Abstract
T cell expression of inhibitory proteins can be a critical component for the regulation of immunopathology due to self-reactivity or potentially exuberant responses to pathogens, but may also limit T cell responses to some malignancies, particularly if the tumor antigen being targeted is a self-protein. We found that the abrogation of SHP-1, in tumor-reactive CD8+ T cells improves the therapeutic outcome of adoptive immunotherapy in a mouse model of disseminated leukemia, with benefit observed in therapy employing transfer of CD8+ T cells alone or in the context of also providing supplemental IL-2. SHP-1−/− and SHP-1+/+ effector T cells were expanded in vitro for immunotherapy. Following transfer in vivo, the SHP-1−/− effector T cells exhibited enhanced short-term accumulation, followed by greater contraction, and ultimately formed similar numbers of long-lived, functional memory cells. The increased therapeutic effectiveness of SHP-1−/− effector cells was also observed in recipients that expressed the tumor antigen as a self-antigen in the liver, without evidence of inducing autoimmune toxicity. SHP-1−/− effector CD8+ T cells expressed higher levels of Eomesodermin, which correlated with enhanced lysis of tumor cells. Furthermore, reduction of SHP-1 expression in tumor-reactive effector T cells by retroviral transduction with vectors that express SHP-1-specific siRNA, a translatable strategy, also exhibited enhanced anti-tumor activity in vivo. These studies suggest that abrogating SHP-1 in effector T cells may improve the efficacy of tumor elimination by T cell therapy without impacting the ability of the effector cells to persist and provide a long-term response.
Introduction
Adoptive immunotherapy by transfer of tumor-reactive T cells has proven successful for treatment of some established malignancies (1, 2), including leukemia (3). Several obstacles can limit the efficacy of this approach, such as the ability of donor T cells to adequately recognize the tumor, and to persist and function (1). Poor recognition often reflects the low avidity of the donor T cells for the targeted tumor cells. Defects in antigen processing and presentation and/or a lack of co-stimulatory molecule expression by the tumor can contribute to a lack of T cell recognition and/or activation. Additionally, if the tumor antigen is not a foreign protein but rather a self-antigen, self/tumor-reactive T cells that develop and express a high affinity TCR are often deleted or rendered tolerant. To overcome this obstacle of inefficient recognition, genetic modification of donor T cells to express high affinity receptors may provide T cells that efficiently recognize the tumor (4, 5). Another common obstacle is poor persistence of transferred T cells and resulting failure to sustain an anti-tumor response, reflecting in part a consequence of the requirement of first having to expand the donor T cells to large numbers in vitro for therapy, which generally produces a population of effector T cells that depend on exogenous cytokines for proliferation and survival. This has been addressed by several strategies, including administration of exogenous IL-2, although this often has toxicities in patients (6), induction of lymphopenia to take advantage of the homeostatic proliferative drive (7, 8), and more recently exploration into the use of other γ-chain cytokines (9–11). An approach our lab is pursuing that can potentially concurrently address these obstacles is to abrogate expression of negative regulators of lymphocyte function prior to T cell transfer, as this may improve responses to antigen stimulation, reduce the threshold for T cell activation and enhance effector T cell function(12–14).
The Src-homology domain-containing protein tyrosine phosphatase-1 (SHP-1) is a negative regulator of signaling expressed in all hematopoietic cells (15). In T cells, SHP-1 recruitment to membrane lipid rafts is inversely correlated with the strength of the antigenic signal, thereby enforcing the discrimination between weak or antagonistic ligands and agonistic ligands (16–20). SHP-1-dependent dephosphorylation of signaling proteins after antigen encounter, including Lck (21, 22), Zap70 (23, 24), Vav (25), PI3K (26) and TCRζ (27), has been shown to limit naive T cell responsiveness. Naive T cells from mice with a loss-of-function mutation in SHP-1 exhibit increased proliferation to antigen and cytolytic activity in vivo after activation compared to wild type T cells(28). In tumor settings, SHP-1 is detected at high levels in tumor infiltrating lymphocytes (TILs) that lack lytic activity, and the abrogation of SHP-1 expression in TILs was found to restore lytic function in vitro (29). Recently, our lab demonstrated that SHP-1 negatively regulates the accumulation of short-lived, antigen-specific effector cells derived from either naïve or memory virus-specific CD8 precursors in response to acute virus infection without impacting memory T cell formation (30). These studies suggest that ablating SHP-1 in tumor-reactive effector cells has the potential to improve anti-tumor activity following T cell therapy by several possible mechanisms.
Many previous studies have assessed the role of SHP-1 in T cells isolated from the motheaten mouse strain, which have a null mutation in SHP-1 protein in all cells, but there are difficulties studying T cells from such mice as T cells develop abnormally in the context of severe autoimmune inflammatory disease (31–34). To overcome this limitation, we have used a conditional knockout of SHP-1 in which mature CD8 T cells lack SHP-1 protein to assess the impact of abrogation of SHP-1 expression in tumor-reactive effector T cells during immunotherapy of disseminated leukemia. We have previously generated a TCR transgenic (tg) mouse strain (TCRgag) with CD8 T cells specific for the immunodominant gag epitope derived from the FMuLV-transformed erythroleukemia, FBL (35), as well as tg mice that express the gag tumor antigen as a self-antigen in the liver (Alb:Gag) (36). Since human adoptive immunotherapy protocols rely on the expansion of effector T cells in vitro prior to transfer, we generated TCRgag mice that had SHP-1 conditionally knocked out specifically in mature T cells, to elucidate if the complete or partial abrogation of SHP-1 regulates the anti-tumor activity of effector T cells. Our results demonstrate that SHP-1 abrogation in in vitro-expanded effector CD8 T cells improves the efficacy of T cells in adoptive immunotherapy of FBL leukemia, and is associated with enhanced function and accumulation of short-lived effector cells in a preclinical setting. This study has implications for the development of effective therapies to treat established tumors, as this strategy, which improves the generation and expansion of short-lived tumor-reactive effector cells in vivo without impacting long-term memory formation or inducing toxicity, increases the in vivo antitumor activity of the infused T cells during the time when the peak response is needed.
Materials and Methods
Mice
SHP-1Flox/Flox mice (37), a gift from L. Pao and B. Neel (Beth Israel Deaconess Medical Center, Boston, MA) and K. Rajewsky (Harvard Medical School, Immune Disease Institute, Boston, MA), were crossed with Lck-Cre mice (dLck-Cre, (38, 39), a gift from P. Fink (University of Washington with permission from N. Killeen), and TCRgag tg mice (35, 36). Alb:Gag tg mice have been previously described (36). C57Bl/6 (B6) mice were purchased from the Jackson Laboratory. Studies were executed according our approved animal protocol and to the policies of the Institute for Animal Care and Use Committee in the Department of Comparative Medicine, University of Washington and mice were maintained under SPF conditions.
Cell lines, antibodies and peptides
The Friend virus-induced erythroleukemia of B6 origin, FBL, expresses the F-MuLV encoded gag epitope (peptide CCLCLTVFL purchased from Pi Proteomics). The fluorochrome-conjugated antibodies CD8α, Thy1.1, CD44, CD25, CD69, CD62L, CD127, KLRG1, CD5, Eomes, Granzyme B, T-bet, Perforin, IL-2 and IFNγ, were purchased from eBiosciences. The antibody to IL-15Rα was purchased from R&D Systems. The Immune Monitoring Core at the Fred Hutchinson Cancer Research Center, Seattle, WA, synthesized the gag/H-2Db tetramer. Neutralizing antibody to mouse IL-7 (M25) was a generous gift from Amgen (Seattle, WA). Human recombinant IL-2 (IL-2, National Institute of Allergy and Infectious Diseases, DAIDS) was used to stimulate cultures.
Intracellular staining for SHP-1
TCRgag SHP-1+/+, SHP-1+/− and SHP-1−/− cells were surface stained for CD8 and Thy1.1 for 30 min at 4° C. Cells were washed two times in FACS buffer (PBS containing 1% FBS) and fixed using BD Phosflow Fix Buffer I at 37° C for 10 min according to BD Biosciences protocol. Cells were washed once with FACS buffer and permeabilized using BD Phosflow Perm Buffer III and incubated for 30 min on ice. Cells were washed and incubated with rabbit anti-mouse SHP-1 (C1486, Cell Signaling Technologies) diluted 1:50 in FACs buffer at 4° C. Cells were washed twice with FACs buffer and incubated with anti-rabbit-547 (Fab2 fragment, Molecular Probes). SHP-1 staining was immediately analyzed on a flow cytometer by gating on CD8+ Thy1.1+ cells.
Expansion of effector TCRgag cells in vitro
TCRgag SHP-1+/+, SHP-1+/− and SHP-1−/− in vitro -derived effector cells were generated as previously described (14). Briefly, TCRgag SHP-1+/+, SHP-1+/− and SHP-1−/− tg cells (1×106) were cultured with irradiated, syngeneic splenocytes cells (5×106), irradiated FBL leukemia (3×106) and IL-2 (20 U/ml) in 10 ml of complete media (RPMI 1640 supplemented with 2 µM glutamine, 100 U/ml penicillin/streptomycin, 10% fetal calf serum, and 30 µM 2-mercapatoethanol). T cells were similarly re-stimulated every 7–10 days. Five days following the 3rd in vitro cycle of antigen stimulation, effector T cells were used in various assays or transferred for adoptive immunotherapy.
T cell proliferation and cytokine production in vitro
The 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) was purchased from Molecular Probes. Transgenic T cells were incubated with 1 µM of CFSE for 20 min at 37°C, and excess CFSE was washed from T cell cultures 3 X using complete media. CFSE-labeled tg T cells (1×105) were incubated in round-bottom, 96-well plates with 5×105 congenic (Thy1.2+) splenocytes pulsed with titrating concentrations of gag peptide. After 4 days, CFSE-dilution of Thy1.1+CD8+ tg T cells was assessed by flow cytometry. Intracellular cytokine staining was performed by incubating tg T cells with congenic (Thy1.2+), peptide-pulsed splenocytes in the presence of Brefeldin-A (BD Biosciences). After 5 h, cells were stained with antibodies to CD8, Thy1.1 and intracellular cytokines according to BD Biosciences protocols.
CFSE-based cytotoxicity assay
EL-4 and FBL tumor were incubated 20 min at 37°C with 0.1 µM or 10 µM CFSE. Excess dye was removed by washing tumor cells in serum-containing media. A 1:1 mixture of EL4 and FBL tumor cells was incubated with titrating numbers of SHP-1+/+, SHP-1+/− or SHP-1−/− in vitro-expanded effector T cells for 5 h in 96-well, round bottom plates at 37°, 5% CO2. FBL lysis was determined by FACs analyses of the number of CFSEhi (FBL) remaining in the well.
Real-time PCR and gene expression analysis
RNA was isolated from 5×106 naïve or effector T cells at various timepoints after activation with antigen ± IL-2 (20 U/ml) using the RNeasy Plus Mini Kit (Qiagen). Reverse transcription to generate cDNA from RNA samples was conducted using the SuperScript First-Strand Synthesis System and Oligo dT primers (Invitrogen). The following primer sequences (Invitrogen) were used: β-actin: 5’-AACTGCAGAGGACTCCTATGTGGGTGACG-3’, 5’-CGGGATCCGATGG CTACGTACATGGCTGG-3’; Eomes: 5’-GCCTACCAAAACACGGATA-3’, 5’TCTGTTGGG GTGAGAGGAG-3’; Runx-3: 5’-TCAAGGTCACTGTGGATGGA-3’, 5’-AGGTCTGAGGAG CCTTGGAT-3’, Granzyme A: 5’-TTTCATCCTGTAATTGGACTAA-3’, 5’-GCGATCTCCAC ACTTCTC-3’; Granzyme B: 5’-TCGACCCTACATGGCCTTAC-3’, 5’-TGGGGAATGCATTT TACCAT-3’; Granzyme C: 5’- TCTCCTGACCCTACTTCTG-3’, 5’-TGTTAGCACGAATTTG TCTC-3’; T-bet: 5’- GTTCCCATTCCTGTCCTTC-3’, 5’-CCTTGTTGTTGGTGAGCTT-3’; Perforin: 5’-GATGTGAACCCTAGGCCAGA-3’, 5’-GGTTTTTGTACCAGGCGAAA-3’. Real-time PCR reactions were in a total volume of 25 µL containing 1X Power SYBR Green PCR Master Mix (Applied Biosystems), 1.25 µL primer mix and 2 µL of cDNA. All reactions were performed in duplicate and each plate contained the endogenous control (β-actin). The ABI-PRISM 7000 Sequence Detection System was used to amplify target genes with the following conditions: denaturing at 95°C for 10 minutes; 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. Relative gene expression was calculated based on the delta delta CT method and was normalized to the endogenous control β-actin and gene expression in naïve or wild type effector T cells as indicated.
Adoptive immunotherapy of disseminated FBL leukemia
B6 or Alb:Gag mice were injected with 5×106 live FBL leukemia intraperitoneally (i.p.) as described (14, 36). After 5 days, after the leukemia had disseminated, mice received cyclophosphamide (Cy, 180 mg/kg) and after 6 h, to permit clearance of the Cy, TCRgag SHP-1+/+, SHP-1+/− and SHP-1−/− effector cells that had undergone 3 cycles of stimulation in vitro were transferred into tumor-bearing mice. Cohorts of recipient mice also received exogenous IL-2 injections (1×104 U/ml/mouse/day i.p.). Mice were monitored for tumor burden (ascites formation, splenomegaly, and nodal masses) and euthanized if the detectable tumor mass reached proportions that predictably lead to mortality within 24–48 h.
LM-gag immunization after transfer of memory TCRgag cells
At 120 days post tumor therapy, splenocytes were harvested from cured mice and sorted for Thy1.1+ CD8+ donor cells. Equivalent numbers of each type of donor cell (1×104) were transferred into B6 recipients. Two days later, mice were immunized with an attenuated (ΔActA) recombinant Listeria monocytogenes engineered to express the gag epitope (3×107 c.f.u.). After 6 days, splenocytes were harvested, counted and stained with antibodies to CD8 and Thy1.1 and various other surface markers and analyzed by flow cytometry.
Administration of the anti-IL-7 monoclonal antibody (M25)
B6 or IL-15−/− mice were injected with irradiated FBL tumor (2×107 cells) and/or Cy (180 mg/kg) 6 h prior to administration of effector T cells and irradiated tumor. Some recipients also received exogenous IL-2 injections (1×104 U/ml/mouse/day i.p.). Effector T cells that had undergone 3 cycles of in vitro expansion with antigen and IL-2 (20 U/ml) were transferred (1×105) into either B6 mice, IL-15−/− mice or B6 and IL-15−/− mice that had been injected with 1 mg of IL-7 depleting M25 antibody (1 mg/mouse/injection i.p.; the M25 antibody was generously provided by Amgen, Seattle, WA) beginning one day prior to and then every other day following effector T cell transfer. After 7 days, the percentage and number of donor cells in the spleen was calculated by counting live splenocytes and FACs analysis of CD8+ Thy1.1+ cells.
Short interfering RNA silencing of SHP-1 in tumor-reactive effector T cells
SHP-1 was targeted with the shRNA 5′-aacgc agctg acatt gagaat-3′ (NM_080549.3), a sequence that is homologous between mice and humans and has been successfully targeted in human (40, 41) and mouse (29) cells. We generated a MigRI retroviral vector that expressed GFP and the SHP-1 specific shRNA under the control of the U6 promoter. A control vector that had a scramble shRNA sequence was used to control for non-specific effects of T cell transduction. Viral supernatant was harvested from transfected packaging cells on days 2 and 3 post transfection. Wild type TCRgag effector cells were transduced by incubating effector T cells with viral supernatant and spinning cells for 90 min, 32°, at 2500 rpm. Transduced cells were purified by sorting for GFP+ CD8+ cells and maintained in vitro by re-stimulating with antigen and IL-2 (20 U/ml) every 7 days.
Statistics
Data in graphs represent the mean ± SEMs. Statistical analyses of the data were performed using a one-way ANOVA followed by Tukey post hoc testing to reduce the risk of Type I errors, with p values <0.05 considered significant for the ANOVA/Tukey testing and p values >0.05 considered not significant (n.s.). For comparisons of only two groups, a student’s T test was used to determine significance. Asterisks in the Figures: * p<0.05, **p<0.005, ***p<0.0005. Analysis of survival curves was performed using a Log-rank Mantel-Cox Test.
Results
Cell intrinsic SHP-1 abrogation lowers the threshold for proliferation of naïve T cells specific to a tumor antigen
To study the T cell intrinsic effects of abrogating SHP-1 in tumor-specific T cells, mice that express a floxed knock-in SHP-1 gene (37, 42) and Cre recombinase under control of the distal Lck (dLck) promoter (39) were crossed to TCR tg mice specific to the gag epitope expressed by FBL leukemia (TCRgag) (35). We have previously shown that this approach permits the study of T cell intrinsic effects of SHP-1 deletion, with nearly all CD8 T cells becoming SHP-1 deficient (>93%) and developing in the absence of the abnormal host environmental milieu and lethal autoimmune disease that occurs if SHP-1 is deleted in all hematopoietic cells (30). The mean fluorescence intensity (MFI) of SHP-1 staining of CD8+ tetramer+ cells demonstrated complete absence of SHP-1 protein in TCRgag SHP-1−/− cells (both alleles floxed) and a 50% reduction in SHP-1 protein levels in SHP-1+/− cells (one allele floxed) compared to SHP-1+/+ cells confirming gene disruption and loss of SHP-1 protein expression in TCRgag cells (Fig. 1A). Expression levels of CD44, CD62L and CD127 of CD8 T cells from TCRgag SHP-1−/− mice were not distinguishable from wild type TCRgag cells, and were consistent with the cells retaining a naïve phenotype despite the loss of the SHP-1 protein (Fig. 1B). The activation markers CD25 and CD69 were not increased on SHP-1−/− T cells further indicating that ablating SHP-1 in mature CD8 T cells does not result in persistent or intermittent T cell activation.
Figure 1.
Cell-intrinsic SHP-1 deficiency does not modify the phenotype of mature, TCRgag cells, but does lower the threshold for activation. A, Splenocytes from naïve TCRgag SHP-1+/+, SHP-1+/− and SHP-1−/− mice were stained with antibodies to CD8 and Thy1.1 and stained intracellularly for SHP-1. The mean fluorescence intensity (MFI) of SHP-1 expression was determined by gating on CD8+ Thy1.1+ cells. Data is pooled from 3 independent experiments. B, Splenocytes isolated from naïve TCRgag SHP-1+/+, SHP-1+/− and SHP-1−/− mice were stained for CD8 and Thy1.1 and the indicated antibodies. Representative histograms of the intensity of antibody staining are gated on CD8+ Thy1.1+ cells. C, Purified CD8 T cells from splenocytes isolated from naïve Thy1.1+ TCRgag SHP-1+/+, SHP-1+/− and SHP-1−/− mice were labeled with CFSE and incubated with Thy1.2+ syngeneic splenocytes pulsed with titrating concentrations of gag peptide. After 4 days, CFSE-dilution of tg cells was determined by FACs analysis of CD8+ Thy1.1+ cells. Data is pooled from two independent experiments. D, The percentage of T cells undergoing apoptosis at 8 days after activation with antigen was determined by analyzing the percentage of CD8+ Thy1.1+ cells that stained positive for Annexin-V and 7AAD by FACs. Statistical analysis between the groups was conducted using a one-way ANOVA followed by a Tukey post-hoc correction.
We previously demonstrated that cell-intrinsic expression of SHP-1 regulates naïve T cell activation toward viral antigen (30), and performed initial studies to verify that SHP-1 deficiency also improves tumor antigen recognition in TCRgag cells. CFSE-labeled naïve TCRgag SHP-1+/+, SHP-1+/− and SHP-1−/− T cells (congenic for Thy1.1) were incubated with syngeneic Thy1.2+ splenocytes pulsed with titrating concentrations of gag peptide. After four days, CFSE dilution of Thy1.1+ CD8+ cells was quantified by flow cytometric analysis (FACs). TCRgag SHP-1−/− cells proliferated in response to a log10 lower concentration of antigen compared to TCRgag SHP-1+/+ and SHP-1+/− cells (Fig. 1C), indicating that complete abrogation of SHP-1 in naïve T cells lowers the threshold for inducing T cell proliferation in response to tumor antigen recognition. Despite the enhanced proliferation of SHP-1−/− cells, the absence of SHP-1 did not enhance T cell apoptosis, as indicated by Annexin-V and 7AAD staining of T cells 8 days after activation (Fig. 1D). The enhanced proliferation and survival in the absence of SHP-1 resulted in ~2-fold increase in the number of SHP-1−/− cells relative to SHP-1+/+ cells (mean T cell number 7 days after activation: SHP-1+/+ = 2.1×107, SHP-1+/− = 2.9×107, SHP-1−/− = 4.3×107).
Functional impact of SHP-1 abrogation of in vitro-expanded effector cytotoxic T cells
The majority of studies evaluating SHP-1 regulation of T cells have assessed naïve T cell responses. Naïve T cells have superior proliferative potential compared to effector T cells, which lose the ability to produce IL-2 and often die or fail to respond after subsequent antigen encounter (43, 44). However, to obtain a sufficient number of T cells with the intended antigen specificity, clinical adoptive immunotherapy protocols require T cells to be expanded in vitro prior to transfer into patients (12, 45). Genetic modification of donor T cells to express an antigen receptor of a defined specificity can reduce the number of in vitro stimulations of T cells needed prior to transfer, but activation and expansion of T cells leading to the differentiation of donor T cells into effector cells is still required (3, 46). Therefore, to model immunotherapy protocols, TCRgag SHP-1+/+, SHP-1+/− and SHP-1−/− cells were expanded in vitro with antigen (irradiated FBL tumor), syngeneic feeder cells and exogenous IL-2 (20 U/ml) for 3 cycles of stimulation (referred to as 3stim effectors). Five days after the 3rd stimulation, SHP-1 protein was readily detectable in wild type TCRgag effector cells, decreased by approximately 50% in SHP-1+/− cells, as indicated by MFI analysis, and absent in SHP-1−/− cells (Fig. 2A). The effector cells expressed similar levels of Vα3 and were CD44hi, CD62Llo and KLRG1hi, indicative of an effector phenotype (Fig. 2B). The TCRgag SHP-1+/+, SHP-1+/− and SHP-1−/− effector cells expressed equivalent and high levels of CD25 (IL-2Rα), IL-15Rα, and downregulated CD127 (IL-7Rα) compared to naïve CD8 T cells (Fig. 2B). The similar cytokine receptor expression in the effector cell groups suggests that all have the ability to initiate signaling through these critical proliferative and survival pathways. To determine the effect of SHP-1 on antigen-dependent expansion of effector cells, we seeded an equal number of effector cells at the start of the 3rd in vitro simulation and quantified the number of live tg cells after 7 days. In the absence of exogenous IL-2, there was a 2-fold increase in the number of live, tg SHP-1−/− compared to SHP-1+/+ effectors, and the partial decrease in SHP-1 in cells from heterozygous mice resulted in a modest increase in cell number (Fig. 2C). Supplementation of exogenous IL-2 significantly enhanced the accumulation of effector T cells, overcoming the impact of SHP-1 deficiency and making the total cell numbers equivalent and independent of SHP-1 levels (Fig. 2C). Seven days after effector cell expansion with antigen, the percentage of effector T cells undergoing apoptosis (Annexin-V+) correlated with the expression of SHP-1 in T cells (Fig. 2D) indicating that the abrogation of SHP-1 improves effector cell survival after antigen encounter. Increased survival of SHP-1−/− and SHP-1+/− effectors correlated with a modest increase in expression of the pro-survival molecule, Bcl-xL (Fig. 2F), with no difference in expression of the pro-survival molecule, Bcl-2 (data not shown). To determine if the abrogation of SHP-1 impacts susceptibility of effector T cells to FasL-induced apoptosis, naïve and 3X in vitro-expanded SHP-1+/+, SHP-1+/− and SHP-1−/− effector T cells were incubated with anti-Fas antibody and a secondary antibody to cross-link Fas as described(47). After 12 h, cells were stained for CD8 and apoptosis markers Annexin-V and 7AAD and analyzed by flow cytometry. Fas cross-linking on naïve T cells did not substantially result in apoptosis (~4%), but did induce apoptosis of the majority (77%) of SHP-1+/+ effector T cells, and this was substantially reduced in the effector T cells that lacked SHP-1 (28%) (Supplementary Figure 1). As there were no detectable differences in Fas expression between SHP-1+/+ and SHP-1−/− cells, these data suggest that one mechanism of increased survival of SHP-1−/− effector T cells may be resistance to Fas signaling.
Figure 2.
SHP-1 abrogation improves cell survival and cytolytic activity of in vitro-expanded effector T cells. Splenocytes from naïve Thy1.1+ TCRgag SHP-1+/+, SHP-1+/− and SHP-1−/− mice were stimulated in vitro with antigen and recombinant human IL-2 (20 U/ml) for 3 cycles of expansion (referred to as 3stim effectors). On day 5 after the 3rd stimulation, T cells were stained for CD8, Thy1.1 and intracellular SHP-1 (A) or the indicated antigens (B) and analyzed by FACs. Representative histograms are gated on CD8+ Thy1.1+ cells. C, On day 7 after the 3rd in vitro stimulation with antigen ± IL-2 (20 U/ml), the number of live, tg T cells in expanded cultures was derived from total cell counts and FACs analysis of CD8+ Thy1.1+ cells. In the absence of exogenous IL-2, the number of SHP-1−/− effector cells was significantly increased compared to SHP-1+/+ cells (P=0.01). The dashed line indicates the number of tg effectors (1×106) at the start of the 3rd stimulation. Data is combined from 4 independent experiments. D, Apoptosis of 3stim effector cells was determined 7 days after the 3rd stimulation of antigen and IL-2 by analyzing the percentage of Annexin V+ 7AAD+ cells of the total tg CD8+ Thy1.1+ cells by FACs. E, Seven days after stimulation with antigen and IL-2, 3stim effectors were stained for intracellular Bcl-xL and the MFI of Bcl-xL staining was determined by FACs analysis of the CD8+ Thy1.1+ cell population. F, 3stim effectors were incubated at a 1:1 ratio with a mixture of FBL targets labeled with 10 µM CFSE and control EL4 tumor cells labeled with 0.1 µM CFSE. After 5 h, the percentage of FBL cells was compared to control tumor by FACs analysis. G, A standard CFSE-based cytoxicity assay as described in (F) was performed using titrating numbers of 3stim effector cells. The percentage lysis was determined by dividing the number of FBL cells incubated with T cells by the number of live FBL cells incubated without T cells. H, CD8+ effectors were stained for the intracellular expression of the indicated proteins and analyzed by FACs. Representative histograms (n=4). I, The percentage of CD8+ tg effectors that express Eomes (left panel) and the MFI of Eomes in CD8 effectors (right panel) was pooled from 4 experiments. J, Relative expression of Eomes and Perforin mRNA was determined by real-time PCR. Data is normalized to the housekeeping gene β-actin and to the gene levels in wild type effectors. Data is pooled from 4 independent experiments. Statistical analysis between the groups was conducted using a one-way ANOVA followed by a Tukey post-hoc correction.
To evaluate if SHP-1 influenced the lytic activity of effector cells, we used a CFSE-based cytotoxicity assay that allows visualization of tumor cell lysis mediated by CTLs. TCRgag SHP-1+/+, SHP-1+/− or SHP-1−/− 3stim effector cells were incubated with a mixture of live FBL tumor (CFSEhi) and control tumor (EL4, CFSEint). After 5 hours, CTL-mediated specific lysis of FBL tumor was assessed by comparing the percentage of FBL (CFSEhi) to the percentage of the control EL4 (CFSEint) remaining in the well by FACs. SHP-1−/− effectors demonstrated a dramatic increase in the ability to specifically lyse FBL tumor cells compared to both SHP-1+/+ and SHP-1+/− cells following incubation at a 1:1 effector to target (ET) cell ratio (Fig. 2F). Using a similar approach, we compared CTL lytic ability at a wide range of cell ratios. At high effector T cell to tumor cell ratios (10:1 and 5:1), SHP-1+/+, SHP-1+/− and SHP-1−/− cells lysed the majority of FBL tumor. However, as the effector to tumor cell ratio decreased, the enhanced cytolytic activity of SHP-1−/− T cells became evident (Fig. 2G). To investigate the mechanism of enhanced CTL activity in the absence of SHP-1, we compared expression of effector molecules, Granzyme B and Perforin, and of transcription factors implicated in effector cell T differentiation and function, Eomesodermin (Eomes) (48, 49), T-bet (50) and Runx-3(49). A higher percentage of SHP-1−/− effectors expressed the transcription factor Eomes (Fig. 2H), and the MFI of Eomes was also significantly higher in SHP-1−/− effector cells, indicating a higher level of Eomes expression on a per cell basis (Fig. 2I). T-bet and Granzyme B protein were expressed at similar levels in SHP-1+/+ and SHP-1−/− effector cells, but a modest significant (P=0.0165, n=4) increase in the expression of Perforin protein was detected in the SHP-1−/− cells as compared to wild type effectors (Fig. 2H). Consistent with the protein analysis, real-time PCR showed that SHP-1−/− effectors expressed 3-fold more Eomes mRNA and 2-fold more Perforin mRNA as compared to SHP-1+/+ effectors (Fig. 2J). Both Runx-3 and T-bet mRNA were increased in wild type and knockout effector T cells as compared to naïve T cells, but expression levels of these genes did not differ between SHP-1−/− and SHP-1+/+ effectors (data not shown). Thus, the enhanced CTL activity of SHP-1−/− effector cells may partially reflect higher expression levels of Eomes, a transcription factor that has been shown to promote the lytic program in CD8 T cells (48, 51).
Abrogating SHP-1 expression increases therapeutic efficacy in adoptive immunotherapy of disseminated leukemia in mice
We utilized our preclinical mouse model of therapy for disseminated FBL leukemia (52, 53) to determine if abrogating SHP-1 in tumor-specific T cells improves therapeutic activity of T cells during adoptive immunotherapy of cancer. B6 mice were injected with a lethal dose of FBL leukemia, which expresses the gag epitope (35), and five days later, after the leukemia is disseminated, cyclophosphamide (Cy, 180 mg/kg) was administered, which reduces tumor burden and renders mice lymphopenic. After 6 h, Thy1.1+ TCRgag SHP-1+/+, SHP-1+/− or SHP-1−/− 3stim effectors (1×105 cells) were transferred into cohorts of tumor-bearing mice. Administration of exogenous IL-2 (104 U/mouse daily X 10 d) following T cell transfer is required to promote in vivo persistence, expansion, and therapeutic activity of wild type effector T cells in the disseminated FBL leukemia model (54). However, transferred TCRgag SHP-1−/− effectors, in distinction to the other cell types, prolonged survival and cured 50% of treated mice in the absence of providing exogenous IL-2 at this cell dose (Fig. 3A). Providing exogenous IL-2 enhanced the therapeutic activity of all of the transferred cells, but mice treated with TCRgag SHP-1−/− effectors still exhibited significantly enhanced survival compared to SHP-1+/+ and SHP-1+/− cells (Fig. 3A, right panel). We stained blood mononuclear cells with antibodies to CD8 and Thy1.1 to determine the percentage of donor T cells in the blood in therapy recipients at 7 days after transfer. Both in the presence and absence of exogenous IL-2, there was an increase in the percentage of TCRgag SHP-1−/− cells in the blood compared to wild type TCRgag cells (Fig. 3B, P<0.001), which correlated with a 2-fold decrease in the percentage of SHP-1−/− effector cells that were Annexin-V positive compared to SHP-1+/+ cells (Fig. 3C). Cells with a partial deficiency in SHP-1 (SHP-1+/−) were indistinguishable from wild type cells indicating that greater than a 50% reduction of SHP-1 in donor T cells is required for the enhanced therapeutic effect.
Figure 3.
Abrogation of SHP-1 in TCRgag in vitro-expanded effector cells improves therapeutic outcome of adoptive immunotherapy of disseminated FBL leukemia. A, B6 mice (Thy1.2) were injected with 5×106 FBL leukemia. After 5 days when the leukemia had disseminated, mice were injected with Cy (180 mg/kg), followed by 1×105 Thy1.1+ TCRgag SHP-1+/+, SHP-1+/− or SHP-1−/− 3stim effector cells. The right panel depicts T cell recipients that also received exogenous IL-2 (104 U/mouse/day i.p. for 10 days beginning on the day of T cell transfer). B, At 7 days after T cell transfer, mononuclear blood cells were isolated from therapy recipients and analyzed for the percentage of donor cells (CD8+ Thy1.1+) by FACs. Numbers in the FACs plots indicate the percentage of CD8+ Thy1.1+ cell of the total percentage of CD8+ cells. Data is representative of 12–15 mice per group. C, At 7 days post T cell transfer, CD8+ Thy1.1+ cells isolated from the blood of Thy1.2+ recipient mice (without exogenous IL-2) were analyzed for apoptosis by Annexin-V staining. Histograms are representative of 3–5 mice per group and gated on CD8+ Thy1.1+ cells. D, The percentage of CD8+ Thy1.1+ cells in the blood of therapy recipients was determined by FACs at the indicated timepoints (n=8–12 mice per group)
We tracked the percentage of donor T cells by staining peripheral blood mononuclear cells with antibodies to CD8 and Thy1.1 to investigate if SHP-1 influenced the contraction and/or persistence of effector T cells. In recipients that did not receive IL-2, the percentage of SHP-1+/+ and SHP-1−/− cells was similar at day 30 and undetectable by FACs by day 60 (Fig. 3D). In mice that received IL-2 injections, there were a significantly higher percentage of TCRgag SHP-1−/− effector cells compared to wild type cells 30 days after T cell transfer (P<0.01), consistent with the enhanced expansion resulting from both abrogation of SHP-1 expression and administration of IL-2 during adoptive immunotherapy (Fig. 3D, right panel). However, even in the presence of exogenous IL-2, donor cells could not be readily detected at 60 days after transfer of 1×105 effectors. Thus, abrogating SHP-1 expression in effector T cells appeared to increase the efficacy of adoptive immunotherapy by enhancing the short-term accumulation of effector cells.
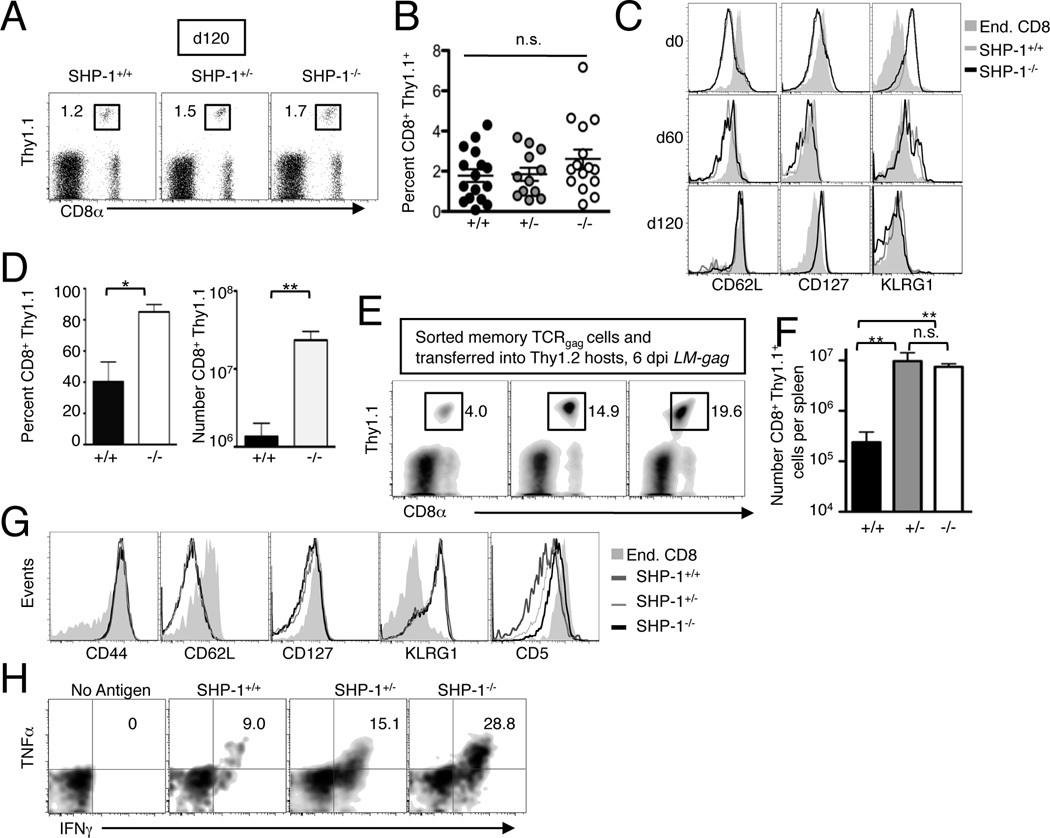
Effector T cells persist long-term and convert to memory T cells independent of SHP-1 during adoptive immunotherapy of cancer
The ability of donor T cells to persist after transfer into tumor-bearing hosts is a major determinant for sustained anti-tumor activity (55) and strategies that enhance T cell persistence during immunotherapy are avidly being pursued (56). The transfer of a 1×105 effector T cells revealed that SHP-1 ablation improves therapeutic efficacy of adoptive immunotherapy of leukemia by enhancing the short-term accumulation of effector T cells (Fig. 3), but did not directly address if SHP-1 abrogation impacts long-term T cell persistence since both wild type and SHP-1−/− cells were largely undetectable at 60 days post transfer. As the ability to detect persisting T cells reflects in part the number of effector cells initially transferred, we infused 50-fold more 3stim effectors (5×106) into tumor-bearing mice treated with Cy prior to transfer, and IL-2 was injected after the cell infusion. With this therapeutic regimen, all recipients survived disseminated tumor. At 120 days post T cell transfer, the CD8+ Thy1.1+ donor T cells in the blood were readily detectable in recipients of either SHP-1+/+, SHP-1+/− or SHP-1−/− cells (Fig. 4A) and there were no significant differences in the percentage (Fig. 4B) or number (data not shown) of T cells. We compared the nature of persisting cells by staining donor cells for markers indicative of effector, effector memory or central memory phenotypes at 60 and 120 days post transfer. Despite initial transfer of a uniform population of phenotypic effector T cells (CD62Llo, CD127lo, KLRG1hi), at 60 days after transfer donor cells expressed intermediate levels of CD62L and CD127 and the majority were KLRG1lo (Fig. 4C). By 120 days, donor T cells were CD62Lhi, KLRG1lo and CD127hi, a phenotype more consistent with central memory cells. To determine if the persisting T cells had the capacity to proliferate to antigen presented in an immunogenic context, we immunized mice that had been cured of tumor with an attenuated recombinant Listeria monocytogenes engineered to express the gag epitope (LM-gag). The percentage of TCRgag SHP-1+/+, SHP-1+/− and SHP-1−/− donor cells (CD8+ Thy1.1+) increased in response to LM-gag immunization, indicating that the memory cells generated following tumor elimination from transferred effectors have the capacity to proliferate upon antigen encounter (Fig. 4D). To determine if SHP-1 regulated the generation of effector cells from these persisting memory cells, we transferred 1×104 purified Thy1.1+ CD8+ SHP-1+/+, SHP-1+/− and SHP-1−/− cells (obtained at 120 days after therapy) into Thy1.2+ hosts. Two days later, these recipients were immunized with LM-gag. At the peak of the response, a significantly higher percentage (Fig. 4E) and number (Fig. 4F) of SHP-1+/− and SHP-1−/− cells compared to wild type T cells were detected in the boosted response indicating that even a 50% reduction in SHP-1 has a biological effect on the response of memory T cells. The vast majority of memory T cells responding to LM-gag immunization acutely converted to an effector phenotype (CD62Llo, KLRG1hi, CD127lo, Fig. 4G) with the only phenotypic difference detected among the groups being slightly higher levels of CD5 expression on SHP-1−/− cells compared to SHP-1+/+ cells (Fig. 4G). A higher percentage of SHP-1−/− cells secreted IFNγ and TNFα compared to wild type cells, indicating that the quality of the expanded effector response post immunization was enhanced in the absence of SHP-1 (Fig. 4H). Thus, SHP-1 ablation does not impact the ability of in vitro-expanded effector T cells to convert to long-lived memory cells following immunotherapy, but does regulate the ability of those memory cells to generate accumulation of effector cells following antigen encounter.
Figure 4.
SHP-1 abrogation does not impact the formation of memory after immunotherapy, but does enhance the expansion of effector cells from memory cells in response to antigen. B6 mice were injected with 5×106 FBL, and after 5 days, received Cy (180 mg/kg) and 6 h later were injected with 5×106 TCRgag SHP-1+/+, SHP-1+/− and SHP-1−/− 3stim effectors and IL-2 (104 U/mouse/day i.p. for 10 days). A, At 120 days after T cell transfer, spleens were harvested from recipient mice and stained for CD8+ Thy1.1+ donor cells and analyzed by FACs. The number in the FACs plots indicates the percentage of donor T cells of total mononuclear splenocytes. B, The percentage of CD8+ Thy1.1+ donor cells in the spleens of recipient mice was determined by FACs (100–120 days after T cell transfer). Each dot represents an individual mouse. C, The phenotype of persisting cells at 60 and 120 days post transfer was determined by staining splenic cells with antibodies to CD8, Thy1.1 and the indicated markers. Histograms are gated on CD8+ Thy1.1+ cells. D, Cured mice (120 days after T cell therapy) were immunized with LM-gag (3×107 c.f.u) and 6 days later, splenocytes were analyzed for the percentage (left panel) and number (right panel) of CD8+ Thy1.1+ T cells. E, Persisting TCRgag SHP-1+/+, SHP-1+/− and SHP-1−/− cells isolated from cured mice were purified using Thy1.1 magnetic beads and aliquots of 1×104 CD8+ Thy1.1+ cells were transferred into naïve B6 recipients. Two days later, recipients were immunized with LM-gag. After 6 days, spleens were harvested and analyzed by FACs for the percentage (E, representative plots) and number (F) of CD8+ Thy1.1+ cells. G, Splenocytes from LM-gag immunized mice in (E) were stained for CD8, Thy1.1 and the indicated antigens to compare the phenotype of expanded effector cells. Histograms are representative mice and are gated on CD8+ Thy1.1+ cells. H, Splenocytes isolated at 6 days post immunization with LM-gag were restimulated with antigen and the protein transport inhibitor Brefeldin-A in vitro. After 5 h, cells were stained for CD8, Thy1.1 and for intracellular expression of IFNγ and TNFα. Plots are gated on CD8+ Thy1.1+ cells. Representative plots shown from n=5. Statistical analysis between the groups was conducted using a one-way ANOVA followed by a Tukey post-hoc correction.
Enhanced short-term accumulation of TCRgag SHP-1−/− cells is not associated with autoimmunity in mice that express the gag tumor antigen as a self-antigen in the liver
We have previously described tg mice that express the FBL gag tumor antigen as a self-antigen in the liver (Alb:Gag mice) (36), and have utilized the Alb:Gag mice as hosts for T cell therapy to better model human cancer in which the tumor antigens are often also self-antigens. Transfer of naïve TCRgag cells into Alb:Gag mice results in the rapid deletion and/or tolerization of the T cells (10, 35, 57), but expression of the gag antigen in the liver does not preclude therapeutic activity when high numbers (1×107) of in vitro-expanded TCRgag effector cells are transferred into Alb:Gag hosts with disseminated leukemia in the presence of exogenous IL-2 (36). Therefore, we transferred 5×106 in vitro-expanded TCRgag SHP-1+/+ and SHP-1−/− effectors into B6 or Alb:Gag mice with disseminated leukemia treated with Cy (as described in Figure 4) and administered exogenous IL-2 after transfer to evaluate if abrogation of SHP-1 in tumor-reactive T cells that can potentially also recognize normal tissues influenced therapeutic efficacy in this setting and/or induced autoimmune toxicity. Seven days after the infusion of effector T cells, we stained blood mononuclear cells from control B6 or Alb:Gag recipients with antibodies to CD8 and Thy1.1. A significantly higher percentage of TCRgag SHP-1−/− cells compared to wild type effectors were found in both B6 and Alb:Gag recipients (Fig. 5A, B). Independent of SHP-1, there was a modest, but not statistically significant, reduction in the percentage of effector T cells in Alb:Gag hosts (Fig. 5B), which correlated with less therapeutic activity (Fig. 5C). TCRgag SHP-1−/− cells had enhanced therapeutic activity compared to TCRgag SHP-1+/+ cells in Alb:Gag mice treated with exogenous IL-2 (Fig. 5C). Additionally, ablating SHP-1 reduced the difference in anti-tumor activity of effector CTL in hosts that express the tumor antigen as a self-antigen as compared to wild type hosts in which the gag tumor antigen is not expressed in self-tissue (Fig. 5C, P<0.05). Thus, while recognition of the self-antigen is likely promoting deletion of a portion of the transferred T cells, the benefit of the abrogation of SHP-1 continues to be sustained in the presence of self-antigen.
Figure 5.
Abrogation of SHP-1 still improves efficacy of adoptive immunotherapy when the tumor antigen is also expressed as a self-antigen in the liver. B6 and Alb:Gag mice (Thy1.2+) were injected with 5×106 FBL leukemia. After 5 days, mice were injected with Cy (180 mg/kg) and 5×106 Thy1.1+ TCRgag SHP-1+/+ or SHP-1−/− 3stim effectors. Recipients also received exogenous IL-2 (104 U/mouse/day i.p. for 10 days). A, At 7 days after T cell transfer, mononuclear blood cells were isolated from therapy recipients and analyzed for the percentage of CD8+ Thy1.1+ cells by FACs. Numbers in the FACs plots indicate the percentage of CD8+ Thy1.1+ cell of the total blood mononuclear cells. Data is representative of 10–12 mice per group. B, The percentage of donor T cells in the blood 7 days after transfer was significantly different between recipients of SHP-1+/+ and SHP-1−/− T cells in both wild type and Alb:Gag mice (P<0.05). C, Survival of B6 and Alb:Gag therapy recipients of TCRgag SHP-1+/+ and SHP-1−/− cells.
The abrogation of SHP-1 in persisting transferred effector CTL could potentially result in autoimmune toxicity to the liver cells that express the gag epitope. However, the Alb:Gag recipients of TCRgag SHP-1−/− cells that were cured of tumor remained healthy without overt clinical systemic signs of autoimmune disease. At the time of T cell transfer, serum levels of the liver enzymes AST and ALT, indicators of liver injury, were found to be ~2-fold elevated in tumor-bearing mice due to the fact that leukemia is disseminated in multiple tissues, including the liver (data not shown). Two days after transfer, liver enzymes were increased 3–4 fold in recipients of either SHP-1+/+ or SHP-1−/− cells, and by 15 days after transfer, the levels of these enzymes had resolved to approximately 1.5 fold as compared to normal Alb:Gag mice. Histological analysis of liver sections on day 12 after T cell transfer also revealed no evidence of lymphocytic cellular infiltration or injury to hepatocytes engineered to express the gag antigen (Supplemental Figure 2). Thus, abrogation of SHP-1 in CTLs still increases the therapeutic efficacy of adoptive immunotherapy in hosts in which the tumor antigen is expressed as a self-antigen in the liver, and does not necessarily increase the likelihood for autoimmune toxicity.
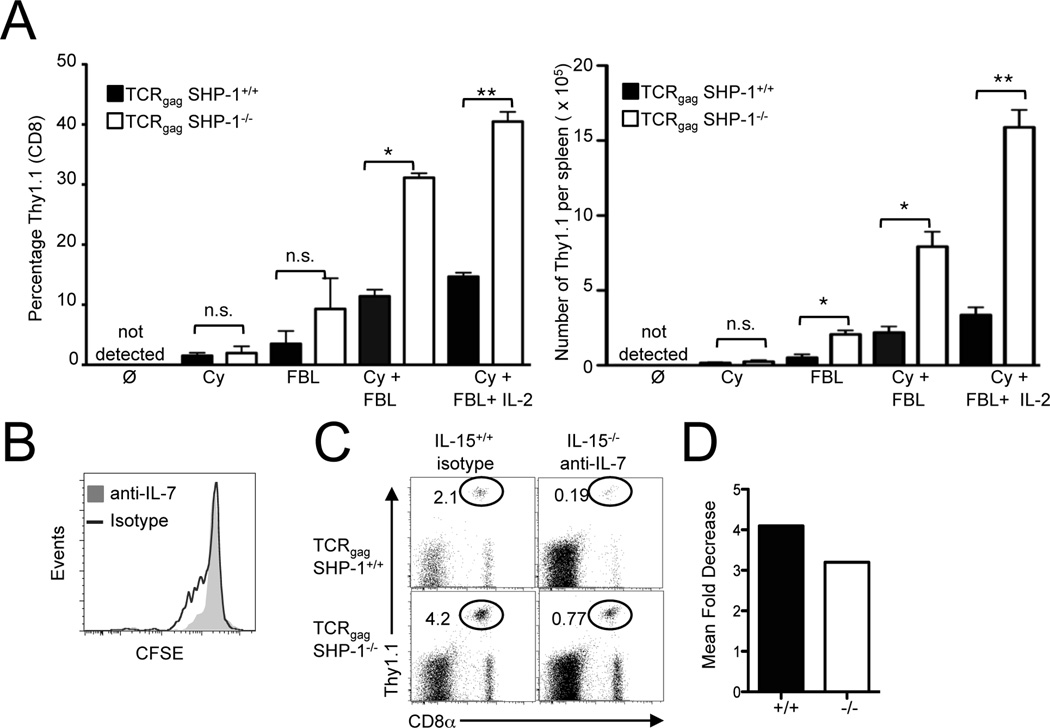
The enhanced accumulation of TCRgag SHP-1−/− effectors during therapy is antigen-dependent and enhanced in mice rendered lymphopenic
The relative differences in the accumulation of SHP-1−/− compared to SHP-1+/+ effectors in vivo were much more pronounced than what we had initially observed in our in vitro studies. This disparity suggested that environmental factors in vivo might be contributing to the increased short-term accumulation of SHP-1−/− effector cells. Lymphodepletion enhances the anti-tumor effects of T cells during T cell-based immunotherapy at least in part, by increasing serum levels of homeostatic cytokines IL-7 and IL-15 in both mouse tumor models and in patients with cancer (4, 7). Since our adoptive immunotherapy protocol transferred T cells into mice rendered lymphopenic by Cy, we hypothesized that abrogation of SHP-1 might also be enhancing signaling from the available cytokines in vivo such as IL-7 and/or IL-15 (58). We transferred 1×105 SHP-1+/+ or SHP-1−/− in vitro-expanded effectors into either naïve B6 mice (negative control), B6 mice rendered lymphopenic from Cy, B6 mice that received irradiated FBL tumor (antigen), or B6 mice that received irradiated FBL antigen in the context of Cy-induced lymphopenia. SHP-1+/+ and SHP-1−/− effectors were not detected 6 days after transfer into naïve B6 mice, but were both detected at similarly low frequencies in the spleen 7 days after transfer into mice rendered lymphopenic by Cy (Fig. 6A). These results suggest that the lymphopenia-dependent accumulation of effector cells in the absence of an antigenic signal, at least in this time frame studied, is not regulated by SHP-1. In contrast, a higher percentage and number of SHP-1−/− effector cells were detected in response to antigen alone compared to SHP-1+/+ T cells resulting in approximately a 3-fold difference in the number of T cells (Fig. 6A). Antigen in a lymphopenic setting amplified the expansion of both SHP-1+/+ and SHP-1−/− T cells, similar to what is observed in a therapeutic setting, and also resulted in a 3-fold increase in the number of SHP-1−/− compared to SHP-1+/+ T cells. Even in the context of antigen and lymphopenia, exogenous IL-2 administration still enhanced proliferation of SHP-1+/+ and SHP-1−/− effector cells (Fig. 6A). To determine if SHP-1−/− effector cells depended on the homeostatic cytokines IL-7 and IL-15 for the enhanced antigen-dependent expansion in a lymphopenic setting, we transferred effector T cells into Cy-treated, FBL bearing IL-15−/− mice that received injections of a mouse anti-human monoclonal (mAb) antibody specific to human IL-7 that cross reacts and neutralizes murine IL-7 (M25) (59). Since IL-7 is critical for naïve T cell homeostasis (38), we first verified that the anti-IL-7 treatment neutralized the biologically active IL-7 in these experiments by transferring naïve, CFSE-labeled Thy1.1+ TCRgag cells into wild type mice treated with either anti-IL-7 or an isotype control. The administration of anti-IL-7 inhibited the low level of homeostatic proliferation of naïve TCRgag cells, as measured by the absence of CFSE-dilution by donor T cells, indicating the dose of anti-IL-7 administered was sufficient to neutralize IL-7 in vivo (Fig. 6B). Therefore, we transferred wild type and SHP-1−/− effector cells into FBL-bearing, Cy-treated IL-15−/− mice that also received anti-IL-7. After seven days, we determined the percentage of donor cells (CD8+ Thy1.1+) in the blood by FACs. The absence of IL-7 and IL-15 reduced the percentage and number of both SHP-1+/+ and SHP-1−/− effector T cells present at day 7 (Fig. 6C, data not shown) resulting in a 3–4 fold decrease in the acute expansion of both wild type and SHP-1−/− cells (Fig. 6D). Thus, similar to wild type cells, SHP-1−/− T cells also depended on IL-7 and IL-15 homeostatic cytokine signals in vivo for their proliferation and survival, suggesting that abrogation of SHP-1 amplifies in vivo responses to antigen but does not bypass the requirement for cytokine signals for anti-tumor activity.
Figure 6.
The enhanced expansion of SHP-1−/− effector T cells in vivo depends on antigen and is enhanced in a lymphopenic setting. A, To temporarily induce lymphopenia, cohorts of B6 mice were administered Cy (180 mg/kg) ± irradiated FBL (1×107) and exogenous IL-2 (104 U/mouse/day) as indicated. Treated mice received 1×105 of TCRgag 3stim SHP-1+/+ or SHP-1−/− effector cells. After 6 days, splenocytes were harvested and the percentage (left panel) and number (right panel) of donor CD8+ Thy1.1+ donor cells was calculated. B, CFSE-labeled, naive Thy1.1+ TCRgag cells (5×106) were transferred into wild type B6 mice treated with the IL-7 depleting monoclonal antibody M25 (1 mg/mouse every other day i.p.), or an isotype control. After 10 days, splenocytes were harvested, stained for CD8 and Thy1.1 and CFSE dilution of the CD8+ Thy1.1+ cells assessed by FACs. C, Wild type and IL-15−/− mice received Cy (180mg/kg), irradiated FBL (1×107 cells per mouse) ± anti-IL-7 depleting antibody (M25) and were administered 1×105 3stim effectors. The percentage of CD8+ Thy1.1+ cells in the spleen 6 days after T cell transfer was determined by FACs. Representative plots are shown (n=5–8 mice per group). D, The relative impact of abrogating homeostatic cytokine signals IL-7 and IL-15 in the expansion of 3stim SHP-1+/+ or SHP-1−/− effectors was determined by calculating the fold decrease in the number of donor T cells recovered from recipients that lacked IL-7 and IL-15 signals as compared to the number of effector cells in control mice (the number of donor T cells from isotype treated B6 mice/ the number of donor T cells from M25 treated IL-15−/− treated mice).
Targeting SHP-1 expression in tumor-reactive T cells by retroviral transduction of SHP-1-specific shRNAs
Translating these findings from the mouse model to clinical studies will require the ability to reduce SHP-1 expression in effector T cells specific to tumor antigens. One approach to reduce SHP-1 protein expression in tumor-reactive T cells for therapy would be to transduce T cells in vitro with retroviral vectors engineered to express shRNAs targeting the specific protein prior to T cell infusion. To investigate the feasibility, efficiency and biologic impact of reducing SHP-1 levels by shRNA-mediated gene silencing in T cells, we targeted abrogation of SHP-1 expression in TCRgag cells by transducing in vitro activated TCRgag effector cells with a retroviral construct that expresses a SHP-1 specific shRNA specific for a conserved sequence in SHP-1. This sequence, when used as an siRNA, has previously been shown to reduce SHP-1 expression in both human and mouse cells (29, 40, 41). Transfection of EL-4 thymoma cells with this specific siRNA sequence effectively knocked down SHP-1 by ~80% (data not shown). Therefore, we constructed a retroviral vector to constitutively express SHP-1 shRNA and green fluorescent protein (GFP) for in vitro sorting and in vivo tracking of transduced effector T cells. Wild type TCRgag cells were stimulated in vitro with peptide-pulsed splenocytes and IL-2 (20 U/ml). After 2 days, during active cell division, the proliferating wild type TCRgag cells were retrovirally transduced and the T cells analyzed for GFP expression (Fig. 7A). By gating on the CD8+ GFP+ cells, we found that cells transduced with the retrovirus that expressed the SHP-1-specific siRNA had reduced levels of SHP-1 (66% average reduction) as compared to cells transduced with a control vector containing a scramble siRNA sequence (Fig. 7B, right panel). On a per cell basis, some GFP+ T cells had greatly reduced SHP-1 expression while others exhibited less reduction of SHP-1 (Fig. 7B), presumably reflecting influences of integration sites and indicating that this approach does not generate a uniform reduction of SHP-1 protein levels. TCRgag cells transduced with SHP-1 shRNA retrovirus displayed an enhanced ability to lyse FBL tumor in vitro using a standard CFSE-based cytotoxicity assay (Fig. 7C). To compare the ability of transduced TCRgag cells to acutely expand in response to FBL in vivo, we transferred 6×104 SHP-1 shRNA transduced effector cells that had been expanded in vitro for an additional 2 cycles of stimulation into recipients that were treated with Cy 5 h prior to T cell transfer and irradiated FBL at the time of T cell transfer. There was a 3-fold increase in the percentage of TCRgag cells expressing the targeted shRNA in the blood compared to TCRgag cells transduced with a control retrovirus that expressed a non-specific scramble siRNA sequence (Fig. 7D). Thus, the retroviral transduction of effector CTL with SHP-1-specific shRNA can improve effector T cell responses to tumors and represents a novel strategy that may improve anti-tumor activity of effector T cells in humans with established malignancies.
Figure 7.
Retroviral transduction of TCRgag cells with SHP-1-specific shRNA reduces SHP-1 expression and improves effector T cell function in response to leukemia. A, Naïve TCRgag cells isolated from wild type tg mice were retrovirally transduced with a MigR1 retroviral vector engineered to express SHP-1-specific shRNA or a control scramble shRNA sequence and GFP under control of the U6 promoter. After 5 days, cells were stained for CD8 and analyzed for expression of GFP. B, SHP-1 levels were determined by FACs analysis of the gated CD8+ GFP+ T cells that had been stained intracellularly with a SHP-1-specific monoclonal antibody. C, Retrovirally transduced TCRgag CD8+ GFP+ 3stim effectors (6×104) were transferred into recipient mice that received Cy (180 mg/kg) and irradiated FBL (1×107 cells) prior to transfer. After 7 days, the percentage of CD8+ Thy1.1+ donor cells of the total mononuclear blood cells was determined by FACs. Each circle represents an individual mouse. Statistical analysis was performed using a student’s T test to compare cell expansion. D, CFSE-based cytotoxicity assays were performed by incubating retrovirally transduced TCRgag 3stim effectors at a 1:1 ratio with a mixture of FBL (CFSEhi) and control tumor EL4 (CFSElo). After 5 h, the ratio of FBL tumor to control tumor was compared by FACs analysis. These data are representative of two independent experiments.
Discussion
This study describes the potential value of disrupting the regulatory signaling pathways mediated by SHP-1 as a strategy to improve the efficacy of adoptive T cell therapy of cancer. We utilized a model of cell-intrinsic SHP-1 deficiency in mature T cells to overcome major limitations of studying T cells derived from mice globally deficient for SHP-1. We found that the abrogation of SHP-1 in tumor-specific, effector T cells significantly improved the efficacy of the T cells in therapy of disseminated leukemia, which in part reflected an increase after transfer in the in vivo expansion and accumulation of short-lived effector T cells. This rapid expansion after transfer of short-lived effector cells derived from the transferred SHP-1-deficient population did not impact the ability of donor T cells to establish a functional long-lived memory T cell population after successful tumor elimination.
A key obstacle to the success of adoptive immunotherapy of cancer is the low level of antigen presentation by tumors. Malignant cells often have defects in antigen processing and presentation (60) including the down-regulation or complete loss of MHC molecules (61), resulting in the lack of T cell recognition. We investigated the impact of abrogating SHP-1 in tumor-reactive T cells, in part, because of the described role for SHP-1 in serving as a rheostat for regulating antigen signaling in T cells. SHP-1 is rapidly recruited to lipid rafts after TCR binding to low avidity peptide/MHC complexes, resulting in the rapid dephosphorylation of Lck and the prevention of potential detrimental activation of T cells directed towards low avidity interactions such as self antigens (17, 18). After TCR binding to higher avidity ligands, SHP-1 is also recruited to the TCR complex potentially turning off prolonged responses to antigen (63). Our observation that naive T cells with cell intrinsic SHP-1 deficiency have enhanced functional avidity, as measured by proliferation in response to a log lower concentration of peptide-loaded targets, suggests that one approach to engineering T cells to respond better to reduced antigen levels would be to ablate SHP-1 expression. These data are consistent with a previous study that demonstrated SHP-1 regulates cell cycle entry after antigen encounter in part by diminishing T cell-APC conjugate formation in vitro (28).
Strategies to enhance T cell avidity and function may overcome obstacles associated with T cell tolerance and dysfunction during adoptive immunotherapy. However, since most tumor antigens are also expressed to some degree in normal tissues, one unintended consequence of increasing T cell function could be immune-mediated toxicity directed at self tissues (62). The death of a patient shortly after receiving T cells engineered to express a high affinity chimeric antigen receptor (CAR) specific to ERBB2 (HER-2/neu), a tumor antigen overexpressed in malignant cells but also expressed at low levels in the lung, was likely the result of T cell recognition of ERBB2 expressed by normal lung cells resulting in a cascading cytokine storm and multiorgan failure (63). The affinity and signaling capabilities of CARs, which are a fusion protein composed of an extracellular Fv fragment and often multiple intracellular signaling domains (CD28, 4-1BB, and CD3ζ), are dramatically different than TCRs. The ablation of SHP-1 in the infused effector CD8 T cells that express TCRs specific to the gag self/tumor antigen enhanced tumor immunity without causing autoimmunity when the self-antigen was expressed in the liver. Similarly, we previously observed that abrogation of another negative regulator of TCR signaling, Cbl-b, in tumor-reactive effector T cells also enhanced therapeutic efficacy of adoptive immunotherapy of FBL leukemia without increasing the likelihood for toxicity in recipient mice that expressed the gag tumor antigen as a self antigen in the liver (14). The lack of autoimmunity cannot be explained by insufficient antigen expression in hepatocytes to be recognized, because naïve TCRgag cells transferred into Alb:Gag mice have been shown to directly recognize hepatocytes, although the outcome of this event is tolerance induction or deletion (57, 64). As hepatocytes are inherently resistant to cytotoxic T cell-mediated killing (65, 66) and the liver may be a more naturally immunosuppressive environment than other organs (67), ablation of SHP-1 might increase autoimmunity if the self/tumor antigen was expressed in tissues other than the liver. However, as autoimmune injury in the liver has been observed in other settings, the liver is not completely resistant to autoimmune damage (68–71). Although additional studies will be required to extrapolate the impact of abrogating SHP-1 during T cell based therapy in settings in which the self/tumor antigen is expressed on other tissues, the current data demonstrate that toxicity is not an inherent consequence of increasing effector T cell function during adoptive immunotherapy, even if the tumor antigen is a self-protein.
Various labs are exploring the impact of inclusion of CD4 T cells in therapeutic efficacy and safety during T cell based therapy of cancer (84). SHP-1 may regulate various functions in CD4 T cells, and abrogation of SHP-1 has been reported to bias naïve CD4 T cells toward a Th2 (85) or Th17 helper phenotype (86). Abrogating SHP-1, or other negative regulators in CD4 helper T cells, would certainly have the potential to further increase the therapeutic activity of CD8 T cells, but may also potentially increase the likelihood for autoimmune injury. A greater understanding of how abrogation of SHP-1 in CD4 T cells specific to tumor antigens influences the therapeutic efficacy and toxicity could potentially lead toward novel strategies that target malignancies.
In order to model human adoptive immunotherapy protocols, we expanded naïve CD8 T cells with antigen and IL-2 for several cycles of stimulation prior to transfer, producing a uniform population of cells that phenotypically resembled short-lived effector cells (CD44hi, CD62Llo, CD127lo, KLRG1hi) independent of SHP-1 expression. Although KLRG-1 has been suggested to be a marker of terminally differentiated short-lived effector cells (SLECs) in mouse models of virus infection(50), we found that KLRG1hi effector T cells can eradicate disseminated tumor. Although the majority of transferred effectors undergo programmed cell death, at least a minor fraction eventually transition to cells with phenotypic and functional characteristics of central memory cells, again independent of SHP-1 expression. Such plasticity of in vitro expanded CD8 effector cells from apparent differentiated effector cells to memory cells has also been reported in humans and animal models (72, 73). The finding that SHP-1 ablation, which increases the sensitivity of TCR signaling to antigen, also increases the abundance of SLECs without impacting memory precursor cells (MPECs) is not entirely consistent with a proposed model suggesting less TCR activation favors memory precursor cell survival and formation, while a stronger TCR signal favors effector differentiation (74). Potentially SHP-1 deficiency may impact subpopulations of the responding cells differently, such as driving an enhanced proliferative response by the population that differentiates to SLECs. The amount of IFNγ production induced shortly after T cell activation (20–24 h) has been shown to depend on the TCR signal strength (ie., the amount of MHC-peptide expressed on the cell surface), and triggering by targets with high antigen densities led to the development of polyfunctional effector cells in the IFNγhi clonal progeny (75). SHP-1−/− effector cells were found to produce ~ 5-fold greater amount of IFNγ 24 h after TCR stimulation as compared to SHP-1+/+ cells (data not shown), suggesting that abrogation of SHP-1 may result in cells with enhanced effector functions.
We observed a modest, but reproducible increase in expression of the transcription factor Eomes in SHP-1−/− effectors shortly after activation, as compared to wild type cells, which may also contribute to the increased cytolytic activity of SHP-1−/− effectors as Eomes has been described to be involved in the cytolytic program of both CD4 and CD8 T cells (48, 49). The increase in Eomes in SHP-1−/− cells was evident in T cells that were stimulated with antigen in the presence and absence of exogenous IL-2, suggesting increased Eomes is not a result of greater proliferation but rather the specific lack of SHP-1 after antigen encounter (data not shown). It is surprising that we did not observe any difference in T-bet levels in SHP-1−/− effectors, at any timepoint tested (days 1, 3, 5 and 7 after primary activation, and day 5 after the 3rd in vitro stimulation with antigen, Fig. 2 and data not shown), yet still observed enhanced IFNγ production in the absence of SHP-1 at early timepoints (day 1 after activation). The enhanced cytolytic capacity of SHP-1−/− effector T cells was apparent at high tumor densities, and correlated with a modest but significant increase in Perforin expression, but did not correlate with an increase in Granzyme A, B or C expression or Fas and FasL expression. These results suggest that while the increase in Perforin expression may contribute to the enhanced lytic capacity of SHP-1−/− effectors, it also remains a likely possibility that the capacity to deliver cytoxic molecules after TCR signaling is also improved in the absence of SHP-1. SHP-1 interfaces with multiple pathways, including key TCR signaling intermediates Lck (21, 22), Zap70 (23, 24), Vav (25), PI3K (26) and TCRζ (27). Defining precisely how the abrogation of SHP-1 enhances cytolytic activity will require further investigation.
The increase in the generation and expansion of SHP-1−/− effector cells derived initially from the transferred tumor-specific in vitro-generated effector cells and subsequently from the in vivo persisting memory cells are consistent with a recent study from our lab showing that SHP-1 limits the accumulation of virus-specific SLECs derived from both naïve and memory precursors after acute LCMV infection in mice (30). The increase in effector cells at peak time points in response to disseminated tumor in the current study could not be attributed to SHP-1-mediated regulation of the differentiation of SLECs from naïve precursors, indicating that SHP-1 can also act at the effector T cell stage to limit cell expansion and survival. In both the setting of LCMV infection and in the current analysis of response to tumor antigen, SHP-1 was found to regulate secondary responses to antigen similar to primary responses (~3-fold increase in the absence of SHP-1). However, in both settings, memory cells partially deficient in SHP-1 (derived from heterozygous SHP-1+/− mice) expanded better in response to antigen as compared to wild type T cells, and similar as compared to SHP-1−/− T cells. These results indicate that memory cells are likely more sensitive to changes in SHP-1 levels than naïve T cells. Preliminary studies with therapy by transferring central memory T cells deficient or heterozygous for SHP-1 deficiency suggest that both populations are better than wild type central memory T cells (unpublished data). Thus, the fact that abrogation of SHP-1 enhances the quantity and quality of effector cells derived from distinct cell subsets suggests that targeting SHP-1 may prove useful independent of the CD8 cell subset used for therapy.
Since SHP-1 has been implicated in a variety of cytokine and costimulatory pathways in addition to TCR signaling (CEACAM (40, 42, 76), BTLA (77), PD-1 (78) CD5 (79), IL-10 (80, 81), and TGF-β (82)), ablating SHP-1 likely influences how effector cells respond to suppressive cytokines and/or inhibitory receptor signaling that can also influence the survival and accumulation of effector T cells during adoptive immunotherapy. TGF-β has been shown to limit the number of SLECs formed in response to infection in vivo (83) and SHP-1 has been implicated in transmitting inhibitory signals by TGF-β binding, suggesting that differential signaling in response to TGF-β may influence the survival of SLECs following transfer. The fact that we did not observe any difference in the survival or accumulation or SHP-1+/+ and SHP-1−/− cells shortly after transfer into lymphopenic hosts in the absence of an antigenic signal indicates that TCR signaling is a necessary component of the differential responses, but does not rule out the contribution of additional pathways. Response to lymphopenic cytokines IL-7 and IL-15, were not impaired in the absence of SHP-1, indicating that the absence of SHP-1 does not perturb these vital pathways that impact T cell activity during adoptive immunotherapy and are also important for memory T cell homeostasis. Although we did not observe an increase in proliferation of SHP-1−/− effectors to antigen and IL-2 in vitro, SHP-1−/− effectors responded better than wild type cells in the presence of antigen and exogenous IL-2 in vivo suggesting that the in vitro conditions do not adequately reflect the extent of the biological impact of SHP-1 ablation. It may be that settings with a high tumor burden, in which effector cells are challenged to function and are being induced to undergo apoptosis or become dysfunctional, provide a more discriminating setting for evaluating the role of SHP-1. Indeed, upregulation of SHP-1 has been observed in dysfunctional T cells isolated from solid tumors in mice, and abrogation of SHP-1 in TILs has been shown to restore T cell lytic function in vitro (29). Preliminary studies in our lab using a solid tumor model have further suggested that abrogating SHP-1 may be useful for overcoming some of the obstacles associated with adoptive immunotherapy of solid tumors, such as effector T cell accumulation.
Precisely how much SHP-1 protein levels will need to be reduced to have a beneficial impact on effector T cell activity is not yet defined. Previous studies have transiently reduced SHP-1 protein levels by transient transfection of T cells (29, 40, 41), but we are currently not aware of any studies that have permanently reduced SHP-1 levels in effector T cells. Our observation that retroviral transduction of tumor-reactive T cells with viral vectors engineered to express a SHP-1-specific shRNA can stably knockdown a sufficient amount of SHP-1 protein in wild type T cells to have a biological impact. Since we obtained a broad range of protein levels in cells in which SHP-1 was reduced after retroviral transduction by expression of an shRNA, we postulate that the T cells that had the most levels of SHP-1 protein reduced are the cells responsible for the increased functional activity. While this study suggests that abrogating SHP-1 for clinical trials should be pursued, translating this strategy would require additional investigation, including the optimization of the retroviral vector and determining if there are more effective SHP-1 sites to target in order to achieve more uniform down-regulation of SHP-1 protein in all transduced T cells. Currently, our laboratory, as well as others, have focused on the genetic modification of T cells to express tumor-reactive antigen receptors (4, 5) to overcome some of the obstacles associated with T cell based therapy of cancer, such as the expression of only low affinity TCRs in naturally derived self/tumor-reactive T cells. The abrogation of SHP-1 in effector T cells is a therapeutic strategy that can be used alone, or in combination with genetic modification of T cells to express antigen receptors, and our data indicate that this approach has the potential to increase therapeutic activity of the T cells in some malignancies.
Supplementary Material
Acknowledgements
We thank L. Pao and B. Neel for the SHP-1 Flox+/+ mice and Amgen (Seattle, WA) for the generous gift of purified M25 monoclonal antibody to IL-7. We thank I. Roberts and H. Nguyen for technical assistance, J. Factor for help preparing the manuscript, and additional members of the Greenberg laboratory for helpful discussion.
1This work was supported by National Institutes of Health/National Cancer Institute Grants R01 CA33084 (to P.D.G), K01 CA117985-01 (to J.N.B. and P.D.G.), and P01 CA18029 (to P.D.G.), a grant from the Leukemia and Lymphoma Society (to P.D.G.), and a grant from the Korea Research Institute of Bioscience and Biotechnology (to P.D.G., T-D.K, and I.C.) 7008-09. I.M.S. received support from the Irvington Institute Fellowship Program of the Cancer Research Institute and a Training Basic and Physician Scientists in Immunology training grant #5T32AI007411.
Abbreviations used in this paper
- SHP-1
Src homology region 2 domain-containing phosphatase-1
- SLEC
short-lived effector cell
- 7-AAD
7-aminoactinomycin D
- dLck
distal Lck
- KLRG1
killer cell lectin-like receptor subfamily G member
- TCRgag
TCR transgenic T cells specific for an epitope derived from the Gag protein of the Friend murine leukemia virus
- FBL
Friend murine leukemia virus
References
- 1.Yee C, Thompson JA, Roche P, Byrd DR, Lee PP, Piepkorn M, Kenyon K, Davis MM, Riddell SR, Greenberg PD. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. J Exp Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitt TM, Ragnarsson GB, Greenberg PD. T cell receptor gene therapy for cancer. Hum Gene Ther. 2009;20:1240–1248. doi: 10.1089/hum.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kammula US, White DE, Rosenberg SA. Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer. 1998;83:797–805. [PubMed] [Google Scholar]
- 7.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, Tan X, Sutton SE, Cooke MP, Ohlen C, Greenberg PD. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 11.Fewkes NM, Mackall CL. Novel gamma-chain cytokines as candidate immune modulators in immune therapies for cancer. Cancer J. 2010;16:392–398. doi: 10.1097/PPO.0b013e3181eacbc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 13.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 14.Stromnes IM, Blattman JN, Tan X, Jeevanjee S, Gu H, Greenberg PD. Abrogating Cbl-b in effector CD8(+) T cells improves the efficacy of adoptive therapy of leukemia in mice. J Clin Invest. 2010;120:3722–3734. doi: 10.1172/JCI41991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009;228:342–359. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson KG, LeRoy FG, Borysiewicz LK, Matthews RJ. TCR signaling thresholds regulating T cell development and activation are dependent upon SHP-1. J Immunol. 1999;162:3802–3813. [PubMed] [Google Scholar]
- 17.Dittel BN, Stefanova I, Germain RN, Janeway CA., Jr Cross-antagonism of a T cell clone expressing two distinct T cell receptors. Immunity. 1999;11:289–298. doi: 10.1016/s1074-7613(00)80104-1. [DOI] [PubMed] [Google Scholar]
- 18.Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 19.Kilgore NE, Carter JD, Lorenz U, Evavold BD. Cutting edge: dependence of TCR antagonism on Src homology 2 domain-containing protein tyrosine phosphatase activity. J Immunol. 2003;170:4891–4895. doi: 10.4049/jimmunol.170.10.4891. [DOI] [PubMed] [Google Scholar]
- 20.Schnell FJ, Alberts-Grill N, Evavold BD. CD8+ T cell responses to a viral escape mutant epitope: active suppression via altered SHP-1 activity. J Immunol. 2009;182:1829–1835. doi: 10.4049/jimmunol.0801798. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz U, Ravichandran KS, Burakoff SJ, Neel BG. Lack of SHPTP1 results in src-family kinase hyperactivation and thymocyte hyperresponsiveness. Proc Natl Acad Sci U S A. 1996;93:9624–9629. doi: 10.1073/pnas.93.18.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang GG, Sefton BM. Specific dephosphorylation of the Lck tyrosine protein kinase at Tyr-394 by the SHP-1 protein-tyrosine phosphatase. J Biol Chem. 2001;276:23173–23178. doi: 10.1074/jbc.M101219200. [DOI] [PubMed] [Google Scholar]
- 23.Plas DR, Johnson R, Pingel JT, Matthews RJ, Dalton M, Roy G, Chan AC, Thomas ML. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 1996;272:1173–1176. doi: 10.1126/science.272.5265.1173. [DOI] [PubMed] [Google Scholar]
- 24.Brockdorff J, Williams S, Couture C, Mustelin T. Dephosphorylation of ZAP-70 and inhibition of T cell activation by activated SHP1. Eur J Immunol. 1999;29:2539–2550. doi: 10.1002/(SICI)1521-4141(199908)29:08<2539::AID-IMMU2539>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Stebbins CC, Watzl C, Billadeau DD, Leibson PJ, Burshtyn DN, Long EO. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol. 2003;23:6291–6299. doi: 10.1128/MCB.23.17.6291-6299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuevas B, Lu Y, Watt S, Kumar R, Zhang J, Siminovitch KA, Mills GB. SHP-1 regulates Lck-induced phosphatidylinositol 3-kinase phosphorylation and activity. J Biol Chem. 1999;274:27583–27589. doi: 10.1074/jbc.274.39.27583. [DOI] [PubMed] [Google Scholar]
- 27.Sozio MS, Mathis MA, Young JA, Walchli S, Pitcher LA, Wrage PC, Bartok B, Campbell A, Watts JD, Aebersold R, Hooft van Huijsduijnen R, van Oers NS. PTPH1 is a predominant protein-tyrosine phosphatase capable of interacting with and dephosphorylating the T cell receptor zeta subunit. J Biol Chem. 2004;279:7760–7769. doi: 10.1074/jbc.M309994200. [DOI] [PubMed] [Google Scholar]
- 28.Sathish JG, Dolton G, Leroy FG, Matthews RJ. Loss of Src homology region 2 domain-containing protein tyrosine phosphatase-1 increases CD8+ T cell-APC conjugate formation and is associated with enhanced in vivo CTL function. J Immunol. 2007;178:330–337. doi: 10.4049/jimmunol.178.1.330. [DOI] [PubMed] [Google Scholar]
- 29.Monu N, Frey AB. Suppression of proximal T cell receptor signaling and lytic function in CD8+ tumor-infiltrating T cells. Cancer Res. 2007;67:11447–11454. doi: 10.1158/0008-5472.CAN-07-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fowler CC, Pao LI, Blattman JN, Greenberg PD. SHP-1 in T cells limits the production of CD8 effector cells without impacting the formation of long-lived central memory cells. J Immunol. 2010;185:3256–3267. doi: 10.4049/jimmunol.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 32.Shultz LD, Rajan TV, Greiner DL. Severe defects in immunity and hematopoiesis caused by SHP-1 protein-tyrosine-phosphatase deficiency. Trends Biotechnol. 1997;15:302–307. doi: 10.1016/S0167-7799(97)01060-3. [DOI] [PubMed] [Google Scholar]
- 33.Khaled AR, Butfiloski EJ, Sobel ES, Schiffenbauer J. Functional consequences of the SHP-1 defect in motheaten viable mice: role of NF-kappa B. Cell Immunol. 1998;185:49–58. doi: 10.1006/cimm.1998.1272. [DOI] [PubMed] [Google Scholar]
- 34.Carter JD, Neel BG, Lorenz U. The tyrosine phosphatase SHP-1 influences thymocyte selection by setting TCR signaling thresholds. Int Immunol. 1999;11:1999–2014. doi: 10.1093/intimm/11.12.1999. [DOI] [PubMed] [Google Scholar]
- 35.Ohlen C, Kalos M, Cheng LE, Shur AC, Hong DJ, Carson BD, Kokot NC, Lerner CG, Sather BD, Huseby ES, Greenberg PD. CD8(+) T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J Exp Med. 2002;195:1407–1418. doi: 10.1084/jem.20011063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohlen C, Kalos M, Hong DJ, Shur AC, Greenberg PD. Expression of a tolerizing tumor antigen in peripheral tissue does not preclude recovery of high-affinity CD8+ T cells or CTL immunotherapy of tumors expressing the antigen. J Immunol. 2001;166:2863–2870. doi: 10.4049/jimmunol.166.4.2863. [DOI] [PubMed] [Google Scholar]
- 37.Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 38.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang DJ, Wang Q, Wei J, Baimukanova G, Buchholz F, Stewart AF, Mao X, Killeen N. Selective expression of the Cre recombinase in late-stage thymocytes using the distal promoter of the Lck gene. J Immunol. 2005;174:6725–6731. doi: 10.4049/jimmunol.174.11.6725. [DOI] [PubMed] [Google Scholar]
- 40.Chen D, Iijima H, Nagaishi T, Nakajima A, Russell S, Raychowdhury R, Morales V, Rudd CE, Utku N, Blumberg RS. Carcinoembryonic antigen-related cellular adhesion molecule 1 isoforms alternatively inhibit and costimulate human T cell function. J Immunol. 2004;172:3535–3543. doi: 10.4049/jimmunol.172.6.3535. [DOI] [PubMed] [Google Scholar]
- 41.Wang N, Li Z, Ding R, Frank GD, Senbonmatsu T, Landon EJ, Inagami T, Zhao ZJ. Antagonism or synergism. Role of tyrosine phosphatases SHP-1 and SHP-2 in growth factor signaling. J Biol Chem. 2006;281:21878–21883. doi: 10.1074/jbc.M605018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagaishi T, Pao L, Lin SH, Iijima H, Kaser A, Qiao SW, Chen Z, Glickman J, Najjar SM, Nakajima A, Neel BG, Blumberg RS. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity. 2006;25:769–781. doi: 10.1016/j.immuni.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 43.Deeths MJ, Kedl RM, Mescher MF. CD8+ T cells become nonresponsive (anergic) following activation in the presence of costimulation. J Immunol. 1999;163:102–110. [PubMed] [Google Scholar]
- 44.Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yee C. Adoptive therapy using antigen-specific T-cell clones. Cancer J. 2010;16:367–373. doi: 10.1097/PPO.0b013e3181eacba8. [DOI] [PubMed] [Google Scholar]
- 46.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miethke T, Vabulas R, Bittlingmaier R, Heeg K, Wagner H. Mechanisms of peripheral T cell deletion: anergized T cells are Fas resistant but undergo proliferation-associated apoptosis. Eur J Immunol. 1996;26:1459–1467. doi: 10.1002/eji.1830260709. [DOI] [PubMed] [Google Scholar]
- 48.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 49.Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, Groner Y, Rao A. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J Exp Med. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qui HZ, Hagymasi AT, Bandyopadhyay S, St Rose MC, Ramanarasimhaiah R, Menoret A, Mittler RS, Gordon SM, Reiner SL, Vella AT, Adler AJ. CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation. J Immunol. 2011;187:3555–3564. doi: 10.4049/jimmunol.1101244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheever MA, Thompson DB, Klarnet JP, Greenberg PD. Antigen-driven long term-cultured T cells proliferate in vivo, distribute widely, mediate specific tumor therapy, and persist long-term as functional memory T cells. J Exp Med. 1986;163:1100–1112. doi: 10.1084/jem.163.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dossett ML, Teague RM, Schmitt TM, Tan X, Cooper LJ, Pinzon C, Greenberg PD. Adoptive Immunotherapy of Disseminated Leukemia With TCR-transduced, CD8(+) T Cells Expressing a Known Endogenous TCR. Mol Ther. 2009;17:742–749. doi: 10.1038/mt.2008.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenberg PD, Cheever MA. Effector mechanisms operative in adoptive therapy of tumor-bearing animals: implications for the use of interleukin-2. J Biol Response Mod. 1984;3:455–461. [PubMed] [Google Scholar]
- 55.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berger C, Turtle CJ, Jensen MC, Riddell SR. Adoptive transfer of virus-specific and tumor-specific T cell immunity. Curr Opin Immunol. 2009 doi: 10.1016/j.coi.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morimoto J, Tan X, Teague RM, Ohlen C, Greenberg PD. Induction of tolerance in CD8+ T cells to a transgenic autoantigen expressed in the liver does not require cross-presentation. J Immunol. 2007;178:6849–6860. doi: 10.4049/jimmunol.178.11.6849. [DOI] [PubMed] [Google Scholar]
- 58.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 59.Grabstein KH, Waldschmidt TJ, Finkelman FD, Hess BW, Alpert AR, Boiani NE, Namen AE, Morrissey PJ. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med. 1993;178:257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Restifo NP, Esquivel F, Kawakami Y, Yewdell JW, Mule JJ, Rosenberg SA, Bennink JR. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol. 2003;195:346–355. doi: 10.1002/jcp.10290. [DOI] [PubMed] [Google Scholar]
- 62.Amos SM, Duong CP, Westwood JA, Ritchie DS, Junghans RP, Darcy PK, Kershaw MH. Autoimmunity associated with immunotherapy of cancer. Blood. 2011;118:499–509. doi: 10.1182/blood-2011-01-325266. [DOI] [PubMed] [Google Scholar]
- 63.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teague RM, Greenberg PD, Fowler C, Huang MZ, Tan X, Morimoto J, Dossett ML, Huseby ES, Ohlen C. Peripheral CD8+ T cell tolerance to self-proteins is regulated proximally at the T cell receptor. Immunity. 2008;28:662–674. doi: 10.1016/j.immuni.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 66.Kafrouni MI, Brown GR, Thiele DL. Virally infected hepatocytes are resistant to perforin-dependent CTL effector mechanisms. J Immunol. 2001;167:1566–1574. doi: 10.4049/jimmunol.167.3.1566. [DOI] [PubMed] [Google Scholar]
- 67.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 68.Peters MG. Animal models of autoimmune liver disease. Immunol Cell Biol. 2002;80:113–116. doi: 10.1046/j.0818-9641.2001.01059.x. [DOI] [PubMed] [Google Scholar]
- 69.Wiede F, Shields BJ, Chew SH, Kyparissoudis K, van Vliet C, Galic S, Tremblay ML, Russell SM, Godfrey DI, Tiganis T. T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice. J Clin Invest. 2011;121:4758–4774. doi: 10.1172/JCI59492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murthy A, Shao YW, Defamie V, Wedeles C, Smookler D, Khokha R. Stromal TIMP3 regulates liver lymphocyte populations and provides protection against Th1 T cell-driven autoimmune hepatitis. J Immunol. 2012;188:2876–2883. doi: 10.4049/jimmunol.1102199. [DOI] [PubMed] [Google Scholar]
- 71.Holdener M, Hintermann E, Bayer M, Rhode A, Rodrigo E, Hintereder G, Johnson EF, Gonzalez FJ, Pfeilschifter J, Manns MP, Herrath MG, Christen U. Breaking tolerance to the natural human liver autoantigen cytochrome P450 2D6 by virus infection. J Exp Med. 2008;205:1409–1422. doi: 10.1084/jem.20071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, Klebanoff CA, Johnson LA, Kerkar SP, Yang S, Muranski P, Palmer DC, Scott CD, Morgan RA, Robbins PF, Rosenberg SA, Restifo NP. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 117:808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arens R, Schoenberger SP. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol Rev. 2010;235:190–205. doi: 10.1111/j.0105-2896.2010.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beuneu H, Lemaitre F, Deguine J, Moreau HD, Bouvier I, Garcia Z, Albert ML, Bousso P. Visualizing the functional diversification of CD8+ T cell responses in lymph nodes. Immunity. 2010;33:412–423. doi: 10.1016/j.immuni.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 76.Chen Z, Chen L, Qiao SW, Nagaishi T, Blumberg RS. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J Immunol. 2008;180:6085–6093. doi: 10.4049/jimmunol.180.9.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 78.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 79.Perez-Villar JJ, Whitney GS, Bowen MA, Hewgill DH, Aruffo AA, Kanner SB. CD5 negatively regulates the T-cell antigen receptor signal transduction pathway: involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol Cell Biol. 1999;19:2903–2912. doi: 10.1128/mcb.19.4.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taylor A, Akdis M, Joss A, Akkoc T, Wenig R, Colonna M, Daigle I, Flory E, Blaser K, Akdis CA. IL-10 inhibits CD28 and ICOS costimulations of T cells via src homology 2 domain-containing protein tyrosine phosphatase 1. J Allergy Clin Immunol. 2007;120:76–83. doi: 10.1016/j.jaci.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 81.Taylor A, Verhagen J, Akkoc T, Wenig R, Flory E, Blaser K, Akdis M, Akdis CA. IL-10 suppresses CD2-mediated T cell activation via SHP-1. Mol Immunol. 2009;46:622–629. doi: 10.1016/j.molimm.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 82.Park IK, Shultz LD, Letterio JJ, Gorham JD. TGF-beta1 inhibits T-bet induction by IFN-gamma in murine CD4+ T cells through the protein tyrosine phosphatase Src homology region 2 domain-containing phosphatase-1. J Immunol. 2005;175:5666–5674. doi: 10.4049/jimmunol.175.9.5666. [DOI] [PubMed] [Google Scholar]
- 83.Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-beta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131–144. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ray S, Chhabra A, Chakraborty NG, Hegde U, Dorsky DI, Chodon T, von Euw E, Comin-Anduix B, Koya RC, Ribas A, Economou JS, Rosenberg SA, Mukherji B. MHC-I-restricted melanoma antigen specific TCR-engineered human CD4+ T cells exhibit multifunctional effector and helper responses, in vitro. Clin Immunol. 2010;136:338–347. doi: 10.1016/j.clim.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kamata T, Yamashita M, Kimura M, Murata K, Inami M, Shimizu C, Sugaya K, Wang CR, Taniguchi M, Nakayama T. src homology 2 domain-containing tyrosine phosphatase SHP-1 controls the development of allergic airway inflammation. J Clin Invest. 2003;111:109–119. doi: 10.1172/JCI15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mauldin IS, Tung KS, Lorenz UM. The tyrosine phosphatase SHP-1 dampens murine Th17 development. Blood. 2012;119:4419–4429. doi: 10.1182/blood-2011-09-377069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.