Abstract
Purpose
The behavior of 64Cu-ATSM in hypoxic tumors was examined through a combination of in vivo dynamic PET imaging, and ex vivo autoradiographic and histological evaluation using a xenograft model of H&N squamous cell carcinoma.
Methods and Materials
64Cu-ATSM was administered during dynamic PET imaging, and temporal changes in 64Cu-ATSM distribution within tumors were evaluated for at least 1 h and up to 18 h. Animals were sacrificed at either 1 h (cohort A) or after 18 h (cohort B) post-injection of radiotracer and autoradiography performed. Ex vivo analysis of microenvironment sub-regions was conducted by immunohistochemical staining for markers of hypoxia (Pimonidazole Hydrochloride) and blood flow (Hoechst-33342).
Results
Kinetic analysis revealed rapid uptake of radiotracer by tumors. The net influx (Ki) constant was twelve-fold that of muscle, while the distribution volume (Vd) was five-fold. PET images showed large tumor-to-muscle ratios, which continually increased over the entire 18 h course of imaging. However, no spatial changes in 64Cu-ATSM distribution occurred in PET imaging after 20 minutes post-injection. Microscopic intratumoral distribution of 64Cu-ATSM and Pimonidazole were not correlated at 1 h or after 18 h post-injection, and neither was 64Cu-ATSM and Hoechst-33342.
Conclusions
The oxygen partial pressures at which 64CuATSM and Pimonidazole are reduced and bound in cells are theorized to be distinct and separable. However, this study demonstrated that microscopic distributions of these tracers within tumors are independent. Nonetheless, researchers have shown 64Cu-ATSM uptake to be specific to malignant expression, and this work has also demonstrated clear tumor targeting by the radiotracer.
Keywords: Hypoxia imaging, Cu-ATSM PET, ATSM
Introduction
As early as 1987, Rasey et al. proposed PET imaging to quantify tumor hypoxia using 18F-fluoromisonidazole (18F-FMISO), a member of the 2′-nitroimidazole family of compounds (1). However, 18F-FMISO has inherent limitations for in vivo hypoxia imaging due noisy images due to slow uptake and blood clearance (2). Thus for hypoxia specificity, late imaging times post-injection are needed, which result in noisy PET images. The desire for a radiotracer that rapidly produces high signal intensity PET images has motivated investigations of the highly lipophilic bis(thiosemicarbazone) compounds, specifically Copper-diacetyl-bis(N4-methylthiosemicarbazone) (Cu-ATSM) (3).
The mechanism of uptake of Cu-ASTM in tumors was believed to be hypoxia specific, as partial dependent Cu-ATSM uptake was reported in 9L gliosarcoma preclinical models pressure of oxygen (pO2) using hydralazine and inhaled oxygen content to manipulate average pO2 values in tumor tissue (4). Moreover, early clinical trials found SUV of Cu-ASTM to be prognostic for radiotherapy outcome in lung (5), cervical (6), and rectal (7) cancers. However, distinct cell-line dependency in 64Cu-ATSM uptake rates have been shown in vitro (8, 9), and tumor model dependencies were also revealed for in vivo PET imaging (10). Study of R3327-AT prostate tumor models showed reverse PET distribution of 64Cu-ATSM versus 18F-FMSIO at early times post-injection, but showed improved agreement between the radiotracers in late images acquired 18 h post-injection of 64Cu-ATSM. Other studies suggest that 64Cu-ATSM may not be hypoxia specific in prostate cancer models in general (9). However, a FaDu H&N tumor model showed close visual agreement between 18F-FMISO and 64Cu-ATSM PET images at both the early and late time-points (10). The apparent agreement between 18F-FMISO and 64Cu-ATSM PET images with little evolution in the macroscopic 64Cu-ATSM distribution led to the interpretation of rapid hypoxia-specific localization of 64Cu-ATSM within these types of cancers (10).
Interpretation of nuclear images of tumor hypoxia may be complicated by difficulties in determining to what extent radiotracer distribution is determined by “supply” (i.e. perfusion, vascularity) or “demand” (i.e. pO2 level). Yet, the rapid cellular uptake of Cu-ATSM, as observed in preclinical and clinical studies, has led to clinical PET protocols adopting early imaging times of between 20 min to 50 min post-injection. However, it remains to be determined whether early Cu-ATSM PET imaging is significantly affected by non-specific radiotracer delivery, and whether delaying acquisition to late time-points, when vascular clearance has completed, would provide a hypoxia specific image. In the FaDu tumor model, the assumption of rapid and hypoxia specific 64Cu-ATSM accumulation had not been validated. The association of Cu-ATSM PET signals with hypoxia specific accumulation or with vascular delivery and perfusion cannot be determined without studying the pharmacokinetics of the radiotracer. Also, the limited spatial resolution of PET did not provide sufficient information about hypoxia specific vs. non-specific accumulation of Cu-ATSM within the tumor microenvironment. To answer these questions, we performed kinetic PET imaging of FaDu tumor models followed by digital autoradiography and immunohistochemical analysis in animals sacrificed at early (1 h) and late (after 18 h) time-points post-injection of 64Cu-ATSM.
Methods and Materials
Tumor models
Subcutaneous xenografts of FaDu human H&N tumors were grown in nude rats in accordance with an Institutional Animal Care Committee (IACUC) protocol. 1×106 tumors cells were implanted in the right hind limb of each animal, and tumors allowed to grow for 2 to 3 weeks until their longest diameters reached 20 mm ± 4 mm standard deviation, with an average tumor volume of approximately 3 cm3. Animals were then divided into equally sized (n = 4) cohorts A and B; each cohort differing only in the duration of PET imaging protocols and time of sacrifice (i.e. early or late times post-injection).
Radiotracer and histochemical marker administration
During in vivo PET imaging, radiolabeled Cu(II)-diacetyl-bis(N(4)-methylthiosemicarbazone) (64Cu-ATSM) was administered, as an intravenous bolus of 43.3 MBq ± 8.4 MBq standard deviation. The injected volume of the radiotracer bolus was approximately 350 μL, and injections were followed by a 500 μL saline flush. The 64Cu-ATSM radiotracer had been synthesized at the Cyclotron-Radiochemistry core facility of the institution with radiochemical purity of 99% as assessed by instant thin layer chromatography. High specific activity 64Cu radioisotope was obtained from Washington University School of Medicine, and ligand synthesis and labeling was done using published methods (3). The longer radiological half-life of 64Cu (τ = 12.6 h) allowed negligible difference in noise between early and late emission images. For subsequent ex vivo immunohistochemical analysis, both cohorts were intravenously administered 80 mg/kg of Pimonidazole Hydrochloride (Pimonidazole) at 3 hours prior to sacrifice, and 15 mg/kg Hoechst-33342 at 5 minutes prior to sacrifice.
PET imaging
All PET imaging was performed on the Focus 120™ (Siemens Medical Systems, USA) preclinical microPET® system and animals were anesthetized by isoflurane inhalation. Cohort A were scanned in a 90-minute dynamic (list mode) PET sequence, and then re-scanned for 10-minute PET acquisitions at 6 h and 18 h after the injection of 64Cu-ATSM. Cohort B underwent a 50-minute dynamic PET sequence only. Cohort A required additional immobilization via a molded foam bed and skin tattooing for consistency in positioning of the tumor between the early and late imaging sessions. PET acquisitions were initiated ~10 s prior to administering the bolus to ensure adequate sampling of the initial venous passage of the radiotracer. The list-mode data then were binned into time-frames designed to finely sample the early phases of the pharmacokinetics. Ordered Subset Expectation Maximization (OSEM) with calculated attenuation-correction was used for image reconstruction.
Pharmacokinetic analysis
Regions of interest (ROI) within the tumor and normal tissue were delineated using PET images acquired at 40 min post-injection. Necrotic tumor tissue was excluded from pharmacokinetic analysis by delineating only voxels ≥ 30% of the maximum tumor signal. The muscle ROI was identified within the left hind leg. The venous ROI was a lateral vein at the base of the tail and delineated in PET images acquired within the first 10 s post-injection. Time activity curves (TAC) were derived from the mean activity concentration within each ROI at each time-frame of the dynamic PET sequence. Tissue activity concentration functions (Ct), the TAC of tumor or muscle ROI, were then compared to venous input functions (Cp), which was estimated as the TAC for whole blood in the venous ROI. Influx-constants or steady-state trapping rates (Ki) were calculated by linear regression analysis of Patlak (11) plots of Ct/Cp ratios versus normalized time integrals of Cp. The distribution volume (Vd) were also calculated by Logan plots (12) of time integrals of Ct and Cp.
Ex vivo autoradiography, histology, and immunohistochemistry
Immediately after sacrifice, a 30 gauge angiocatheter was placed in a central tumor location perpendicular to the PET imaging plane, as a fiducial marker for ex vivo image registration. The tumors were removed with the angiocatheter in situ, embedded in specimen mounting medium, frozen, and sliced into 10-μm-thick tissue sections. Two tissue sections per animal were used for microscopic imaging, which correspond to PET image locations with the anterior and posterior or the superior and inferior halves of the tumor.
Digital autoradiographs of 64Cu-ATSM microdistributions were obtained by exposing tissue sections to a phosphor imaging screen for 12 h at −20°C. Immunofluorescence staining of Pimonidazole and Hoechst-33342 was carried out, and the tissue sections imaged by fluorescence microscopy. General histology was also assessed by hematoxylin and eosin (H&E) staining. Autoradiographic and microscopic images were co-registered using image processing software by aligning the fiducial marks and tissue boundaries. Image registrations were evaluated to maximize the normalized mutual information criterion between the microscopic images. Co-registered microscopic images were then resampled down to the 50-μm pixel-size of autoradiographs, using a box filter to retain contrast and relative pixels intensities.
Spatial analysis
None of the extrinsic markers was observed to pool in necrotic regions, and co-distributions within muscle were not relevant to this study. Therefore, analysis of spatial correlation was selectively restricted to viable tumor as identified by H&E histology, excluding necrosis, muscle and other non-tumor tissue. The relationships between microdistributions of 64Cu-ATSM, Pimonidazole, and Hoechst-33342 within viable tumor regions were quantified by Spearman rank correlation coefficients (Spearman’s ρ). To facilitate visualization of scattergrams and to evaluate statistical trends, the several thousand pixel-values of each microdistribution image were binned into deciles (13).
Results
PET images indicated significantly higher accumulation of 64Cu-ATSM in tumors than in muscle tissue (p < 0.01). Figure 1 shows a sequence of PET images of a typical tumor, and spans the time-frame from 20 min to 18 h post-injection. At 20 min, tumors exhibited four-fold higher 64Cu-ATSM uptake than muscle, and tumor-to-muscle ratios continued to increase over time, reaching twelve-fold at 18 h. Due to hepatobiliary clearance of 64Cu-ASTM metabolites, gut uptake became apparent in later PET images and peaked at 6 h post-injection in figure 1.
Figure 1.
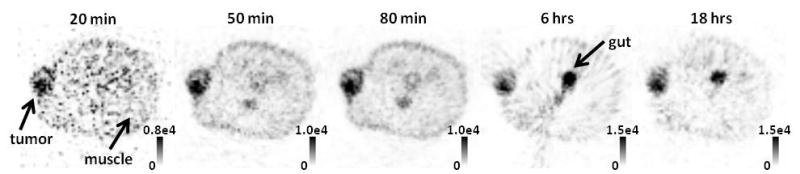
Dynamic PET image sequence showing an axial slice though a subcutaneous FaDu tumor. Tumor-to-muscle ratio of 64Cu-ATSM accumulation continually increased over the 90-minute imaging sequence; but spatial distribution within the tumor was unchanged. At 6 h and 18 h, hepatobiliary excretion of 64Cu-ATSM metabolites can be noted within the liver (not shown) and gut.
Digital autoradiography confirmed higher accumulation of radiotracer within tumor cells as compared to muscle. In figure 2, autoradiographs were compared to histology, and show an example of microdistributions of 64Cu-ATSM within a tissue section. Qualitatively, 64Cu-ASTM concentrations in viable (non-necrotic) tumor tissue were visibly higher than the surrounding muscle. Additionally, microdistributions of radiotracer were heterogeneous within the viable tumor region.
Figure 2.
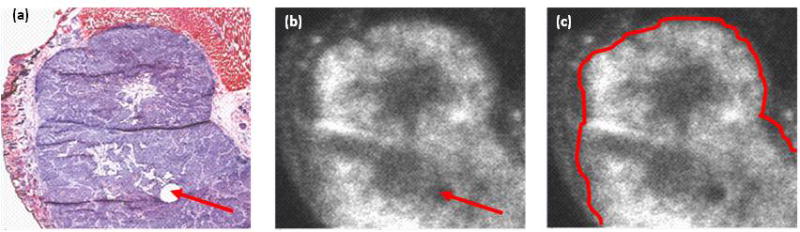
Histology of FaDu tumor and microdistribution of 64Cu-ATSM. Figures show H&E stained tissue section (a), and autoradiograph of the same section (b), with the tumor delineated (c). Arrows indicate a fiducial mark used for image registration.
The mean activity within PET voxels, PETmean, of tumor and muscle regions was used to quantify net 64Cu-ATSM retention in the tissues. TACs in figure 3(a) was generated by plotting the PETmean values of dynamic PET images after correcting for radiological decay between the imaging time-points. PETmean values within muscle remained statistically constant (p > 0.01) after 20 min post-injection. In contrast, PETmean in tumors rapidly increased within the first minute, reaching a pseudo-plateau then continued to increase at a much slower rate, indicating that net 64Cu-ATSM retention within tumors did not reach an absolute steady state even at many hours post-injection. Despite continual increase in PETmean, macroscopic distributions of radiotracer within the PET images remained stable, with high spatial correlation (Pearson’s r > 0.6) between PET images of the tumors acquired at 18 h and at 20 min post-injection. The stable macroscopic distributions were also apparent in figure 1.
Figure 3.
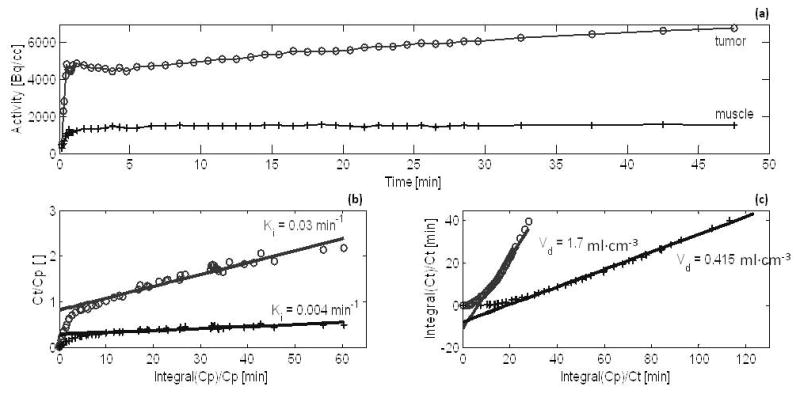
Graphical pharmacokinetic analysis of the behavior of 64Cu-ATSM within tumors (o), and normal muscle tissues (+) over time. Figures show time activity curves (a), Patlak plots (b), and Logan plots (c) from the analysis of one representative animal.
Pharmacokinetic analysis
Patlak and Logan plots were generated for each rat, and linear regression analysis was used to fit data points that corresponded to a time interval spanning 3 min through 50 min post-injection. The time interval for regression analysis was selected because the sharply rising initial segment of Patlak plots flattened after 3 min and dynamic data for cohorts B only extended to 50 min post-injection. Examples of Patlak and Logan plots and linear regression fits are shown in figure 3(b, c), while table 1 detailed tumor-to-muscle ratios of the pharmacokinetic parameters.
Table 1.
Tumor-to-muscle ratios of 64Cu-ATSM pharmacokinetic parameters and mean PET values.
| Tumor-to-muscle ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vd | χ2 | Ki | PETmean (20min) | PETmean (30min) | PETmean (40min) | PETmean (80min) | PETmean (18h) | ||
| cohort A | Rat01 | 4.16 | 0.70 | 6.12 | 3.72 | 4.08 | 4.16 | 4.92 | 17.07 |
| Rat02 | 6.70 | 1.01 | 7.40 | 6.47 | 6.58 | 6.86 | 7.49 | 11.41 | |
| Rat03 | 4.18 | 0.88 | 4.79 | NA† | 4.18 | 4.35 | 10.70 | ||
| Rat04 | 3.48 | 0.82 | 4.29 | 3.37 | 3.41 | 3.58 | 3.98 | 9.50 | |
| cohort B | Rat06 | 4.22 | 0.44 | 14.54 | 4.28 | 4.64 | 4.94 | NA‡ | |
| Rat07 | 7.00 | 0.63 | 12.77 | 6.86 | 7.58 | 7.84 | |||
| Rat08 | 4.45 | 0.42 | 9.60 | 4.15 | 4.30 | 4.64 | |||
| Rat09 | 4.07 | 0.61 | 7.60 | 3.92 | 4.35 | 4.48 | |||
| sample statistics | mean | 4.78 | 0.69 | 8.39 | 4.68 | 4.99 | 5.09 | 5.18 | 12.17 |
| ± std error | ± 0.46 | ± 0.07 | ± 1.30 | ± 0.53 | ± 0.57 | ± 0.52 | ± 0.79 | ± 1.68 | |
| p value | 2.E-04 | 2.E-02* | 2.E-04 | 8.E-06 | 5.E-06 | 7.E-07 | 5.E-04 | 4.E-03 | |
NA† indicates missing data where the dynamic scan of Rat03 was briefly interrupted by a software failure.
NA‡ indicates unavailable data at the late time-points for cohort B (sacrificed at 1 h).
indicates a lack of statistical significance.
Regression analysis of the Patlak plots often showed marginal to poor fits to a linear line. This can be observed as divergent data points in figure 3(b) and was indicated by low r2 regression values, especially for muscle ROIs. This indicates that steady state was not reached at 50 min post-injection and that trapping was not irreversible. As a consequence of the poor Patlak fits, the estimate of influx rate, Ki, had wide 95% confidence intervals. For tumors, the 95% confidence intervals about the mean Ki was [2.8×10−2 min−1 to 3.5×10−2 min−1]. For muscle the Patlak fits were slightly worse with a wider 95% confidence interval of [3.0×10−3 min−1 to 5.0×10−3 min−1] about the mean Ki value. In contrast, Logan plots showed distinct linear trends, with r2 regression values well above 0.9 for both tumor and muscle ROIs; see figure 3(c). Likewise, the pharmacokinetic parameters were better estimated from these plots. The 95% confidence intervals about the mean volume of distribution, Vd, was [1.9 ml·cm−3 to 2.1 ml·cm−3] for tumors, and were [0.42 ml·cm−3 to 0.44 ml·cm−3] for muscle.
As shown in table 1, all PET values and most pharmacokinetic parameters were significantly greater within tumor than in muscle (p < 0.01). At the 95% confidence interval, the Ki values for tumors of 64Cu-ATSM was five-were estimated to be between seven-fold to eleven-fold that of muscle, while Vd fold greater for tumors than muscle tissue. Within the 50 min post-injection interval, PETmean within tumors were also five-fold greater. However, the effective washout rate of 64Cu-ATSM, χ2, was indistinguishable (p = 0.02) between tumor and muscle. Even though effective washout rates were similar, 64Cu-ATSM concentration within tumors were observed to increase steadily over time. This was indicated by the slope of TACs in figure 3(a), and in table 1, ratios of PETmean within tumors versus muscle continually increased reaching twelve-fold at 18 h.
Spatial analysis
Scattergram analysis of autoradiographs and immunohistochemical slides showed a random relationship between microdistribution of 64Cu-ATSM and accumulation of Pimonidazole, within tumors. Macroscopic PET images in figure 1 were similar over time; nonetheless, figure 4 and figure 5 illustrate that changes did occur in 64Cu-ATSM microdistributions between early and late time-points.
Figure 4.
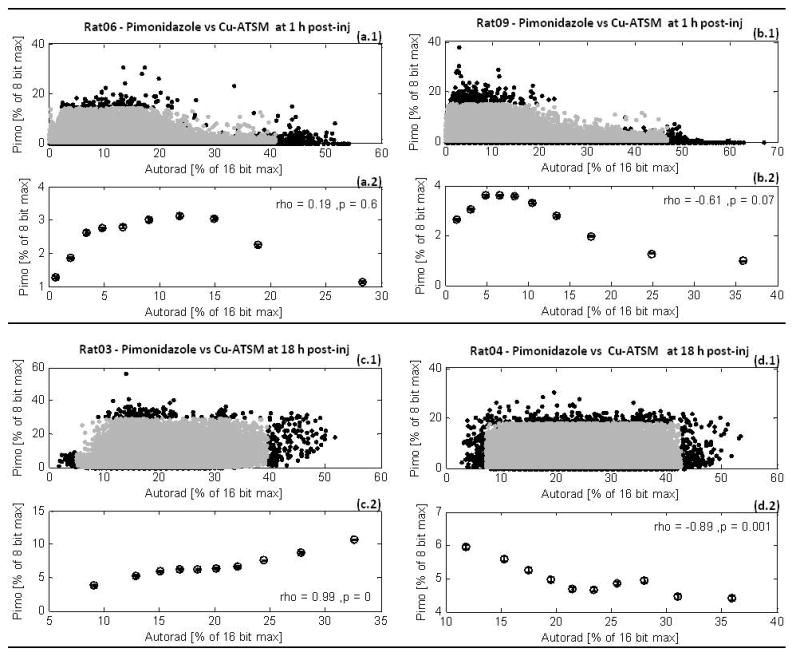
Scattergrams and correlation analysis of Pimonidazole microscopy, Pimo, vs. 64Cu-ATSM autoradiography, Autorad, in four representative tumors. Figures (a) and (b) show Pimo vs. early Autorad at 1 h post-injection of 64Cu-ATSM. Figures (c) and (d) show Pimo vs. late Autorad at 18 h. The central 99% of pixel values from scattergrams (a.1), (b.1), (c.1), and (d.1) were binned into deciles for analysis of spatial correlation between the Pimo vs. Autorad microdistributions. This correlation analysis is shown in the plots correspondingly labeled (a.2), (b.2), (c.2), and (d.2).
Figure 5.
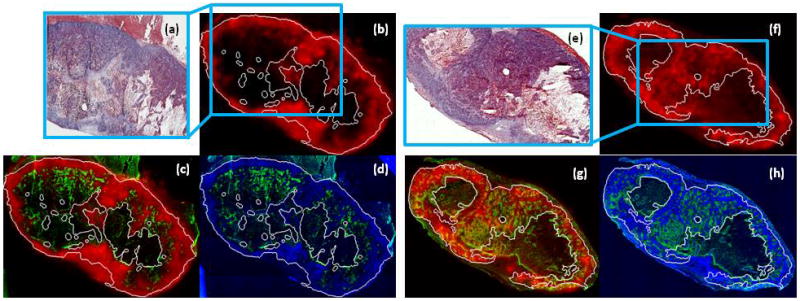
Microenvironmental features at early and late time-points post injection of 64Cu-ATSM. Figures (a) and (e) are H&E stained tumor histology tissue sections of two FaDu tumors. The viable tumor regions were contoured on H&E histology and are indicated by white lines in the corresponding immunohistochemical and autoradiographic images. Early and late 64Cu-ATSM autoradiographs, at 1 h and after 18 h post-injection, are shown in (b) and (f). Spatial microdistribution of Pimonidazole [green] with the early 64Cu-ATSM [red] is shown in figure (c) and with the late 64Cu-ATSM in figure (g). Figures (d) and (h) show Pimonidazole [green] and Hoechst-33342 [blue] microdistributions.
In figure 4, no significant trend was found between 64Cu-ATSM and Pimonidazole at either time-point. Across both cohorts, Spearman’s ρ values were not significantly greater than zero (p > 0.05) for seven of the sixteen tumor sections analyzed. Of the nine data sets that showed significant Spearman’s ρ values (p ≤ 0.05), six showed negative correlation while the other three showed positive correlation. Figures 4(c), 4(d) show examples of bimodal behavior observed between Pimonidazole and 64Cu-ATSM microdistributions at early time-points, with a positive slope at very low 64Cu-ATSM values followed by a negative slope. Figures 4(a) and 4(b) are examples of the monotonic trends at late time-points, but note that both positive and negative correlations were observed. Inconsistent spatial relationships between late 64Cu-ATSM and Pimonidazole were even evident among different tissue sections of the same tumor. Likewise, no visual pattern can be observed between Pimonidazole fluorescence images and 64Cu-ATSM autoradiographs at the early time-points of figure 5(c), or the late time-points of figure 5(g). Strong anti-correlation was found between Pimonidazole and Hoechst-33342 microdistributions (Spearman’s ρ > −0.9, p ≪ 0.05), and this can also be observed in figures 5(d) and 5(h). A significant positive relationship was found between early 64Cu-ATSM and Hoechst-33342 (Spearman’s ρ > 0.9, p ≪ 0.05); however, no correlation was found after 18 h post-injection. In figure 5, early 64Cu-ATSM appears co-distributed with the blood flow marker.
No relationship was found between elevated 64Cu-ATSM signal within the tumor and the hypoxic fraction measured by Pimonidazole positive staining. Corrected for necrosis, the hypoxic fraction within the tumor sections was calculated as the fraction of the viable tumor that was Pimonidazole positive. Positive staining was determined by visual inspection, and corresponded to a color threshold of 1/3 of the 8-bit pixel maximum. Likewise, elevated 64Cu-ATSM signal was defined as autoradiography signals greater than 1/3 of pixel saturation level. The hypoxic fraction of the sixteen tumor samples was 28% and ranged from 16% to 51%. At 1 h, the average 64Cu-ATSM signal in the tumor sections was 55%, and at 18 h, average 64Cu-ATSM signal was 79%. There was no correlation to hypoxic fractions at the 1 h (Spearman’s ρ = 0.33, p = 0.4), or 18 h (Spearman’s ρ = −0.24, p = 0.6) time-points.
Discussion
Using a xenograft model of H&N squamous cell carcinoma, we observed rapid tumor uptake and retention of radiotracer. Tumor-to-muscle ratios were 4:1 within 20 min post- injection and were still increasing at 18 hr post injection. Yet, this H&N tumor model did not exhibit an evolution of the macroscopic radiotracer distributions in PET imaging, which had been observed in other tumors (10). Comparative autoradiography and immunohistochemical analysis revealed that sub-tumoral distribution of 64Cu-ATSM was not easily interpreted. 64Cu-ATSM and nitroimidazole-based hypoxia tracers (exemplified here by Pimonidazole) have quite different uptake dependencies. At 1 h post injection, the early 64Cu-ATSM showed no correlation to Pimonidazole, but strong positive correlations with Hoechst-33342, indicative of highly perfused regions. Correlation to Hoechst-33342 was not observed with the late 64Cu-ATSM after 18 h; however, there was still no clear correlation to the Pimonidazole hypoxia tracer.
Because of the electrochemical properties of Cu-ATSM (3, 14), quantification of Cu-ATSM PET signals is assumed to provide direct indication of pO2 levels and hypoxia in tumors. However, the precise mechanism of in Cu-ATSM accumulation remains largely unknown, as Cu-ATSM accumulation occurs under normoxic conditions (8, 15) and is strongly influenced by genetic characteristics that are independent of pO2 status (8, 9, 16). As such, there is a need to further investigate the pathways of Cu-ATSM transport, to determine if active influx and efflux of Cu-ATSM and its metabolites (e.g. protein bound 64Cu) are likely to out-compete pO2 dependent retention mechanisms. Strong evidence of active influx and efflux of Cu-ATSM by cell membrane proteins has been found in human cancer cells (17), and the expressions of proteins involved in Cu-ATSM transport pathways are likely specific to the cell type. In vivo copper homeostasis results in the active transport of protein bound Cu from plasma into tissue within 30 min or less (18), so during a typical PET scan Cu-ATSM metabolites may enter the copper regulation cycle and be actively transported into and out of tumor cells.
Nonetheless, Cu-ATSM PET may provide clinical benefits for tumor demarcation due to large tumor-to-muscle ratios at early times post-injection. Autoradiographs confirmed higher Cu-ATSM levels in viable tumor cells versus stromal and muscle tissue. Cu-ATSM PET has also shown prognostic strengths (5–7), which may be powered by a number of hypoxia-independent factors. Copper homeostasis in malignant cells is often altered to maintain significantly higher copper levels (19), which is linked to cancer progression (20). The potential utility of Cu-ATSM as a tumor imaging agent may lie with its relationship to these characteristics.
Acknowledgments
Grant support: NIH R01 CA84596, R24 CA83084, P01 CA115675 and P50 CA86438; Geoffrey Beene Cancer Research Foundation
Nicholas Ramos of the Lewis Research Laboratory, and the Cyclotron-Radiochemistry Core Facility, Pat Zanzonico, PhD and Valerie Longo of the Animal Imaging Core Facility, Memorial Sloan-Kettering Cancer Center
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rasey JS, Grunbaum Z, Magee S, et al. Characterization of Radiolabeled Fluoromisonidazole as a Probe for Hypoxic Cells. Radiation Research. 1987;111:292–304. [PubMed] [Google Scholar]
- 2.Grunbaum Z, Freauff SJ, Krohn KA, et al. Synthesis and Characterization of Congeners of Misonidazole for Imaging Hypoxia. Journal of Nuclear Medicine. 1987;28:68–75. [PubMed] [Google Scholar]
- 3.Fujibayashi Y, Taniuchi H, Yonekura Y, et al. Copper-62-ATSM: A New Hypoxia Imaging Agent with High Membrane Permeability and Low Redox Potential. Journal of Nuclear Medicine. 1997;38:1155–1160. [PubMed] [Google Scholar]
- 4.Lewis JS, Sharp TL, Laforest R, et al. Tumor uptake of copper-diacetyl-bis(N(4)-methylthiosemicarbazone): effect of changes in tissue oxygenation. Journal of Nuclear Medicine. 2001;42:655–661. [PubMed] [Google Scholar]
- 5.Dehdashti F, Mintun MA, Lewis JS, et al. In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. European Journal of Nuclear Medicine and Molecular Imaging. 2003;30:844–850. doi: 10.1007/s00259-003-1130-4. [DOI] [PubMed] [Google Scholar]
- 6.Dehdashti F, Grigsby PW, Mintun MA, et al. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM: Relationship to therapeutic response—a preliminary report. International Journal of Radiation Oncology, Biology, Physics. 2003;55:1233–1238. doi: 10.1016/s0360-3016(02)04477-2. [DOI] [PubMed] [Google Scholar]
- 7.Dietz D, Dehdashti F, Grigsby P, et al. Tumor Hypoxia Detected by Positron Emission Tomography with 60Cu-ATSM as a Predictor of Response and Survival in Patients Undergoing Neoadjuvant Chemoradiotherapy for Rectal Carcinoma: A Pilot Study. Diseases of the Colon & Rectum. 2008;51:1641–1648. doi: 10.1007/s10350-008-9420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgman P, O’Donoghue JA, Lewis JS, et al. Cell line-dependent differences in uptake and retention of the hypoxia-selective nuclear imaging agent Cu-ATSM. Nuclear Medicine and Biology. 2005;32:623–630. doi: 10.1016/j.nucmedbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 9.V3vere AL, Lewis JS. Examining the relationship between Cu-ATSM hypoxia selectivity and fatty acid synthase expression in human prostate cancer cell lines. Nuclear Medicine and Biology. 2008;35:273–279. doi: 10.1016/j.nucmedbio.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Donoghue JA, Zanzonico P, Pugachev A, et al. Assessment of regional tumor hypoxia using 18F-fluoromisonidazole and 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) positron emission tomography: Comparative study featuring microPET imaging, Po2 probe measurement, autoradiography, and fluorescent microscopy in the R3327-AT and FaDu rat tumor models. International Journal of Radiation Oncology*Biology*Physics. 2005;61:1493–1502. doi: 10.1016/j.ijrobp.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 11.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical Evaluation of Blood-to-Brain Transfer Constants from Multiple-Time Uptake Data. Journal of Cerebral Blood Flow and Metabolism. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 12.Logan J. Graphical analysis of PET data applied to reversible and irreversible tracers. Nuclear Medicine and Biology. 2000;27:661–670. doi: 10.1016/s0969-8051(00)00137-2. [DOI] [PubMed] [Google Scholar]
- 13.Carlin S, Pugachev A, Sun X, et al. In vivo characterization of a reporter gene system for imaging hypoxia-induced gene expression. Nuclear Medicine and Biology. 2009;36:821–831. doi: 10.1016/j.nucmedbio.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obata A, Yoshimi E, Waki A, et al. Retention mechanism of hypoxia selective nuclear imaging/radiotherapeutic agent Cu-diacetyl-bis-methylthiosemicarbazone) (Cu-ATSM) in tumor cells. Annals of Nuclear Medicine. 2001;15:499–504. doi: 10.1007/BF02988502. [DOI] [PubMed] [Google Scholar]
- 15.Yuan H, Schroeder T, Bowsher JE, et al. Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) Journal of Nuclear Medicine. 2006;47:989–998. [PubMed] [Google Scholar]
- 16.Liu J, Hajibeigi A, Ren G, et al. Retention of the Radiotracers 64Cu-ATSM and 64Cu-PTSM in Human and Murine Tumors Is Influenced by MDR1 Protein Expression. Journal of Nuclear Medicine. 2009;50:1332–1339. doi: 10.2967/jnumed.109.061879. [DOI] [PubMed] [Google Scholar]
- 17.Price KA, Crouch PJ, Volitakis I, et al. Mechanisms Controlling the Cellular Accumulation of Copper Bis(thiosemicarbazonato) Complexes. Inorganic Chemistry. 2011;50:9594–9605. doi: 10.1021/ic201334q. [DOI] [PubMed] [Google Scholar]
- 18.Turnlund J. Human whole-body copper metabolism. The American Journal of Clinical Nutrition. 1998;67:960S–964S. doi: 10.1093/ajcn/67.5.960S. [DOI] [PubMed] [Google Scholar]
- 19.Margalioth EJ, Schenker JG, Chevion M. Copper and Zinc levels in normal and malignant tissues. Cancer. 1983;52:868–872. doi: 10.1002/1097-0142(19830901)52:5<868::aid-cncr2820520521>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 20.Turski ML, Thiele DJ. New Roles for Copper Metabolism in Cell Proliferation, Signaling, and Disease. Journal of Biological Chemistry. 2009;284:717–721. doi: 10.1074/jbc.R800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]


