Abstract
Background
Launaea procumbens (L.) is traditionally used in the treatment of various human ailments including pulmonary damages. The present study was arranged to evaluate the role of Launaea procumbens methanol extract (LME) against carbon tetrachloride (CCl4) induced oxidative pulmonary damages in rat.
Methods
36 Sprague–Dawley male rats (170-180 g) were randomly divided into 06 groups. After a week of acclamization, group I was remained untreated while group II was given olive oil intraperitoneally (i.p.) and dimethyl sulfoxide (DMSO) orally, groups III, IV, V and VI were administered CCl4, 3 ml/kg body weight (30% in olive oil i.p.). Groups IV, V were treated with 100 mg/kg, 200 mg/kg of LME whereas group VI was administered with 50 mg/kg body weight of rutin (RT) after 48 h of CCl4 treatment for four weeks. Antioxidant profile in lungs were evaluated by estimating the activities of antioxidant enzymes; catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), glutathione-S-transferase (GST), glutathione reductase (GSR), glutathione peroxidase (GSH-Px), quinone reductase (QR) and reduced glutathione (GSH). CCl4-induced lipid peroxidation was determined by measuring the level of thiobarbituric acid reactive substances (TBARS) with conjugation of deoxyribonucleic acid (DNA) damages, argyrophilic nucleolar organizer regions (AgNORs) counts and histopathology.
Results
Administration of CCl4 for 6 weeks significantly (p < 0.01) reduced the activities of antioxidant enzymes and GSH concentration while increased TBARS contents and DNA damages in lung samples. Co-treatment of LME and rutin restored the activities of antioxidant enzymes and GSH contents. Changes in TBARS concentration and DNA fragmentation were significantly (p < 0.01) decreased with the treatment of LME and rutin in lung. Changes induced with CCl4 in histopathology of lungs were significantly reduced with co-treatment of LME and rutin.
Conclusion
Results of present study revealed that LME could protect the lung tissues against CCl4-induced oxidative stress possibly by improving the antioxidant defence system.
Keywords: Launaea procumbens, Lungs, CCl4, Antioxidant enzymes, TBARS, GSH
Background
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced as byproducts during normal metabolism, exposure of sunlight, ultraviolet light, ionizing radiation and toxic chemicals [1]; probably causes chronic diseases and pulmonary damages [2]. Various reports revealed that pneumotoxic substances such as carbon tetrachloride [3], paraquat [4] and bleomycin [5] play a critical role in pulmonary injury. Carbon tetrachloride (CCl4) is a potent environmental hepatotoxin, used as industrial organic solvents [6] cause liver, kidney and lungs dysfunction in workers [7]. It has been established that trichloromethyl (CCl3) radical and chloride (Cl) are formed as a result of the metabolic conversion of CCl4 by cytochrome P-450 [8]. These free radicals react with polyunsaturated fatty acids (PUFA) of lung membranes and enhance lipid peroxidation (TBARS), DNA fragmentation [9], deplete activities of antioxidant enzymes such as CAT, SOD, GSH-Px, GSR and amount of tissue soluble proteins [10]. Medicinal plants and polyphenolic compounds isolated from medicinal plants play important role in the treatment of various human ailments and in the detoxification of the products (the intermediate and final) of oxidative stress [11] as well as ameliorate lungs oxidative damages in experimental rats [3,12].
Launaea procumbens (LP) is one of important medicinal plant widely spread in waste places, vacant lots and in cultivated fields through out Pakistan. Ayurvedic and herbal medicine prepared from this plant promote self healing, good health and longevity, as well as used as a food ingredient [13]. It has been used in the treatment of nephritis, pulmonary fibrosis, hormonal balance and sexual diseases by local healer in Pakistan [14]. Phytochemistry of LP revealed the presence of salicylic acid, vanillic acid, 2-methyl-resercinol and gallic acid [15]. These compounds have spasmogenic, cardiovascular, anti-carcinogenic, anti-inflammatory, and antioxidant properties to scavenge reactive oxygen species. The present study was therefore arranged to investigate the protective effects of LP on lungs against CCl4-induced oxidative damage in rats.
Methods
LP collection and extraction
Aerial parts of LP were collected during June 2010, identified and a specimen was submitted at Herbarium of Pakistan, Quaid-i-Azam University (QAU) Islamabad, Pakistan. Leaves were shade dried at room temperature and ground mechanically. 2 kg of the powder was extracted twice in 5 liter of methanol with random shaking for a week and evaporated through rotary evaporator after filtration by Whatmann filters paper No. 45; to get crude methanolic extract (LME). LME is stored at 4°C for in vivo studies.
Animals and experimental design
36 Sprague–Dawley male rats (170-1800 g) were purchased from NIH, Islamabad, Pakistan and brought to animal house of Quaid-i-Azam University Islamabad. After one week of acclamization under standard laboratory conditions (12 h light/darkness; at 25 ± 3°C), with free access of diet and water, they were randomly divided into 06 groups according to study protocol as approved by ethical committee of Quaid-i-Azam University, Islamabad. Group I remained untreated (control) while group II was given olive oil intraperitoneally and DMSO orally, groups III-VI were administered CCl4, 3 ml/kg body weight (30% in olive oil i.p.). Groups IV and V were treated with 100 mg/kg and 200 mg/kg of LME while group VI was treated with 50 mg/kg body weight of RT after 48 h of CCl4 treatment. These treatments were carried out twice a week for four weeks. After 24 h of the last treatment, all the animals were weighted, sacrificed; their lungs were removed, weighted and perfused in ice-cold saline solution. Half of lung tissue was treated with liquid nitrogen for further enzymatic and DNA damage analysis while the other portion was processed for histology.
Assessment of antioxidant status
Tissue of lungs was homogenized in phosphate buffer (pH 7.4) and centrifuged at 12,000 × g at 4°C for 30 min to get tissue homogenate. Tissue soluble protein concentration of lung homogenate was obtained [16] while antioxidant status was determined by estimation of antioxidant activities of CAT and POD [17], SOD [18], GST [19], GSR [20], GSH-Px [21], H2O2 concentration [22] and activity of QR [23]. Oxidative status was determined using estimation of GSH [24] while lipid peroxidation (TBARS) was [25] in lung homogenates.
Nitrite assay
Griess reagent was used for determination of nitrite contents. The reagent includes 0.3 M NaOH and 5% ZnSO4 used as griess reagent. Concentration of nitrite contents was expressed using sodium nitrite standard curve.
DNA ladder assay
Protocol of Wu et al. [26] was used for isolation of DNA to determine DNA damages. 5 μg DNA extracted from each sample of different groups separately loaded in 1.5% agarose gel for 45 min and photographed, using digital camera under gel doc system.
% Quantification of DNA fragmentation
Quantification of % DNA fragmentation was carried out using tris triton EDTA (TTE), trichloro acetic acid (TCA) and diphenylamine (DPA) as regents. OD of DNA was checked with a spectrophotometer (Smart spec TM Plus, catalog # 170–2525) at 600 nm [26].
AgNORs count
Silver staining technique was used according to [27]. During NORs staining, unstained fixed slides were dewaxed with xylene and hydrated in decrease ethanol concentration (90, 70 and 50%) and washed. After drying slides were treated with one drop of colloidal solution (2% gelatin and 1% formic acid) and two drops of 50% AgNO3 solution onto the slide and incubated at 35°C for 8–12 min. The progressive staining was followed under light microscope to get golden colored nuclei and brown/black NORs at 100 × magnification and counted number of NORs per cell.
Morphological study of lungs
Microscopic studies of lung tissues were carried out by the protocol as used by Khan et al. [3] with some modifications.
Statistical analysis
Data were expressed as mean and standard error (SE) and ANOVA test were used to analyze the difference among various treatments, with least significance difference (LSD) at 0.05 and 0.01 as a level of significance. SPSS ver. 14.0 (Chicago, IL, USA) and Microsoft Excel 2007 (Roselle, IL, USA) were used for the statistical and graphical evaluations.
Results
Effect of LME on lungs protein and antioxidant enzymes in rat
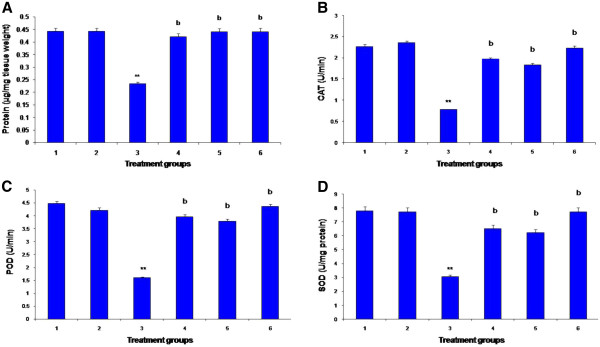
Figure 1 shows changes in lung protein and activities of antioxidant enzymes in all the experimental groups of rat. Administration of CCl4 significantly (p < 0.01) decreased the tissue soluble protein, activities of CAT, POD and SOD as compared to normal rats. Treatment with LME markedly augmented (p < 0.01) the effects of CCl4 intoxicity, and restored the amount of tissue soluble protein, activities of CAT, POD and SOD in lung tissues in a dose dependent way. Treatment of RT also showed significant protection (p < 0.01) in the improvement of tissue protein and activities of antioxidant enzymes comparatively to control group.
Figure 1.
(A-D) Effect of LME treatments on tissue protein (A), antioxidant enzymes; catalase (CAT) (B), peroxidase (POD) (C) superoxide dismutase (SOD) (D) in lungs of various groups in rat. I: control; II: vehicle (olive oil + DMSO); III: CCl4 3 ml/kg; IV: CCl4 + rutin 50 mg/kg; V: CCl4 + LME 100 mg/kg; VI: CCl4 + LME 200 mg/kg; ** (P < 0.01) show significance from the control group. b (P < 0.01) show significance from the CCl4 group. Mean ± SE (n = 06 rats).
Effect of LME on lungs GSH-Px, GST, QR and GSR
Changes in the activities of different enzymes viz; GST, GSR, GSH-Px and QR are shown in Table 1. CCl4 treatment to rats considerably (p < 0.01) depleted the activities of GSR, GST, GSH-Px and QR. Treatment of rats with different doses of LME alleviated the toxic effects of CCl4 by increasing the activity of GSH-Px, GSR, QR and GST as compared to the CCl4 group. Similar observations were deliberated in rats having a dose (50 mg/kg b.w.) of RT used in this experiment.
Table 1.
Effect of LME on lungs GSH-Px, GSR, GST and QR
| Treatment | GSH-Px (nM/mg protein) | GSR (nM/min/mg protein) | GST (nM/min/mg protein) | QR (nM/min/mg protein) |
|---|---|---|---|---|
| Control |
103.17 ± 0.60++ |
65.83 ± 1.33++ |
54.83 ± 10.1++ |
81.00 ± 1.65++ |
| Olive oil + DMSO |
99.000 ± 0.577++ |
64.17 ± 1.33++ |
52.667 ± 8.2++ |
80.00 ± 1.71++ |
| 3 ml/kg CCl4 |
54.500 ± 0.764** |
29.3 ± 0.494** |
35.500 ± 7.4** |
39.33 ± 1.73** |
| 100 mg/kg LME + CCl4 |
77.500 ± 0.764++ |
54.3 ± 0.88++ |
45.333 ± 7.1++ |
67.5 ± 1.9++ |
| 200 mg/kg LME + CCl4 |
98.567 ± 0.464++ |
62.1 ± 1.3++ |
51.42 ± 11.4++ |
77.58 ± 1.71++ |
| 50 mg/kg RT + CCl4 | 96.333 ± 0.882++ | 62.17 ± 1.62++ | 51.33 ± 13.3++ | 77.33 ± 1.89++ |
Mean ± SE (n = 6 number).
** indicate significance from the control group at p < 0.01 probability level.
++ indicate significance from the CCl4 group at p < 0.01 probability level.
Effect of LME on lung GSH, TBARS, H2O2 and nitrite contents in rat
Changes in the content of GSH, TBARS, H2O2 and nitrite in lungs of rat are shown in Table 2. Administration of CCl4 significantly depleted (p < 0.01) the GSH while increased markedly (p < 0.01) the tissue nitrite, TBARS and H2O2 contents as compared to normal rats. Administration of LME and RT in CCl4 treated rats considerably (p < 0.01) augmented the toxic effects of CCl4, restored the GSH, TBARS, nitrite and H2O2 contents towards the non treated control group.
Table 2.
Effect of LME on lungs TBARS, H2O2, GSH and nitrite contents
| Treatment | TBARS (nM/min/mg protein) | H2O2(nM/min/mg tissue) | GSH (μM/g tissue) | Nitrite (μM/ml) |
|---|---|---|---|---|
| Control |
19.51 ± 0.17++ |
5.71 ± 0.41++ |
0.53 ± 0.014++ |
30.36 ± 0.99++ |
| Olive oil + DMSO |
19.83 ± 0.30++ |
5.32 ± 0.71++ |
0.52 ± 0.010++ |
31.8 ± 1.23++ |
| 3 ml/kg CCl4 |
39.16 ± 0.70** |
14.11 ± 0.31** |
0.25 ± 0.004** |
59.4 ± 1.37** |
| 100 mg/kg LME + CCl4 |
24.25 ± 0.62++ |
6.21 ± 0.61++ |
0.53 ± 0.009++ |
33.5 ± 0.69++ |
| 200 mg/kg LME + CCl4 |
20.50 ± 0.42++ |
6.52 ± 0.41++ |
0.52 ± 0.007++ |
34.0 ± 1.11++ |
| 50 mg/kg RT + CCl4 | 25.66 ± 0.33++ | 5.92 ± 0.31++ | 0.50 ± 0.01++ | 35.2 ± 1.38++ |
Mean ± SE (n = 6 number).
** indicate significance from the control group at p < 0.01 probability level.
++ indicate significance from the CCl4 group at p < 0.01 probability level.
Effect of LME on body weight, lung weight, relative lung weight, AgNORs count and % DNA fragmentation in rat
Toxic effects of CCl4 administration in rat on % changes in body weight, lung weight, relative lung weight, AgNORs count and % DNA fragmentation are presented in Table 3. Administration of CCl4 significantly increased (p < 0.01) lung weight, relative lung weight, AgNORs count and %DNA fragmentation while decreased the % body weight of rats as compare to normal rats. Post-treatment of LME at 100 mg/kg b.w and 200 mg/kg b.w, dose improved the CCl4 intoxication and extensively reduced (p < 0.01) the lung weight, relative lung weight, % DNA damages and AgNORs count as compare to CCl4 group.
Table 3.
Effect of LME on lung weight, relative lung weight, AgNORs count and DNA damages in rat
| Treatment | % increase in Body weight | Lung weight (g) | Relative lung weight | DNA Fragmentation% | AgNORs (NORs/cell) |
|---|---|---|---|---|---|
| Control |
26.0 ± 0.80++ |
2.56 ± 0.006++ |
0.026 ± 0.00006++ |
7.07 ± 0.43++ |
1.26 ± 0.15++ |
| Olive oil + DMSO |
25.9 ± 0.63++ |
2.55 ± 0.008++ |
0.025 ± 0.00008++ |
6.93 ± 1.42++ |
1.19 ± 0.07++ |
| 3 ml/kg CCl4 |
18.6 ± 0.72** |
3.89 ± 0.020** |
0.038 ± 0.00020** |
29.80 ± 1.41** |
7.8 ± 0.52** |
| 100 mg/kg LME + CCl4 |
22.8 ± 0.81++ |
2.91 ± 0.025++ |
0.029 ± 0.00025++ |
18.07 ± 1.33++ |
3.5 ± 0.13++ |
| 200 mg/kg LME + CCl4 |
25.6 ± 0.73++ |
2.56 ± 0.007++ |
0.026 ± 0.00007++ |
7.40 ± 1.56++ |
2.95 ± 0.06++ |
| 50 mg/kg RT + CCl4 | 24.5 ± 0.46++ | 2.09 ± 0.020++ | 0.020 ± 0.00020++ | 7.10 ± 1.50++ | 2.03 ± 0.09++ |
Mean ± SE (n = 6 number).
** indicate significance from the control group at p < 0.01 probability level.
++ indicate significance from the CCl4 group at p < 0.01 probability level.
Effect of LME on lung DNA damages in rat
DNA damages in the lung tissues of rats in different experimental groups are shown in Figure 2. DNA ladder assay in control group (Lane 1–4) show no changes while extensive DNA damages were found in CCl4 group as depicted by (Lane 5,6). Post-administration of 100 mg/kg, 200 mg/kg b.w, LME and 50 mg/kg b.w, rutin reduced the DNA damages, dose dependently, as shown by DNA band pattern of different groups comparatively to CCl4 group (Lane 7–10), (Lane 11–14) and (Lane 15–18) respectively.
Figure 2.
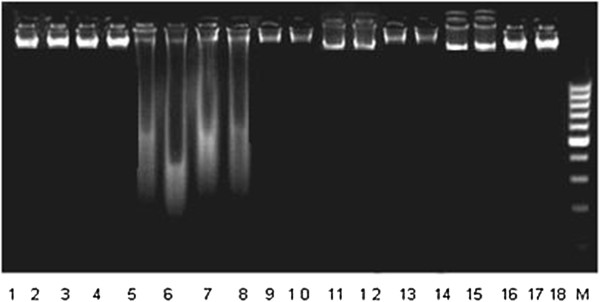
Agarose gel showing DNA damage by CCl4 and preventive effect of Launaea procumbens extracts in different groups. Lanes (from left), Control (1–4), CCl4 (5–8), 100 mg/kg LME (9, 12) 200 mg/kg LME (13, 16), 50 mg/kg RT (17, 18), DNA marker (M).
Effect of LME on lung morphology
The thin sections of control having normal alveoli with thin intralveolar septum, type I and type II pneumocytes were also clearly observed (Figure 3A). The alveolar macrophages were also prominent and the alveolar bronchioles show their normal shape with inner epithelium. Treatment of CCl4 in the lungs induced the degeneration of the alveolar septa, disruption of the connective tissues, elastic fibers and the congestion of the blood capillaries which were also blocked with large aggregation of the blood cells (Figure 3B & C). Administrations of 100 mg/kg, 200 mg/kg LME and 50 mg/kg b.w. RT reduced the toxic effects of CCl4 and reduced injuries were observed in the lung tissues of these groups. The ameliorating effects of the fractions were more pronounced at the higher dose of LME (Figure 3D, E & F). Most areas of the lungs showed the normal alveolar spaces, alveolar and bronchioles with minor cell degeneration, PNI, PNII but a less marked thickening was still observed in the intralveolar septum (Table 4).
Figure 3.
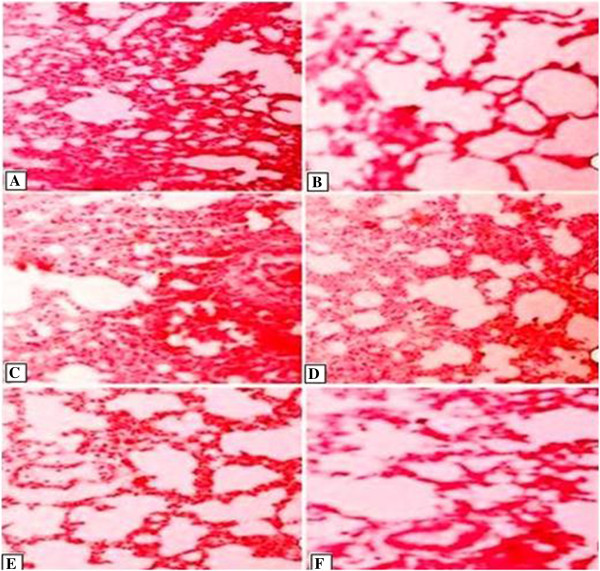
Effect of LME on lung morphology; Slides (from left) Control (A), CCl4 (B, C), 100 mg/kg LME (D) 200 mg/kg LME (E) 50 mg/kg RT (F).
Table 4.
Effect of LME on lung morphology
| Treatment | A b | DCT | DEF | CBC | ABC | PE | PF |
|---|---|---|---|---|---|---|---|
| Control |
- |
- |
- |
- |
- |
- |
- |
| Olive oil + DMSO |
- |
- |
- |
- |
- |
- |
- |
| 3 ml/kg CCl4 |
+++ |
+++ |
+++ |
+++ |
+++ |
+++ |
++ |
| 100 mg/kg LME + CCl4 |
−/+ |
- |
- |
−/+ |
- |
- |
- |
| 200 mg/kg LME + CCl4 |
- |
- |
- |
- |
- |
−/+ |
- |
| 50 mg/kg RT + CCl4 | −/+ | - | - | - | - | - | - |
-, normal; +/−, mild; +++, severe disruption.
AB (alveolar breakage), DCT (degeneration of connective tissue), DEF (damages of elastic fiber), CBC (congestion of blood capillaries), ABC (aggregation of blood cells), DIEAB (disorganized inner epithelium of alveolar bronchiole, PE (pulmonary edema), PF (pulmonary fibrosis.
Discussion
Metabolism is a necessary process of living organisms for energy production; however normal metabolisms produce various reactive oxygen species (ROS) such as superoxide radicals (O2·-), hydrogen peroxide (H2O2) and hydroxyl radicals (OH·). In small amounts, these ROS are beneficial in signal transduction and growth regulation. However, large amount of ROS produced oxidative stress, attack various biomolecules [28]. ROS produced from CCl4 like trichloromethyl radical (·CCl3) and peroxy trichloromethyl radical (·OOCCl3) cause oxidative damages in lungs rats probably disturbing antioxidant status [3]. Antioxidant enzymes such as CAT, POD and SOD play key role in detoxification and protect lungs tissue from oxidative damages [29]. Data of the present study revealed that administration of CCl4 depleted activity of antioxidant enzymes; CAT, POD and SOD. Co-administration of various concentration of LME ameliorated the activities of antioxidant enzymes dose dependently, might be due to the presence of phenolic and polyphenolic compounds. Various other studies revealed similar reports [30,31]. Toxic metabolites of drugs and xenobiotic are metabolized by glutathione system (reduced glutathione, glutathione reductase, glutathione peroxidase and glutathione-S-transferase). In the present study we get marked decreased in activities of GST, GSR, GSH-Px and QR. Administration LME ameliorated the CCl4 toxicity, thereby increases the activity of GST, GSR, GSH-Px and QR. Adewole et al. [8] reported similar observations during administration of melatonin against CCl4 induced oxidative stress. Free radicals cause lipid peroxidation, elevate TBARS and deplete tissue GSH contents [32]. In the present study contents of GSH were considerably depleted while amplified the TBARS and H2O2 contents by induction of CCl4 comparatively to the control group in this study. Administration of different concentrations of LME extensively increased the GSH contents and decreased the TBARS and H2O2 contents. Similar observations were reported during co-treatment of plant extracts against CCl4 induced damages in rats [33]. Lipid peroxidation induced by CCl4 not only disturbs protein but diffuse into nucleic acid causes DNA fragmentation [34,35] which might lead to pulmonary damages. In the present study DNA fragmentation induced by CCl4 are ameliorated, with LME significantly as was reported by Khan et al. [9]. Quantification of AgNORs proteins per cell has been used in the diagnosis of oxidative lesions [36,37]. In this study co-administration of LME significantly augmented NORs/cell as was altered with treatment of CCl4 in lungs. Khan et al. [9] reported similar results during administration Digera muricata in rats.
Extensive variations were observed during histopathalogical study of rat lungs. Treatment of CCl4 caused destruction of alveolar septa and congestion of blood capillaries. As a result blood cells and collagen fibers are accumulated at various places leads to endemic condition. Similar observation was found in other study during CCl4 administraion in rat lungs [3,38]. Co-treatment with LME repaired the pulmonary damages; showing normal spaces in alveoli, reduced cellular degeneration of alveoli and bronchioles as well as normalized pneumocytes as were reported by Khan et al. [3] during Sonchus asper administration against CCl4-induced injuries in rats.
Conclusion
Our results propose that LME comprised of bioactive compounds; presenting protective effects against CCl4 induced toxic affects in lungs of rat. Further studies of isolation and purification of these constituents are in progress in our lab.
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
RAK made significant contribution to acquisition of data, analysis, conception, design and drafting of the manuscript. The authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
References
- Cerutti PA. Oxidant stress and carcinogenesis. Eur J Clin Invest. 1991;21:1–5. doi: 10.1111/j.1365-2362.1991.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Brennan FM, Maini RN, Feldmann M. Cytokine expression in chronic inflammatory disease. British Med Bull. 1995;51:368–384. doi: 10.1093/oxfordjournals.bmb.a072967. [DOI] [PubMed] [Google Scholar]
- Khan RA, Khan MR, Sahreen S. Protective effect of Sonchus asper extracts against experimentally-induced lung injuries in rats: A novel study. Exp Toxicol Pathol. [DOI] [PubMed]
- Dinis-Oliveira RJ, Remiao F, Duarte JA, Ferreira R, Sánchez Navarro A, Bastos ML, Carvalho F. P-glycoprotein induction: an antidotal pathway for paraquat-induced lung toxicity. Free Rad Biol Med. 2006;41:1213–1224. doi: 10.1016/j.freeradbiomed.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Keogh KA, Standing J, Kane GC, Terzic A, Limper AH. Angiotensin II antagonism fails to ameliorate bleomycin-induced pulmonary fibrosis in mice. The European Respiratory J. 2005;25:708–714. doi: 10.1183/09031936.05.00090204. [DOI] [PubMed] [Google Scholar]
- Abraham P, Wilfred G, Cathrine SP. Oxidative damage to lipids and proteins of the lungs, testis and kidney of rats during CCl4 intoxication. Clinic Chimica Acta. 1999;289:177–179. doi: 10.1016/S0009-8981(99)00140-0. [DOI] [PubMed] [Google Scholar]
- Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- Adewole SO, Salako AA, Doherty OW, Naicker T. Effect of melatonin on carbon tetrachloride-induced kidney injury in Wistar rats. Afr J Biomed Res. 2007;10:153–164. [Google Scholar]
- Khan MR, Rizvi W, Khan GN, Khan RA, Shaheen S. Carbon tetrachloride induced nephrotoxicity in rat: Protective role of Digera muricata (L.) Mart. J Ethnopharmacol. 2009;122:91–99. doi: 10.1016/j.jep.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Khan RA, Khan MR, Sahreen S. Evaluation of Launaea procumbens use in renal disorders: a rat model. J Ethnopharmacol. 2010;128:452–461. doi: 10.1016/j.jep.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Sahreen S, Khan MR, Khan RA. Hepatoprotective effects of methanol extract of Carissa opaca leaves on CCl4-induced damage in rat. BMC Compl Alter Med. 2011;11:48. doi: 10.1186/1472-6882-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HK, Kim SJ, Kwon DY, Park JH, Kim YC. Protective effect of quercetin against paraquat-induced lung injury in rats. Life Sci. 2010;87:181–186. doi: 10.1016/j.lfs.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Wazir SM, Saima S, Dasti AA, Subhan S. Ethnobotnical importance of salt range species of district karak, Pakistan. Pakistan J Plant Sci. 2007;13:29–31. [Google Scholar]
- Ahmad M, Khan MA, Manzoor S, Zafar M, Sultana S. Check list of medicinal flora of Tehsil Isakhel, District Mianwali Pakistan. Ethnobotanical Leaflets. 2006;10:41–48. [Google Scholar]
- Shaukat SS, Siddiqui IA, Nasim AI. Nematocidal, allelophatic and antifugal potential of Launaea procumbens. Pakistan J Plant Pathol. 2003;2:181–193. [Google Scholar]
- Lowry OH, Rosenberg NJ, Farr AL, Randall RJ. Protein measurement with Folin Phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- Chance B, Maehly AC. Assay of catalase and peroxidases. Methods Enzymol. 1955;11:764–75. [Google Scholar]
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dimutase. Ind J Biochem Biol. 1984;21:130–132. [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Carlberg I, Mannervik EB. Glutathione level in rat brain. J Biol Chem. 1975;250:4475–80. [PubMed] [Google Scholar]
- Mohandas J, Marshal JJ, Duggin GG, Horvath JS, Tiller DJ. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem Pharmacol. 1984;33:1801–1807. doi: 10.1016/0006-2952(84)90353-8. [DOI] [PubMed] [Google Scholar]
- Pick A, Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages—induction by multiple nonphagocytotic stimuli. Cell Immunol. 1981;59:301–18. doi: 10.1016/0008-8749(81)90411-1. [DOI] [PubMed] [Google Scholar]
- Benson AM, Hunkeler MJ, Talalay P. Increase of NADPH, quinone reductase activity by dietary antioxidant: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci USA. 1980;77:5216–20. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Zampaglione N, Gillete JR. Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as a hepatotoxic metabolite. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Iqbal M, Sharma SD, Zadeh HR, Hasan N, Abdulla M, Athar M. Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate (Fe-NTA) mediated hepatic injury. Redox Rep. 1996;2:385–91. doi: 10.1080/13510002.1996.11747079. [DOI] [PubMed] [Google Scholar]
- Wu B, Ootani A, Iwakiri R, Sakata Y, Fujise T, Amemori S, Yokoyama F, Tsunada S, Fujimoto K. T cell deficiency leads to liver carcinogenesis in azoxymethane treated rats. Exp Biol Med. 2005;231:91–8. doi: 10.1177/153537020623100111. [DOI] [PubMed] [Google Scholar]
- Trere D, Zilbering A, Dittus D, Kim P, Ginsberg PC, Daskal I. AgNOR quantity in needle biopsy specimens of prostatic adenocarcinomas: correlation with proliferation state, Gleason score, clinical stage, and DNA content. J Clin Pathol. 1996;49:209–213. doi: 10.1136/mp.49.4.M209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Brit J Pharmacol. 2004;142:231–55. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganie SA, Haq E, Hamid A, Qurishi Y, Mahmood Z, Zargar BA, Masood A, Zargar MA. Carbon tetrachloride induced kidney and lung tissue damages and antioxidant activities of the aqueous rhizome extract of Podophyllum hexandrum. BMC Compl Alter Med. 2011;11:17. doi: 10.1186/1472-6882-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna P, Sinha M, Sil PC. Aqueous extract of Terminalia arjuna prevents carbon tetrachloride induced hepatic and renal disorders. BMC Compl Alter Med. 2006;6:33. doi: 10.1186/1472-6882-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan SA, Rizk MZ, El-Sharkawi F, Badary O, Kadry MO. The possible synergistic role of phytic acid and catechin in ameliorating the deteriorative biochemical effects induced by carbon tetrachloride in rats. J Appl Sci Res. 2007;3:1449–1459. [Google Scholar]
- Ohkawa H, Ohishi N, Yogi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Sreelatha S, Padma PR, Umadevi M. Protective effects of Coriandrum sativum extracts on CCl4-induced hepatotoxicity in rats. Food Chem Toxicol. 2008;48:702–708. doi: 10.1016/j.fct.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Marnett JL. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–70. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- Sahreen S, Khan MR, Khan RA. Hepatoprotective effects of methanol extract of Carissa opaca leaves on CCl4-induced damage in rat. BMC Compl Alter Med. 2011;11:48. doi: 10.1186/1472-6882-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazizadeh M, Sasaki Y, Araki T. Prognostic value of proliferative activity of ovarian carcinoma as revealed by PCNA and AgNOR analyses. Am J Clin Pathol. 1997;107:451–458. doi: 10.1093/ajcp/107.4.451. [DOI] [PubMed] [Google Scholar]
- Irazusta SP, Vassallo J, Magna LA. The value of PCNA and AgNOR staining in endoscopic biopsies of gastric mucosa. Pathol Res Pract. 1998;194:33–39. doi: 10.1016/S0344-0338(98)80009-5. [DOI] [PubMed] [Google Scholar]
- Zakaria I, Khalik IAE, Selim ME. The protective effects of curcumin against carbon tetrachloride induced pulmonary injury in rats. Egypt J Med Lab Sci. 2004;13:1–15. [Google Scholar]