Abstract
Objective
The purpose of the research study was to examine the manifestation of variability in reaction times (RT) in children with Attention Deficit Hyperactivity Disorder (ADHD) and to examine whether RT variability presented differently across a variety of neuropsychological tasks, was present across the two most common ADHD subtypes, and whether it was affected by reward and event rate (ER) manipulations.
Method
Children with ADHD-Combined Type (n=51), ADHD-Predominantly Inattentive Type (n=53) and 47 controls completed five neuropsychological tasks (Choice Discrimination Task, Child Attentional Network Task, Go/No-Go task, Stop Signal Task, and N-back task), each allowing trial-by-trial assessment of reaction times. Multiple indicators of RT variability including RT standard deviation, coefficient of variation and ex-Gaussian tau were used.
Results
Children with ADHD demonstrated greater RT variability than controls across all five tasks as measured by the ex-Gaussian indicator tau. There were minimal differences in RT variability across the ADHD subtypes. Children with ADHD also had poorer task accuracy than controls across all tasks except the Choice Discrimination task. Although ER and reward manipulations did affect children’s RT variability and task accuracy, these manipulations largely did not differentially affect children with ADHD compared to controls. RT variability and task accuracy were highly correlated across tasks. Removing variance attributable to RT variability from task accuracy did not appreciably affect between-group differences in task accuracy.
Conclusions
High RT variability is a ubiquitous and robust phenomenon in children with ADHD.
Keywords: attention deficit hyperactivity disorder, intra-individual variability, reward, event rate, subtypes
Typical outcomes from neuropsychological tests include measures of accuracy and/or reaction time speed (Lezak, 1995). Using such outcomes, a plethora of research has shown that children with Attention Deficit Hyperactivity Disorder (ADHD) demonstrate neuropsychological deficits across a wide range of cognitive skills including response inhibition, working memory, delay aversion, and attention (Nigg, 2005; Solanto, et al., 2001; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). Based on observed error and/or reaction time (RT) deficits among patients with ADHD, multiple theories and hypotheses have been proposed to explain the etiology of ADHD (Barkley, 1997; Douglas & Peters, 1978; Quay, 1988; Sagvolden, et al., 2005; Sonuga-Barke, 2005).
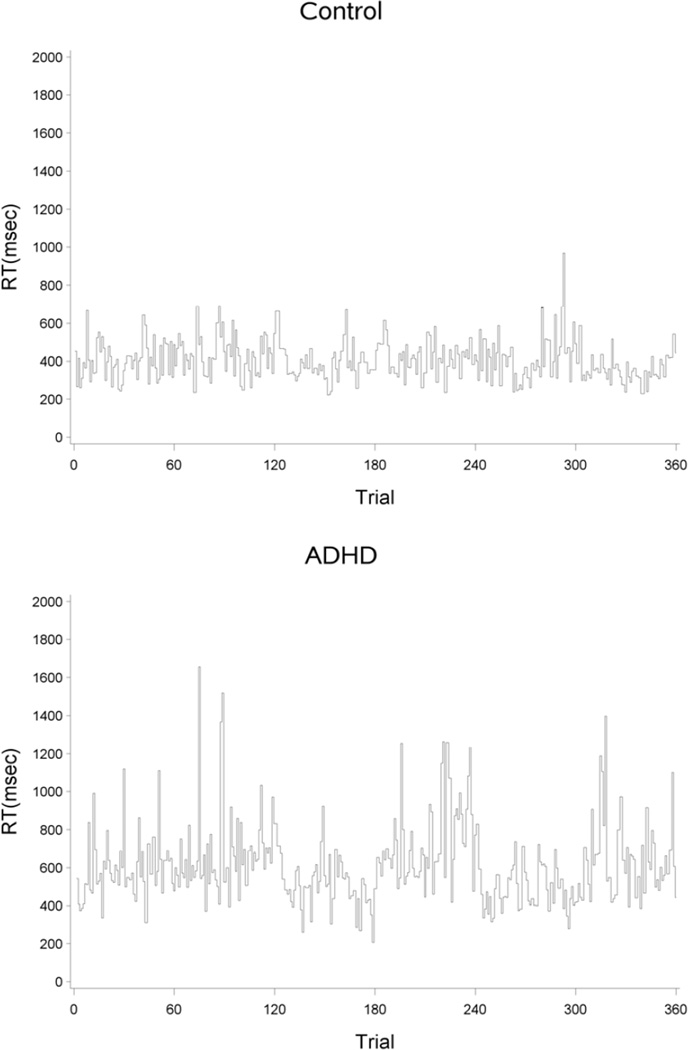
Within this extensive neuropsychological literature demonstrating between-group performance differences on construct-specific cognitive tasks, investigators have increasingly begun to note that one of the most consistent and most discriminating ADHD-associated neuropsychological deficit is the inability for children with ADHD to maintain a regular rhythm of responses to task stimuli (Mirsky, Pascualvaca, Duncan, & French, 1999; See Figure 1). This variability in reaction times is referred to as intra-individual variability in RTs or RT variability. Since RT variability could potentially affect performance, both in terms of RT and accuracy, investigators have begun to question whether some of the core areas of deficit identified on neuropsychological tests (i.e., response inhibition, working memory) may be an artifact of increased RT variability (Castellanos & Tannock, 2002; de Zeeuw, et al., 2008).
Figure 1.
Step graphs illustrating trial by trial reaction times (RT) for a sample child with ADHD and a sample control. The goal of this study is to examine the observed differences in RT variability across children with ADHD and controls.
Elevated RT variability among patients with ADHD appears to be a robust phenomenon. Children with ADHD demonstrate elevated RT variability across a wide variety of neuropsychological tests and test parameters (Castellanos, et al., 2005; de Zeeuw, et al., 2008; Hervey, et al., 2006; Johnson, et al., 2007; Klein, et al., 2006; Kuntsi, et al., 2001; Leth-Steensen, Elbaz, & Douglas, 2000; Lijffijt, Kenemans, Verbaten, & vanEngeland, 2005; Mullins, Bellgrove, Gill, & Robertson, 2005; Rubia, et al., 2001; Schachar, Tannock, Marriott, & Logan, 1995; Shanahan, Pennington, & Willcutt, 2008; Uebel, et al., 2010; Vaurio, et al., 2009). RT variability in ADHD is not merely a widening of the RT distribution but appears to have specific properties that define its manifestation. Using ex-Gaussian indicators to define the RT distribution, ADHD-related RT variability appears to be the result of an increase in the exponential component or positive skew in the RT distribution, signified by the indicator tau (Hervey, et al., 2006; Leth-Steensen, et al., 2000; Vaurio, et al., 2009). Higher values of tau are caused by an increased frequency and higher magnitude of exceedingly long RTs. Thus, RT variability in patients with ADHD appears to be defined by periodic long RTs that occur throughout the RT stream. These long RTs are not predictable by task events (Epstein, et al., 2009) though they may be predictable in terms of oscillation frequency (Castellanos, et al., 2005; Johnson, et al., 2007; Vaurio, et al., 2009).
Though advanced analytic methods (e.g., ex-Gaussian indicators, Fast Fourier Transform analysis) are allowing for a more defined characterization of RT variability manifestation in ADHD, an understanding of the cause of RT variability in children with ADHD is uncertain. To date, many explanations have been put forth to explain increased RT variability. These include a deficit in motor timing (Rubia, et al., 2001), problems with top-down attentional control (Bellgrove, Hester, & Garavan, 2004), subcortically mediated problems in state regulation (Kuntsi, Oosterlaan, & Stevenson, 2001; Scheres, et al., 2001; Sergeant, et al., 2003), deficient attentional processes (Leth-Steensen, et al., 2000), and insufficient suppression of the default mode network (Sonuga-Barke & Castellanos, 2007) among others.
One method for increasing our understanding of the nature of RT variability in ADHD is to investigate variables that attenuate or exacerbate RT variability. Such variables may be contextual factors related to the task or may be individual factors reflecting subject characteristics. Some possible contextual factors that have been assessed in regards to their effects on RT variability include type of task, event rate (ER), and reward. As noted above, RT variability has been observed across a wide variety of neuropsychological tasks designed to assess a range of cognitive skills including response inhibition (de Zeeuw, et al., 2008; Hervey, et al., 2006; Klein, et al., 2006; Rubia, Russell, et al., 2001; Uebel, et al., 2010; Vaurio, et al., 2009), working memory (Buzy, Medoff, & Schweitzer, 2009; Karatekin, 2004; Klein, et al., 2006), attention (Johnson, et al., 2007), and simple choice tasks (Andreou, et al., 2007. Although RT variability effects manifest across tasks, there have been some findings that suggest that RT variability among patients with ADHD is positively correlated with task complexity (Geurts, et al., 2008; Klein, et al., 2006). Speeding the ER also appears to decrease RT variability (see reviews by Sergeant, et al., 2003; Sergeant & Sergeant, 2005; Sonuga-Barke, Wiersema, van der Meere, & Roeyers, 2010; van der Meere, Marzocchi, & De Meo, 2005) in children with ADHD possibly suggesting that state arousal has an effect on RT variability presentation. Finally, there has been a small and inconsistent literature examining the effects of reward on RT variability with some studies showing that reward improves RT variability (Andreou, et al., 2007; Kuntsi, et al., 2009; Slusarek, Velling, Bunk, & Eggers, 2001; Uebel, et al., 2010) and others suggesting no effect of reward on RT variability (Luman, Ooserlaan, & Sergeant, 2008; Shanahan, et al., 2008). Similar to ER, a beneficial effect of reward on RT variability among patients with ADHD would suggest that modifying a patient’s state of arousal can normalize performance. Moreover, combining fast ER and reward has the potential to synergistically improve RT variability (Andreou, et al., 2007; Kuntsi, et al., 2009).
Regarding patient-related characteristics that may affect manifestation of RT variability, the existing research on these variables has been limited. A few studies have examined the relationship between ADHD subtype and RT variability. Some studies have found no differences between the ADHD-Predominantly Inattentive Type (ADHD-I) and ADHD-Combined (ADHD-C) subtypes (Nigg, Blaskey, Huang-Pollock, & Rappley, 2002; Shanahan, et al., 2008; Simmonds, et al., 2007; Vaurio, et al., 2009) while others have found that patients with ADHD-C display increased RT variability compared to patients with ADHD-I (de Zeeuw, et al., 2008; Mullins, et al., 2005) and still others have found that patients with ADHD-I have higher RT variability than patients with ADHD-C (Desman, et al., 2008).
With a few exceptions, our knowledge about RT variability in children with ADHD has been derived from an increasingly large number of single task studies that have occasionally manipulated task parameters or used sample sub-groups (e.g., ADHD subtype) to examine task- and patient-related factors that affect RT variability. The current study was designed to comprehensively investigate RT variability in children with ADHD. A neuropsychological battery was developed to assess a variety of neuropsychological constructs (i.e., response inhibition, working memory, attention) with varied complexity. On each task, within-task manipulations (i.e., ER and reward) were conducted to examine the effects of task-related factors on the presentation of RT variability. Also, the possible moderating effects of patient variables on RT variability were examined by comparing the performance of the two most prevalent ADHD subtypes (i.e., ADHD-I and ADHD-C) to each other and to a control group. Finally, several variables that have been shown to affect neuropsychological performance (i.e., comorbidity and IQ; see (Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005) were examined to determine whether these variables moderate the relationship between ADHD and RT variability.
Based on the existing literature, we predicted that children with both subtypes of ADHD would demonstrate higher levels of RT variability than controls across all cognitive tasks. We did not expect that there would be differences in RT variability across subtypes nor did we expect that any other patient-related variable (i.e., comorbidity or IQ) would moderate the relationship between ADHD and RT variability. Further, we predicted that task manipulations targeted at modifying arousal state (i.e., speeding ER and adding reward) would attenuate the magnitude of RT variability deficits in children with ADHD. Finally, Klein et al. (2006) found that between-group differences in task accuracy remained after controlling for RT variability. Consistent with Klein et al. (2006), we predict that ADHD-related neuropsychological deficits on accuracy will exist after controlling for RT variability.
Method
Participants
One hundred and fifty-one children aged 7–11 participated in the present study. The study included 104 children who met diagnostic criteria for ADHD (53 ADHD-I; 51 ADHD-C) and 47 controls. Children with a Full Scale IQ below 80 as estimated by the Wechsler Abbreviated Scale of Intelligence (WASI) were excluded from participation in the study. Children with standardized achievement scores below 80 on the Wechsler Individual Achievement Test, Second Edition (WIAT-II) Word Reading or Numerical Operations subtests were also excluded from the study in order to rule out children with possible learning disorders. Children were also excluded from the study if their medical history suggested brain injury such as head trauma with loss of consciousness, seizure disorder, or history of infarction.
ADHD Participants
Medication naïve children with ADHD were recruited through multiple community and clinical sources, including schools and local practitioners. Diagnostic status was determined using methodology similar to that employed by the Multimodal Treatment Study of ADHD (MTA Cooperative Group, 1999). Specifically, children were considered to have met criteria for a symptom domain (i.e., inattention and/or hyperactivity/impulsivity) if the parent on the Diagnostic Interview Schedule for Children-Parent Report (DISC-P) and the teacher on the Vanderbilt ADHD Teacher Rating Scale reported 6 non-overlapping symptoms in a symptom domain and both parent and teacher reported at least 4 symptoms in that domain. Children who met these criteria for both inattention and hyperactivity/impulsivity were enrolled in the ADHD-C group, while children who met symptom criteria for inattention but not hyperactivity/impulsivity were enrolled in the ADHD-I group. Children also needed to meet DSM-IV criteria for age of onset, pervasiveness, and impairment as reported by the parent on the DISC-P. All children had to be medication naïve with no prior psychoactive medication treatment. Children in the ADHD-I and ADHD-C groups demonstrated comparable demographic profiles and symptom severity for the inattentive symptom domain. However, children in the ADHD-C group demonstrated significantly greater severity of hyperactive/impulsive symptoms (see Table 1), as would be predicted based on ADHD subtype diagnosis.
Table 1.
Demographic and Clinical Characteristics of the ADHD-Combined Type (ADHD-C), ADHD-Predominantly Inattentive Type (ADHD-I) and Control (C) Samples
| ADHD-C (n = 51) |
ADHD-I (n = 53) |
Control (n = 47) |
Group Comparisons | |
|---|---|---|---|---|
| Mean (SD) age in years | 7.90 (1.11) |
8.35 (1.31) |
8.33 (1.35) |
ns |
| Number male | 41 | 34 | 31 | ns |
| Number of each ethnicity (percentage) |
||||
| Caucasian | 33 | 42 | 38 | (comparing proportion of Caucasians to minorities) ADHD-C > ADHD-I* |
| African American | 16 | 2 | 7 | |
| Hispanic (non-Black) | 0 | 2 | 1 | |
| Asian | 0 | 2 | 0 | |
| Mixed | 0 | 0 | 0 | |
| American Indian | 1 | 1 | 0 | |
| WASI full scale IQ (SD) | 104.88 (11.79) |
105.51 (13.34) |
116.11 (14.14) |
ADHD-C < C** ADHD-I < C** |
| Parent Vanderbilt Scores (SD) | ||||
| Inattention Symptom Score | 7.54 (2.03) |
7.57 (1.82) |
0.06 (0.25) |
ADHD-C > C** ADHD-I > C** |
| Hyperactivity/Impulsivity Symptom Score |
7.06 (2.23) |
4.23 (2.76) |
0.02 (0.15) |
ADHD-C > C** ADHD-I > C** ADHD-C > ADHD-I** |
| Total Symptom Score | 14.60 (3.88) |
11.79 (3.83) |
0.09 (0.28) |
ADHD-C > C** ADHD-I > C** ADHD-C > ADHD-I** |
| Teacher Vanderbilt Scores (SD) | ||||
| Inattention Symptom Score | 7.46 (1.97) |
7.04 (1.98) |
0.19 (0.58) |
ADHD-C > C** ADHD-I > C** |
| Hyperactivity/Impulsivity Symptom Score |
7.12 (1.76) |
2.28 (2.14) |
0.28 (0.77) |
ADHD-C > C** ADHD-I > C** ADHD-C > ADHD-I** |
| Total Symptom Score | 14.58 (2.94) |
9.32 (3.07) |
0.47 (1.25) |
ADHD-C > C** ADHD-I > C** ADHD-C > ADHD-I** |
| Number with specified comorbid psychological disorder from DISC-P |
||||
| Oppositional Defiant Disorder | 22 | 16 | 0 | ADHD-C > C** ADHD-I > C* |
| Conduct Disorder | 4 | 0 | 0 | ADHD-C > C* ADHD-I > C* ADHD-C > ADHD-I* |
| Any Anxiety Disorder | 18 | 20 | 2 | ADHD-C > C** ADHD-I > C** |
| Any Mood Disorder | 1 | 1 | 0 | ns |
Note:
p<.05;
p<.01;
ns = none significant; all three comparisons were run for all variables. Only significant differences are presented.
Controls
Controls were matched to the ADHD sample according to age, gender and ethnicity. Children in the control group were recruited primarily through schools and other community settings. Controls were only admitted to the study if they demonstrated minimal symptoms of ADHD (≤3 symptoms in either ADHD symptom domain as reported on the DISC-P) and did not meet criteria for any other behavioral disorder as assessed by the DISC-P. Children in the control group demonstrated significantly higher intelligence scores than did children in either of the ADHD groups (see Table 1).
Measures
Clinical Measures
Diagnostic Interview Schedule for Children – Parent Version 4.0 (DISC-P; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000)
The DISC-P is a commonly-used structured diagnostic interview designed for use in epidemiological and clinical studies. It contains algorithms to generate diagnoses, based on rules similar to those published in the Diagnostic and Statistical Manual, Fourth Edition (American Psychiatric Association, 2000), The DISC-P has demonstrated substantial reliability and validity across a multitude of studies (Shaffer, et al., 2000) and was used as the primary diagnostic instrument of ADHD in the present study.
Vanderbilt ADHD Rating Scales (VARS; (Wolraich, Feurer, Hannah, Baumgaertel, & Pinnock, 1998)
The VARS are DSM-IV-based scales with teacher-report (VADTRS) and parent-report (VADPRS; (Wolraich, et al., 1998) forms. Parents and teachers are asked to rate DSM-IV criteria for ADHD on a four point scale. The VARS yields a symptom count score, with symptoms rated as “often” or “very often” counted as “present” (Wolraich, et al., 1998). Each form yields parallel DSM-IV based subscales of Inattention and Hyperactivity/Impulsivity, as well as an impairment scale that assesses the impact of ADHD symptoms on social, behavioral, and academic functioning. The VARS has demonstrated excellent reliability and substantial validity in previous studies (Wolraich, et al., 1998). The VARS was used in the present study as the primary means of assessing teacher-report of ADHD symptoms, and as a supplemental means of assessing parent-report of ADHD.
Wechsler Abbreviated Scale of Intelligence (WASI)
The WASI is an abbreviated standardized measure that provides IQ estimates for individuals between the ages of 6 and 89 years. It contains four subtests that provide an estimate of full-scale IQ. Studies have indicated that the WASI provides a reliable estimate of expanded intellectual performance. The full-scale IQ was used in the current study to screen out children with intellectual disability (WASI IQ < 80).
Wechsler Individual Achievement Test, Second Edition (WIAT-II)
The WIAT-II provides standardized norm-referenced academic achievement scores across a variety of subjects for individuals between the ages of 4 and 85. The Word Reading and Numerical Operations subtests were administered in the present study to provide a brief estimate of academic achievement. Children were screened out in the present study if their grade-referenced Word Reading or Numerical Operations standard scores (SS) indicated a potential learning disability (SS <80).
Neuropsychological Assessment Measures
Participants completed five computer-based neuropsychological tasks (Choice Discrimination Task, Child Attentional Network Task, Go/No-Go task, Stop Signal Task, and N-back task) that assessed a range of neuropsychological domains (e.g., response inhibition, working memory, and attention). Tasks were programmed using E-prime 1.2, and were administered on a desktop computer with a 17” monitor and response pad (Cedrus RB-834). Incentive was manipulated within tasks for all participants, such that participants were able to earn points for performance on half of the trials of each task. The incentive condition was blocked so that the child either received incentives on the 1st half of each task or the 2nd half of the task. For each task, participants were explicitly informed on which half of the trials they were able to earn points for accurate responses through both verbal (“you will earn points for this section”) and visual prompts (i.e., a green border appeared around the screen on incentive trials). At the end of each task, the number of points earned during the task was reported to the child. Participants were informed that they would be able to use the points to “purchase” incentives (i.e., toys, games, school supplies, etc.) following completion of all tasks.
Three different ERs were used within each task. Stimulus presentation was held constant at 500 msec for each task. Although some of the tasks had inter-stimulus events with varying events (e.g., warning cues during the Attentional Network Task [ANT]), the inter-stimulus intervals (ISI) were held constant across tasks so that there was either 1 sec, 3 sec, or 5 sec between stimulus presentations. Hence, the three different ERs were 1.5 sec, 3.5 sec, and 5.5 sec. Each task was divided into six continuous “blocks” of trials, with event rate (ER) varying across blocks. The three ER blocks were randomized within tasks to ensure that all three ERs occurred in a random order during the first half, or first 3 blocks, and again in a different random order the second half, or final 3 blocks. Both the ER and incentive condition order was counterbalanced across subjects. Excluding the practice trials, each task took 21 min to complete except for the Child ANT task which took 14 min, 48 sec to complete.
Choice Discrimination Task
Participants observed a continuous stream of individually-presented stimuli (i.e., circles and squares), and were asked to push a specific key for circle and another key for square. A target stimulus was presented followed by presentation of a fixation cross for the duration of the ISI. Following a 20-trial practice block, participants were presented with 6 blocks of 60 trials apiece, for a total of 360 trials. Each block contained an equal proportion of circles and squares, and the order of stimulus presentation within blocks was randomly determined. ER was varied across blocks. During incentive conditions, participants were notified that they would receive 1 point for each correct response and lose 1 point for each incorrect response.
Child Attentional Network Task (ANT; Rueda, et al., 2004)
Participants were presented with a target stimulus (a fish) either individually or in the center of a horizontal row of 5 distractor stimuli (identical fish). The row of fish either appeared on the upper portion of the screen (50%) or lower portion of the screen (50%). The task included congruent trials (target facing same direction as distractors), incongruent trials (target facing opposite direction as distractors), and neutral trials (target presented by itself). Within each condition, participants were instructed to indicate the direction of the target stimulus. Prior to each trial, participants were provided with one of four target cues: 1) a central cue (i.e., in the center of the screen), 2) a double cue (i.e., above and below the center of the screen), 3) a spatial cue (i.e., in the location in which the target will appear), or 4) no cue. See Rueda et al. (2004) for a more comprehensive description and depiction of the task. Each cue was maintained for 150 msec, followed by a 450 msec presentation of a fixation cross, followed by a 500 msec stimulus presentation, followed by presentation of a fixation cross for the duration of the ISI. Following a 20-trial practice block, participants were presented with 6 blocks of 48 trials apiece, for a total of 288 trials. Equivalent proportions of each target condition (33% congruent, 33% incongruent, 33% neutral) and cue condition (25% central cue, 25% double cue, 25% spatial cue, 25% no cue) were present within each block, with cues distributed equally among the targets. ER was varied across blocks. During the incentive condition, participants were instructed that they would receive a point for each correct response and lose a point for each incorrect response.
Go/No-Go task
The Go/No-Go task required participants to respond (i.e., pressing the spacebar) to a variety of non-target stimuli (i.e., individually presented letters on a screen) while inhibiting their response to a specific target stimuli (the letter ‘X’). Target and non-target stimuli appeared individually on the computer screen for 500 msec followed by presentation of a fixation cross for the duration of the ISI. Participants were initially presented with 20 practice stimuli, and then completed 360 trials organized in 6 continuously-presented blocks of 60 trials with ER varying across blocks. A ratio of 10% target stimuli and 90% non-target stimuli was maintained within each block. During the incentive condition, participants were informed that they would receive 1 point for each accurate response and lose 5 points for each commission error (i.e., pressing the response key in response to the letter ‘X’).
Stop-Signal task (Logan, 1994)
A fixation cross was presented in the center of a computer screen for 500 msec followed by a 500 ms presentation of a target stimulus (an airplane) facing to either the left or right. Participants were provided with a response pad, and asked to press the button that corresponded to the direction that the target stimulus was facing. However, an auditory “stop signal” (1000 Hz tone) was presented on 25% of trials within each block that required participants to inhibit their response to the visual stimulus (stop trials). The delay between presentation of the target stimulus and the tone began at 250 msec and varied according to the participant’s performance. Successful inhibition resulted in increases of 50 msec and unsuccessful inhibition resulted in decreases of 50 msec so that the rate of inhibition was controlled to approximate 50%. Following three practice blocks of 20 trials each (one block without stop-signal, two with stop-signal), participants completed 6 blocks consisting of 60 trials apiece for a total of 360 trials. ER was varied across blocks. During incentive conditions, children were instructed that they would earn 1 point for each successful response on non-stop trials and lose 4 points for each incorrect response on non-stop trials. Reaction time was recorded only for non-stop trials.
N-back task
The present study employed a 1-back design, where participants were instructed to push one button if the currently-presented letter was identical to the previous (1-back) and another button if the letter was different from the previous one. Letters were presented on the screen continuously for 500 msec followed by a fixation cross for the duration of the ISI. Following a 20-trial practice block, participants were presented with 6 blocks of 60 trials apiece, for a total of 360 trials. The target condition (identical letter as 1-back trial) was present in 30% of the trials within each block, and the three ER conditions were varied across the 6 blocks. During the incentive condition, participants were instructed that they would earn 1 point for each stimulus they correctly identified and lose 1 point for every incorrect response.
Procedures
This study was approved by the Cincinnati Children's Hospital Institutional Review Board. Participants and their parents completed three visits including one screening visit and two assessment visits. At the screening visit, informed consent was performed. Parents then completed the DISC-P. During the screening visit, children were also administered the WASI and WIAT to asses for the presence of intellectual or learning disabilities. To ensure that symptoms were present across multiple settings, teachers of participants also completed the VATRS. Neuropsychological assessment then occurred over the course of two visits to minimize the effects of participant fatigue. Tasks were administered in a counterbalanced fashion. Children were all medication naïve and remained so during all three sessions.
Statistical Analyses
For all tasks, if the percentage of omission errors exceeded 50%, performance on that task was omitted from all analyses. In order to ensure that children who had omitted data did not differ from those with a full complement of data, we separately compared children in the ADHD and control groups with omitted data to children in the ADHD and control groups with a full complement of data on the following variables: age, sex, race, WASI full scale IQ, ODD, conduct disorder, anxiety disorder, mood disorder, or parent- or teacher-rated ADHD symptom scores. Children with omitted data did not differ from those whose data was used in the analyses (all ps>.05).
Summary statistics were created as follows. For computation of summary statistics involving RTs, only RTs on successful trials were utilized. Also, all RTs less than 100 msec were excluded since the non-decision portion of simple RT is approximately 100 msec (Luce, 1986). Mean RT for each participant was computed by averaging RTs. RT standard deviation (RT SD) was derived by computing the sd of each individual’s RTs. Coefficient of variation (CV) for each participant was computed by dividing the standard deviation of the RTs by the mean RT which provides a measure of RT variability while controlling for RT speed. Percent accuracy was calculated by computing the number of correct responses divided by the number of trials. RTSYS 1.0 (Heathcote, 1996) was used to provide ex-Gaussian estimates. The Ex-Gaussian distribution has three parameters. Mu (μ) and sigma (σ) represent the mean (mu) and standard deviation (sigma) of the normal component of the distribution respectively. Tau (τ) represents the exponential component of the distribution or positive skew. For each of the five cognitive tasks, all indicators were calculated within each subject and were stratified by the three ER conditions (1.5, 3.5, and 5.5 sec ER) and two reward conditions (reward and no reward) for a total of six summary variables.
Linear mixed models were conducted using SAS PROC MIXED to test for between-subjects effects (Group; ADHD-C, ADHD-I, and controls) and within-subjects effects (ER and Reward) as well as their interactions for each of the five summary measures (RT mean, RT SD, CV, mu, sigma, and tau). These models were run separately for each of the five cognitive tasks. Linear mixed models account for the expected correlation among data points collected from the same individual. Only interactions with group were included in the model (i.e., the reward × ER interaction was not included in the models). In addition, the three-way interaction between Group, ER, and Reward was not significant in all models and thus was subsequently removed as a modeled interaction. The above analyses were considered our primary analyses. In order to control for multiple testing (i.e., multiple outcomes for each task) in our primary analyses, a false discovery rate correction (FDR) was used (Benjamini & Hochberg 1995). All p values presented for these primary analyses are the FDR corrected p values. For analyses that generated significant effects post-hoc Tukey-Kramer tests were conducted to identify differences among the three groups and effect sizes (Cohen’s d) were computed. Tables 2–6 are limited to descriptives and parametric testing for group differences for mean RT, RT SD, CV, tau, and percent accuracy outcomes across the five tasks. Supplemental tables are available online at the journal website that report descriptives and parametric test results for all outcomes across all task conditions.
Table 2.
Group Means, Standard Errors, and Group Differences for Reaction Time Mean Across the 5 Tasks
| df | ADHD-C | ADHD-I | Controls | F | p | Group Comparisons (ES) | |
|---|---|---|---|---|---|---|---|
| Choice | 141 | 742.53 (26.37) | 767.49 (32.06) | 702.81 (34.46) | 0.95 | 0.50 | ADHD-C=C (.19) ADHD-I=C (.28) ADHD-C=ADHD-I (.12) |
| ANT | 136 | 874.52 (42.31) | 767.43 (39.69) | 754.91 (41.84) | 2.46 | 0.18 | ADHD-C=C (.42) ADHD-I=C (.04) ADHD-C=ADHD-I (.37) |
| Go/No Go | 141 | 640.9 (23.56) | 617.70 (25.05) | 528.47 (27.70) | 5.08 | 0.02 | ADHD-C<C** (.64) ADHD-I<C* (.49) ADHD-C=ADHD-I (.14) |
| Stop Signal | 138 | 892.78 (48.53) | 805.80 (45.59) | 898.91 (48.52) | 1.25 | 0.41 | ADHD-C=C (.02) ADHD-I=C (.28) ADHD-C=ADHD-I (.26) |
| N-back | 116 | 896.78 (34.52) | 872.81 (29.54) | 828.83 (32.42) | 1.08 | 0.46 | ADHD-C=C (.30) ADHD-I=C (.20) ADHD-C=ADHD-I (.11) |
Note: ADHD-C = ADHD-Combined Type; ADHD-I = ADHD-Predominantly Inattentive Type; ES = Effect size (Cohen’s d); ANT = Child Attention Network Task; Group comparisons are Tukey tests;
p<.05;
p<.01;
p<.001;
p<.0001
Table 6.
Group Means, Standard Errors, and Group Differences for Percent Accuracy Across the 5 Tasks
| df | ADHD-C | ADHD-I | Controls | F | p | Group Comparisons (ES) | |
|---|---|---|---|---|---|---|---|
| Choice | 141 | 84.29(1.79) | 82.83(1.83) | 88.48(1.83) | 2.7 | 0.15 | ADHD-C=C (.34) ADHD-I=C (.46) ADHD-C= ADHD-I (.12) |
| ANT | 139 | 71.51(2.66) | 75.56(2.58) | 83.05(2.72) | 4.6 | 0.01 | ADHD-C<C** (.63) ADHD-I=C (.29) ADHD-C=ADHD-I (.33) |
| Go/No Go | 141 | 91.38(0.86) | 90.54(0.82) | 94.63(0.88) | 6.3 | 0.009 | ADHD-C<C* (.55) ADHD-I<C** (.69) ADHD-C= ADHD-I (.14) |
| Stop Signal | 141 | 79.08(2.31) | 83.87(2.24) | 90.37(2.39) | 5.8 | 0.01 | ADHD-C<C** (.71) ADHD-I=C (.40) ADHD-C=ADHD-I (.30) |
| N-back | 116 | 68.40(2.44) | 72.07(2.05) | 81.90(2.25) | 9.22 | 0.0009 | ADHD-C<NC***(.85) ADHD-I<C** (.66) ADHD-C=ADHD-I (.23) |
Note: ADHD-C = ADHD-Combined Type; ADHD-I = ADHD-Predominantly Inattentive Type; ES = Effect size (Cohen’s d); ANT = Child Attention Network Task; Group comparisons are Tukey tests;
p<.05;
p<.01;
p<.001;
p<.0001
In a set of secondary analyses testing for potential moderator variables (i.e., IQ and Anxiety Disorders/Depressive Disorders), each of these variables was added to the linear mixed models described above, one at a time. Anxiety Disorders/Depressive Disorders were dichotomous variables indicating whether the child met criteria for either of these disorders based on the DISC-P. Note that since the control group did not include any children with ODD/CD, this potential moderator could not be tested in our analyses. Significant interactions of these moderator variables with Group or higher order interactions with Group × ER or Group × Reward would indicate that between-group differences vary according to the presence of the moderating variable.
Since two of the tasks have unique variables not captured by RT and accuracy alone, we also conducted a set of secondary analyses using linear mixed models on these variables to examine main effects of Group, ER, and reward and their interactions. On the SST, Stop Signal RT was computed by subtracting the average stop signal delay from the mean hit reaction time (Logan, 1994). On the ANT task, alerting, orienting, and conflict variables were computed as follows: alerting score = median RT for no cue condition − median RT for double cue condition; orienting score = median RT for central cue condition − median RT for spatial cue condition; conflict score = median RT for incongruent cue condition − median RT for congruent cue condition (Rueda, et al., 2004).
Finally, the relationship between percent accuracy and RT variability was examined by first performing Pearson correlation coefficients between one of the RT variability indicators (i.e., CV) and percent accuracy for each of the five tasks. We selected CV as the indicator of RT variability since it inherently controls for RT speed. The entire sample of ADHD and controls was used for these correlational analyses. Then, a linear mixed model was computed examining whether any significant Group main effects on the percent accuracy variable persisted after removing variance attributable to RT variability. For all secondary analyses, a p value of .05 was used to test for significance.
Results
Reaction Time
Across the five cognitive tasks, there was only a difference in RTs across the three groups on the Go/No-Go task. See Table 2. On this task, both the ADHD-C (p<.01) and ADHD-I (p<.05) groups had slower mean RTs than children in the control group. No differences were observed between the ADHD-I and ADHD-C groups. There were no group differences in RT on the other four tasks. There was a main effect of ER across all five tasks (all ps<.001) with shorter RTs at faster ERs. On the Go/No-Go task, there was an interaction of group × ER (F(4,141)=3.51, p=.03). This interaction resulted from the ADHD-I group showing incrementally less slowing from the 3.5-sec ER (M=646.9, SE=23.2) to the 5.5-sec ER conditions (M=742.7, SE=47.1) compared to the ADHD-C group (3.5-sec: M=651.6, SE=23.2; 5.5 sec: M=816.4, SE=42.9). This interaction effect was not evident across the other tasks. No main effect of reward or group × reward interaction was significant across any of the tasks.
RT Standard Deviation
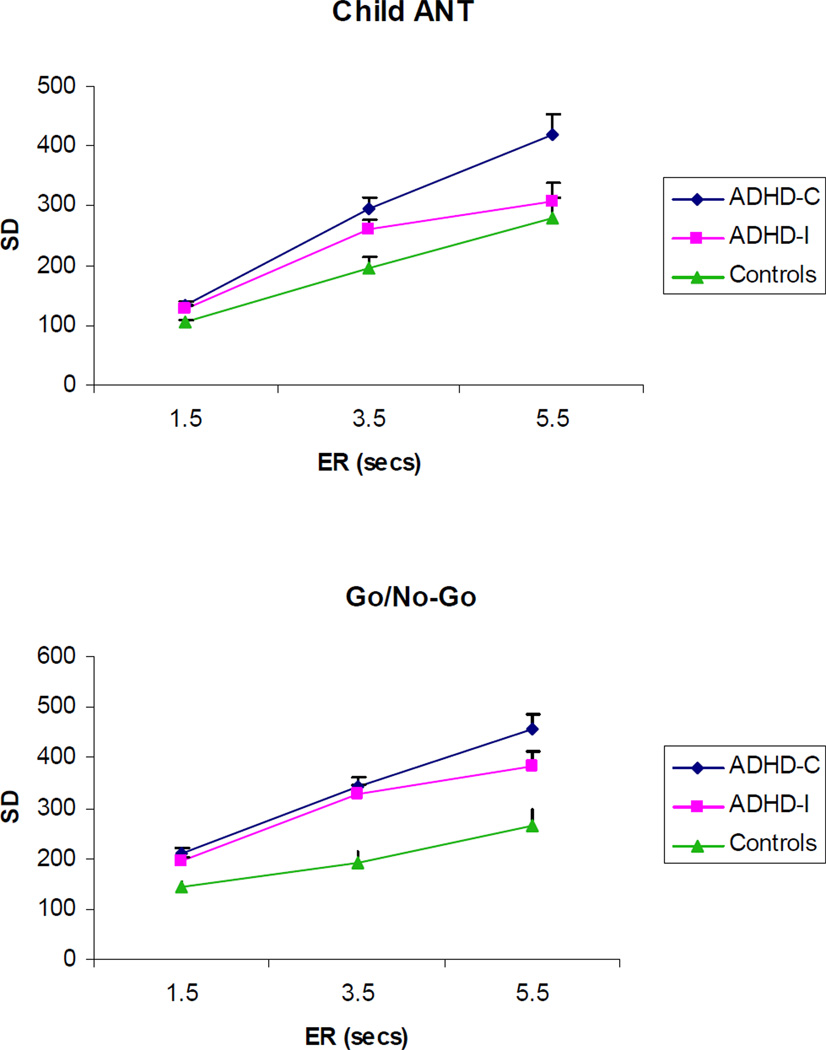
Significant group effects on SD were observed on the Choice, ANT, Go/No-Go, and N-back tasks. See Table 3. Post-hoc tests showed that the ADHD-C group had larger SDs than controls across all four tasks (all ps<.05). The ADHD-I group was also more variable than controls on the Go/No-Go task (p<.001) and Choice task (p<.05). The ADHD subtype groups did not differ across any of the tasks. Across all tasks there was a significant main effect of ER (all ps<.001). There was a significant interaction of group × ER on the ANT (F(4,136)=3.39, p=.03) and the Go/No-Go task (F(4,141)=5.00, p=.004). See Figure 2. Both interactions were a result of children with ADHD-C demonstrating a greater incremental increase in RT SD between the 3.5-sec and 5.5-sec ER conditions as compared to children with ADHD-I. No other main effects of reward or two-way interactions were significant across tasks.
Table 3.
Group Means, Standard Errors, and Group Differences for Reaction Time Standard Deviation Across the 5 Tasks
| df | ADHD-C | ADHD-I | Controls | F | p | Group Comparisons (ES) | |
|---|---|---|---|---|---|---|---|
| Choice | 141 | 245.29 (10.16) | 243.71 (12.28) | 200.20 (13.20) | 4.20 | 0.05 | ADHD-C>C* (.56) ADHD-I>C* (.49) ADHD-C=ADHD-I (.02) |
| ANT | 136 | 283.05 (15.90) | 232.15 (14.91) | 193.42 (15.72) | 8.08 | 0.002 | ADHD-C>C*** (.84) ADHD-I=C (.36) ADHD-C=ADHD-I (.47) |
| Go/No Go | 141 | 336.71 (17.35) | 300.81 (17.33) | 200.18 (18.89) | 14.93 | 0.0005 | ADHD-C>C**** (1.11) ADHD-I>C*** (.80) ADHD-C=ADHD-I (.29) |
| Stop Signal | 138 | 326.50 (17.94) | 276.11 (16.85) | 268.17 (17.94) | 3.15 | 0.11 | ADHD-C>C (.48) ADHD-I = C (.07) ADHD-C= ADHD-I (.41) |
| N-back | 116 | 323.06 (14.95) | 290.54 (12.73) | 257.49 (13.97) | 5.14 | 0.02 | ADHD-C>C** (.67) ADHD-I = C (.36) ADHD-C = ADHD-I (.33) |
Note: ADHD-C = ADHD-Combined Type; ADHD-I = ADHD-Predominantly Inattentive Type; ES = Effect size (Cohen’s d); ANT = Child Attention Network Task; Group comparisons are Tukey tests;
p<.05;
p<.01;
p<.001;
p<.0001
Figure 2.
Group (ADHD-Combined Type, ADHD-Inattentive Type, and Control) by event rate (ER; 1.5 sec, 3.5 sec, 5.5 sec) interaction effect on reaction time standard deviation (SD) indicator for the Child Attentional Network Test (ANT) and Go/No-Go task.
Coefficient of Variation
Significant group effects on CV were observed on the Choice, ANT, Go/No-Go, and N-back (all ps < .05). There was a subthreshold group effect on the SST (p = .08). See Table 4. Post-hoc tests revealed significantly higher variation in the ADHD-C group than the control group (all ps<.05). The ADHD-I group had higher variation than the control group on the Choice, ANT, Go/No-Go tasks (all ps<.05). There were no differences between the ADHD-C and ADHD-I groups. Across all tasks except the SST, there was a significant effect of ER (all ps <.05) with children demonstrating less variable RTs on the faster ER trials across all tasks. There was also a significant main effect of reward on the Go/No-Go (F(1,141)=10.32, p=.006; Cohen’s d=.38) and SST tasks (F(1,138)=17.73, p=.0005; Cohen’s d=.51) with lower CV on reward trials compared to non-reward trials. There was also a Group × Reward interaction on the SST task (F(2,138)=4.19, p=.047). Children with ADHD-C (reward: M=34.2, SE=1.7; no reward: M=38.9, SE=1.6; p=.005) and ADHD-I (reward: M=32.4, SE=1.6; no reward: M=36.7, SE=1.5; p=.005) demonstrated more variability in the no reward condition compared to the reward condition. There was no difference in performance between the reward and no reward conditions for children in the control group (reward: M=31.0, SE=1.7; no reward: M=31.0, SE=1.6; p=.99). No other main effects of reward or two-way interactions were significant across tasks.
Table 4.
Group Means, Standard Errors, and Group Differences for Coefficient of Variation Indicator Across the 5 Tasks
| df | ADHD-C | ADHD-I | Controls | F | p | Group Comparisons (ES) | |
|---|---|---|---|---|---|---|---|
| Choice | 141 | 34.44(.94) | 31.34(.90) | 27.36(.96) | 13.95 | 0.0005 | ADHD-C>C**** (1.10) ADHD-I>C** (.62) ADHD-C = ADHD-I (.48) |
| Go/No Go | 141 | 47.87(1.68) | 44.44(1.61) | 35.43(1.73) | 14.07 | 0.0005 | ADHD-C>C**** (1.07) ADHD-I>C*** (.64) ADHD-C=ADHD-I (.25) |
| Stop Signal | 138 | 36.53(1.52) | 34.557(1.42) | 30.99(1.52) | 3.44 | 0.08 | ADHD-C>C* (.54) ADHD-I=C (.35) ADHD-C=ADHD-I (.19) |
| ANT | 136 | 31.69(1.12) | 28.47(1.05) | 24.79(1.11) | 9.59 | 0.0005 | ADHD-C>C**** (.91) ADHD-I>C* (.49) ADHD-C=ADHD-I (.42) |
| N-back | 116 | 36.17(1.32) | 33.20(1.10) | 30.99(1.21) | 4.20 | 0.05 | ADHD-C>C* (.60) ADHD-I=C (.27) ADHD-C=ADHD-I (.35) |
Note: ADHD-C = ADHD-Combined Type; ADHD-I = ADHD-Predominantly Inattentive Type; ES = Effect size (Cohen’s d); ANT = Child Attention Network Task; Group comparisons are Tukey tests;
p<.05;
p<.01;
p<.001;
p<.0001
Ex-Gaussian Indicators
Mu
The only significant effect for the ex-Gaussian indicator mu was for the main effect of ER across all five tasks (all ps<.05). As ER slowed, mu increased. No other main or interaction effects were significant.
Sigma
The only significant effect for the ex-Gaussian indicator sigma was for the main effect of ER on all tasks (all ps < .05) except for the Choice task (p=.09). As ER slowed, sigma increased. No other main or interaction effects were significant across tasks.
Tau
There were significant main effects of Group across all five tasks. See Table 5. Children in the ADHD-C group had higher tau estimates than controls across all five tasks (all ps<.01). The ADHD-I group had higher tau estimates than controls on all tasks except the ANT (all ps<.05). The only task that showed a difference between the ADHD-C and ADHD-I group was on the ANT task (p<.05) where the ADHD-C group had higher estimates than the ADHD-I group. Similarly there were significant main effects of ER across the five tasks (all ps < .001). There was a significant main effect of reward on the Go/No-Go (F(1,141)=8.70, p=.01; Cohen’s d=.35), SST (F(1,138)=6.44, p=.04; Cohen’s d=.31), and N-back tasks (F(1,116)=6.13, p=.04; Cohen’s d=.33) indicating lower indicators of tau on reward trials. The main effect of reward on the Choice Discrimination (F(1,141)=6.26, p=.09) was marginally significant. The only significant interaction effect was the group × ER interaction on the ANT task (F(4,135)=3.88, p=.02). The interaction effect was similar at that found for the group × ER interaction on the ANT task for the RT SD indicator (see Figure 2). In summary, the three groups performed similarly in the 1.5-sec ER condition (ADHD-C: M=53.1, SE=3.6; ADHD-I: M=57.4, SE=3.4; Controls: M=49.6, SE=3.7) but the ADHD-C group showed increasingly more variability as indicated by tau than the controls at the 3.5-sec (ADHD-C: M=248.9, SE=15.9; ADHD-I: M=208.1, SE=14.6; Controls: M=173.9, SE=15.6) and 5.5-sec ER conditions (ADHD-C: M=332.5, SE=21.6; ADHD-I: M=257.5, SE=20.0; Controls: M=208.7, SE=21.5). The ADHD-I group’s tau scores fell between the ADHD-C and control groups’ tau scores.
Table 5.
Group Means, Standard Errors, and Group Differences for Ex-Gaussian tau Indicator Across the 5 Tasks
| df | ADHD-C | ADHD-I | Controls | F | p | Group Comparisons (ES) |
|
|---|---|---|---|---|---|---|---|
| Choice | 141 | 192.32(8.18) | 188.05(8.73) | 138.91(9.36) | 10.79 | 0.0005 | ADHD-C>C****(.90) ADHD-I>C***(.78) ADHD-C=ADHD-I (.07) |
| ANT | 135 | 211.5(11.18) | 174.35 (10.40) | 144.03(11.12) | 9.18 | 0.0009 | ADHD-C>C*** (.89) ADHD-I = C (.41) ADHD-C>ADHD-I* (.49) |
| Go/No Go | 141 | 243.77(13.77) | 223.61(13.73) | 160.97(14.68) | 9.06 | 0.0009 | ADHD-C>C**** (.86) ADHD-I>C** (.63) ADHD-C= ADHD-I (.21) |
| Stop Signal | 138 | 251.65(15.75) | 225.50(14.76) | 161.51(15.66) | 8.78 | 0.001 | ADHD-C>C*** (.85) ADHD-I>C** (.61) ADHD-C=ADHD-I (.24) |
| N-back | 116 | 247.36(18.20) | 224.61(15.31) | 157.72(16.67) | 7.47 | 0.004 | ADHD-C>C** (.76) ADHD-I>C* (.60) ADHD-C=ADHD-I (.19) |
Note: ADHD-C = ADHD-Combined Type; ADHD-I = ADHD-Predominantly Inattentive Type; ES = Effect size (Cohen’s d); ANT = Child Attention Network Task; Group comparisons are Tukey tests;
p<.05;
p<.01;
p<.001;
p<.0001
Accuracy
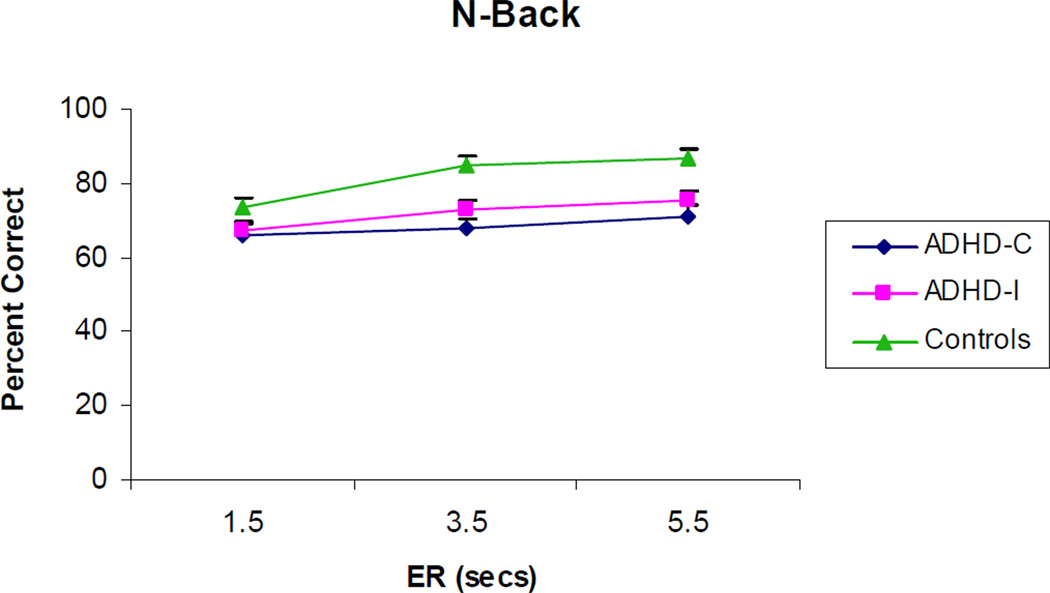
Significant main effects of Group on the accuracy variable were observed on the ANT, Go/No-Go, SST, and N-back tasks. Children in the ADHD-C group were less accurate than controls across these four tasks (all ps < .05; See Table 6). Children in the ADHD-I group were less accurate than controls on two of the tasks (i.e., Go/No-Go & SST; both ps<.05). There were no differences between the ADHD subtypes across tasks. Significant main effects of ER were also observed across all of the tasks (all ps < .001) except the Go/No-Go task. Children made more errors as ER became faster. A main effect of reward was found on the ANT (F(1,139)=7.04, p=.03; Cohen’s d=.24), Go/No-Go (F(1,141=7.25, p=.03; Cohen’s d=.38) and SST tasks (F(1,141)=20.76, p=.0005; Cohen’s d=.51). The main effect of reward on the Choice Discrimination task (F(1,141)=5.04, p=.07) was marginally significant. Accuracy improved with reward presence. Finally, there was a significant group × ER interaction on the N-back task (F(4,116)=3.28, p=.04). See Figure 3. Children in the control group demonstrated a greater benefit at slower ERs compared to children in the ADHD groups. No other interaction effects were statistically significant.
Figure 3.
Group (ADHD-Combined Type, ADHD-Inattentive Type, and Control) by event rate (ER; 1.5 sec, 3.5 sec, 5.5 sec) interaction effect for task accuracy on the N-back task.
Other Performance Indicators
The SST and ANT tasks have additional outcome measures that were estimated in the context of this study. Stop Signal Reaction Time (SSRT) was computed on the SST. A main effect of group (F(2,140)=3.37, p=.04) was found. The ADHD-C group had a longer SSRT than the control group (p=.03). There was also a main effect of reward (F(1,140)=4.30, p=.04) and ER (F(2,140)=61.56, p<.0001) due to children having shorter SSRTs during the reward condition and as ER became faster.
On the ANT task, scores were computed for alerting, orienting, and conflict. For the alerting variable, there was only a main effect of ER (F(2,138)=41.13, p<.0001) indicating a lesser effect of alerting with decreasing ER. For the orienting variable, there were no significant main or interaction effects. Finally, for the conflict variable, there was a main effect of group (F(2, 138)=3.77, p=.03) and a main effect of ER (F(2,138)=34.42, p<.0001). The main effect of group resulted from higher conflict scores in the ADHD-C group compared to controls. The main effect of ER was significant because conflict scores decreased as ER became faster. No interaction effects were significant.
Do patient characteristics moderate between-group differences?
Separate models were conducted controlling for gender, IQ, and anxiety/depression comorbidity. For any significant main effect of group or interaction effect reported above, none of the aforementioned moderator variables significantly interacted with the reported effects.
Does CV account for ADHD-related deficits in accuracy?
Measures of accuracy and RT variability (i.e., CV) were significantly correlated across all tasks (Choice Discrimination task: r=−.27; ANT task: r=−.17; Go/No-Go task: r=−.48, all ps<.0001; Stop-Signal task: r=−.44; N-back task: r=−.21; all ps<.0001). Mixed effect analyses were conducted testing between-group differences on the accuracy measures after variance attributable to RT variability, was removed. For the four tasks that displayed between-group differences in accuracy performance (i.e., ANT Go/No-Go, N-Back, and SST), significant between group differences in accuracy remained after removing variance attributable to RT variability for the Go/No-Go task (F(2,141)=5.98, p=.003), SST (F(2,138)=5.00, p=.008) and the N-back task (F(2,116)=8.98, p=.0002). However, there was no longer a significant between group difference on the ANT (F(2,136)=2.17, p=.12) after this variance was removed. Between-group differences in accuracy remained non-significant for the Choice Discrimination (F(2,141)=2.24, p=.11).
Discussion
Consistent with predictions, children with ADHD demonstrated significant elevations in RT variability compared to controls. Increased variability among children with ADHD was evident across a range of cognitive tasks assessing a variety of cognitive skills (i.e., working memory, response inhibition, and attention). Contrary to our predictions, ADHD-related RT variability elevations were not consistently moderated by task or subject variables. Children with ADHD demonstrated increased RT variability across a variety of ER and reward conditions designed to enhance subject arousal. Further, subject variables that could possibly have affected RT variability, such as ADHD subtype, psychiatric comorbidity, and intelligence did not moderate between-group differences in RT variability across tasks. The robust nature of RT variability elevations observed in children with ADHD suggests dysfunction in a core cognitive process that is utilized across cognitive skills and is not consistently affected by experimental manipulations that attempted to target arousal.
Multiple indicators of RT variability were used including RT SD, CV, and tau. While the CV and RT SD indicators demonstrated larger magnitude between-group effect sizes than tau on some tasks (see Go/No-Go task), tau was the only RT variability indicator that identified group differences across all tasks. The ubiquitous across-task between-group differences on the tau variable is consistent with the previous literature where tau has been shown to be sensitive to ADHD-related between group differences on choice discrimination tasks (Leth-Steensen, et al., 2000) and Go/No-Go tasks (Hervey, et al., 2006; Vaurio, et al., 2009). Also, the magnitude of between-group effect sizes on the tau indicator were comparable to effect sizes of tau in previous studies (e.g., Hervey, et al., 2006). The present study extends the utility of the ex-Gaussian tau indicator to other response inhibition tasks (e.g., SST) as well as attentional network tasks and working memory tasks. It appears that irrespective of the cognitive construct being targeted on speeded (i.e., event rate less than 6 sec) cognitive tasks, children with ADHD will demonstrate an irregular pattern of RTs characterized by periodic long RTs. While some have posited that these intermittent long RTs may represent attentional lapses (Hervey, et al., 2006; Leth-Steensen, et al., 2000), the nature of these long RTs continues to be unknown. Research examining behavioral correlates of RT variability may help in better understanding RT variability (Rapport, Kofler, Alderson, Timko, & DuPaul, 2009). In addition, more psychophysiological research is needed to identify the neural correlates of RT variability (Bellgrove, et al., 2004; Weissman, Roberts, Visscher, & Woldorff, 2006).
The effect of ER on performance was mostly consistent across tasks and across performance indicators. Faster ER decreased RT variability across tasks for all children. This pattern is consistent with the neuropsychological literature (Sanders, 1983; Sergeant, 2005). In our analyses examining for any differential effect of ER across groups, we did not find significance on the vast majority of our models. Where effects were found (i.e., Mean RT on Go/No-Go, RT SD on ANT and Go/No-Go, and tau on ANT), the interaction effects were largely attributable to the ADHD groups performance at the slower ERs. That is, at faster ERs, children in the ADHD and control groups performed similarly. This may be attributable to the use of a 1.5-sec ER which may have restricted RTs during these blocks. But as ER slowed, children in the ADHD groups demonstrated slower RTs and more RT variability on the respective tasks. Interestingly, the deleterious effects of the slowest ER seemed to have a greater effect on children in the ADHD-C group than children in the ADHD-I group (see Figure 2). Much of the existing research literature demonstrates that slowing ER has a more detrimental effect on RT variability in children with ADHD compared to controls (see Sonuga-Barke, et al., 2010 for review; also see Wiersema, et al., (Wiersema, van der Meere, Antrop, & Roeyers, 2006 for exceptions). One explanation for our failure to obtain significant group × ER interaction effects is the range of ERs used in this study. Although the 1.5 sec lower end of the ER manipulation is likely as fast an ER as can be tolerated, the upper bound ER used in this study was 5.5 sec. This “slow” ER may not have fully tested the upper bound effects of slow ER on performance. Indeed, several studies have used ERs as long as 8 sec and found increasingly longer RT variability in children with ADHD compared to controls at these long ERs (Andreou, et al., 2007). Another methodological explanation for the lack of group × ER interaction effects is that ER varied across blocks (i.e., every 60 trials) in the present study as opposed to having a long stimulus array with the same ER. Research has shown that varying ER frequently within task (i.e., jittering) both improves RT variability across all children (Wodka, Simmonds, Mahone, & Mostofsky, 2009) and differentially improves RT variability in children with ADHD compared to controls (Ryan, Martin, Denckla, Mostofsky, & Mahone, 2010). Hence, the present study may have inadvertently produced a “jittering effect” and attenuated any detrimental effect of long ERs on our RT variability outcomes. Future research studies will need to utilize a greater range of ERs in order to more fully test the effects of long ERs on RT variability and task performance. However, given that optimal ER and individual’s perceptions of ERs may differ across individuals and across tasks, some have argued that what is needed are studies that adjust ER in response to performance in order to identify optimal and sub-optimal states (Sonuga-Barke, et al., 2010).
We found that reward did have some effect on RT variability as measured by CV and tau indicators on some of the tasks. In particular, reward appeared to decrease RT variability on the Go/No-Go, SST, and N-back tasks. Tau values across all groups tended to be highest on these three tasks (see Table 5) suggesting that these tasks were most susceptible to intermittent long RTs for all children. All three of these tasks require higher order executive functioning (e.g., response inhibition, working memory) for successful task performance. Whereas the two tasks where reward main effects on RT variability were not observed (i.e., Choice and ANT) primarily involve non-executive functioning brain processes (Cabeza, & Nyberg, 2000). This pattern of findings may indicate that reward is effective at reducing periodic long RTs on tasks involving higher order executive functioning. Alternatively, ceiling effects on the tau variable on the Choice and ANT tasks may have prevented detection of reward effects. We found one group × reward interaction for RT variability indicators across tasks (i.e., CV on SST). On this task, the two ADHD groups demonstrated a greater benefit of reward than the controls. In fact, when reward was introduced, their RT variability as indicated by CV was comparable to the control group. This is the type of benefit that was predicted and has been previously reported (Andreou, et al., 2007; Kuntsi, et al., 2009; Slusarek, et al., 2001; Uebel, et al., 2010). But this effect was only observed on one indicator on one task. Hence, our results seem to support the several studies that have not found a differential effect of reward on RT variability across groups (Desman, et al., 2008; Luman, et al., 2008; Shanahan, et al., 2008). However, it is possible that in our study the focus and/or saliency of the reward condition attenuated our ability to detect effects of reward and interaction with group. Participants were told that rewards were based on task accuracy (i.e., earn and lose points for correct and incorrect responses respectively). Changing the focus of the rewards to RT speed or RT variability may have altered the outcome variable where reward effects were observed. In fact, Andreou et al. (2007) demonstrated that conditionally rewarding participants based on RT variability produced significant reductions in RT variability, particularly among children with ADHD. Our reward condition may also have lacked saliency for the participants with ADHD. Certainly, several prevailing ADHD theoretical models emphasize the need for immediate versus delayed rewards for children with ADHD (Barkley, 1997; Sagvolden, et al., 2005). However, empirical tests of this supposition have not always supported this hypothesis (e.g., Michel, Kerns, & Mateer, 2005). In this study, children earned points to be applied towards the purchase of a material reward that they would receive in the future. It is possible that the schedule of delayed rewards may have attenuated the effects of reward for children with ADHD and may explain why we found no significant group × reward interaction effects.
We found minimal differences between the children with ADHD-C and ADHD-I on RT variability. In general, however, effect sizes between ADHD-C group and controls tended to be larger in magnitude than effect sizes between ADHD-I and controls. Also, there were more significant differences between ADHD-C and controls than between ADHD-I and controls. However, when directly comparing the subtypes on the various indicators, there was a significant difference between the subtypes only on one comparison (i.e., the tau indicator for the ANT task). There were also no subtype differences on RT speed or accuracy. The lack of differences between the ADHD-C and ADHD-I groups on RT speed, RT variability, and accuracy was predicted and is consistent with many previous studies examining these differences (Nigg, et al., 2002; Shanahan, et al., 2008; Simmonds, et al., 2007; Vaurio, et al., 2009). The few studies that have found differences have shown that children diagnosed with ADHD-C have greater RT variability than children with ADHD-I (de Zeeuw, et al., 2008; Mullins, et al., 2005). However, Mullins et al. (2005) found differences on a time reproduction task which was not used in the present study. The subtype differences demonstrated by de Zeeuw et al. (2008) were with a SST similar to the one used in this study. There were some minor differences in the way the current sample subtypes were defined across these two studies. The discrepant results regarding subtype differences across these two studies are ultimately unclear.
Using the same tasks, we also examined whether children with ADHD demonstrate deficits in performance accuracy. On all tasks except the Choice Discrimination task, children with ADHD demonstrated higher rates of errors than controls. The Choice Discrimination task and the SST both involve a forced choice discrimination yet children with ADHD only showed a deficit in task accuracy on the SST. The primary difference between the two tasks is that the SST additionally requires participants to inhibit responding to selected trials. It is possible that requiring additional executive processing during a task exacerbates error rates for children with ADHD. Increasing task difficulty by speeding ER had a detrimental effect on error rates across all tasks but only on the N-back task did ER interact with group status. On the N-back task, children in all groups demonstrated similar accuracy rates in the fast ER condition. However, when ER slowed, the control group’s accuracy improved whereas the children in the ADHD groups did not benefit from the slower ERs. Based on accuracy rates across tasks, the N-back was the most difficult task for children across all groups. The observed lack of benefit from slowing ER on the N-back combined with what were the largest between group effect sizes for the ADHD-C and ADHD-I groups compared to the control group may confirm the observed large and pervasive ADHD-related deficits in working memory (Martinussen, Hayden, Hogg-Johnson, & Tannock, 2005). Finally, the presence of reward, which improved accuracy across groups across some of the tasks (i.e., ANT, Go/No-Go, and SST) did not differentially affect the ADHD and control groups. Again, the lack of moderating effects of ER or reward on group differences must be interpreted in the context of the limitations of the ER and reward manipulations discussed above.
Finally, consistent with Klein et al. (2006), we found that task accuracy and RT variability were highly correlated but that task accuracy seemed to account for mostly unique between-group variance. That is, on three (i.e., Go/No-Go, SST, and N-back) of the four tasks where group differences in accuracy were found, these difference remained after partialing out variance attributable to RT variability. On the ANT task, however, a different pattern was observed, whereby between-group differences were no longer significant once variance attributable to RT variability was partialled out. In comparison, Klein et al. (Klein, et al., 2006) found that between-group differences in error rates remained after controlling for RT variability on a continuous performance task (CPT). It is unclear why a different pattern emerges between two purported attentional tasks (i.e., ANT and CPT) across the two studies. Differences in study methods may account for differences in results across studies. In particular, Klein et al.’s (2006) sample primarily consisted of children with ADHD-C. Findings from our study suggest that between-group effect sizes comparing ADHD subtypes to the control group on measures of accuracy were larger for the ADHD-C group than the ADHD-I group (see Table 6). Hence, between-group effect sizes on accuracy were larger in Klein et al.’s (2006) study (e.g., .85 for CPT commission errors) compared to our results which could have contributed to their findings of persistent between-group effect on accuracy outcomes even after controlling for RT variability. Overall, the prevalent pattern of findings across the two studies suggests that RT variability and task accuracy both uniquely account for between-group differences. This is consistent with a myriad of research demonstrating that not all children with ADHD have the same pattern of cognitive impairments (Solanto, et al., 2001). Indeed, ADHD-related deficits are likely multi-dimensional (Nigg, 2005; Willcutt, et al., 2005) and there are likely multiple pathways to ADHD manifestation (Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005; Solanto, et al., 2001; Sonuga-Barke, 2003).
This set of interpretations must, of course, be viewed in the context of the current study’s limitations. We have noted above some of the limitations with our task manipulations, including the limited saliency of our reward condition, and limited range of ER manipulations. We also acknowledge that although we included five different tasks which target a range of neuropsychological functions, there are other task paradigms that have been used in the existing literature (e.g., continuous performance tasks; Klein et al., 2006) that were not used in the present study. There are also study limitations related to our group variable. First, the ADHD group included only two of the three ADHD subtypes. The ADHD Predominantly Hyperactive-Impulsive subtype was not included in this study due to the low prevalence rate of this subtype (Froehlich, et al., 2007) and the questionable diagnostic stability of this subtype (Lahey, Pelham, Loney, Lee, & Willcutt, 2005). Nevertheless, excluding this subtype precludes drawing conclusions across all three of the ADHD subtypes. Also, we attempted to recruit a comparable group of healthy controls by matching to our ADHD group on age, gender, and ethnicity. Ultimately, the control group had IQs that were both above normal (mean IQ = 116) and higher than children in the ADHD groups. This discrepancy in IQ scores across groups may have exacerbated between-group differences. Many neuropsychological studies comparing children with ADHD to controls find IQ discrepancies (see Barkley, 2006 for review). Whether to control for IQ differences is controversial since poor performance on IQ tests is likely affected by ADHD-related cognitive deficits (e.g., inattention) while performing IQ tests (Willcutt, et al., 2005). Another limitation of our control group is that we used only typically-developing children and did not include a group with psychopathology. As a result, we are not able to conclude whether the between-group differences are unique to the ADHD condition. In a recent study, Geurts et al. (2008) showed that children with ADHD and comorbid autism spectrum disorders had elevated RT variability. In this study, we did not measure ASD and thus cannot replicate this finding in our analyses. It is also possible that other disorders comorbid with ADHD, such as ODD/CD which could not be tested in our analyses, may have contributed to the observed between-group differences.
In summary, the present study found robust between-group differences indicating that children with ADHD exhibit significantly more RT variability than controls on a variety of neuropsychological tasks. In general, these effects were task-independent and not moderated by any task factor or moderating variable including ADHD subtype. This pattern of results was demonstrated in a medication-naïve sample of children with ADHD suggesting that higher RT variability is likely attributable to the ADHD condition as opposed to any possible effects of medication history on brain function (Andersen, 2005).
Supplementary Material
Acknowledgments
Funding for this study was provided by NIH (R01MH074770). This research was also supported by a Mid-Career Investigator Award in Patient Oriented Research (PI: Epstein; K24 MH064478) and two Mentored Patient-Oriented Research Career Development Awards from the National Institute of Mental Health (PIs: Brinkman & Froehlich; K23 MH083881 & K23 MH083027).
References
- Andersen SL. Stimulants and the developing brain. Trends in Pharmacological Sciences. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, et al. Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychological Medicine. 2007;37(12):1703–1715. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (IV-Text Revision) Washington, DC: Author; 2000. [Google Scholar]
- Barkley R. ADHD and the Nature of Self-control. New York: Guilford Press; 1997. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York: The Guilford Press; 2006. [Google Scholar]
- Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: Evidence from a response inhibition task. Neuropsychologia. 2004;42:1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57(1):298–300. [Google Scholar]
- Buzy W, Medoff DR, Schweitzer JB. Intra-individual variability among children with ADHD on a working memory task: An ex-Gaussian approach. Child Neuropsychology. 2009;2:1–19. doi: 10.1080/09297040802646991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Scheres A, Di Martino A, Hyde C, Walters JR, et al. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biological Psychiatry. 2005;57(11):1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of Attention-Deficit/Hyperactivity Disorder: The search for endophenotypes. Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- de Zeeuw P, Aarnoudse-Moens C, Bijlhout J, Konig C, Post Uiterweer A, Papanikolau A, et al. Inhibitory performance, response speed, intraindividual variability, and response accuracy in ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(7):808–816. doi: 10.1097/CHI.0b013e318172eee9. [DOI] [PubMed] [Google Scholar]
- Desman C, Petermann F, Hampel P. Deficit in response inhibition in children with attention deficit/hyperactivity disorder (ADHD): impact of motivation? Child Neuropsychology. 2008;14(6):483–503. doi: 10.1080/09297040701625831. [DOI] [PubMed] [Google Scholar]
- Douglas V, Peters K. Toward a clearer definition of the attentional deficit of hyperactive children. In: Hale G, Lewis M, editors. Attention and the development of cognitive skills. New York: Plenum Press; 1978. pp. 173–248. [Google Scholar]
- Epstein JN, Hwang ME, Antonini T, Langberg JM, Altaye M, Arnold LE. Examining predictors of reaction times in children with ADHD and normal controls. Journal of the International Neuropsychological Society. 2009;16:1–10. doi: 10.1017/S1355617709991111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich T, Lanphear B, Epstein J, Barbaresi W, Katusic S, Kahn R. Prevalence and treatment of Attention-Deficit/Hyperactivity Disorder in a national sample of U.S. children. Archives of Pediatrics and Adolescent Medicine. 2007;161:857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Grasman RP, Verte S, Ooserlaan J, Roeyers H, van Kammen SM, et al. Intra-individual variability in ADHD, autism spectrum disorders and Tourette's Syndrome. Neuropsychologia. 2008;46:3030–3041. doi: 10.1016/j.neuropsychologia.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Heathcote A. RTSYS: A DOS application for the analysis of reaction time data. Behavior Research Methods, Instruments, & Computers. 1996;28:427–445. [Google Scholar]
- Hervey A, Epstein JN, Curry JF, Tonev S, Arnold LE, Conners CK, et al. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychology. 2006;12:125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Kelly SP, Bellgrove MA, Barry E, Cox M, Gill M, et al. Response variability in Attention Deficit Hyperactivity Disroder: Evidence for neuropsychological heterogeneity. Neuropsychologia. 2007;45:630–638. doi: 10.1016/j.neuropsychologia.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Karatekin C. A test of the interity of the components of Baddeley's model of working memory in attention-deficit/hyperactivity disorder (ADHD) Journal of Child Psychology and Psychiatry. 2004;45:912–926. doi: 10.1111/j.1469-7610.2004.t01-1-00285.x. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60(10):1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Oosterlaan J, Stevenson J. Psychological mechanisms in hyperactivity. I. Response inhibition deficit, working memory impairment, delay aversion, or something else? Journal of Child Psychology and Psychiatry. 2001;42:199–210. [PubMed] [Google Scholar]
- Kuntsi J, Wood AC, Van Der Meere J, Asherson P. Why cognitive performance in ADHD may not reveal true potential: findings from a large population-based sample. Journal of the International Neuropsychological Society. 2009;15(4):570–579. doi: 10.1017/S135561770909081X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Lee S, Willcutt E. Instability of the DSM-IV subtypes of ADHD from preschool through elementary school. Archives of General Psychiatry. 2005;62:896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol (Amst) 2000;104(2):167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychologial Assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, vanEngeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: Deficient inhibitory motor control? Journal of Abnormal Psychology. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: A users' guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. San Diego: Academic Press; 1994. pp. 189–239. [Google Scholar]
- Luce RD. Response times: Their role in inferring elementary mental organization. New York: Oxford University Press; 1986. [Google Scholar]
- Luman M, Ooserlaan J, Sergeant J. Modulation of response timein in ADHD, effects of reinforcement valence and magnitude. Journal of Abnormal Child Psychology. 2008;36:445–456. doi: 10.1007/s10802-007-9190-8. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(4):377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Michel JA, Kerns KA, Mateer CA. The effect of reinforcement variables on inhibition in children with ADHD. Child Neuropsychology. 2005;11:295–302. doi: 10.1080/092970490911270. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Pascualvaca DM, Duncan CC, French LM. A model of attention and its relation to ADHD. Mental Retardation & Developmental Disabilities Research Reviews. 1999;5:169–176. [Google Scholar]
- MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 1999;56(12):1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Mullins C, Bellgrove MA, Gill M, Robertson IH. Variability in time reproduction: Difference in ADHD Combined and Inattentive subtypes. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:169–176. doi: 10.1097/00004583-200502000-00009. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: The state of the field and salient challenges for the coming decade. Biological Psychiatry. 2005;57:1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV ADHD subtypes. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(1):59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt E, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: Do we need neuropsychologically impaired subtypes. Biological Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Quay HC. Attention deficit disorder and the behavioral inhibition system: The relevance of the neuropsychological theory of Jeffrey A Gray. In: Sergeant LMBJ, editor. Attention deficit disorder: Criteria, cognition, and intervention. Oxford, England: Pergamon Press; 1988. [Google Scholar]
- Rapport MD, Kofler MJ, Alderson RM, Timko TM, DuPaul GJ. Variability of attention processes in ADHD: Observations from the classroom. Journal of Attention Disorders. 2009;12:563–573. doi: 10.1177/1087054708322990. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, et al. Mapping motor inhibition Conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rubia K, Taylor E, Smith AB, Oksanen H, Overmeyer S, Newman S. Neuropsychological analyses of impulsiveness in childhood hyperactivity. British Journal of Psychiatry. 2001;179:138–143. doi: 10.1192/bjp.179.2.138. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, et al. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Ryan M, Martin R, Denckla MB, Mostofsky SH, Mahone EM. Interstimulus jitter facilitates response control in children with ADHD. Journal of the International Neuropsychological Society. 2010;16:388–393. doi: 10.1017/S1355617709991305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA, Sagvolden T, Johansen EB, et al. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences. 2005;28(3):397–419. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Sanders A. Towards a model of stress and human performance. Acta Psychologia. 1983;53:61–97. doi: 10.1016/0001-6918(83)90016-1. [DOI] [PubMed] [Google Scholar]
- Schachar R, Tannock R, Marriott M, Logan G. Deficient Inhibitory Control in Attention Deficit Hyperactivity Disorder. Journal of Abnormal Child Psychology. 1995;23:411–437. doi: 10.1007/BF01447206. [DOI] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Sergeant JA. Response execution and inhibition in children with AD/HD and other disruptive disorders: the role of behavioural activation. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2001;42(3):347–357. [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. The top and the bottom of ADHD: a neuropsychological perspective. Neuroscience and Biobehavioral Reviews. 2003;27(7):583–592. doi: 10.1016/j.neubiorev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Sergeant JA. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biological Psychiatry. 2005;57(11):1248–1255. doi: 10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shanahan MA, Pennington BF, Willcutt EF. Do motivational incentives reduce inhibition deficits in ADHD? Developmental Neuropsychology. 2008;33:137–159. doi: 10.1080/87565640701884238. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH, et al. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45(9):2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Slusarek M, Velling S, Bunk D, Eggers C. Motivational effects on inhibitory control in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(3):355–363. doi: 10.1097/00004583-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, et al. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. Journal of Abnormal Child Psychology. 2001;29(3):215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neuroscience and Biobehavioral Reviews. 2003;27(7):593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biological Psychiatry. 2005;57(11):1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosciences and Biobehavioral Reviews. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Wiersema JR, van der Meere JJ, Roeyers H. Context-dependent dynamic processes in Attention Deficit/Hyperactivity Disorder: Differentiating commong and unique effects of state regualtion deficits and delay aversion. Neuropsychology Review. 2010;20:86–102. doi: 10.1007/s11065-009-9115-0. [DOI] [PubMed] [Google Scholar]
- Uebel H, Albrecht B, Asherson P, Borger NA, Butler L, Chen W, et al. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. The Journal of Child Psychology and Pscyhiatry. 2010;51:210–218. doi: 10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meere J, Marzocchi GM, De Meo T. Response inhibition and Attention Deficit Hyperactivity Disorder with and without Oppositional Defiant Disorder screened from a community sample. Developmental Neuropsychology. 2005;28:459–472. doi: 10.1207/s15326942dn2801_1. [DOI] [PubMed] [Google Scholar]
- Vaurio RG, Simmonds DJ, Mostofsky SH. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47(12):2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses of attention. Nature Neuroscience. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wiersema JR, van der Meere J, Antrop I, Roeyers H. State regulation in adult ADHD: An event-related potential study. Journal of Clinical and Experimental Neuropsychology. 2006;7:1113–1126. doi: 10.1080/13803390500212896. [DOI] [PubMed] [Google Scholar]
- Wiersema R, van der Meere J, Roeyers H, Van Coster R, Baeyens D. Event rate and event-related potentials in ADHD. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47(6):560–567. doi: 10.1111/j.1469-7610.2005.01592.x. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wodka EL, Simmonds DJ, Mahone EM, Mostofsky SH. Moderate variability in stimulus presenation improves motor response control. Journal of Clinical and Experimental Neuropsychology. 2009;31:483–488. doi: 10.1080/13803390802272036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolraich ML, Feurer ID, Hannah JN, Baumgaertel A, Pinnock TY. Obtaining systematic teacher reports of disruptive behavior disorders utilizing DSM-IV. Journal of Abnormal Child Psychology. 1998;26:141–152. doi: 10.1023/a:1022673906401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.