Abstract
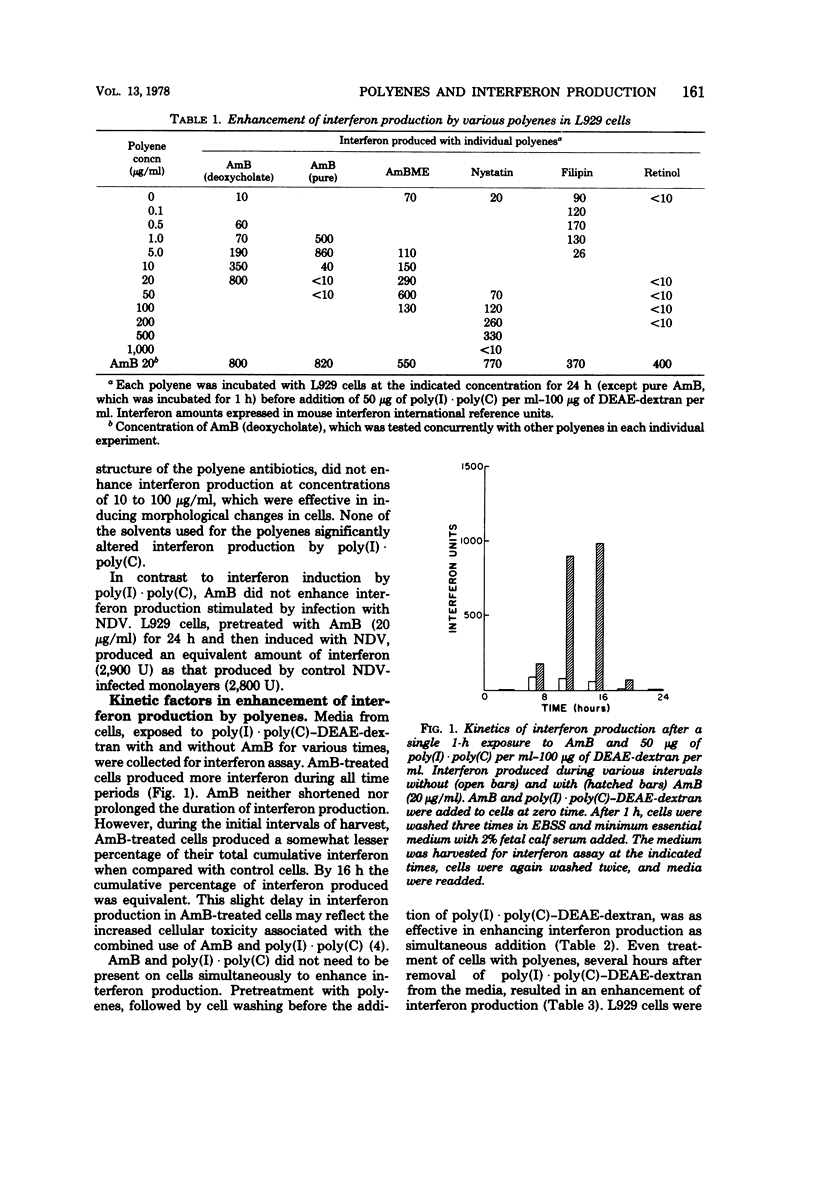
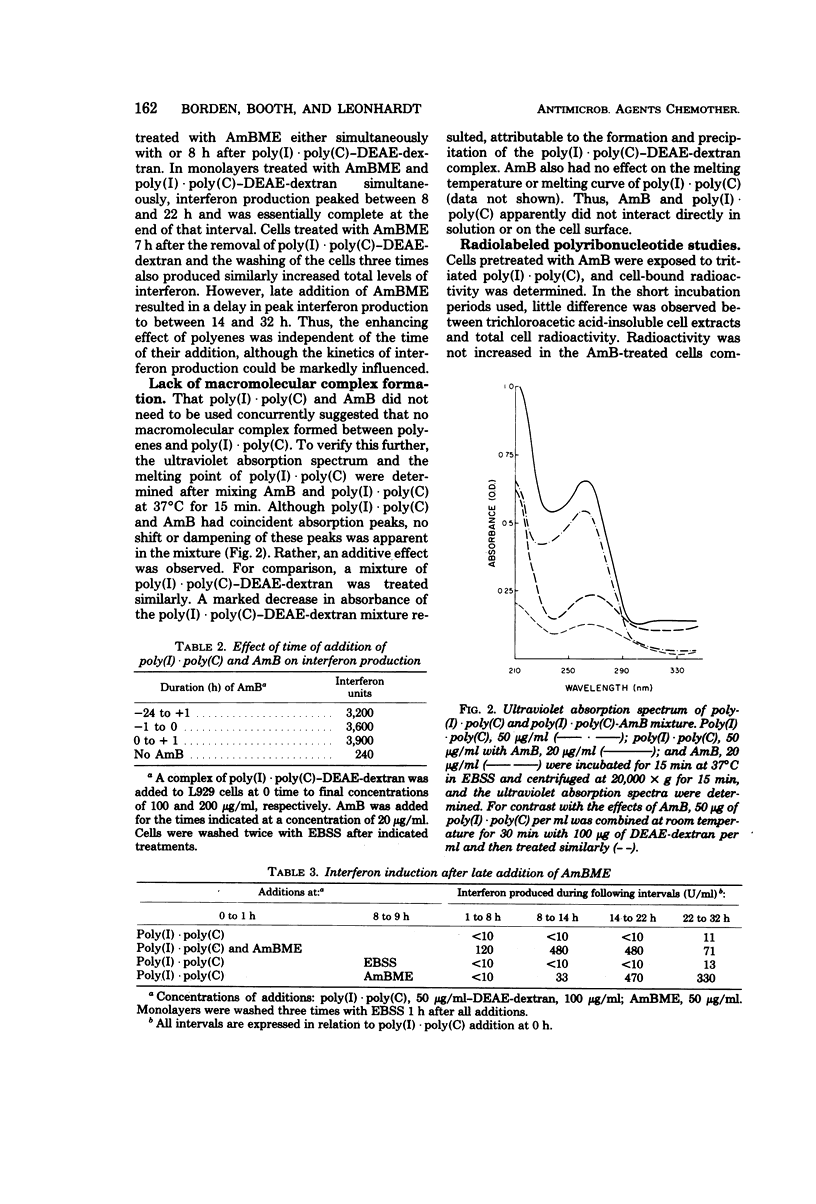
The production of interferon by polyriboinosinic-polyribocytidylic acid [poly(I) · poly(C)] and poly(I) · poly(C)-diethylaminoethyl-dextran in L929 cells was enhanced from 10 to 100 times by polyene macrolides, including amphotericin B (AmB), AmB methyl ester, nystatin, and filipin. AmB and its water-soluble methyl ester were the most effective; retinol, a nonmacrolide polyene, was ineffective. Interferon induction by Newcastle disease virus was not enhanced by AmB. The kinetics of interferon production were not markedly altered by AmB. Polyenes and poly(I) · poly(C)-diethylaminoethyl-dextran did not need to be present on cells simultaneously to enhance interferon production. Pretreatment with polyenes was as effective as simultaneous addition. Even treatment of washed cells, several hours after removal of poly(I) · poly(C)-diethylaminoethyl-dextran, resulted in enhancement of interferon production. AmB did not appear to form a macromolecular complex with poly(I) · poly(C) in that neither the ultraviolet absorption spectrum nor the melting point of poly(I) · poly(C) was altered by mixing with AmB. Isotopic studies indicated that AmB did not enhance binding of poly(I) · poly(C) to cells. Since the macrolide polyenes have been demonstrated to bind to cell membrane sterols with subsequent alterations in membrane permeability barriers, they may enhance interferon production by increasing cell penetration of poly(I) · poly(C).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli T. E. On the anatomy of amphotericin B-cholesterol pores in lipid bilayer membranes. Kidney Int. 1973 Nov;4(5):337–345. doi: 10.1038/ki.1973.126. [DOI] [PubMed] [Google Scholar]
- Bausek G. H., Merigan T. C. Cell interaction with a synthetic polynucleotide and interferon production in vitro. Virology. 1969 Nov;39(3):491–498. doi: 10.1016/0042-6822(69)90097-x. [DOI] [PubMed] [Google Scholar]
- Borden E. C., Leonhardt P. H. Aquanititave semimicro, semiautomated colorimetric assay for interferon. J Lab Clin Med. 1977 May;89(5):1036–1042. [PubMed] [Google Scholar]
- Carter W. A., Pitha P. M., Marshall L. W., Tazawa I., Tazawa S., Ts'o P. O. Structural requirements of the rI n -rC n complex for induction of human interferon. J Mol Biol. 1972 Oct 14;70(3):567–587. doi: 10.1016/0022-2836(72)90560-8. [DOI] [PubMed] [Google Scholar]
- Colby C., Chamberlin M. J. The specificity of interferon induction in chick embryo cells by helical RNA. Proc Natl Acad Sci U S A. 1969 May;63(1):160–167. doi: 10.1073/pnas.63.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianzani F., Cantagalli P., Gagnoni S., Rita G. Effect of DEAE-dextran on production of interferon induced by synthetic double-stranded RNA in L cell cultures. Proc Soc Exp Biol Med. 1968 Jul;128(3):708–710. doi: 10.3181/00379727-128-33105. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Holz R. Aqueous pores created in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. Membranes. 1973;2:377–408. [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Fungal sterols and the mode of action of the polyene antibiotics. Adv Appl Microbiol. 1974;17(0):109–134. doi: 10.1016/s0065-2164(08)70556-2. [DOI] [PubMed] [Google Scholar]
- Kinsky S. C. Antibiotic interaction with model membranes. Annu Rev Pharmacol. 1970;10:119–142. doi: 10.1146/annurev.pa.10.040170.001003. [DOI] [PubMed] [Google Scholar]
- Lampson G. P., Tytell A. A., Field A. K., Nemes M. M., Hilleman M. R. Influence of polyamines on induction of interferon and resistance to viruses by synthetic polynucleotides. Proc Soc Exp Biol Med. 1969 Oct;132(1):212–218. doi: 10.3181/00379727-132-34182. [DOI] [PubMed] [Google Scholar]
- Medoff G., Kwan C. N., Schlessinger D., Kobayashi G. S. Permeability control in animal cells by polyenes: a possibility. Antimicrob Agents Chemother. 1973 Mar;3(3):441–443. doi: 10.1128/aac.3.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G., Kwan C. N., Schlessinger D., Kobayashi G. S. Potentiation of rifampicin, rifampicin analogs, and tetracycline against animal cells by amphotericin B and polymyxin B. Cancer Res. 1973 Jun;33(6):1146–1149. [PubMed] [Google Scholar]
- Medoff G., Schlessinger D., Kobayashi G. S. Brief communication: polyene potentiation of antitumor agents. J Natl Cancer Inst. 1973 Apr;50(4):1047–1050. doi: 10.1093/jnci/50.4.1047. [DOI] [PubMed] [Google Scholar]
- Medoff G., Schlessinger D., Kobayashi G. S. Sensitivity of transformed and nontransformed cells to amphotericin B and several rifamycin derivatives. Cancer Res. 1974 Apr;34(4):823–826. [PubMed] [Google Scholar]
- Medoff G., Valeriote F., Lynch R. G., Schlessinger D., Kobayashi G. S. Synergistic effect of amphotericin B and 1,3-bis(2-chloroethyl)-1-nitrosourea against a transplantable AKR leukemia. Cancer Res. 1974 May;34(5):974–978. [PubMed] [Google Scholar]
- Rice J. M., Turner W., Chirigos M. A., Spahn G. Dose responsiveness and variation among inbred strains of mice in production of interferon after treatment with poly (I)-poly (C) (poly-D-lysine) complexes. Appl Microbiol. 1971 Sep;22(3):380–386. doi: 10.1128/am.22.3.380-386.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. H., Armstrong J. A., Ke Y. H., Ho M. Regulation of cellular interferon production: enhancement by antimetabolites. Proc Natl Acad Sci U S A. 1970 Sep;67(1):464–471. doi: 10.1073/pnas.67.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]


