Abstract
Pancreatic cancer (PC) is the most aggressive malignant disease, ranks as the fourth most leading cause of cancer related death among men and women in the United States. We present here that plumbagin (PL), a quinoid constituent isolated from the roots of the medicinal plant Plumbago zeylanica L, inhibits the growth of PC cells both in vitro and in vivo model systems. PL treatment induces apoptosis and inhibits cell viability of PC cells (PANC1, BxPC3, and ASPC1). In addition, i.p administration of PL (2 mg/kg body weight, 5 days a week) in SCID mice beginning 3 days after ectopic implantation of PANC1 cells resulted in a significant (P<0.01) inhibition of both tumor weight and volume. PL treatment inhibited 1) constitutive expression of EGFR, pStat3Tyr705, pStat3Ser727, 2) DNA binding of Stat3, and 3) physical interaction of EGFR with Stat3, in both cultured PANC1 cells and their xenograft tumors. PL treatment also inhibited phosphorylation and DNA-binding activity of NF-κB in both cultured PC cells (PANC1, ASPC1) and in PANC1 cells xenograft tumors. Downstream target genes (cyclin D1, MMP9, and Survivin) of Stat3 and NF-κB were similarly inhibited. These results suggest that PL may be used as a novel therapeutic agent against human PC.
Keywords: Plumbagin, EGFR, NF-κB and Stat3
Introduction
Pancreatic cancer (PC) is one of the most fatal of all cancers and is ranked as the fourth most common cause of cancer related deaths among both men and women in the United States.1 It has been estimated that 44,030 cases of PC will be diagnosed in theUnited States alone in 2011 and 37,660 cancer related deaths are projected.1 Despite these alarming statistics and the increase incidence of PC over the past several decades, the molecular and biochemical determinants of the disease remain poorly understood and no effective therapeutic regimen exists to significantly ameliorate the clinical course or prognosis of this disease.1 The difficulties in treating PC partly arise due to the de novo chemoresistant behavior of PC cells to cytotoxic chemotherapeutic agents and/or radiotherapy. Therefore, it is necessary to intensify our efforts for a better understanding of this disease and for the development of novel therapeutic strategies for its prevention and treatment.
Several molecular signaling pathways including epidermal growth factor receptor (EGFR), signal transducer and activator of transcription factor 3 (Stat3), and nuclear factor kappaB (NF-κB) play an important role in cell survival, proliferation, chemoresistance, angiogenesis, promotion, and metastasis of PC.2,3 EGFR is a member of the ErbB family of receptor kinases, which is overexpressed in at least one-half of all PC4,5, and correlates with poor prognosis.6,7 It has been reported that EGFR physically interacts and activates Stat3 in various types of cancers including PC.8,9 Constitutive activation of Stat3 has been reported in PC cells and tissues, and blocking Stat3 via ectopic expression of dominant-negative Stat3 led to a significant reduction in tumor growth and angiogenesis in an experimental model.10 Evidence indicates that inactivation of IL-6/Stat3 signaling inhibits pancreatic intraepithelial neoplasia (PanINs) progression and reduces the development of PC.11 Also, a recent study has demonstrated the role of Stat3 in pancreatitis-accelerated pancreatic ductal adenocarcinoma formation, cell proliferation, metaplasia-associated inflammation, and enforced MMP7 expression during neoplastic development.12 Interleukin 6 (IL-6), Janus-activated kinases (JAK), EGFR, and Src family kinases are among the activators of Stat3. They all phosphorylate Stat3 at the critical tyrosine residue (705), leading to Stat3 dimerization, nuclear translocation, and binding to DNA response elements in the promoter region of target genes.13,14 It has been demonstrated functional cooperation between EGFR, Src, and Stat3 in promoting PC.15 A recent study suggests that nuclear heteromeric EGFR, Src and Stat3 complex regulates the oncogene c-Myc expression in PC. 16
NF-κB is another transcription factor which is constitutively activated in most human PC cells and PC tissues, but not in normal pancreatic tissues.17,18 Other studies suggest that NF-κB signaling contributes to the chemoresistance of PC.19,20 It has also been reported that constitutive activation of NF-κB requires Stat3, since Stat3 prolongs the retention of NF-κB in the nucleus, which occurs through p300-mediated acetylation of RelA/65.21 NF-κB is also involved in the activation of Stat3 as it upregulates the expression of IL-6 which initiates activation of Stat3 signaling via paracrine mechanism.22 Therefore, we need to develop an agent which could inhibit the growth of PC via targeting or interrupting these inter-connecting signaling pathways.
Plumbagin (PL) (5-hydroxy-2-methyl-1,4-napthoquinone) was isolated from the roots of the medicinal plant Plumbago zeylanica L. (also known as Chitrak).23 The roots of plumbago zeylanica have been used in Indian medicine for more than 2,500 years for the treatments of various ailments. PL is also present in black walnut and other various medicinal plants.23 PL has been shown to exert its medicinal properties including anticancer potential against various types of cancers.24 PL, fed in the diet (200 ppm), inhibited azoxymethane-induced intestinal tumors in rats.25 PL inhibits ectopic growth of breast cancer MDA-MB-231 cells.26 non-small cell lung cancer A549 cells,27 and melanoma A375-S2 cells in athymic nude mice.28 It has been illustrated that PL treatment of prostate cancer cells induces apoptosis.29 Our laboratory has also shown the potential anti-tumor activity of PL against prostate cancer.30 A recent study has demonstrated its anti-cancer activity against PC.31 However, the molecular mechanisms associated with the prevention of PC remain elusive. In this study, we report that PL significantly prevents the growth of PC cells xenograft tumors in SCID mice, which is, in part, due to the inhibition of EGFR, Stat3 and NF-κB signaling pathways.
Materials and Methods
Cell lines
PC cell lines (PANC1, and BxPC3) cells were obtained from American Type Culture Collection and were cultured in DMEM high glucose and RPMI-1640 medium containing 10% fetal bovine serum and 1% antibiotics (penicillin and streptomycin) respectively. ASPC1 cells were a kind gift from Prof. Fazlul H. Sarkar (Wayne State University, Detroit, Michigan) and cultured in the same medium as PANC1 cells.
Chemicals and antibodies
Monoclonal or polyclonal antibodies specific for actin, cyclin D1, Cdc25A, EGFR, GAPDH, total NF-κB/p65, MMP9, PCNA, ki67, total Stat3, survivin, and VEGF were purchased from Santa Cruz Biotechnology, (Santa Cruz, CA). Blocking peptides for EGFR and Stat3 antibodies and mouse IgG were also procured from Santa Cruz Biotechnology. Monoclonal antibodies specific for pStat3Tyr705, and pStat3Ser727 were obtained from BD Biosciences (San Jose, CA). Antibody specific to IKKγ, pIκBα, total IκBα, pNF-κB/p65Ser536, was purchased from Cell Signaling Technology. PARP antibody was purchased from Promega (Madison, WI).
Cell viability
PC cells (PANC1, BxPC3, and ASPC1) were treated with PL (5–20 μM) for 12, 24 and 48 h. MTT assay was performed as described earlier.32
Apoptosis assay
PC cells (PANC1 and BxPC3) were treated with PL (5–20 μM) for 24 h and the percent of cells undergoing apoptosis was determined by enhanced AnnexinV staining as described previously.32
In vitro chemoinvasion assay
Approximately 70% confluent cells were treated with PL (5–15 μM) for 12 h. Viable cells of each group were counted by the trypan blue exclusion method. All of the procedures were followed according to the manufacturer’s instructions (Chemicon International, Temecula, CA (Cat # ECM551)). In brief, 200 μl serum-free medium containing 2×105 cells were seeded into the invasion chamber and placed into the 24 well plate containing 500 μl complete medium. After 24 h incubation at 37°C, media was removed from the chamber, and cells were stained by putting the chamber in staining solution for 20 min at room temperature. Non-invaded cells were carefully removed from the top-side of the chamber with the help of cotton-tipped swab. Stained chamber was inserted to a clean well containing 200 μl of extraction buffer for 15 min at room temperature. 100 μl extracted stained solution from the chamber was transferred into the 96 well plate and optical density was measured at 490 nm at spectrophotometer.
Xenograft study
Six week old SCID male mice, purchased from Taconic laboratory (Rensselaer, NY), were housed under pathogen-free environment with a 12 h light/12 h dark schedule and fed with an autoclaved diet and water ad libitum. To establish tumor xenografts in mice, PANC1 cells (2.5 × 106) were suspended in 1:1 medium fixed with Matrigel and were subcutaneously implanted on both flanks of the mice. Three days later, the animals (n=10) were treated with an i.p injection of PL (2mg/kg body weight in 0.1 ml PBS, 5 days per week. Control animals (n=10) were treated same with vehicle (0.1 ml PBS). Mice were weighed and tumors were measured weekly by caliper. Tumor volumes were calculated by the formula 0.5238 X L X W X H, where L is length, W is width, and H is the height of the tumor. All the animals were euthanized when tumors of the control group reached the targeted volume 900 mm3.
IL-6 ELISA assay
IL-6 levels were determined from mouse serum by using specific ELISA kit for mouse IL-6 (eBioscience, Inc. San Diego, CA). All of the procedures were followed according to the manufacturer’s protocol.
Western blot analysis
Total cell lysate from the control, PL treated PC cells, and part of the excised tumor tissues of both control and PL treated groups were made for western blot analysis as described previously.30 The Western blots were quantitated by densitometric analysis using TotalLab Nonlinear Dynamic Imageanalysis software (Nonlinear USA, Inc., Durham, NC).
Electrophoretic mobility shift assay (EMSA)
PC cells were treated with PL (5–20 μM) for 12 h and PL (15μM) for 3–24h for dose and time-dependent manner respectively. Nuclear extracts were prepared as described previously.30 EMSA was performed for DNA binding activities of Stat3 and NF-κB as described previously.30
Immunohistochemistry
Part of the excised xenograft tumors from control and PL treated mice were fixed in 10% neutral buffered formalin, transferred to PBS (pH 7.4), and then sent to the technician for 4μm thick sectioning. These section slides were used for immunohistochemistry of PCNA, ki67, and VEGF as described previously.30
Immunoprecipitation/blotting
Total cell lysates prepared from control and PL-treated PANC1 cells and whole tumor tissue lysates obtained from excised tumors of control and PL treated animals were used for immunoprecipitation/blotting study. Detailed procedures are described in our previously published report.33
Immunofluorescence of EGFR, Stat3 and NF-κB in PC cells and excised tumor tissues
Paraffin-fixed xenograft tumors sections (4-μm thick) from control and PL treated mice were used to determine the co-localization of EGFR and Stat3. After antigen retrieval by incubating samples at 95°C in Tris-urea solution (pH 9.5) for 30 min, the tissue slides were incubated with normal horse serum (1:10 dilution) for 30 min to block nonspecific binding of the antibodies. Subsequently, the slides were incubated overnight with a mixture of EGFR (1:50 dilution) and Stat3 (1:50 dilution) primary antibodies in a humidified chamber. The mixture of antibodies was decanted and the slides were washed thrice in 1X TBS (pH 7.4). The slides were incubated with Alexa fluor 488 mouse antibody for EGFR detection and Alexa fluor 594 rabbit antibody for Stat3 detection (Invitrogen) for 1 h at room temperature in the dark. The solution of secondary antibodies was decanted and the slides were washed thrice with TBS for 5 min intervals each in the dark. For double immunofluorescence of Stat3 and EGFR, cells were fixed with ice-cold methanol for 15 minutes, washed 3 times in 1X PBS, permeabilized with 0.2% Triton X-100 for 10 min, and further washed 3 times with PBS. Specimens were blocked in 1% bovine serum albumin (BSA) for 30 min, and then incubated with primary and secondary antibodies as described before. Finally, the specimens were mounted with coverslips using a drop of mounting medium containing DAPI (Vector Lab, Inc., Burlingame, CA), Coverslips were sealed with nail polish to prevent drying and movement under the microscope. All sections were examined with an Olympus Microscope attached with fluorescence detector.
Statistical analysis
Student’s t-test was performed to determine the significance. P value <0.05 was considered as significant.
Results
PL induces apoptosis and inhibits the cell viability of PC cells
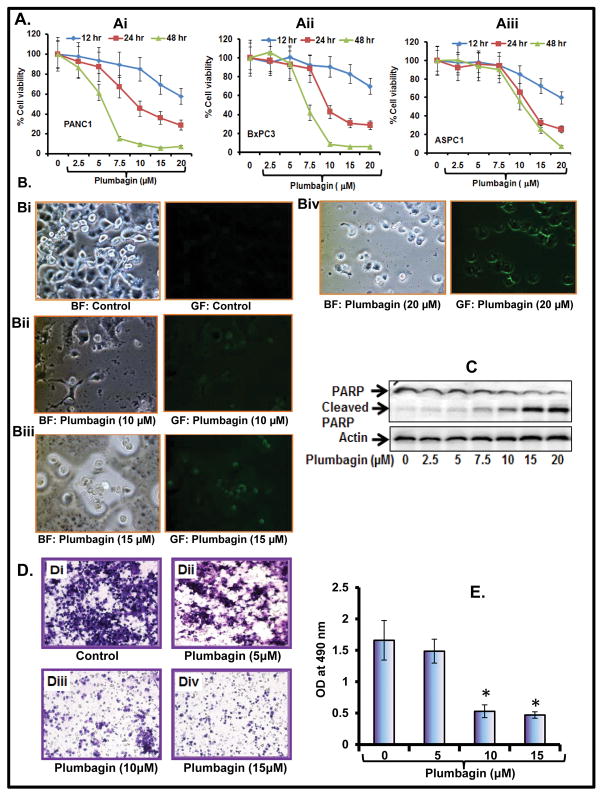
To determine whether PL inhibits the PC (PANC1, BxPC3 and ASPC1) cells viability, we performed a MTT assay. Data illustrated that PL treatment of PC cells significantly decreased the viability in a dose and time dependent manner (Figure 1Ai–Aiii). IC50 of PL in PANC1 and BxPC3 cells was 10 and 6 μM respectively after 24 and 48 post-treatment (Figure 1Ai–Aii), whereas IC50 for ASPC1 cells was 15 and 10 μM after 24 and 48 h post-treatment respectively (Figure 1Aiii). Next, we determined the apoptosis inducing potential of PL in PC cells. We observed that PL treatment (10–20 μM) of PANC1 cells induces apoptosis as determined by enhanced annexin V staining (Figure 1Bi–Biv). Cleavage in PARP protein is considered as one of the biomarkers of apoptosis. We further confirmed the effect of PL on PARP protein by Western blot analysis. Results demonstrated that PL treatment of PC cells dose-dependently enhances cleavage of PARP protein as evident from enhanced expression of cleaved product of PARP protein and a decreased in total PARP protein (Figure 1D). These results suggest that PL treatment induces apoptosis and inhibits the growth of PC cells.
Figure 1. PL inhibits cell viability, cell invasion and induces apoptosis in PC cells.
A. Effect of PL on viability of PANC1 (Ai), BxPC3 (Aii) and ASPC1 (Aiii) cells as determined by MTT assay. The values are represented as percent viable cells as compared to the vehicle treated cells. Each value is the mean± SE of each group. B. PL induces apoptosis in PANC1 cells as observed by Annexin V staining (Bi–Biv), and the images were captured with a fluorescent microscope. GF (green field) represents Annexin V staining while BF (bright field) pictures represent the total number of cells from the same field. C. PL induces PARP cleavage as determined by Western blot analysis. D. Effect of PL on cell invasion of PANC1 cells. Representative photographs of invaded cells of untreated (Di) and PL treated (Dii–iv) cells. E. Number of invading cells was determined by colorimetric measurement at 490 nM according to the manufacturer’s instructions. Each indicated group value in the graph is the mean± S.E from 3 separate wells.
PL inhibits the cell invasion of PC cells
To determine whether PL inhibits the PC cells invasion, we performed in vitro chemo-invasion assay through artificial matrix membrane (Millipore). Cells were treated with different doses of PL for 12 h. Cells viability was determined by trypan blue exclusion method, and viable cells were taken for the assay. We observed that PL treatment significantly inhibits the cell invasion of PANC1 cells (Figure 1E–F). This result demonstrates that PL has the potential for the inhibition of PC cell invasion.
PL inhibits the physical association and activation of EGFR and Stat3 in PC
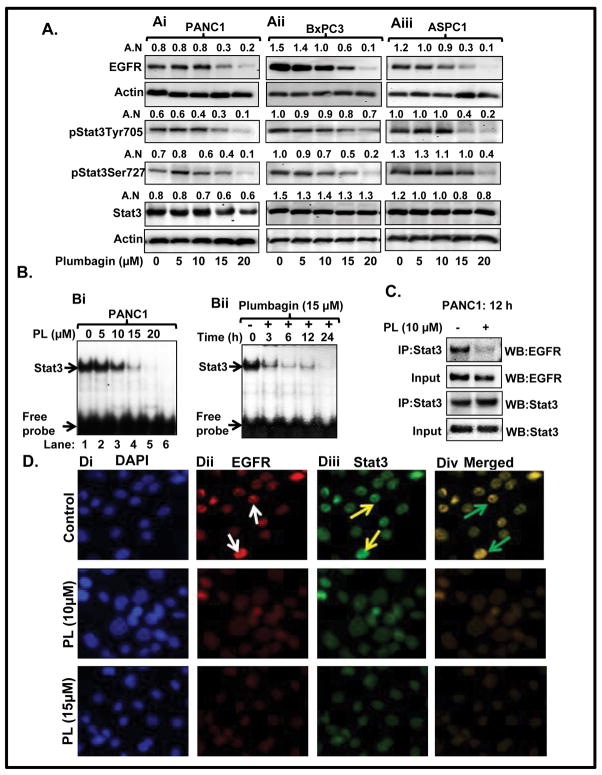
Next, we observed that PL-treatment inhibits EGFR as well as phosphorylation of Stat3 proteins in PANC1, BxPC3 and ASPC1 cells (Figure 2Ai–Aiii), while total Stat3 protein level remained unaffected (Figure 2Ai–Aiii). We further assessed the effect of PL on DNA binding activity of Stat3 by EMSA. As shown in Figure 2Bi–Bii, PL-treatment of PANC1 cells inhibited Stat3 DNA binding activity in a dose and time dependent manner. Importantly, PL-treatment of PC cells inhibited the physical association of Stat3 with EGFR in PC cells (Figure 2C), as evident from reciprocal and immunoblotting experiment. Double immunofluorescence data illustrated a strong association of Stat3 protein with EGFR in untreated PC cells (Figure 2Div, upper panel), whereas association of these proteins was significantly decreased in PL-treated PC cells (Figure 2Div, lower panel).
Figure 2. PL inhibits both Stat3 activation and dissociates Stat3-interaction with EGFR.
A. PL inhibits protein level of EGFR, pStat3Tyr705, pStat3Ser727 in PANC1 (Ai) BxPC3 (Aii) and ASPC1 (Aiii) cells as determined by Western blot analysis. Equal loading of protein was determined by stripping and reprobing the blots with actin antibody. Values in arbitrary number (A.N) shown above the immunoblots represent densitometer quantitation of band normalized to actin. B. Dose (Bi) and time-dependent (Bii) effect of PL on Stat3 DNA binding activity in PANC1 cells as determined by EMSA. In the blot (Bi) Lane 6 represents the mutant consensus sequence of Stat3. C. Effect of PL on physical association of EGFR with Stat3 in PANC1 cells as determined by immunoprecipitation and blotting experiment. D. Effect of PL on co-localization of EGFR and Stat3 in PANC1 cells as determined by double immunofluorescence. White arrows indicate the expression of EGFR, yellow arrows indicate expression of Stat3, and green arrows denote co-localization of EGFR with Stat3.
PL inhibits the activation of NF-κB signaling in PC cells
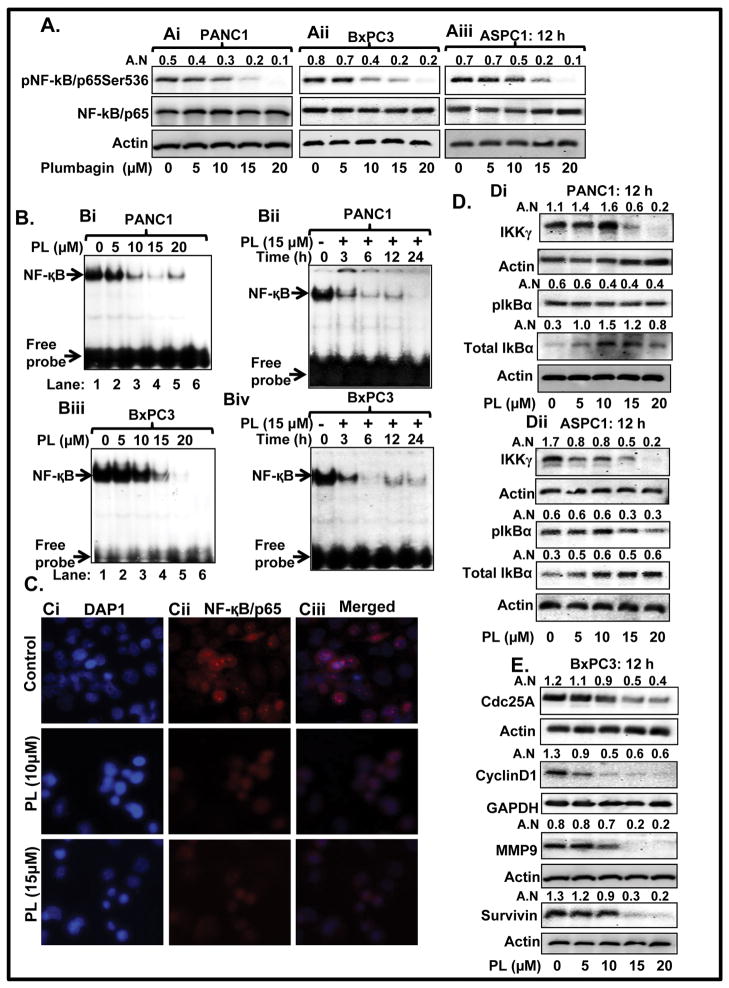
It has been demonstrated that NF-κB activates IL-6, which is a ligand for activation of the classical JAK/Stat3 signaling pathway (22). We observed that PL-treatment of PC cells inhibited phosphorylation of NF-κB in PANC1, BxPC3, and ASPC1 cells (Figure 3Ai–Aiii). EMSA results demonstrated that PL-treatment of PANC1 cells significantly inhibited DNA binding activity of NF-κB in a dose and time-dependent manner (Figure 3Bi–Bii). Similar effects were observed in BxPC3 cells (Figure 3Biii–Biv). We further performed immunocytochemistry of pNF-κB/p65 in control and PL treated PANC1 cells. Results illustrated a higher expression of pNF-κB/p65 in the nucleus of control PANC1 cells (Figure 3Cii–Ciii: upper panel), while PL-treated cells demonstrated decreased expression of pNF-κB/p65 in the nucleus (Figure 3Cii–Ciii lower panel). These data suggest NF-κB is another molecular target of PL in PC cells. PL-treatment inhibited phosphorylation of IkBα in ASPC1 cells (Figure 3Dii), but not in PANC1 (Figure 3Di) cells. However, PL-treatment enhanced total protein levels of IkBα in both PANC1 (Figure 3Di) and ASPC1 (Figure 3Dii) cells. IkB is regulated by upstream kinases IKKγ (NEMO), which phosphorylates IkB. We also observed that PL-treatment inhibits protein levels of IKKγ in both PANC1 (Figure 3Di) and ASPC1 (Figure 3Dii) cells.
Figure 3. PL inhibits activation of NF-κB in PC cells.
A. Expression level of pNF-κB/p65Ser536 and total NF-κB/p65 in PANC1 (Ai) BxPC3 (Aii) and ASPC1 (Aiii) cells as determined by Western blot analysis. B. EMSA assays represent dose and time-dependent effect of PL on DNA binding activity of NF-κB in PANC1 (Bi–Bii) and BxPC3 (Biii–Biv) cells. Lane 6 of Bi and Biii EMSA blot represent mutant consensus sequence of NF-κB. C. Localization of NF-κB/p65 in PANC1 cells as determined by immunocytochemistry. Representing pictures illustrating DAPI (Ci), NF-κB/p65 (Cii) staining and merged images of DAPI and NF-κB/p65 (Ciii) in control (upper panel) and PL treated cells (Lower panel). D. Effect of PL on protein levels of IKKγ, pIkBα, and total IkBα in PANC1 (Di) and ASPC1 (Dii) cells as determined by Western blot analysis. (E) Expression levels of Cdc25A, Cyclin D1, MMP9, and survivin as determined by Western blot analysis.
PL inhibits the expression of Cdc25A, cyclin D1, MMP9 and survivin
We determined the effect of PL on some of the common downstream target genes of both Stat3 and NF-κB. Results demonstrated that PL-treatment of PC cells inhibited protein levels of Cdc25A, cyclin D1, MMP9, and survivin (Figure 3Di–ii).
PL-treatment inhibits the growth of PC cells xenograft tumors
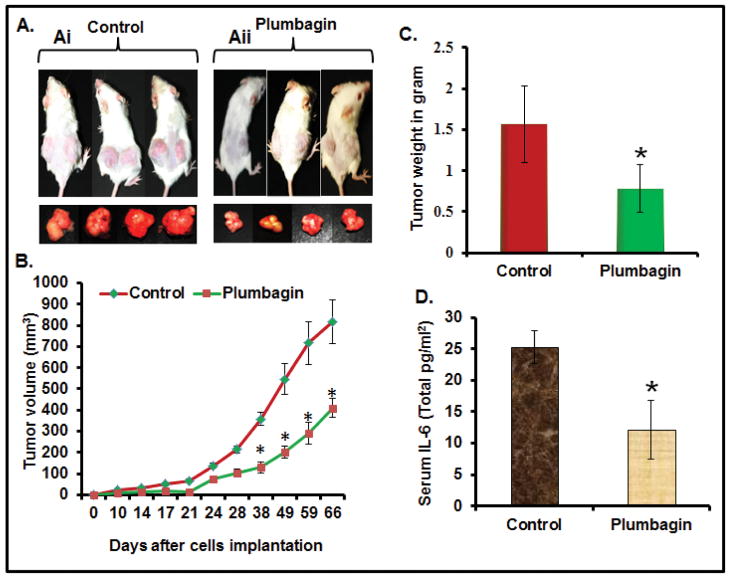
Because we observed that PL induces apoptosis and inhibits the growth and invasion of PC cells in vitro, we next examined whether these results could be translated into an in vivo xenograft mouse model. We determined the effect of PL on PANC1 cells ectopic xenograft tumors in SCID mice. PL administration (2mg/kg, body weight, i.p) in SCID mice did not cause any loss in body weight and food intake (data not shown) suggesting no apparent toxicity. PL treatment prevented the growth of PANC1 cells xenograft tumors in SCID mice as determined by a significant decrease in tumor volume (Figure 4B) and tumor weight (Figure 4C) compared to vehicle treated animals. The average volume of tumors in vehicle treated animals reached the targeted volume 900 mm3 after 66 days post-inoculation. However, PL-treated mice the average tumor volume was only 400 mm3 (Figure 4B). The observed differences in tumor development was statistically significant (P<0.01; Figure 4B) starting from day 38 to day 66. From this data, we conclude that PL is an effective anti-cancer agent that has the potential to prevent the tumorigenicity of PANC1 cells in SCID mice.
Figure 4. PL-treatment inhibits the growth of ectopically xenograft PANC1 cells tumors (A–B) in SCID mice and decreases serum IL-6 levels (D).
A. Representative photographs of the SCID mice bearing PANC1 xenograft and excised tumors of control (Ai) and PL-treated group (Aii) after 66 days post-implantation. B. Average tumor volume of vehicle or PL-treated animals was plotted over days. Each value on the graph represents the mean and S.E of 16 tumors. C. Effect of PL on tumor weight of control and PL-treated animals after 66 days post-implantation of PC cells. Values in bar graph represent mean±SE of 16 tumors of each group. Asterisks represent level of significance (P<0.01). D. Effect of PL on serum IL-6 levels as determined by ELISA assay. Values in bar graph represent mean±SE from 8 animals.
PL inhibits the serum IL-6 levels
A clinical study has observed elevated serum IL-6 in PC patients, which was further correlated with the PC metastasis into the liver.34,35 Other studies suggest autocrine IL-6 is implicated as an important activator of Stat3 in PC.36 Therefore, we determined the effect of PL-administration on serum IL-6 levels. Results demonstrated a significant (P<0.05) decrease in the serum IL-6 levels in PL-treated animals compared to vehicle treated animals (Figure 4D).
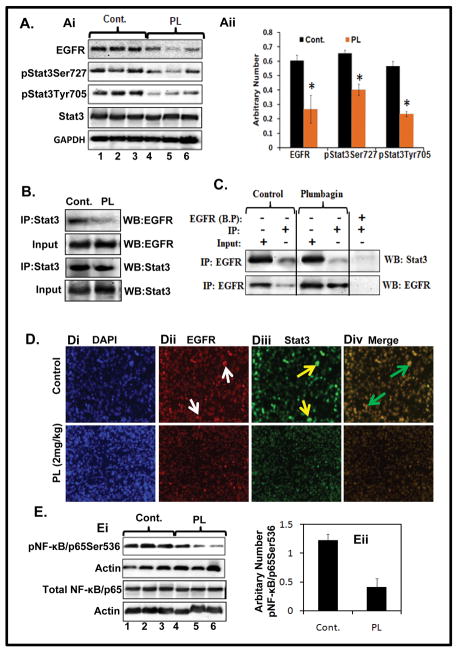
PL inhibits the activation of EGFR, Stat3 and NF-κB signaling pathways in PANC1 xenograft tumors
Because we observed that PL targets these signaling pathways in PC cells under in vitro condition, we examined the effect of PL-administration on these signaling molecules in excised xenograft tumors. Results from Western blot analysis demonstrated decreased constitutive expression of EGFR protein in PL-administered xenograft tumors compared to vehicle treated group (Figure 5Ai–Aii). Next, we determined the effect of PL on Stat3 activation. Western blot results illustrated inhibition of phospho-Stat3 protein at both Ser727 and Tyr705 residues in xenograft tumors but no change in total Stat3 (Figure 5Ai–Aii). Importantly, PL-administration inhibited the association of EGFR with Stat3 in xenograft tumors as determined by reciprocal immunoprecipitation and Western blot analysis (Figure 5B–C). As shown in Figure 5D, PL-treated xenograft tumors exhibited inhibition of EGFR and Stat3 co-localization compared to control xenograft tumors. These results demonstrate that PL-treatment inhibits association of Stat3 with EGFR in PC cells xenograft tumors. We further determined the effect of PL on NF-κB activation in xenograft tumors. Western blot analysis exhibited a decrease in protein levels of pNF-κB/p65Ser536 (Figure 5Ei–Eii), while no change was observed in total NF-κB/p65protein when compared with control. Overall these results demonstrate that PL targets EGFR, Stat3 and NF-κB pathways in PC cells xenograft tumors.
Figure 5. PL treatment inhibits EGFR, Stat3 and NF-κB/p65 expression in PC cell xenograft tumors.
A. Effect of PL on the expression levels of EGFR, pStat3Tyr705, pStat3Ser727 in PC cells xenograft tumors as determined by Western blot analysis. Expression of EGFR, pStat3Ser727, pStat3Tyr705 and total Stat3 (Ai). Each lane represents pooled samples of two animal xenograft tumors. Lane 1, 2, and 3 denote control, whereas lane 4, 5, and 6 represent PL-treated group. Bar graph values represent the arbitrary number normalized with GAPDH (Aii). B–C. Effect of PL on physical interaction of Stat3 with EGFR as determined by reciprocal immunoprecipitation and Western blot analysis. BP in Fig. C denotes to blocking peptide of EGFR. D. Effect of PL on co-localization of EGFR and Stat3 as determined by double immunofluorescence. Representative images of DAPI staining (Di), EGFR staining (Dii), Stat3 staining (Diii) and co-localization of EGFR and Stat3 (Div) of control (upper panel) or PL-treated (lower panel) xenograft tumors. E. Effect of PL on activation of NF-κB in PC cells xenograft tumors. Ei. Western blot analysis of pNF-κB/p65Ser536 in control and PL-treated xenograft tumors. Bar graph values represent the arbitrary number normalized with actin (Eii).
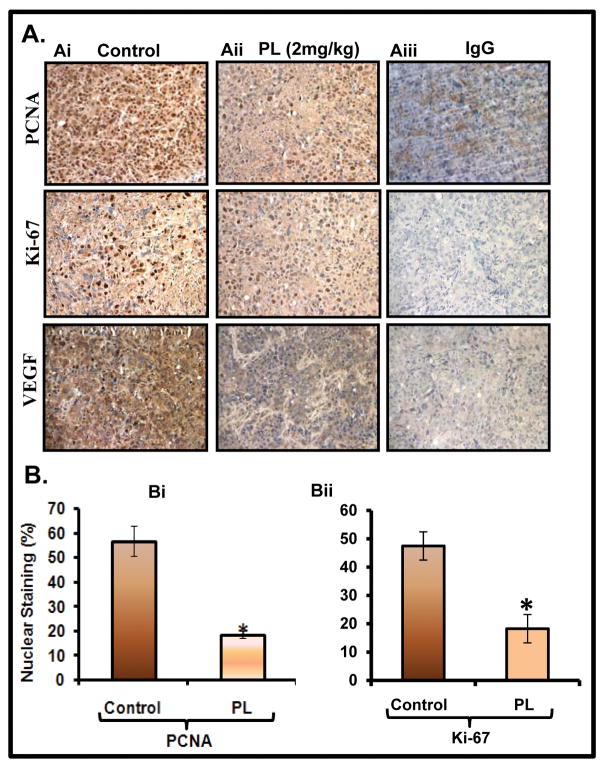
PL-administration inhibits the marker of proliferation and metastasis
Because, we observed that PL-treatment of PC cells inhibited cell invasion under in vitro chemo-invasion assay, we next examined the effect of PL on cell proliferative (PCNA, ki67) and metastasis (VEGF) markers in excised xenograft tumors. Immunohistochemistry results exhibited a significant decrease in the expression of PCNA (Figure 6Ai–Aii upper panel, Bi), ki67 (Figure 6Ai–Aii middle panel, Bii), and VEGF (Figure 6Ai–Aii, lower panel) in excised tumors of PL-treated mice compared to control animals.
Figure 6. PL-treatment inhibits markers of cell proliferation and metastasis in PC cell xenograft tumors.
Expression levels of PCNA (Ai–Aii upper panel), ki67 (Ai–Aii middle panel), and VEGF (Ai–Aii lower panel) in control and PL-treated xenograft tumors as determined by immunohistochemistry. Aiii: Negative control by using IgG. Bi–Bii: Bar graphs represent quantification of PCNA nuclear staining in control and PL-treated xenograft tumors. Student t-test was performed to analyze nuclear staining difference (P<0.05).
Discussion
Treatment of human PC remains a major challenge because of inadequate therapeutic strategies. Despite a greater understanding of the molecular pathways involved in PC development and metastasis, the use of individual targeted agents, have failed to provide significant improvements in the survival of PC patients. Numerous studies suggest the involvement of multiple signaling pathways play a pivotal role in the development, progression, and metastasis of PC.3 It is evident that natural products individually and or in combination with known chemotherapeutic drugs have a role in the prevention of PC via targeting multiple signaling molecules37,39. We present here that PL, a quinoid constituent isolated from the roots of medicinal plant Plumbago zeylanica L. (also known as Chitrak), induces apoptosis (Figure 1B–C) and inhibits invasion of PC cells (Figure 1D–E). Administration of PL (2mg/kg body weight) significantly (P<0.01) inhibits the growth of human PC cell xenograft tumors via targeting EGFR, Stat3, and NF-κB signaling pathways (Figure 4A–C).
During apoptosis, cells die in a controlled, regulated fashion, which makes them distinct from other forms of cell death. However, cancer cells proliferate in an uncontrolled manner and are resistant to apoptosis. We observed that PL has the capacity to sensitize PC cells towards apoptosis (Figure 1B–C). These results are consistent with previously published reports including our previous study suggesting the apoptosis inducing potential of PL in various other cancer cell lines under in vitro and in vivo model systems.23,27–30
EGFR is overexpressed in at least one-half of all PC4,5 and correlates with poor prognosis.6,7 It has been reported that EGFR activates Stat3 in various types of cancers including PC.8,9 Stat3 has been linked to the PC development and metastasis through the induction of various genes responsible for tumor cell proliferation, cell survival, and carcinogenesis.12,13,14 Maximum activation of Stat3 requires its phosphorylation at both of the residues (Tyr705 and Ser727). Stat3 phosphorylation at tyrosine residue (705) induces its dimerization through phosphotyrosine-SH-2 domain interaction,39 while Stat3 transcriptional activity and DNA binding activity is further enhanced through its phosphorylation at Ser727 residue.40 We observed that PL-treatment inhibits both Tyr705 and Ser727 residues of Stat3 in PC cells under in vitro cell culture (Figure 2Ai–Aiii) and in vivo xenograft tumors (Figure 5Ai–Aii). These findings clearly demonstrate that PL targets Stat3 and diminishes its activation in PC under in vitro and in vivo model systems. A previous study suggests that PL inhibits the activation of Stat3 via inducing the expression of protein tyrosine phosphatase, SHP-1.41 It is possible that PL suppresses Stat3 activation via a similar mechanism in PC. Association of nuclear EGFR and Stat3 has been shown in PC, which involve maximum activation of c-Myc oncogene.15,16 Interestingly, we observed that PL inhibits the association of Stat3 with EGFR in PC cells (Figure 2C–D) and in xenograft tumors (Figure 5B–C). These results may be due to the inhibition of Stat3 phosphorylation by PL. It has been revealed that Stat3 controls the constitutive activation of NF-κB by prolonging the retention of NF-κB in the nucleus, which occurs through p300-mediated acetylation of RelA/65.21 NF-κB is also involved in the activation of Stat3 as it up-regulates the expression of IL-6, which initiates activation of Stat3 signaling via paracrine mechanism.22 Elevated serum IL-6 levels have also been reported in patients with PC (42). These studies suggest cross talk between NF-κB and IL-6/Stat3 pathways. Numerous studies indicate that NF-κB has a key role in various types of cancers including pancreatic carcinogenesis.43, 44 Constitutive activation of NF-κB has been reported in various human PC cells and PC tissues, but not in non-tumorigenic pancreatic epithelial cells, or normal pancreatic tissues.17,18 It has been shown that suppression of NF-κB DNA binding activation restores the sensitivity of apoptosis in PC cells.45 However, various NF-κB inhibitors or transfection of IκBα super-repressors strongly enhanced the apoptosis effects of etoposide, or doxorubicin in resistant PC cells.46 Another study has demonstrated that inhibition of constitutive NF-κB activation by I kappa B alpha mutant (IκBαM) suppresses pancreatic carcinogenesis.47 Our data illustrated that PL-treatment inhibits NF-κB activation in PC cells (Figure 3A–D) and in xenograft tumors (Figure 5D) as evident from a decrease in NF-κB/p65 phosphorylation. Interestingly, we also observed that PL inhibits serum IL-6 levels in xenograft animals. Taken together, this data suggests that PL also has the potential to inhibit the Stat3 and NF-κB inter-connecting signaling pathways. It has been reported that both transcription factors share some of the common downstream target genes such as Cdc25A, cyclin D1, survivin, and MMP9, which collectively force the cancer cells to proliferate and resist apoptosis. PL treatment illustrated inhibition in the expression of these downstream target genes in PC cells. PL reduces the proliferation of PC cells under in vivo conditions as is evident from the data that shows a significant decrease in the expression of proliferative markers such as PCNA and ki67 in xenograft tumors (Figure 6A, Bi–Bii).
Various growth factors, their receptors, and matrix metalloproteases have been reported to be involved during the progression and metastasis of various types of cancers.48 It is well documented that VEGF promotes endothelial cell proliferation and survival by binding to VEGFR-1 and VEGFR-2 endothelial cell membrane receptors. It has been documented that overexpression of VEGF is associated with liver metastasis and a poor prognosis for patients with PC.49 A recent study has demonstrated that PL inhibits tumor angiogenesis and tumor growth via targeting VEGFR2-mediated Ras signaling.50 In our study, PL inhibited VEGF expression in PC xenograft tumors (Figure 6A). It is noteworthy that PL inhibited PC cell invasion (Figure 1E–F). The molecular mechanism linked to PL-induced inhibition of PC cells invasion may involve the decreased expression of VEGF and MMP9 (Figure 3D, Figure 6A). Because induction of apoptosis and inhibition in growth and invasion of PC cells was observed under in vitro conditions, we asked whether these findings could be translated into in vivo situations. An i.p administration of PL (2mg/kg body weight) to the SCID mice showed significant (P<0.01) inhibitory effects against human PC cells xenograft tumors (Figure 4A–C). These in vivo growth inhibitory effects of PL could be explained by the biochemical mechanisms observed in the present study. These data are significant because the SCID mouse model is extremely useful to study the therapeutic potential of anti-cancer agents.
In summary, PC is a highly aggressive malignant disease, which is currently treated with chemo and radiation therapy. Thus, there is an urgent need to discover novel targeting agents, which could be used in the treatment of human PC. Our data suggests that PL has potential anti-cancer activity against PC, which is, in part, due to the inhibition of EGFR, Stat3, and NF-κB signaling pathways. Based on our promising data, we suggest that PL is a potential anti-cancer agent and it may be used for the prevention and/or treatment of human PC. Further studies are warranted to test the chemopreventive potential of PL in appropriate animal models with relevance to human PC.
Novelty and Impact Statements.
Plumbagin (PL) induces apoptosis and inhibits invasion of human pancreatic cancer (PC) cells. PL targets EGFR, Stat3 and NF-κB signaling networks in PC cells and inhibits the growth of PC cells derived xenograft tumors in SCID mice. Importantly, PL inhibits the association of EGFR with Stat3 in both in vitro and in vivo model systems. These data suggest that PL could be used as a novel therapeutic agent against human PC.
Acknowledgments
We acknowledge NIH CA35368 and DHO pilot project grants. We are also thankful to Nancy E. Dreckschmidtfor her technical support in EMSA experiments.
Abbreviations
- PL
Plumbagin
- PC
Pancreatic cancer
- EGFR
Epidermal growth factor receptor
- Stat3
signal transducers and activators of transcription3
- NF-κB
Nuclear factor kappa-B
- IL-6
interleukin-6
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–69. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 3.Gupta SC, Kim JH, Kannappan R, Reuter S, Dougherty PM, Aggarwal BB. Role of nuclear factor-{kappa}B-mediated inflammatory pathways in cancer-related symptoms and their regulation by nutritional agents. Exp Biol Med. 2011;236(6):658–71. doi: 10.1258/ebm.2011.011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dancer J, Takei H, Ro JY, Lowery-Nordberg M. Coexpression of EGFR and HER-2 in pancreatic ductal adenocarcinoma: a comparative study using immunohistochemistry correlated with gene amplification by fluorescencent in situ hybridization. Oncol Rep. 2007;18(1):151–5. [PubMed] [Google Scholar]
- 5.Tsiambas E, Karameris A, Lazaris AC, Talieri M, Triantafillidis JK, Cheracakis P, Manaios L, Gerontopoulos K, Patsouris E, Lygidakis NJ. EGFR alterations in pancreatic ductal adenocarcinoma: a chromogenic in situ hybridization analysis based on tissue microarrays. Hepatogastroenterology. 2006;53 (69):452–7. [PubMed] [Google Scholar]
- 6.Smeenk HG, Erdmann J, van Dekken H, van Marion R, Hop WC, Jeekel J, van Eijck CH. Long-term survival after radical resection for pancreatic head and ampullary cancer: a potential role for the EGF-R. Dig Surg. 2007;24(1):38–45. doi: 10.1159/000100917. [DOI] [PubMed] [Google Scholar]
- 7.Ueda S, Ogata S, Tsuda H, Kawarabayashi N, Kimura M, Sugiura Y, Tamai S, Matsubara O, Hatsuse K, Mochizuki H. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004;29(1):e1–8. doi: 10.1097/00006676-200407000-00061. [DOI] [PubMed] [Google Scholar]
- 8.Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, Abbruzzese JL, Hortobagyi GN, Hung MC. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67(19):9066–76. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeArmond D, Brattain MG, Jessup JM, Kreisberg J, Malik S, Zhao S, Freeman JW. Autocrine-mediated ErbB-2 kinase activation of STAT3 is required for growth factor independence of pancreatic cancer cell lines. Oncogene. 2003;22(49):7781–95. doi: 10.1038/sj.onc.1206966. [DOI] [PubMed] [Google Scholar]
- 10.Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL, Xie K. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22 (3):319–29. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 11.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S, Schmid RM, Algül H. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19(4):456–69. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda A, Wang SC, Morris JP, 4th, Folias AE, Liou A, Kim GE, Akira S, Boucher KM, Firpo MA, Mulvihill SJ, Hebrok M. Stat3 and MMP7 Contribute to Pancreatic Ductal Adenocarcinoma Initiation and Progression. Cancer Cell. 2011;19(4):441–55. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Germain D, Frank DA. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res. 2007;13(19):5665–9. doi: 10.1158/1078-0432.CCR-06-2491. [DOI] [PubMed] [Google Scholar]
- 14.Darnell JE. Validating Stat3 in cancer therapy. Nat Med. 2005;11(6):595–6. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- 15.Jaganathan S, Yue P, Turkson J. Enhanced sensitivity of pancreatic cancer cells to concurrent inhibition of aberrant signal transducer and activator of transcription 3 and epidermal growth factor receptor or Src. J Pharmacol Exp Ther. 2010;333(2):373–81. doi: 10.1124/jpet.109.162669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaganathan S, Yue P, Paladino DC, Bogdanovic J, Huo Q, Turkson J. A functional nuclear epidermal growth factor receptor, SRC and Stat3 heteromeric complex in pancreatic cancer cells. PLoS One. 2011;6(5):e19605. doi: 10.1371/journal.pone.0019605. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Chandler NM, Canete JJ, Callery MP. Increased expression of NF-kappa B subunits in human pancreatic cancer cells. J Surg Res. 2004;118(1):9–14. doi: 10.1016/S0022-4804(03)00354-8. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5(1):119–27. [PubMed] [Google Scholar]
- 19.Arlt A, Gehrz A, Müerköster S, Vorndamm J, Kruse ML, Fölsch UR, Schäfer H. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22(21):3243–51. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 20.Dong QG, Sclabas GM, Fujioka S, Schmidt C, Peng B, Wu T, Tsao MS, Evans DB, Abbruzzese JL, McDonnell TJ, Chiao PJ. The function of multiple IkappaB NF-kappaB complexes in the resistance of cancer cells to Taxol-induced apoptosis. Oncogene. 2002;21(42):6510–19. doi: 10.1038/sj.onc.1205848. [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15(4):283–93. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bollrath J, Greten FR. IKK/NF-kappaB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009;10(12):1314–9. doi: 10.1038/embor.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandur SK, Ichikawa H, Sethi G, Ahn KS, Aggarwal BB. Plumbagin (5-hydroxy-methyl-1,4-naphthoquinone) suppresses NF-kappaB activation and NF-kappaB-regulated gene products through modulation of p65 and lkappaBalpha kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J Biol Chem. 2006;281:17023–33. doi: 10.1074/jbc.M601595200. [DOI] [PubMed] [Google Scholar]
- 24.Padhye S, Dandawate P, Yusufi M, Ahmad A, Sarkar FH. Perspectives on medicinal properties of plumbagin and its analogs. Med Res Rev. 2010 doi: 10.1002/med.20235. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Sugie S, Okamoto K, Rahman KM, Tanaka T, Kawai K, Yamahara J, Mori H. Inhibitory effects of plumbagin and juglone on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Lett. 1998;127(1–2):177–83. doi: 10.1016/s0304-3835(98)00035-4. [DOI] [PubMed] [Google Scholar]
- 26.Kuo PL, Hsu YL, Cho CY. Plumbagin induces G2-M arrest and autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol Cancer Ther. 2006;5(12):3209–21. doi: 10.1158/1535-7163.MCT-06-0478. [DOI] [PubMed] [Google Scholar]
- 27.Hsu YL, Cho CY, Kuo PL, Huang YT, Lin CC. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) induces apoptosis and cell cycle arrest in A549 cells through p53 accumulation via c-Jun NH2-terminal kinase-mediated phosphorylation at serine 15 in vitro and in vivo. J Pharmacol Exp Ther. 2006;318(2):484–94. doi: 10.1124/jpet.105.098863. [DOI] [PubMed] [Google Scholar]
- 28.Wang CC, Chiang YM, Sung SC, Hsu YL, Chang JK, Kuo PL. Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human melanoma A375.S2 cells. Cancer Lett. 2008;259:82–98. doi: 10.1016/j.canlet.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Powolny AA, Singh SV. Plumbagin-induced apoptosis in human prostate cancer cells is associated with modulation of cellular redox status and generation of reactive oxygen species. Pharm Res. 2008;25:2171–80. doi: 10.1007/s11095-008-9533-3. [DOI] [PubMed] [Google Scholar]
- 30.Aziz MH, Dreckschmidt NE, Verma AK. Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res. 2008;68:9024–32. doi: 10.1158/0008-5472.CAN-08-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CA, Chang HH, Kao CY, Tsai TH, Chen YJ. Plumbagin, isolated from Plumbago zeylanica, induces cell death through apoptosis in human pancreatic cancer cells. Pancreatology. 2009;9(6):797–809. doi: 10.1159/000210028. [DOI] [PubMed] [Google Scholar]
- 32.Hafeez BB, Imtiaz IA, Asim M, Malik A, Afaq F, Adhami VM, Saleem M, Mukhtar H. Delphinidin, a major anthocyanidin present in pigmented fruits and vegetables inhibits the prostate cancer cells xenograft in athymic nude mice. Cancer Research. 2008;15; 68(20):8564–72. [Google Scholar]
- 33.Aziz MH, Manoharan HT, Verma AK. Protein kinase C epsilon, which sensitizes skin to sun’s UV radiation-induced cutaneous damage and development of squamous cell carcinomas, associates with Stat3. Cancer Res. 2007;67 (3):1385–94. doi: 10.1158/0008-5472.CAN-06-3350. [DOI] [PubMed] [Google Scholar]
- 34.Talar-Wojnarowska R, Gasiorowska A, Smolarz B, Romanowicz-Makowska H, Kulig A, Malecka-Panas E. Clinical significance of interleukin-6 (IL-6) gene polymorphism and IL-6 serum level in pancreatic adenocarcinoma and chronic pancreatitis. Dig Dis Sci. 2009;54(3):683–9. doi: 10.1007/s10620-008-0390-z. [DOI] [PubMed] [Google Scholar]
- 35.Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101(12):2727–36. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 36.Bharadwaj U, Marin-Muller C, Li M, Chen C, Yao Q. Mesothelin Overexpression Promotes Autocrine IL-6/sIL-6R Trans-signaling to stimulate Pancreatic Cancer Cell Proliferation. Carcinogenesis. 2011;32(7):1013–24. doi: 10.1093/carcin/bgr075. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahu RP, Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J Natl Cancer Inst. 2009;101(3):176–93. doi: 10.1093/jnci/djn470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banerjee S, Wang Z, Kong D, Sarkar FH. 3,3′-Diindolylmethane enhances chemosensitivity of multiple chemotherapeutic agents in pancreatic cancer. Cancer Res. 2009;69 (13):5592–600. doi: 10.1158/0008-5472.CAN-09-0838. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7(6):454–65. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 40.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82(2):241–50. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 41.Sandur SK, Pandey MK, Sung B, Aggarwal BB. 5-hydroxy-2-methyl-1,4-naphthoquinone, a vitamin K3 analogue, suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase, SHP-1: potential role in chemosensitization. Mol Cancer Res. 2010;8(1):107–18. doi: 10.1158/1541-7786.MCR-09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C, Yang G, Jiang T, Huang K, Cao J, Qiu Z. Effects of IL-6 and AG490 on regulation of Stat3 signaling pathway and invasion of human pancreatic cancer cells in vitro. J Exp Clin Cancer Res. 2010;29:51. doi: 10.1186/1756-9966-29-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z, Rigas B. NF-kappaB, inflammation and pancreatic carcinogenesis: NF-kappaB as a chemoprevention target (review) Int J Oncol. 2006;29(1):185–92. Review. [PubMed] [Google Scholar]
- 45.Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4(8):641–8. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- 46.Arlt A, Vorndamm J, Breitenbroich M, Fölsch UR, Kalthoff H, Schmidt WE, Schäfer H. Inhibition of NF-kappaB sensitizes human pancreatic carcinoma cells to apoptosis induced by etoposide (VP16) or doxorubicin. Oncogene. 2001;20(7):859–68. doi: 10.1038/sj.onc.1204168. Erratum in: Oncogene 2002, 21(16) 2611. [DOI] [PubMed] [Google Scholar]
- 47.Fujioka S, Sclabas GM, Schmidt C, Niu J, Frederick WA, Dong QG, Abbruzzese JL, Evans DB, Baker C, Chiao PJ. Inhibition of constitutive NF-kappa B activity by I kappa B alpha M suppresses tumorigenesis. Oncogene. 2003;22(9):1365–70. doi: 10.1038/sj.onc.1206323. [DOI] [PubMed] [Google Scholar]
- 48.Itakura J, Ishiwata T, Friess H, Fujii H, Matsumoto Y, Büchler MW, Korc M. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res. 1997;3(8):1309–16. [PubMed] [Google Scholar]
- 49.Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88(10):2239–45. doi: 10.1002/(sici)1097-0142(20000515)88:10<2239::aid-cncr6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 50.Lai L, Liu J, Zhai D, Lin Q, He L, Dong Y, Zhang J, Lu B, Chen Y, Yi Z, Liu M. Plumbagin inhibits tumour angiogenesis and tumour growth through the Ras signalling pathway following activation of the VEGF receptor-2. Br J Pharmacol. 2012 Feb;165(4b):1084–96. doi: 10.1111/j.1476–5381.2011.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]