1 mutant rats. A and B thymus; C and D spleen; E and F lymph nodes. H/E staining.
Disruption of wild type Rag1
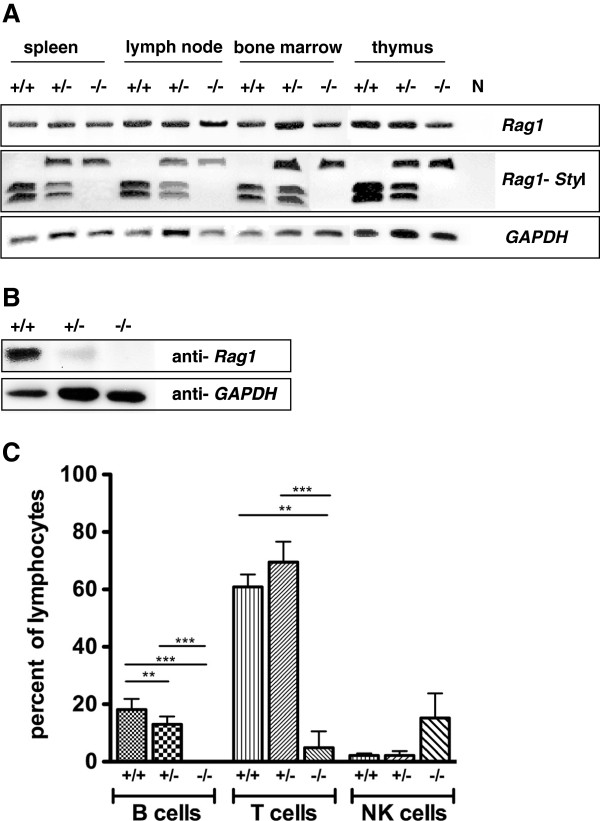
Molecular characterization by RT-PCR showed a shift from the expression of wild type Rag1 mRNA in the lymphatic organs of wild type rats to the exclusive expression of mutated Rag1 in knockout rats. In heterozygous animals wild type and mutated Rag1 were transcribed equally (Figure 6A). Western Blot analysis confirmed the loss of full length Rag1 protein in the thymus of homozygous mutant rats, while heterozygous animals exhibited a significant reduction of the Rag1 protein concentration as compared to the strong translation of Rag1 in wild type rats (Figure 6B).
Figure 6.
Analysis of Rag1 expression in lymphatic organs. RT-PCR of wild type, heterozygous and Rag1 mutant rats: The Rag1 PCR product (upper panel) was digested with StyI-HF to distinguish between translation of wild type and mutated Rag1 (mid panel). GAPDH used as housekeeping gene (lower panel). B Western Blot analysis: Translation of Rag1 in the thymus from wild type, heterozygous and Rag1 mutant rats compared to GAPDH as housekeeping protein. C Comparison of NK cells, B and T cell ratio in the peripheral blood of wild type, heterozygous and Rag1 mutated rats determined by FACS analysis. *** P < 0.0001; ** P < 0.005.
Lymphocyte depletion in Rag1 mutant rats
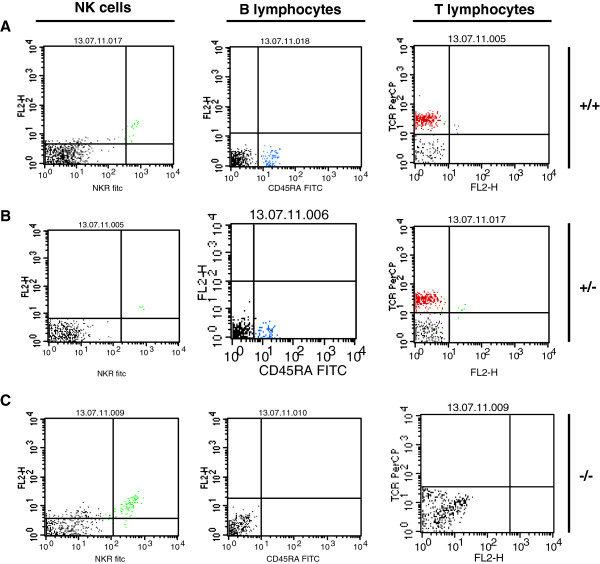
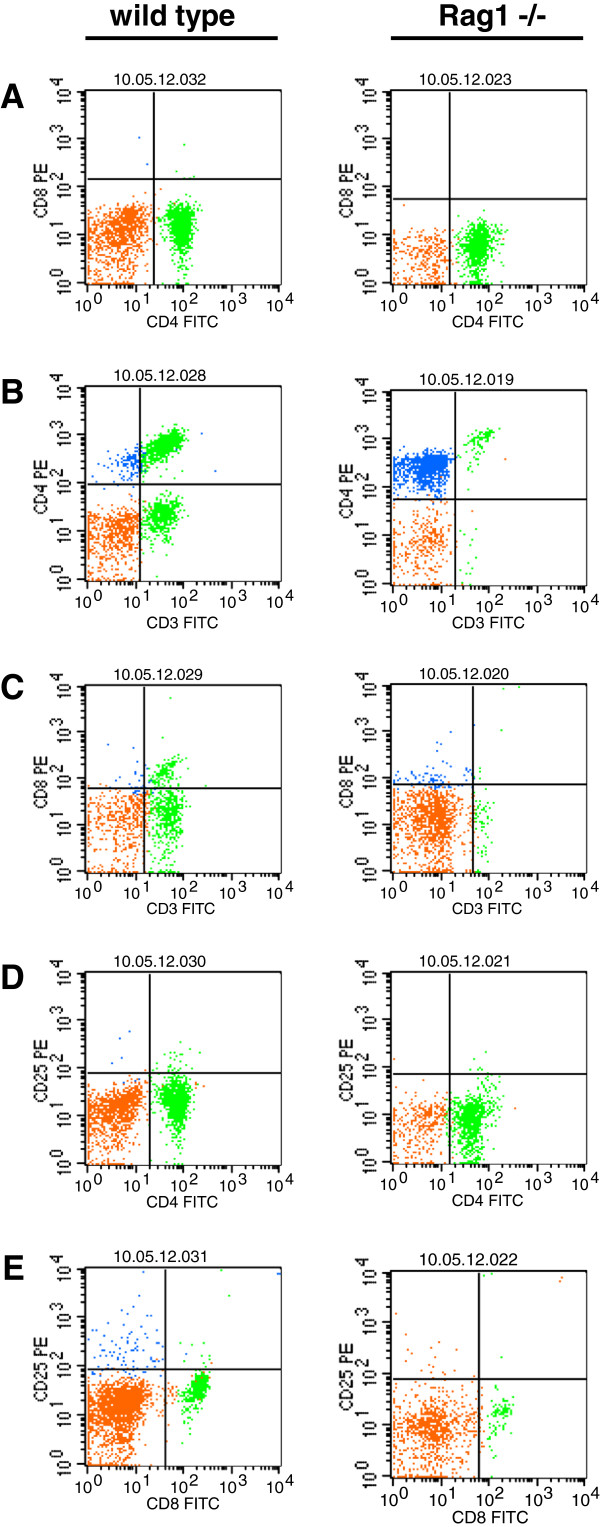
The quantity of B and T lymphocytes, as well as NKC was determined by FACS analysis. The fraction of B cells in peripheral blood was almost identical in wild type and heterozygous animals with a mean of 18,1% (SD ± 3,4%) and 13% (SD ± 2,5%). B cells were absent in Rag1 homozygous mutant rats (Figure 6C; 7A-C). Further, the ratio of T cells was almost the same in wild type and heterozygous rats with 60,9% (SD ± 4,1%) and 69,5% (SD ± 6,9%), however in rats with deficiency of full length Rag1 T cells were significantly reduced with 4,6 (SD± 5,7%) (Figure 6C; 7A-C). Inverse ratios were found when counting NKC. While in wild type and heterozygous rats only 2,2% (SD ± 0,7%) respectively 2,2% (SD ± 1,2%) of NKC could be detected, the peripheral blood of Rag1 mutant rats contained 15,3 ± 7,5% NKC suggesting a compensatory increase of natural killer cells (Figure 6Cx; 7A-C). Further analysis revealed that the T cell population isolated from the peripheral blood of rats with homozygous Rag1 frame shift mutation consisted of mature CD4+/CD3+/TCRαβ+ and CD8+/CD3+/TCRαβ+ thymocytes (Figure 8).
Figure 7.
FACS analysis of NKC, B and T lymphocytes in the blood: A wild type, B heterozygous and C Rag1 mutant rat; blue: CD161 positive NKC; green: CD45RA positive B cells; red: T cell receptor positive lymphocytes.
Figure 8.
Mature CD4 or CD8 positive T lymphocytes in the peripheral blood of Rag1 mutant rats. A Absence of CD4+/CD8+ lymphocytes in wild type and Rag1 mutant rats. B and C Reduction of CD4+/CD3+ and CD8+/CD3+ T cells. D and E While there are no differences in the population of CD25+/CD4+ T cells the number of CD25+/CD8+ lymphocytes was reduced. orange: autofluorescent cells; green: FITC positive lymphocytes; blue: PE positive lymphocytes.
Discussion
Low efficiency of Rag1 specific ZFNs in LEW/Ztm rats
The most striking advantage of the ZFN technology is the capability to manipulate embryos of any species. Nevertheless, the efficiency of the genetic manipulation may be modulated by strain variations and species differences, as well as locus specific chromatin structure and technical setup. In LEW/Ztm 71,3% of the injected embryos were still viable and resulted in 13,3% live births. However, only 1,7% carried the expected Rag1 mutation. In contrast to this the workgroup at the SAGE Lab, which used the identical ZFN mRNAs for the disruption of Rag1 in SD rats achieved a ratio of 20% of Rag1 mutated rats (5 out of 24 pups born, personal communication).
The efficiency of 3% (1 out of 31 born animals carrying the mutation) seen in the targeting of the Renin gene in inbred SS (salt-sensitive) rats [30] was at a similar low level as the ablation of Rag1 in the LEW/Ztm background. Guerts and coworkers found survival rates comparable to the LEW rat with 56% to 91% after microinjection of ZFN encoding plasmids or ZFN mRNA in the SD background. The ratio of live-born pups born (13 - 20%) also correlates with our results, however, the efficiency of the manipulation was much higher in the SD rats up to 75% mutants [27] instead of 1,7% on the LEW background. Nevertheless, Mashimo and colleagues detected high average ratios of born and mutated animals with 24,3% and 24,1% after micromanipulation of zygotes from inbred F344/Stm or TM/Kyo strains to inactivate the IL2rg gene [28]. However, comparing the efficiency of the Rag1 mutation in outbred SD and inbred LEW suggests that the different genetic backgrounds are an important determinant in the ratio of transgenesis, even though it also depends on the accessibility of the gene locus due to the chromatin structure [41].
Further, the conditions of the technical engineering determined the success of the ZFN mediated gene targeting. Guerts and coworkers showed that the stepwise increase of the concentration of the injected mRNAs from 0.4 μg/μl to 2μg/μl and then to 10 μg/μl correlates with reduced ratios of born embryos. Among these the number of mutant offspring increased with the injection of higher mRNA concentrations in SD rats [27]. Therefore, the functionality of the individual pair of ZFNs and the technical setup of the experiment in combination with the stress sensitive and delicate LEW embryos might be responsible for the low outcome of Rag1 mutated rats in this study [42]. Future comparison of identical ZFN in several rat inbred strains will be necessary to validate strain dependent variations of ZFN mediated manipulation efficiencies.
High fidelity of the Rag1 ZFN
The introduction of genetic modifications in the mammalian genome harbors the risk of causing unwanted, random or off-target mutation. It is well known that the Cre recombinase widely used for the conditional deletion of genomic sequences recognizes cryptic “pseudo-loxP” sites, initiates chromosomal rearrangements and infertility in mice [43,44]. Despite the high binding specificity of ZFN to their specific target sequence additional off-target mutations at homologous loci must be excluded. Neither in our LEW-Rag1 mutant rat, nor in any other published knockout rat model created by ZFN-mediated genome modification, off-target mutations have been detected so far, thereby demonstrating the high fidelity of ZFN binding and activity [21,26-30].
Leakiness of LEW/Ztm-Rag1em1Ztm
The phenotype of the LEW-Rag1 mutant rat was characterized by a substantial decrease of Rag1 translation in heterozygous animals and by a complete loss of the Rag1 protein in the homozygous mutant rats suggesting a biallelic expression of Rag1 in wild type rats. The absence of dose dependent effects in the heterozygous animals emphasized that the translation of Rag1 from only one allele was sufficient for a functional immune system while homozygous Rag1 mutation was associated with a reduction of T cells, complete ablation of B cells and an elevation of NK cell number in peripheral blood demonstrated by FACS analysis. The repression of lymphocyte development due to the lack of V(D)J recombination was associated with a hypoplastic thymus and the loss of follicular structures associated with lymphocyte maturation in thymus, spleen and lymph nodes. The underdevelopment of the thymus in the LEW-Rag1 mutant rat correlates with the reduced size of the thymus in the Rag1 knockout mouse, where the substitution of a central part of the coding sequence in exon 2 through a neomycin resistance cassette led to the complete functional inactivation of Rag1[37]. In contrast to the non-leaky Rag1 knockout mouse we found an average of 4,6 % T cell receptor positive thymocytes while no B cells were found in the LEW-Rag1 mutant rat. Furthermore, in the SD-Rag1 mutant rat [45] nearly 11% CD3 positive T cells and some CD45RA expressing B cells were detected. Rag1 deficiency excludes lymphocyte maturation as shown in the Rag1 knockout mouse. T cell precursors mature only up to the double negative CD4-CD8-IL2R-α positive stage in the absence of murine Rag1[35,37]. Therefore, the origin of mature T lymphocytes in the Rag1 knockout rat is still unknown as extrathymic thymocyte maturation, such as intestinal intraepithelial TCRγδ+ T lymphocytes (IEL) in BB rats, CD2+CD7+ preTCRα+ T cells in the adult liver or CD2+CD7+CD3- T cells in the human small intestine, always require the expression of functional Rag1[46-48]. A similar frame shift mutations like in the LEW/Ztm-Rag1em1Ztm were observed in the human Rag1 gene in two patients with Omenn’s syndrome carrying an adenosine deletion at position 877 of the Rag1 cDNA also introducing a premature stop codon in front of the nonamer binding region (NBR) [49,50]. Santagata and colleagues showed that in response to the frame shift mutation truncated Rag1 protein had been translated from an internal methionine without the N-terminal BIIA nuclear localization signal. Like in LEW/Ztm-Rag1em1Ztm homozygous rats, in these patients differentiation of mature, activated but oligoclonal T cells with impaired function took place due to cytoplasmic localization of the truncated Rag1 and reduced recombinational activity [49,50]. In the future cloning of the LEW/Ztm-Rag1em1Ztm allele followed by in vivo translation and recombination should demonstrate the translation, cellular localization and functionality of the mutated Rag1 protein in the LEW/Ztm rats.
Functional compensatory NK cell increase
The elevated NK cell number in the peripheral blood of LEW/Ztm-Rag1em1Ztm rats correlates with the observation of an increase in NKC population in spleen, lymph nodes, lung, and liver of Rag1 knockout mice [36]. Grundy and Sentman speculate that the NKCs substitute T lymphocytes by migration along chemokine gradients into these organs. The increase in NK cell concentration in the blood of LEW/Ztm-Rag1em1Ztm mutant rats was also associated with a reduction of thymocytes, but the molecular pathways involved in this compensatory process are still unknown.
Further characterization of the LEW/Ztm-Rag1em1Ztm strain as well as xenotransplantation experiments of cells and tissues will be performed to establish the Rag1 mutant rat as a innovative model for studies in autoimmune diseases, transplantation approaches and carcinogenesis as well as cancer therapy.
Methods
Animals
All rats were bred and maintained at the Central Animal Facility of the Hannover Medical School, Carl-Neuberg-Strasse 1, 30625 Hannover, Germany (subline code: Ztm: http://www.mh-hannover.de/einrichtungen/tierlabor). All handling of animals has been conducted in accordance with German law for animal protection and with the European Communities Council Directive 2010/63/EU for the protection of animals used for experimental purposes. All experiments have been approved by the Local Institutional Animal Care and Research Advisory committee and permitted by the local government (Lower Saxony State Office for Consumer Protection, Food Safety, and Animal Welfare Service – LAVES; Az.10/0051).
Husbandry
LEW, LEW/Ztm-Rag1em1Ztm, and (LEW x WKY)F1 rats were maintained under standardized conditions at a temperature of 22 ± 2°C, relative humidity of 55±5%, and artificial light for 14h. Commercial softwood granulate bedding was sterilized (Lignocel, Altromin; Lage, Germany). They received an autoclaved commercial pelleted diet (Altromin 1314) and water ad libitum. The rats were kept as pairs or in sibling groups. Microbiological status was monitored according to FELASA recommendations [51]. The rats were positive for parvovirus and apathogenic protozoa.
Zinc-finger nucleases
Rag1 specific ZFN consisting of 5 zinc-finger modules fused to the endonuclease domain of the restriction endonuclease FokI were designed and functionality evaluated by Sigma Advanced Genetic Engineering Labs using the CompoZr® ZFN technology. The forward ZFN recognized the DNA sequence GTCTACTGCCCAAGG between positions 5233-5247, while the reverse ZFN detected the DNA segment GACCGTGGAGTGGCA located at 5253-5267 in Exon 2 of genomic Rag1 (Rattus norvegicus strain BN/SsNHsdMCW chromosome 3, Rnor_5.0: NCBI Reference Sequence: NC_005102.3). The ZFN binding sites were separated by the 6 bp cut site. ZFN mRNAs were translated in vitro using the MessageMax T7 ARCA-Capped Message Transcription Kit (#MMA60710, Epicentre Biotechnologies, Madison, WI) and the Poly (A) Polymerase tailing kit (#PAP5104H, Epicentre Biotchnologies, Madison WI) following the manufacturer’s recommendations. mRNA quantity was measured using the Nanodrop spectrophotometer (Peqlab, Erlangen, Germany) and sufficient quality was confirmed by agarose gel electrophoresis.
Embryo collection and manipulation
LEW/Ztm female were hyperovulated as described earlier [52]. Equal amounts of both ZFN mRNAs were mixed and diluted with injection buffer (1mM Tris-HCl, pH 7.4; 0.25 mM EDTA) to a final concentration of 5 ng/μl [21]. Estrous in LEW/Ztm was induced by injection of 50μg LHRH (L 4513, Sigma-Aldrich, Seelze, Germany) 4 days prior to mating. Zygotes were harvested on day 0.5 of pregnancy from the ampullae of oviducts in M2 medium (M7176, Sigma-Aldrich, Seelze, Germany) containing 2.2 mg/ml hyaluronidase (9978.1, Carl Roth, Karlsruhe, Germany) to detach the cumulus cells. Cumulus cell free zygotes were washed several times in M2 and than transferred to modified M16 medium supplemented with 1% non-essential amino acids (M7145, Sigma-Aldrich, Seelze, Germany) and covered with mineral oil (Light Mineral Oil EmbryoMax®, Millipore, Schwalbach, Germany). ZFN mRNAs were microinjected with glas capillaries (inner diameter of 1.2 μm, Pronucleus PI-1.2, BioMedical Instruments, Zöllnitz, Germany) into the cytoplasm of the zygotes with an injection pressure of 120 hPA (Ph: 80 hPA) using an Axiovert 135 microscope (Zeiss, Göttingen, Germany) with TransferMan NK2 and FemtoJet (Eppendorf, Hamburg, Germany). Injected zygotes were incubated over night in an incubator at 37°C, 5% CO2 and 95% humidity. Morphologically intact one-cell and two-cell embryos were transferred the next day into the oviducts of day 0.5 pseudopregnant (LEW x WKY) F1 rats [42].
Mutation detection
Rag1 from the putative founder animals and from wild type LEW rats was amplified with the Optimase® Polymerase (Transgenomic, Glasgow, UK) and the primer pair Cel-I forward 5’-CTCATTGCCAGAGTTTTCCG-‘3 and Cel-I reverse 5’-TGCTGACCCTAGCCTGAGTT-‘3 using the following PCR protocol: 95°C for 5 min, 34 cycles: 95°C for 30 sec, 60°C for 30 sec, 72°C for 1 min); 72°C for 5 min, 4°C ∞. The resulting 310 bp fragments covering the ZFN cut site was hybridized and digested with the SURVEYOR® Mutation Detection Assay (Transgenomic, Glasgow, UK) following the supplier’s instruction. Cel-I digestion of heteroduplex DNA derived from Rag1 mutated animals resulted in cleavage of the 310 bp PCR product into 174 bp and 136 bp fragments. Rag1 mutation was confirmed by sequencing of genomic DNA and PCR products with the ABI 310 Genetic Analyzer (Applied Biosystems, Darmstadt, Germany) using the BigDye® Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Darmstadt, Germany).
Performing blast search 10 genomic loci showing strong homology to the ZFN binding and cut site of Rag1 were identified. Off-target mutations at these loci were excluded by PCR amplification at an annealing temperature of 58°C followed by Cel-I digest using the SURVEYOR® Mutation Detection Assay (Transgenomic, Glasgow, UK) (Oligonucleotides see Additional file 1.
Genotyping
The genotype of LEW/Ztm-Rag1em1Ztm rats was determined by PCR amplification of a 1052 bp fragment of Rag1 with the primer pair Rag1for1: 5’-AGGTAGCTTCGCCAAA.
ATGG-‘3 and Rag1rev1: 5’-TCAGAAAGGACTTGACCGGA-‘3 using the HotStarTaq Master Mix Kit (Qiagen, Hilden, Germany) following the manufacturer’s recommendations. PCR were performed in a volume of 50 μl according to the following protocol: 95°C for 15 min; 40 cycles: 95°C for 15 sec, 60°C for 30 sec, 72°C for 1 min; 72°C for 5 min; 4°C ∞. After reaction clean up with the Qiaquick Gel Extraction Kit (Qiagen, Hilden, Germany) the PCR products containing the Rag1 mutation were cleaved by StyI-HF (NEB, Frankfurt, Germany) into 603 bp and 449 bp, while the wild type Rag1 fragments remained unchanged.
RNA and RT-PCR
Total RNA was isolated from spleen, thymus, bone marrow and lymph nodes (mesenteric and cervical) from wild type LEW/Ztm rats, heterozygous and homozygous Rag1 mutant rats (n=10) using the RNeasy Mini Kit (Qiagen, Hilden, Germany) after homogenization of the tissues with the UP100H ultrasonic device (Hielscher, Teltow, Germany) and Qiashredder (Qiagen, Hilden, Germany). RNA concentrations were determined with the Nanodrop spectrophotometer (Peqlab, Erlangen, Germany). 2 μg of pooled RNA were transcribed to cDNA with the Omniscript RT Kit (Qiagen, Hilden, Germany), Random Hexamers (Fermentas, St. Leon-Rot, Germany) and Protector Rnase Inhibitor (Roche, Mannheim, Germany). Rat GAPDH was amplified with the following primers 5’-AGGGCTGCCTTCTCTTGTGAC-‘3 and 5’-CCGTGGGTAGAGTCATAC TGG-‘3 with an annealing temperature of 57°C for 25 cycles using the HotStarTaq Master Mix Kit (Qiagen, Hilden, Germany). Expression of wild type and mutated Rag1 was determined using the genotyping protocol.
Western blot
Whole cell protein extracts were isolated from spleen, thymus, bone marrow and lymph nodes (mesenteric and cervical) from wild type, heterozygous, and Rag1 deficient rats (n=10) with NP40 buffer supplemented with Complete Mini Protease Inhibitor Cocktail Tablets (Roche, Mannheim, Germany), sodium orthovanadate (NEB, Frankfurt, Germany), PMSF (Sigma-Aldrich, Seelze, Germany) and DTT (Sigma-Aldrich, Seelze, Germany). Bradford Assay served to estimate the protein concentration. 50 μg of protein supplemented with 5x LSB loading dye was separated in a 10% gel by SDS-PAGE and transferred to Optitran BA-S85 membrane by semi-dry blotting. After blocking for 1 hour at room temperature with blocking solution (5% dry milk (Carl Roth, Karlsruhe, Germany) and 2,5% BSA, fraction V (Sigma-Aldrich, Seelze, Germany) in 3% TBS-Tween, pH 7,4) the membrane was incubated with the first antibody diluted in blocking solution at 4°C over night. Incubation with the secondary, HRP-conjugated antibodies was done for 1 hour at room temperature in 3% TBS-Tween, pH 7,4. Roti-Lumin® was used as chemoluminescence substrate. For detection of the Rag1 protein a 1:300 dilution of the RAG-1 antibody (sc-5599, SCBT, LaJolla, CA) was sufficient followed by incubation with a 1:2500 dilution of a donkey anti rabbit secondary antibody (ab16284, Abcam, Cambridge, UK). GAPDH expression was demonstrated with a 1:300 dilution of the anti-GAPDH antibody (OBT1636, AbD Serotec, Düsseldorf, Germany) in combination with a 1:5000 dilution of the rabbit anti mouse antibody (STAR13B, AbD Serotec, Düsseldorf, Germany).
Histology and thymus weight
Tissues were fixed in 4% formaldehyde solution for 3 days, paraffin-embedded and cut into 4 μm slices before staining with hematoxylin and eosin. Microphotographs were taken using a Zeiss AxioCam MRc camera and analyzed histologically. Thymus weight was measured using a LA230S scale (Sartorius, Göttingen, Germany). One-way analysis of variance (ANOVA) and Bonferroni's Multiple Comparison Test were performed using Graph Pad Prizm Software (Version 5.0a) to determine statistical significances with a threshold of P < 0.05.
FACS analysis
Blood was harvested from wild type, heterozygous and Rag1 deficient rats (n=10), and lymphocytes were isolated following standard procedures. Cells were incubated for 30 min at 4°C with a 1:10 dilution of the anti-CD45RA FITC (sc-53048, SCBT, LaJolla, CA) antibody to detect B cells, while the number of T cells and natural killer cells were determined by double staining with 1:10 dilutions of the PerCP mouse anti-rat αβ T-cell receptor (BD Pharmingen, Heidelberg, Germany) and the mouse anti rat CD161:FITC antibody (AbD Serotec, Düsseldorf, Germany). Double stainings were performed using mouse anti rat CD3:FITC/CD4:RPE dual color reagent (DC041), mouse anti rat CD3:FITC/CD8:RPE (DC042) and mouse anti rat CD4/CD25 (DC040) (Abd Sertec). For CD4/CD8 double staining the antibodies mouse anti rat CD4-Fitc (554843, BD Pharmingen) and mouse anti rat CD8A-PE (554857, BD Pharmingen) were used. Stained cells were measured together with unstained cells and isotype controls stained with 1:50 dilutions of the PerCP Mouse IgG1 κ isotype control antibody (BD Pharmingen, Heidelberg, Germany) or the Mouse IgG (FITC) antibody (ab37356, Abcam, Glasgow, UK) with the FACSCalibur flow cytometer (BD Pharmingen, Heidelberg, Germany). ARCSIN transformation, One-way analysis of variance (ANOVA) and Tukey’s Multiple Comparison Test were performed using Graph Pad Prizm Software (Version 5.0a) to determine statistical significances with a threshold of P < 0.05.
Abbreviations
ZFN: Zinc-finger nucleases; DSB: DNA double strand breaks; NHEJ: Non-homologous end joining; TALEN: Transcription activator-like effector nuclease; SCID: Severe combined immunodeficiency; NKC: Natural killer cells; ZDD: Zinc-binding dimerization domain; NBR: Nonamer binding region; PALS: Periarteriolar lymphoid sheaths.
Competing interests
EJW and XC are full-time of employees of SAGE Labs of Sigma-Aldrich Corporation. NHZ, SG, DW, MD and HJH declare that they have no competing interests.
Authors’ contributions
NHZ carried out the microinjection, genotyping, molecular biological analysis and drafted the manuscript. SG performed the histopathological examinations. DW was responsible for the FACS analysis. EJW and XC validated the Rag1 ZFNs and provided technical advice. MD and HJH participated in the study design. All authors read and approved the final manuscript.
Supplementary Material
Off-target gene loci:RAG1 ZFN mutagenesis.
Contributor Information
Nils-Holger Zschemisch, Email: Zschemisch.Nils-Holger@mh-hannover.de.
Silke Glage, Email: Glage.Silke@mh-hannover.de.
Dirk Wedekind, Email: Wedekind.Dirk@mh-hannover.de.
Edward J Weinstein, Email: Edward.Weinstein@sial.com.
Xiaoxia Cui, Email: Xiaoxia.Cui@sial.com.
Martina Dorsch, Email: Dorsch.Martina@mh-hannover.de.
Hans-Jürgen Hedrich, Email: Hedrich.Hans@mh-hannover.de.
Acknowledgements
We have to thank Regina Eisenblätter, Elena Wiebe, Sieglinde Egthessadi, Isabell Wittur, Cindy Elfers and Silke Glowotz for their technical lab assistance. The article processing charge was funded by means of the DFG-Project „Open Access Publishing“ by the German Research Council.
References
- Charreau B, Tesson L, Soulillou JP, Pourcel C, Anegon I. Transgensis in rats: technical aspects and models. Transgenic Res. 1996;5:223–234. doi: 10.1007/BF01972876. [DOI] [PubMed] [Google Scholar]
- Jang CW, Behringer RR. Transposon-mediated transgensis in rats. CSH Protoc. 2007. [DOI] [PubMed]
- Murphy D. Production of transgenic rodents by microinjection of cloned DNA into fertilized one-celled eggs. Methods Mol Biol. 2008;461:71–109. doi: 10.1007/978-1-60327-483-8_7. [DOI] [PubMed] [Google Scholar]
- Pfeifer A. Lentiviral transgenesis. Transgenic Res. 2004;13:513–522. doi: 10.1007/s11248-004-2735-5. [DOI] [PubMed] [Google Scholar]
- Tesson L, Cozzi J, Menoret S, Remy S, Usal C, Fraichard A, Anegon I. Transgenic modifications of the rat genome. Transgenic Res. 2005;14:531–546. doi: 10.1007/s11248-005-5077-z. [DOI] [PubMed] [Google Scholar]
- Ivics Z, Izsvak Z, Medrano G, Chapman KM, Hamra FK. Sleeping Beauty transposon mutagenesis in rat spermatogonial stem cells. Nat Prot. 2011;6:1521–1535. doi: 10.1038/nprot.2011.378. [DOI] [PubMed] [Google Scholar]
- Kitada K, Keng VW, Takeda J, Horie K. Generating mutant rats using the Sleeping Beauty transposon system. Methods. 2009;49:236–242. doi: 10.1016/j.ymeth.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Li P, Tong C, Mehrian-Shai R, Jia L, Wu N, Yan Y, Maxson RE, Schulze EN, Song H, Hsieh C-L, Pera MF, Ying Q-L. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, McLay R, Hall J, Ying Q-L, Smith A. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Meek S, Buehr M, Sunderlamd L, Thompson A, Mullins JJ, Smith AJ, Burdon T. Efficient gene targeting by homologous recombination in rat embryonic stem cells. PlosOne. 2010;5:e14225. doi: 10.1371/journal.pone.0014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C, Li P, Wu NL, Yan Y, Ying Q-L. Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature. 2010;467:211–213. doi: 10.1038/nature09368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Chang T, Kumbhani DS, Ashton C, Yan H, Ying Q-L. Beyond knockout rats. New insights into finer genome manipulation in rats. Cell Cycle. 2011;10:1059–1066. doi: 10.4161/cc.10.7.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Nakata M, Sasada R, Ooshima Y, Yano T, Shinozawa T, Tsukimi Y, Takeyama M, Matsumoto Y, Hashimoto T. Derivation of rat embryonic stem cells and generation of protease-activated recepror-2 knockout rats. Transgenic Res. 2011. epud anhead of print. [DOI] [PubMed]
- Urnov FD, Rebar FJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engenireed zinc finger nucleases. Nat Rev Genet. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- Li T, Huang S, Zhao X, Wright DA, Carpenter S, Spalding MH, Weeks DP, Yang B. Modularly assembled designer TAL effector nucleases for targeted gene knockout and gene replacement in eukaryotes. Nucleic Acids Res. 2011;39:6315–6325. doi: 10.1093/nar/gkr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy S, Tesson L, Menoret S, Usal C, Scharenberg AM, Anegon I. Zinc-finger nucleases: a powerful tool for genetic engineering of animals. Transgenic Res. 2010;19:363–371. doi: 10.1007/s11248-009-9323-7. [DOI] [PubMed] [Google Scholar]
- Caroll D. Genome engineering with zinc-finger nucleases. Genetics. 2011;188:773–782. doi: 10.1534/genetics.111.131433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly JP, Barker JC, Pruett-Miller S, Porteus MH. Gene correction by homologous recombination with zinc finger nucleases in primary cells from a mouse model of a generic recessive genetic disease. Mol Therapy. 2010;18:1103–1110. doi: 10.1038/mt.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehle EA, Rock JM, Lee Y-L, Jouvenot Y, DeKelver RC, Gregory PD, Urnov FD, Holmes MC. Targeted gene addition into a specified location in the human genome using designed zinc finger nucleases. Proc Natl Acad Sci USA. 2007;104:3055–3060. doi: 10.1073/pnas.0611478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago Y, Chan E, Liu P-Q, Orlando S, Zhang L, Urnov FD, Holmes MC, Guschin D, Waite A, Miller JC, Rebar EJ, Gregory PD, Klug A, Collingwood TN. Targeted gene knockout in mammalian cells using engineered zinc-finger nucleases. Proc Natl Acad Sci USA. 2008;105:5809–5814. doi: 10.1073/pnas.0800940105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbery ID, Ji D, Harrington A, Brown V, Weinstein EJ, Liaw L, Cui X. Targeted genome modification in mice using zinc-finger nucleases. Genetics. 2010;186:451–459. doi: 10.1534/genetics.110.117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciu X, Ji D, Fisher DA, Wu Y, Briner DM, Weinstein EJ. Targeted integration in rat and mouse embryos with zinc-finger nucleases. Nat Biotech. 2011;29:64–67. doi: 10.1038/nbt.1731. [DOI] [PubMed] [Google Scholar]
- Meyer M, Hrabe De Angelis M, Wurst W, Kühn R. Gene targeting by homologous recombination in mouse zygotes mediated by zinc-finger nucleases. Proc Natl Acad Sci USA. 2010;107:15022–15026. doi: 10.1073/pnas.1009424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods IG, Schier AF. Targeted mutagenesis in zebrafish. Nat Biotech. 2008;26:650–651. doi: 10.1038/nbt0608-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild J, Petersen B, Santiago Y, Queisser A-L, Carnwath JW, Lucas-Hahn A, Zhang L, Meng X, Gregory PD, Schwinzer R, Cost GJ, Niemann H. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proc Natl Acad Sci USA. 2011;108:12013–12017. doi: 10.1073/pnas.1106422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu X, Zhang Z, Yabut J, Horwitz S, Levorse J, LI XQ, Zhu L, Ledermann H, Ortiga R, Strauss J, Li X, Owens KA, Dragovic J, Vogt T, Evers R, Shin MK. Characterization of multidrug resistance 1a/P-glycoprotein knockout rats generated by zinc finger nucleases. Mol Pharmacol. 2012;81:220–227. doi: 10.1124/mol.111.074179. [DOI] [PubMed] [Google Scholar]
- Guerts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Menoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo T, Takizawa A, Voigt B, Yoshimi K, Hiai H, Kuramoto T, Serikawa T. Generation of knockout rats with X-linked severe combined immunodeficiency (X-SCID) using zinc-finger nucleases. PlosONE. 2010;5:e8870. doi: 10.1371/journal.pone.0008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menoret S, Iscache A-L, Tesson L, Remy S, Usal C, Osborn MJ, Cost GJ, Brüggemann M, Buelow R, Anegon I. Characterization of immunoglobulin heavy chain knockout rats. Eur J Immunol. 2010;40:2932–2941. doi: 10.1002/eji.201040939. [DOI] [PubMed] [Google Scholar]
- Moreno C, Hoffman M, Stodola TJ, Didier DN, Lazar J, Guerts AM, North PE, Jacob HJ, Greene AS. Creation and characterization of a Renin knockout rat. Hypertension. 2011;57:614–9. doi: 10.1161/HYPERTENSIONAHA.110.163840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Ann Rev Immunol. 2003;22:625–655. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- Niehues T, Perez-Becker R, Schuetz C. More than just SCID- the phenotypic range of combined immunodeficiencies associated with mutations in the recombinase activating genes (RAG) 1 and 2. Clin Immunol. 2010;135:183–192. doi: 10.1016/j.clim.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Notarangelo LD, Villa A, Schwarz K. RAG and RAG defects. Curr Opin Immunol. 1999;11:435–442. doi: 10.1016/S0952-7915(99)80073-9. [DOI] [PubMed] [Google Scholar]
- Santagata S, Villa A, Sobacchi C, Cortes P, Vezzoni P. The genetic and biochemical basis of Omenn syndrome. Immunol Rev. 2000;178:64–74. doi: 10.1034/j.1600-065X.2000.17818.x. [DOI] [PubMed] [Google Scholar]
- Falk I, Potocnik AJ, Barthlott T, Levelt CN, Eichmann K. Immature T cells in peripheral lymphoid organs of the recombinase activating gene-1/-2- deficient mice. J Immunol. 1996;156:1362–1368. [PubMed] [Google Scholar]
- Grundy MA, Sentman CL. Immunodeficient mice have elevated numbers of NK cells in non-lymphoid tissues. Exp Cell Res. 2006;312:3920–3926. doi: 10.1016/j.yexcr.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-G. [DOI] [PubMed] [Google Scholar]
- De P, Rodgers KK. Putting the pieces together: identification and characterization of structural domains in the V(D)J recombination protein RAG1. Immunlogical Reviews. 2004;200:70–82. doi: 10.1111/j.0105-2896.2004.00154.x. [DOI] [PubMed] [Google Scholar]
- Oleykowski CA, Bronson Mullins CR, Godwin AK, Yeung AT. Mutation detection using a novel plant endonuclease. Nucleic Acids Res. 1998;26:4597–4602. doi: 10.1093/nar/26.20.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulinski J, Besack D, Oleykowski CA, Godwin AK, Yeung AT. CEL I enzymatic mutation detection assay. Biotechniques. 2000;29(44–6):48. doi: 10.2144/00291bm07. [DOI] [PubMed] [Google Scholar]
- Recillas-Targa F. Multiple strategies for gene transfer, expression, knockdown, and chromatin influence in mammalian cell lines and transgenic animals. Mol Biotechnol. 2006;34:337–354. doi: 10.1385/MB:34:3:337. [DOI] [PubMed] [Google Scholar]
- Filipiak WA, Saunders TL. Advances in transgenic rat production. Transgenic Res. 2006;15:673–686. doi: 10.1007/s11248-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Sempirini S, Troup TJ, Kotelevtseva N, King K, Davis JR, Mullins LJ, Chapman KE, Dunbar DR, Mullins JJ. Cryptic loxP sites in mammalian genomes: genome-wide distribution and relevance for the efficiency of BAC/PAC recombineering techniques. Nucleic Acid Res. 2007;35:1402–1410. doi: 10.1093/nar/gkl1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EE, Taylor DS, Prigge JR, Barnett S, Carpecchi MR. Illegitimate Cre-dependent chromosome rearrangement in transgenic mouse spermatids. Proc Natl Acad Sci USA. 2000;97:13702–13707. doi: 10.1073/pnas.240471297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigma Advanced Genetic Engineering Labs. http://www.sageresearchmodels.com/research-models/knockout-rats/Rag1-knockout-rat).
- Bas A, Hammarström SG, Hammarström ML. Extrathymic TCG gene rearrangement in human small intestine: identification of new splice forms of recombinant activating gene-1 mRNA with selective tissue expression. J Immunol. 2003;171:3359–3371. doi: 10.4049/jimmunol.171.7.3359. [DOI] [PubMed] [Google Scholar]
- Collins C, Norris S, McEntee G, Traynor O, Bruno L, von Boehmer H, Hegarty J, Farrelly O. RAG1, RAG2 and pre-T cell receptor alpha chain expression by adult human hepatic T cells: evidence for extrathymic T cell maturation. Eur J Immunol. 1996;26:3114–3118. doi: 10.1002/eji.1830261243. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Marandi L, Poussier P. Evidence for the extrathymic origin of intestinal TCRγδ+ T cells in normal rats and for an impairment of this differentiation pathway in BB rats. J Immunol. 2002;168:2182–2187. doi: 10.4049/jimmunol.168.5.2182. [DOI] [PubMed] [Google Scholar]
- Santagata S, Gomez CA, Sobacchi C, Bozzi F, Abium M, Pasic S, Cortes P, Vezzoni P, Villa A. N-terminal RAG1 frameshift mutations in Omenn’s syndrome: Internal methionine usage leads to partial V(D)J recombination activity and reveals a fundamental role in vivo for the N-terminal domains. Proc Natl Acad Sci USA. 2000;97:14572–14577. doi: 10.1073/pnas.97.26.14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobacchi C, Marella V, Rucci F, Vezzoni P, Villa A. RAG-dependent primary immunodeficiencies. Hum Mutat. 2006;12:1174–1184. doi: 10.1002/humu.20408. [DOI] [PubMed] [Google Scholar]
- Nicklas W, Baneux P, Boot R, Decelle T, Deeny AA, Fumanelli M, Illgen-Wilcke B. FELASA (Federation of European Laboratory Animal Science Associations Working Group on Health Monitoring of Rodent and Rabbit Colonies) Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab Anim. 2002;6:20–42. doi: 10.1258/0023677021911740. [DOI] [PubMed] [Google Scholar]
- Hamilton GC, Armstrong DT. The superovulation of synchronous adult rats using follicle-stimulation hormone delivered by continuous infusion. Biol Reprod. 1991;44:851–856. doi: 10.1095/biolreprod44.5.851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Off-target gene loci:RAG1 ZFN mutagenesis.