Abstract
Gegen-Qinlian-Tang (GQT), a popular Chinese medicine prescription, consists of Puerariae Radix, Scutellariae Radix, Coptidis Rhizoma, and Glycyrrhizae Radix. This study investigated the pharmacokinetics of GQT in rats and compared the bioavailability between two dosage forms, that is, traditional decoction (TD) and concentrated powder (CP). Rats were given TD and CP of GQT in a crossover design, and blood samples were withdrawn at predetermined time points. The quantitation methods of ten constituents in two dosage forms of GQT and in serum specimen using HPLC were developed and validated in this study. The pharmacokinetic parameters were calculated using noncompartment model. The results showed that daidzein, baicalein, wogonin, berberine, palmatine, and coptisine were not found in the circulation, whereas the sulfates/glucuronides of daidzein, baicalein, and wogonin were the major forms after oral administration of GQT. Comparison between two dosage forms indicated that the AUC0–t of daidzein sulfates/glucuronides after administration of CP was significantly lower than that of TD by 28.9%, whereas the bioavailabilities of baicalin/baicalein and wogonoside/wogonin were comparable between two dosage forms. In conclusion, the major flavonoids of GQT were extensively metabolized into sulfates/glucuronides and present as the major molecules in the circulation. TD of GQT revealed higher bioavailability of daidzin/daidzein than CP.
1. Introduction
Gegen-Qinlian-Tang (GQT) was a famous prescription firstly described in Shang-Han-Lun (also called “treatise on febrile diseases”) and commonly used to treat virus diarrhea, bacillary dysentery, and general fever clinically [1]. GQT is composed of four Chinese herbs, including Puerariae Radix (roots of Pueraria lobata (Willd.) Ohwi, PR), Scutellariae Radix (roots of Scutellaria baicalensis Georgi SR), Coptidis Rhizoma (rhizomes of Coptis chinensis Franch, CR), and honey-processed Glycyrrhizae Radix (roots of Glycyrrhiza uralensis Fisch, GR). Recently, numerous studies have reported beneficial effects of these four herbs and their constituents, such as anti-inflammation [2], neuroprotection [3, 4], and anticancer activities [5–7].
The chemical constituents in a Chinese herbal prescription are extraordinarily complex. Understanding the pharmacokinetics of the major constituents in GQT would be helpful to explore the rationale of its clinical implication. The major constituents of GQT include flavonoids such as puerarin, daidzin, daidzein, baicalin, baicalein, and wogonin; alkaloids such as coptisine, palmatine, and berberine; and triterpene glycosides such as glycyrrhizin (chemical structures shown in Figure 1). Based on recent findings of polyphenol pharmacokinetics, it has been generally recognized that most polyphenols are present predominantly as sulfates/glucuronides rather than their parent forms in the bloodstream [8–10]. Besides, the alkaloids, such as berberine, coptisine, and palmatine, the constituents of CR, have been reported to be transformed to sulfates and glucuronides after being absorbed and metabolized by dealkylation [10]. Lately, many endogenous sulfates and glucuronides as well as the conjugated metabolites of xenobiotics have been reported as substrates of multidrug resistance-associated proteins (MRPs) [11, 12] and organic anion transporters (OATs) [12, 13]. We speculate that OATs and MRPs may be associated with the disposition of polyphenol metabolites after oral administration of GQT. Glycyrrhizin, the major constituent of GR, has been found existing as glycyrrhetic acid in the circulation, which was also a putative substrate of OATs and MRPs [14]. Therefore, we hypothesized that the metabolites of GQT might compete with each other for the anion transport mediated by OATs and/or MRPs in intestine, liver, and/or kidney. We thus suspected that the pharmacokinetics and bioavailability of GQT might be different from those of the individual component herbs, for example, PR and SR [8, 9, 14, 15].
Figure 1.
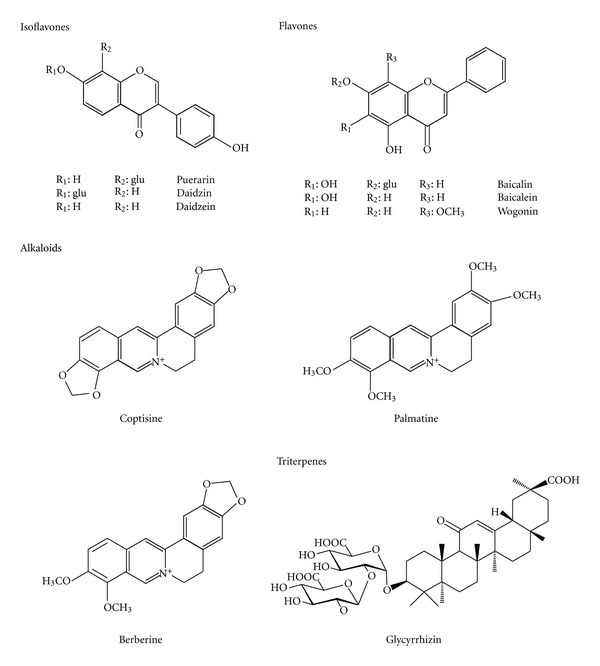
Chemical structures of major constituents in GQT.
For a long tradition, Chinese medicines are generally prepared by extracting crude drugs with boiling water and administered as the water extract so called traditional decoction (TD). Nowadays, the dosage form of concentrated powders (CPs) is more popularly prescribed for patients by Chinese medical doctors in Taiwan. Till now, the pharmacokinetics information of both dosage forms of GQT is still lacking, and their bioequivalence remained unknown. Therefore, this study investigated the pharmacokinetics of GQT in rats and, furthermore, the relative bioavailability between TD and CP was evaluated.
2. Materials and Methods
2.1. Materials and Reagents
The crude drugs, TD and CP of GQT, were supplied by Sun Ten Pharmaceutical Co., Taipei, Taiwan. Briefly, the crude drugs were boiled with water for 4 h and filtered while hot to afford TD. Half volume of TD was then concentrated by spray-drying process with starch as excipient to obtain CP. The origins of SR, PR, GR, and CR were identified by Dr. Yu-Chi Hou and voucher specimens were deposited in China Medical University. Baicalin, baicalein, wogonin, daidzin, and coptisine were supplied by Wako (Osaka, Japan) and their purities were 98%. Berberine (purity 98%) and amyl paraben were obtained from Tokyo Pure Chemical Industries (Tokyo, Japan). Puerarin (purity 80%), daidzein (purity 98%), glycyrrhizin (purity 75%), β-glucuronidase (type B-1 from bovine liver), and sulfatase (type H-1 from Helix pomatia, containing 14,400 units/g of sulfatase and 574,000 units/g of β-glucuronidase) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Palmatine (purity 97%) was supplied by Aldrich Chemical Co. (Milwaukee, WI, USA). Acetonitrile and methanol (LC grade) were purchased from ECHO Chemical Co. (Miaoli, Taiwan). Ethyl acetate (LC grade) was supplied by Mallinckrodt Baker, Inc. (Phillipsburg, NJ, USA). L(+)-Ascorbic acid was obtained from RdH Laborchemikalien GmbH & Co. KG (Seelze, Germany). Other reagents were HPLC grade or analytical reagent grade. Milli-Q plus water (Millipore, Bedford, MA, USA) was used throughout this study.
2.2. Quantitation of the Major Constituents in TD and CP of GQT
The TD (200 μL) containing 1 g/mL of crude drugs was diluted with 200 μL of water and mixed with MeOH (v/v, 3 : 7). After centrifuged, the supernatant (160 μL) was added with 40 μL of amyl paraben solution (100 μg/mL in methanol as internal standard) and 20 μL was subjected to HPLC analysis. The instrumentation included a pump (LC-10ATVP, Shimadzu, Japan), a diode array detector (SPD-M10AVP, Shimadzu, Japan), and an automatic injector (SiL-10AF, Shimadzu, Japan). An Apollo C18 column (4.6 × 250 mm, 5 μm) was equipped with a guard column (4.6 × 50 mm, 5 μm) (Alltech Associates Inc., USA). The mobile phase consisted of acetonitrile (A) and 0.1% phosphoric acid (B). A gradient elution was programmed as follows: A/B: 11/89 (0–10 min), 18/82 (12–25 min), 30/70 (45 min), 43/57 (54 min), 46/54 (59 min), 65/35 (65 min), and 11/89 (70–75 min). The detection wavelength was set at 250 nm and the flow rate was 1.0 mL/min.
For the quantitation of CP, the powders (100 mg) equivalent to 171 mg of crude drugs were accurately weighed and extracted twice with 10 mL of 70% methanol by ultrasonic shaking for 30 min each time and filtered. Sufficient amount of 70% methanol was added to the combined filtrates to make 20 mL. After properly diluted with methanol, the sample was subjected to the HPLC analysis described above.
2.3. Animals and Drug Administration
Sprague-Dawley rats were supplied by National Laboratory Animal Center (Taipei, Taiwan) and housed in a 12 h light-dark cycle, constant temperature environment at the Animal Center of China Medical University (Taichung, Taiwan) prior to study. Six male rats weighing 325–490 g were fasted for 12 h before drug administration and food was withheld for another 3 h. Rats were randomly divided into two groups. A single dose of TD and CP of GQT equivalent to 6 g/kg of crude drugs was given to rats via gastric gavage in a crossover design. Water was supplied ad libitum. The animal study adhered to “The Guidebook for the Care and Use of Laboratory Animals” published by the Chinese Society for the Laboratory Animal Science, Taiwan. The Institutional Animal Care and Use Committee in China Medical University approved this animal protocol.
2.4. Blood Collection
Blood samples (1.0 mL) were collected via cardiac puncture at 15, 30, 60, 120, 360, 720, 1440, 2160, 2880, and 4320 min after dosing and centrifuged at 10,000 g to obtain serum, which was stored at −30°C for later analysis.
2.5. Development and Validation of Quantitaton Method for Serum
The serum specimens were analyzed by HPLC method before and after treatments with β-glucuronidase and sulfatase. For the quantitation of glucuronides (G), 200 μL of serum was mixed with 50 μL of β-glucuronidase solution (1000 units/mL in pH 5 acetate buffer), 50 μL of ascorbic acid (200 mg/mL) in light protected test tube and incubated at 37°C for 2 h under anaerobic condition, which had been determined by a preliminary study. After hydrolysis, the serum was acidified with 50 μL of 0.1 N HCl and partitioned with 350 μL of ethyl acetate (containing 1 μg/mL of propyl paraben as internal standard). The ethyl acetate layer was evaporated under N2 gas to dryness and reconstituted with 50 μL of methanol, then 20 μL was subjected to HPLC analysis. For the quantitation of sulfates/glucuronides, 200 μL of serum was mixed with 50 μL of sulfatase (1000 units/mL, containing 39,861 units/mL of β-glucuronidase in pH 5 acetate buffer) and incubated at 37°C for 30 min under anaerobic condition, which had been determined by a preliminary study. The following procedures were the same as that described above for glucuronides.
An LC-2010C (Shimadzu, Japan) system was used for the analysis and the mobile phase consisted of acetonitrile (A) and 0.1% phosphoric acid (B). A gradient elution was programmed as follows: A/B: 28/72 (0–12 min), 42/58 (28–35 min), 28/72 (40–45 min). The detection wavelength was set at 250 nm and the flow rate was 1 mL/min.
For the assay of polyphenol free forms, 200 μL of serum sample was subjected to the process described above except the treatments with sulfatase or β-glucuronidase. For calibrator preparation, 200 μL of serum was spiked with various concentrations of standards including daidzein, baicalein, and wogonin. The later procedure followed that described above for serum specimen. The calibration graph was plotted by linear regression of the peak area ratios (each standard to internal standard) against concentrations of individual standard.
2.6. Validation of the Assay Methods
The system suitability was evaluated through intraday and interday analysis of precision and accuracy. The recoveries of each compound from serum were determined by comparing the peak area of extracted serum standards to those of standards spiked in extracted serum. The LLOQ (lower limit of quantitation) represents the lowest concentration of analyte in a sample that can be determined with acceptable precision and accuracy, whereas LOD (limit of detection) represents the lowest concentration of analysis in a sample that can be detected (with S/N > 3).
2.7. Data Analysis
The pharmacokinetic parameters were calculated using noncompartment model with the aid of WinNonlin (version 1.1, SCI software, Statistical Consulting, Inc., Apex, NC). The area under the serum concentration-time curve to the last point (AUC0–t) was calculated using trapezoidal rule. The peak plasma concentration (C max) and the time to peak concentration (T max) were obtained from experimental measurement. Paired Student's t-test was used for statistical analysis with significance level set at P < 0.05.
3. Results
3.1. Quantitation of the Major Constituents in TD and CP of GQT
Figure 2 showed the HPLC chromatogram of ten major constituents in GQT decoction, which were satisfactorily resolved within 75 min by a gradient elution. Table 1 listed the contents of each constituent in TD and CP equivalent to 6 g of GQT. Our quantitation results showed that the contents of ten major constituents in TD and CP were comparable.
Figure 2.
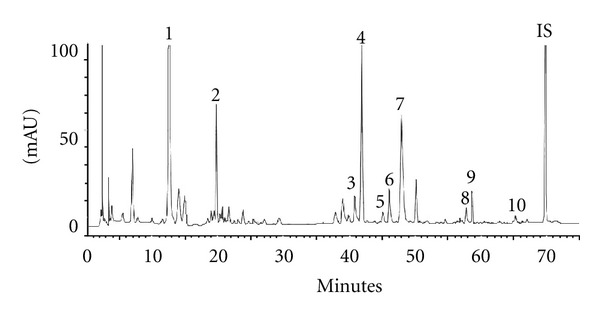
HPLC chromatogram of GQT decoction. 1: puerarin, 2: daidzin, 3: coptisine, 4: baicalin, 5: daidzein, 6: palmatine, 7: berberine, 8: baicalein, 9: glycyrrhizin, 10: wogonin, IS: amyl paraben.
Table 1.
The contents (mg) of various constituents in traditional decoction (TD) and concentrated powder (CP) equivalent to 6 g of GQT.
| Herb | Compound | TD | CP |
|---|---|---|---|
| Puerarin | 71.1 | 55.6 | |
| Puerariae Radix | Daidzin | 12.5 | 10.6 |
| Daidzein | 1.3 | 1.4 | |
|
| |||
| Bailcalin | 80.6 | 80.4 | |
| Scutellariae Radix | Baicalein | 3.5 | 3.6 |
| Wogonin | 1.9 | 2.3 | |
|
| |||
| Berberine | 50.4 | 43.3 | |
| Coptidis Rhizoma | Coptisine | 22.7 | 17.6 |
| Palmatine | 14.0 | 14.3 | |
|
| |||
| Glycyrrhizae Radix | Glycyrrhizin | 18.1 | 16.1 |
3.2. Development and Validation of Quantitaton Method for Serum
The quantitation method for serum specimen was developed and validated in this study. Before enzymatic hydrolysis, the free forms of puerarin, daidzin, daidzein, baicalein, wogonin, coptisine, palmatine, berberine, and glycyrrhizin were not detected. Upon treatment with sulfatase and glucuronidase, daidzein, baicalein, and wogonin emerged. For quantitation of daidzein, baicalein, and wogonin in serum, the calibration curves were in good linearity. The coefficients of variation were less than 9% and the relative errors were below 9% for intraday and interday analysis. The recoveries of daidzein, baicalein, and wogonin from serum were 96.8~100.0%, 83.0~92.3%, and 98.7~106.8%, respectively. The LLOQs were 0.3, 0.2, and 0.1 μg/mL for daidzein, baicalein, and wogonin, respectively, and their LODs were 0.04 μg/mL. Typical HPLC chromatograms of a serum specimen before and after hydrolysis with glucuronidase and sulfatase/glucuronidase are shown in Figure 3.
Figure 3.
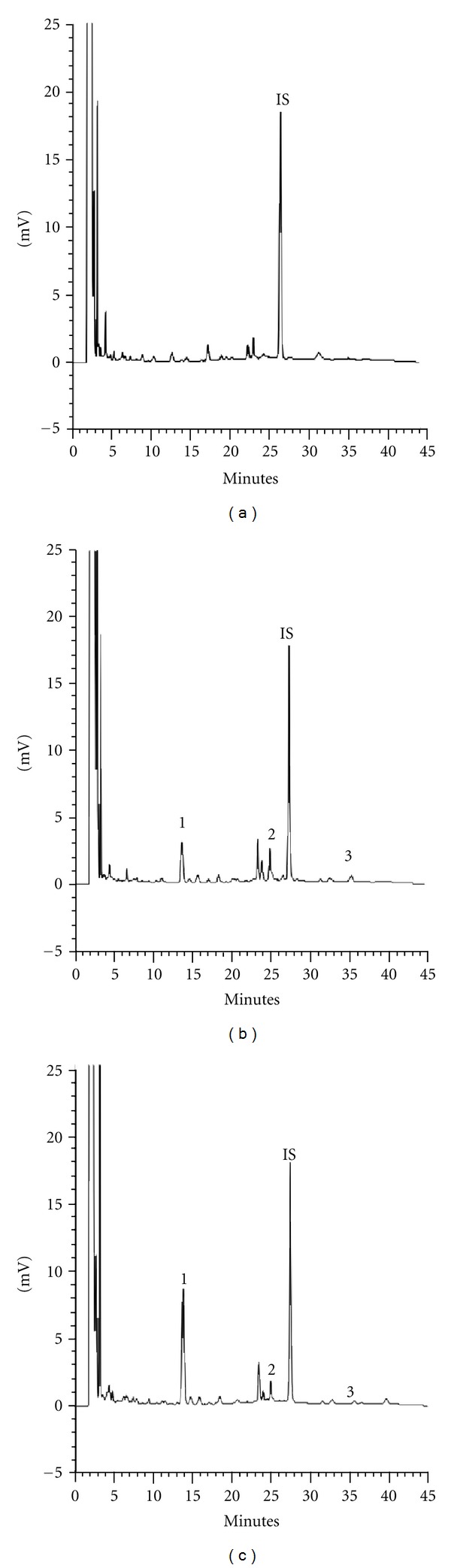
Typical HPLC chromatograms of serum specimens after administration of GQT to rat. (a) Serum specimen, (b) serum specimen after hydrolyzed with glucuronidase, (c) serum specimen after hydrolyzed with sulfatase/glucuronidase. 1: daidzein, 2: baicalein, 3: wogonin and IS: propyl paraben.
3.3. Pharmacokinetic Parameters of the Conjugated Metabolites of Daidzein, Baicalein, and Wogonin after Administration of GQT in Rats
Figure 4 depicts the serum concentration-time profiles of sulfates/glucuronides (S/G) and glucuronides (G) of daidzein, baicalein, and wogonin in rats after oral administration of TD and CP of GQT. The pharmacokinetic parameters are listed in Table 2. The C max and AUC0–t of the S/G of daidzein, baicalein, and wogonin were ranked in the order of daidzein S/G > baicalein S/G > wogonin S/G for both dosage forms. The AUC0–t of daidzein S/G after administration of CP was significantly lower than that of TD by 28.9%, whereas the AUC0–t of baicalein S/G and wogonin S/G after administration of CP and TD were comparable. The relative bioavailabilities of flavonoids between administrations of GQT and PR or SR in rats are shown in Table 3. The AUC0–t/dose of daidzin/daidzein and baicalin/baicalein in GQT was comparable with those in PR and SR, respectively.
Figure 4.
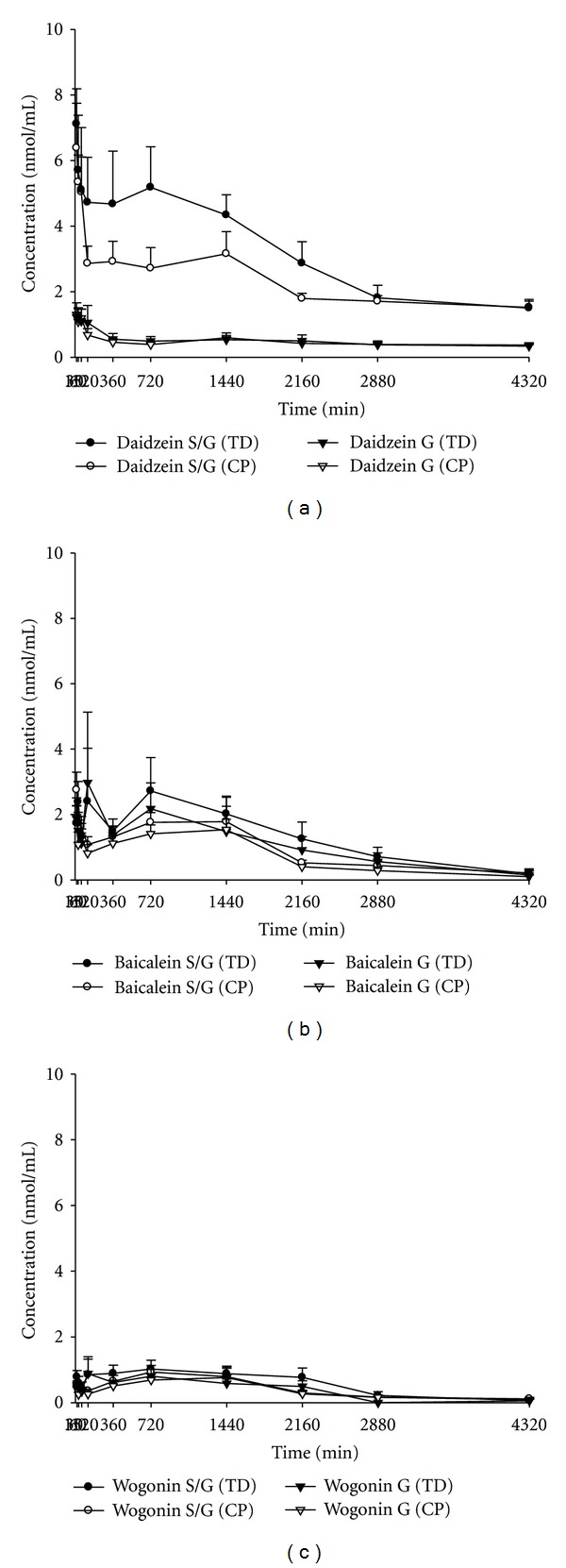
Mean (±S.E.) serum concentration: time profiles of the sulfates/glucuronides (S/G) and glucuronides (G) of daidzein (a), baicalein (b), and wogonin (c) after oral administration of traditional decoction (TD) and concentrated powder (CP) of GQT in six rats.
Table 2.
Comparison of pharmacokinetic parameters between traditional decoction (TD) and concentrated powder (CP) of GQT.
| Metabolites | Dosage form |
C
max
(nmol/mL) |
AUC0–4320
(nmol·min/mL) |
MRT0–4320
(min) |
|---|---|---|---|---|
| Daidzein S/G | TD | 8.9 ± 1.2 | 13669.7 ± 1704.7a | 1647.4 ± 103.3 |
| CP | 7.0 ± 0.9 | 9720.4 ± 853.4b
(−28.9%) |
1842.7 ± 100.8 | |
|
| ||||
| Baicalein S/G | TD | 4.7 ± 1.3 | 5445.1 ± 1212.9 | 1513.7 ± 209.8 |
| CP | 3.2 ± 0.5 | 3949.7 ± 666.2 | 1451.3 ± 85.2 | |
|
| ||||
| Wogonin S/G | TD | 1.7 ± 0.4 | 2314.3 ± 495.4 | 1409.7 ± 163.9 |
| CP | 1.1 ± 0.3 | 1816.2 ± 319.7 | 1476.5 ± 100.9 | |
Data expressed as mean ± S.E.
Means in a column without a common superscript difference, P < 0.05.
C max: peak serum level.
AUC0–4320: area under the serum concentration-time curve to 4320 min.
MRT: mean residence time.
Table 3.
Comparison of bioavailabilities of flavonoids from GQT, PR and SR in rats.
| Flavonoids | Treatments | AUC0–t
(mean ± S.E.) |
Dose (μmol/kg) |
AUC0–t/dose |
|---|---|---|---|---|
| Daidzin/daidzein | GQT (n = 6) | 11.3 ± 1.5 | 35.2 | 0.32 ± 0.04 |
| PR (n = 7) | 8.9 ± 1.1 | 26.6 | 0.33 ± 0.04 | |
|
| ||||
| Baicalin/baicalein | GQT (n = 6) | 5.0 ± 1.3 | 193.0 | 0.03 ± 0.01 |
| SR (n = 6) | 4.2 ± 0.7 | 250.9 | 0.02 ± 0.00 | |
AUC0–t (μmol·min/mL): area under the serum concentration-time curve of sulfates/glucuronides of daidzein and baicalein from zero to the last point.
4. Discussions
This study is the first work investigating the pharmacokinetics of GQT in rats. The analytical method of serum specimens after administration of GQT was established in this study. The validity of the method was shown by acceptable precision, accuracy, and satisfactory recoveries.
When GQT decoction was orally administered to rats, none of the parent forms of puerarin, daidzin, daidzein, baicalein, wogonin, berberine, palmatine, coptisine, and glycyrrhizin have been detected in serum specimens, indicating that these compounds were not absorbed per se. This fact suggested that the in vitro bioactivities of those constituents in GQT reported in literature might not predict the in vivo effects [16, 17]. What appeared in the circulation was the S/G of daidzein, baicalein, and wogonin, which were found emerging rapidly in serum, indicating that these flavonoids were absorbed quickly and extensively metabolized by conjugation reaction during the first pass [18, 19]. These results were consistent with our previous studies unraveling the pharmacokinetics of PR and SR [8, 9]. The absence of puerarin, daidzin, and glycyrrhizin in serum, which were abundant glycosides in nature, can be explained by their poor lipophilicity which limited their passive diffusion across cell membrane of intestine. Of the isoflavone glycosides, the sugar moiety of daidzin, an O-glycoside of daidzein, was easily hydrolyzed in small intestine to form daidzein, which was able to permeate through the cell membrane of enterocytes [20–22]. As to the metabolic fate of puerarin, a C-glycoside of daidzein, our feces studies have shown that it is hardly hydrolyzed by the gut microflora of rats, rabbits, or humans (data not shown). Besides, the absence of berberine, palmatine, and coptisine in serum specimens can be accounted for by the extensive first pass effect, including dealkylation and conjugation reactions [10]. The pharmacokinetics of the metabolites of these alkaloids has not been investigated because of the unavailability of authentic standards. As to glycyrrhizin, it has been found gradually hydrolyzed to form glycyrrhetic acid, an absorbable aglycone by intestinal bacteria [23]. However, glycyrrhetic acid was not detected in the serum, which might be due to the small dose of GR in GQT and low metabolic rate of glycyrrhizin to form glycyrrhetic acid [14].
Owing to the sulfatase used in this study contained considerable amount of glucuronidase, hydrolysis with sulfatase resulted in the release of both sulfates and glucuronides. The AUC0–t of daidzein S/G was 6.5 folds of daidzein G, implying that daidzein S was the major conjugated metabolite of daidzein in blood. This discovery is in good agreement with our previous findings in rats after administration of PR decoction [8]. Interestingly, this was contrary to the metabolic fates of daidzin after intake of soy products, which presented mainly daidzein G in the circulation of female rats, pigs, monkeys, and women [24, 25]. We speculate that the matrix difference between PR and soy products might affect the selective kinetics of conjugation reaction for daidzein in rats. The comparable amounts of baicalein and wogonin liberated between treatments with sulfatase/glucuronidase and glucuronidase in all serum specimens indicated that baicalein G and wogonin G were the major conjugated metabolites, which was consistent with our previous findings in the pharmacokinetic study of SR decoction in rats [15].
When the AUC0–t of the S/G of three flavonoids were compared, the systemic exposure was ranked as daidzein S/G ≫ baicalein S/G > wogonin S/G. However, when the ratios of AUC0–t of the S/G of each flavonoid to the intake doses including aglycone and glycosides were calculated and compared, their order became daidzin/daidzein > wogonoside/wogonin > baicalin/baicalein, implying that daidzin/daidzein had the highest bioavailability, whereas baicalin/baicalein was the least bioavailable. This fact could be in part accounted for by better dissolution of daidzein than baicalein or wogonin in gastrointestine juice based on its shorter retention time in reversed-phase HPLC chromatogram. On other hand, one possible reason for the poor bioavailability of baicalin/baicalein might be attributed to the poor chemical stability of baicalein, which contains a catechol structure and has been found more degradable than daidzein and wogonin in the artificial intestine juice [20].
When the bioavailabilities of each flavonoid were compared between two dosage forms, TD and CP, the significantly lower ratio of AUC0–t of daidzein S/G to the intake dose of daidzin/daidzein after administration of CP than that of TD indicated that daidzin/daidzein in TD afforded higher bioavailability. As to the relative bioavailability of baicalin/baicalein and wogonoside/wogonin in two dosage forms, CP and TD were bioequivalent. We thus suggest that, for achieving clinical efficacy, the TD dosage form of GQT is a better choice.
For the past years, there have been a growing number of in vitro bioactivity assays reporting on polyphenols which were commercially available or isolated by phytochemists. For instance, several studies on in vitro beneficial activities of the free forms of daidzein, baicalein, and wogonin have been reported [5, 26]. However, based on our pharmacokinetic results showing that the sulfates of daidzein and the glucuronides of baicalein and wogonin were the major forms in the blood, it is likely that the conjugated metabolites of flavonoid play more important role for the pharmacological effects in vascular system than their free forms. In literature, the sulfates/glucuronides of daidzein and glucuronides of baicalein have been shown to exhibit promising activities on breast cancer [26, 27] and bladder cancer [28]. We thus speculate that the clinical efficacy of GQT may be attributed to the bioactivities of these conjugated metabolites of flavonoids.
A few previous studies have reported that herb-herb interaction may occur in Chinese medicine prescription, especially in the decocting process and during absorption or disposition in vivo [29, 30]. In this study, in order to assess the relative bioavailability of each flavonoid from GQT, PR and SR, the AUC0–t/dose of each flavonoid was calculated and the results showed no significant differences between GQT and PR or SR. These facts indicated that the bioavailabilities of these flavonoids from GQT, PR and SR were comparable. We can thus infer that no significant pharmacokinetic herb-herb interaction occurred in the prescription formula of GQT.
5. Conclusions
In conclusion, sulfates/glucuronides of daidzein, baicalein, and wogonin were the major forms exposed systemically in rats after oral administration of GQT. Comparison of two dosage forms indicated that TD has higher bioavailability of daidzin/daidzein than CP.
Acknowledgments
This work was in part supported by National Science Council, Taiwan (NSC 99-2320-B-039-017-MY3, NSC99-2628-B-039-005-MY3) and China Medical University, Taichung, Taiwan (CMU100-S-05 and CMU100-S-17).
References
- 1.Wang Y, Yao Y, An R, You L, Wang X. Simultaneous determination of puerarin, daidzein, baicalin, wogonoside and liquiritin of GegenQinlian decoction in rat plasma by ultra-performance liquid chromatography-mass spectrometry. Journal of Chromatography B. 2009;877(20-21):1820–1826. doi: 10.1016/j.jchromb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 2.Huang WH, Lee AR, Yang CH. Antioxidative and anti-inflammatory activities of polyhydroxyflavonoids of Scutellaria baicalensis GEORGI. Bioscience, Biotechnology and Biochemistry. 2006;70(10):2371–2380. doi: 10.1271/bbb.50698. [DOI] [PubMed] [Google Scholar]
- 3.Xu X, Zhang S, Zhang L, Yan W, Zheng X. The neuroprotection of puerarin against cerebral ischemia is associated with the prevention of apoptosis in rats. Planta Medica. 2005;71(7):585–591. doi: 10.1055/s-2005-871261. [DOI] [PubMed] [Google Scholar]
- 4.Ye M, Fu S, Pi R, He F. Neuropharmacological and pharmacokinetic properties of berberine: a review of recent research. Journal of Pharmacy and Pharmacology. 2009;61(7):831–837. doi: 10.1211/jpp/61.07.0001. [DOI] [PubMed] [Google Scholar]
- 5.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treatment Reviews. 2009;35(1):57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Tang J, Feng Y, Tsao S, Wang N, Curtain R, Wang Y. Berberine and Coptidis Rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. Journal of Ethnopharmacology. 2009;126(1):5–17. doi: 10.1016/j.jep.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Hawthorne S, Gallagher S. Effects of glycyrrhetinic acid and liquorice extract on cell proliferation and prostate-specific antigen secretion in LNCaP prostate cancer cells. Journal of Pharmacy and Pharmacology. 2008;60(5):661–666. doi: 10.1211/jpp.60.5.0013. [DOI] [PubMed] [Google Scholar]
- 8.Chiang HM, Yeh YR, Chao PDL, et al. Metabolic pharmacokinetics of isoflavones in the roots of Pueraria lobata in rats. Mid-Taiwan Journal of Medicine. 2005;10(2):57–64. [Google Scholar]
- 9.Lai MY, Hsiu SL, Chen CC, Hou YC, Chao PDL. Urinary pharmacokinetics of baicalein, wogonin and their glycosides after oral administration of Scutellariae Radix in humans. Biological and Pharmaceutical Bulletin. 2003;26(1):79–83. doi: 10.1248/bpb.26.79. [DOI] [PubMed] [Google Scholar]
- 10.Liu YT, Hao HP, Xie HG, et al. Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metabolism and Disposition. 2010;38(10):1779–1784. doi: 10.1124/dmd.110.033936. [DOI] [PubMed] [Google Scholar]
- 11.Chu XY, Huskey SEW, Braun MP, Sarkadi B, Evans DC, Evers R. Transport of ethinylestradiol glucuronide and ethinylestradiol sulfate by the multidrug resistance proteins MRP1, MRP2, and MRP3. Journal of Pharmacology and Experimental Therapeutics. 2004;309(1):156–164. doi: 10.1124/jpet.103.062091. [DOI] [PubMed] [Google Scholar]
- 12.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacological Reviews. 2010;62(1):1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You G. Structure, function, and regulation of renal organic anion transporters. Medicinal Research Reviews. 2002;22(6):602–616. doi: 10.1002/med.10019. [DOI] [PubMed] [Google Scholar]
- 14.Lin SP, Tsai SY, Hou YC, Chao PDL. Glycyrrhizin and licorice significantly affect the pharmacokinetics of methotrexate in rats. Journal of Agricultural and Food Chemistry. 2009;57(5):1854–1859. doi: 10.1021/jf8029918. [DOI] [PubMed] [Google Scholar]
- 15.Hou YC, Lin SP, Tsai SY, Ko MH, Chang YC, Chao PDL. Flavonoid pharmacokinetics and tissue distribution after repeated dosing of the roots of Scutellaria baicalensis in rats. Planta Medica. 2011;77(5):455–460. doi: 10.1055/s-0030-1250433. [DOI] [PubMed] [Google Scholar]
- 16.Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radical Research. 2004;38(8):771–785. doi: 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]
- 17.Walle T. Absorption and metabolism of flavonoids. Free Radical Biology and Medicine. 2004;36(7):829–837. doi: 10.1016/j.freeradbiomed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Lee Chao PD, Hsiu SL, Hou YC. Flavonoids in herbs: biological fates and potential interactions with xenobiotics. Journal of Food and Drug Analysis. 2002;10(4):219–228. [Google Scholar]
- 19.Hollman PCH. Absorption, bioavailability, and metabolism of flavonoids. Pharmaceutical Biology. 2004;42:74–83. [Google Scholar]
- 20.Lin YT, Hsiu SL, Hou YC, Chen HY, Chao PDL. Degradation of flavonoid aglycones by rabbit, rat and human fecal flora. Biological and Pharmaceutical Bulletin. 2003;26(5):747–751. doi: 10.1248/bpb.26.747. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson AP, Gee JM, Dupont MS, et al. Hydrolysis by lactase phlorizin hydrolase is the first step in the uptake of daidzein glucosides by rat small intestine in vitro. Xenobiotica. 2003;33(3):255–264. doi: 10.1080/0049825021000058088. [DOI] [PubMed] [Google Scholar]
- 22.Simons AL, Renouf M, Hendrich S, Murphy PA. Human gut microbial degradation of flavonoids: structure-function relationships. Journal of Agricultural and Food Chemistry. 2005;53(10):4258–4263. doi: 10.1021/jf0500177. [DOI] [PubMed] [Google Scholar]
- 23.Ploeger B, Mensinga T, Sips A, Meulenbelt J, DeJongh J. A human physiologically-based model for glycyrrhzic acid, a compound subject to presystemic metabolism and enterohepatic cycling. Pharmaceutical Research. 2000;17(12):1516–1525. doi: 10.1023/a:1007661209921. [DOI] [PubMed] [Google Scholar]
- 24.Doerge DR, Chang HG, Churchwell MI, Holder CL. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metabolism and Disposition. 2000;28(3):298–307. [PubMed] [Google Scholar]
- 25.Gu L, House SE, Prior RL, et al. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. Journal of Nutrition. 2006;136(5):1215–1221. doi: 10.1093/jn/136.5.1215. [DOI] [PubMed] [Google Scholar]
- 26.Lin YJ, Hou YC, Lin CH, et al. Puerariae radix isoflavones and their metabolites inhibit growth and induce apoptosis in breast cancer cells. Biochemical and Biophysical Research Communications. 2009;378(4):683–688. doi: 10.1016/j.bbrc.2008.10.178. [DOI] [PubMed] [Google Scholar]
- 27.Wang N, Tang LJ, Zhu GQ, et al. Apoptosis induced by baicalin involving up-regulation of P53 and bax in MCF-7 cells. Journal of Asian Natural Products Research. 2008;10(11-12):1129–1135. doi: 10.1080/10286020802410664. [DOI] [PubMed] [Google Scholar]
- 28.Ikemoto S, Sugimura K, Yoshida N, et al. Antitumor effects of Scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology. 2000;55(6):951–955. doi: 10.1016/s0090-4295(00)00467-2. [DOI] [PubMed] [Google Scholar]
- 29.Lu T, Song J, Huang F, et al. Comparative pharmacokinetics of baicalin after oral administration of pure baicalin, Radix scutellariae extract and Huang-Lian-Jie-Du-Tang to rats. Journal of Ethnopharmacology. 2007;110(3):412–418. doi: 10.1016/j.jep.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 30.Shi R, Zhou H, Liu Z, et al. Influence of Coptis chinensis on pharmacokinetics of flavonoids after oral administration of radix Scutellariae in rats. Biopharmaceutics & Drug Disposition. 2009;30(7):398–410. doi: 10.1002/bdd.674. [DOI] [PubMed] [Google Scholar]


