Abstract
Rationale
Adenosine A2A antagonists can reverse many of the behavioral effects of dopamine antagonists, including actions on instrumental behavior. However, little is known about the effects of selective adenosine antagonists on operant behavior when these drugs are administered alone.
Objective
The present studies were undertaken to investigate the potential for rate-dependent stimulant effects of both selective and nonselective adenosine antagonists.
Methods
Six drugs were tested: two nonselective adenosine antagonists (caffeine and theophylline), two adenosine A1 antagonists (DPCPX and CPT), and two adenosine A2A antagonists (istradefylline (KW6002) and MSX-3). Two schedules of reinforcement were employed; a fixed interval 240-s (FI-240 sec) schedule was used to generate low baseline rates of responding and a fixed ratio 20 (FR20) schedule generated high rates.
Results
Caffeine and theophylline produced rate-dependent effects on lever pressing, increasing responding on the FI-240 sec schedule but decreasing responding on the FR20 schedule. The A2A antagonists MSX-3 and istradefylline increased FI-240 sec lever pressing but did not suppress FR20 lever pressing in the dose range tested. In fact, there was a tendency for istradefylline to increase FR20 responding at a moderate dose. A1 antagonists failed to increase lever pressing rate, but DPCPX decreased FR20 responding at higher doses.
Conclusions
These results suggest that adenosine A2A antagonists enhance operant response rates, but A1 antagonists do not. The involvement of adenosine A2A receptors in regulating aspects of instrumental response output and behavioral activation may have implications for the treatment of effort-related psychiatric dysfunctions, such as psychomotor slowing and anergia in depression.
Keywords: Activation, Anergia, Psychomotor slowing, Depression, Dopamine, Parkinsonism
Introduction
There is considerable interest in the behavioral actions of drugs that modulate adenosine receptor function. Nonselective adenosine antagonists such as caffeine act as minor stimulants and are commonly consumed by humans drinking coffee, tea, and “energy drinks” (Antoniou et al. 2005; Ferré 2008; Ferré et al. 2008a; Reissig et al. 2009). Moreover, adenosine A2A antagonists are being intensively studied because of their potential utility for the treatment of various conditions, including idiopathic Parkinson’s disease (Ferré et al. 1997, 2001, 2004; Hauber et al. 2001; Wardas et al. 2001; Morelli and Wardas 2001; Carta et al. 2002; Jenner 2005; Pinna 2010; Salamone 2010), the parkinsonian side effects of antipsychotic drugs (Correa et al. 2004; Ishiwari et al. 2007; Salamone et al. 2008; Varty et al. 2008), and energy-related symptoms such as psychomotor slowing, anergia, and fatigue in patients with depression and other disorders (Farrar et al. 2007, 2010; Salamone et al. 2007, 2009, 2010; Nunes et al. 2010). Striatal areas that are rich in dopamine (DA), including neostriatum and nucleus accumbens, also have a high concentration of adenosine A2A receptors (Jarvis and Williams 1989; Schiffmann et al. 1991; DeMet and Chicz-DeMet 2002; Ferré et al. 2004). Moreover, there is a functional interaction between DA D2 and adenosine A2A receptors, which are co-localized on enkephalin-containing medium spiny neurons (Fink et al. 1992; Ferré 1997; Ferré et al. 1997, 2008b; Hettinger et al. 2001; Chen et al. 2001; Hillion et al. 2002; Fuxe et al. 2003). Consistent with these anatomical data, a large body of evidence indicates that adenosine A2A antagonists can reverse the effects of DA antagonists, particularly those of D2 family antagonists such as haloperidol and eticlopride (Worden et al. 2009; Salamone et al. 2009; Nunes et al. 2010). Thus, adenosine A2A antagonists have been shown to attenuate the effects of D2 antagonists on tremor (Correa et al. 2004; Salamone et al. 2008; Betz et al. 2009), locomotor suppression (Correa et al. 2004; Salamone et al. 2008; Collins et al. 2010B), and various aspects of instrumental behavior, including lever pressing response rate and instrumental response selection in studies involving effort-related choice behavior (Farrar et al. 2007, 2010; Worden et al. 2009; Salamone et al. 2009; Mott et al. 2009; Nunes et al. 2010).
Several previous studies of adenosine antagonists in rodents have focused upon their ability to stimulate locomotor activity. Consistent with its profile as a minor stimulant, caffeine has been shown repeatedly to enhance locomotor activity in rats and mice (Garrett and Holtzman 1994; Daly and Fredholm 1998; Karcz-Kubicha et al. 2003; Antoniou et al. 2005). Several studies have demonstrated that adenosine A2A antagonists, including istradefylline (KW6002), MSX-3, and SCH 58261, also can increase locomotion (Popoli et al. 1998; Antoniou et al. 2005; Collins et al. 2010B). The locomotor stimulant effects of A1 antagonists appear to be more variable and may depend upon the particular drug; CPT (8-cyclopentyltheophylline) has been shown in several studies to induce locomotion in rodents (Marston et al. 1998; Popoli et al. 1998; Karcz-Kubicha et al. 2003), while DPCPX (8-cyclopentyl-1,3-dipropylxanthine), which is more selective for A1 receptors than CPT, generally does not stimulate locomotion (Marston et al. 1998; Collins et al. 2010B). Yet despite the large number of studies of the effects of adenosine A2A antagonists on locomotion and the growing body of evidence indicating that these drugs can reverse the suppression of lever pressing induced by DA antagonism, little is known about the effects of adenosine A2A antagonists on operant behavior when they are administered in the absence of other drugs. Moderate doses of MSX-3 and istradefylline were reported not to have much effect on lever pressing rates with rats responding on either a fixed ratio 5 (FR5) schedule (Farrar et al. 2007; Salamone et al. 2009) or the FR10 component of a drug discrimination procedure (Justinova et al. 2003). More recently, it was reported that high doses of MSX-3 and CPT (i.e., 30.0 mg/kg) decreased lever pressing rates on the FR10 component of a drug discrimination task in rats trained to discriminate nicotine (Justinova et al. 2009). However, in the behavioral pharmacology literature, it is well known that the rate-enhancing effects of stimulant drugs depend greatly upon the schedule of reinforcement (Dews 1955, 1958). Thus, with major stimulants such as amphetamine, enhanced rates of lever pressing are most readily demonstrated when animals are responding on schedules that generate low rates of responding (e.g., fixed interval schedules), and response rates tend to be decreased when animals are responding on schedules that generate high baseline rates of responding (Dews 1958; Sanger and Blackman 1974; Wenger and Dews 1976). Studies of the rate-dependent effects of caffeine have yielded mixed results; some papers have indicated that caffeine can produce rate-dependent effects on operant responding (Davis et al. 1973; McKim 1980), while other research failed to find this result (Harris et al. 1978). Moreover, it is uncertain if adenosine antagonists with different receptor selectivity profiles would also produce this pattern of effects.
The present studies were undertaken to investigate the potential for rate-dependent stimulant effects of both selective and non-selective adenosine antagonists. Six drugs were tested: two nonselective adenosine antagonists (caffeine and theophylline), two adenosine A2A antagonists (MSX-3 and istradefylline (KW6002)), and two adenosine A1 antagonists (DPCPX and CPT). Two schedules of reinforcement were employed; a fixed interval 240-s (FI-240 sec) schedule was used to generate low baseline rates of responding and a fixed ratio 20 (FR20) schedule generated very high rates of lever pressing.
Materials and methods
Animals
Male Sprague–Dawley rats (Harlan, Indianapolis, IN, USA) were used in all of these experiments. The animals were food-restricted to 85% of their free feeding weight for initial lever pressing training but they were then allowed modest growth throughout the course of the study. The animals weighed 350–400 g at the time of testing. All rats were housed in a climate-controlled colony maintained at 23°C on a 12-h light/dark cycle (lights on 07:00 h) with access to water ad libitum in their home cages. All protocols were approved by the University of Connecticut Institute for Animal Care and Use Committee and studies were conducted in accordance with NIH guidelines.
Behavioral procedures
Behavioral sessions were conducted in operant chambers (28×23×23 cm, Med Associates). Rats were initially trained to lever press on a continuous reinforcement schedule (30-min sessions and 45 mg Bioserve Pellets, Frenchtown, NJ, USA, were used for all operant behavior tests). For the FI-240 training, rats then were shifted to a series of interval schedules, in which the first response after the time interval elapsed was reinforced by the delivery of a single 45-mg pellet. Rats were trained on a FI-10 s schedule for 5 days, FI-30 s for 5 days, FI-60 s for 5 days, FI-120 s for 5 days, and finally FI-240 sec for the duration of the experiment. Rats were trained on the FI-240 sec schedule for several weeks until they reached stable baseline rates of lever pressing (consistent responding of less than 120 lever presses in 30 min), after which drug testing began. On all baseline days and over weekends, rats training on the FI 240-s schedule received supplemental feeding in order to maintain body weight. For FR20 training, rats were shifted from continuous reinforcement to a series of ratio schedules: FR5 for 5 days, FR10 for 5 days, and finally FR20 for the duration of the experiment. Rats were trained until they reached stable baseline rates of lever pressing (consistent responding over 3,000 lever presses in 30 min), after which drug testing began. On all baseline days and over weekends, rats received supplemental feeding in order to maintain body weight.
Pharmacological agents and selection of doses
Caffeine and theophylline (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in a 0.9% saline solution, which also was used as the vehicle control for these drugs. MSX-3 was provided by Christa Müller (University of Bonn, Bonn, Germany) and was dissolved in 0.9% saline solution and then pH-adjusted with 1.0M NaOH solution to the range of 7.2–7.4; a 0.9% saline solution also served as the vehicle control for MSX-3. Istradefylline (KW6002) was provided by Lundbeck Pharmaceuticals (Copenhagen, Denmark) and was dissolved in dimethyl sulfoxide (DMSO, Fisher Scientific, Pittsburgh, PA, USA) and Tween 80 (Fisher Scientific, Pittsburgh PA) mixed with 0.9% saline (10:10:80% mixture; see Nunes et al. (2010)). This mixture also served as the vehicle control for istradefylline. DPCPX (8-cyclopentyl-1,3-dipropylxanthine) and CPT (8-cyclopentyltheophylline) were obtained from Tocris Bioscience (Ellisville, MO, USA). DPCPX was dissolved in 10% ethanol vehicle (Collins et al. 2010b; Nunes et al. 2010). CPT was dissolved in 0.9% saline. All drugs were administered IP, and all doses and injection times were selected based on previously published work (Salamone et al. 2009; Collins et al. 2010, b; Nunes et al. 2010) and pilot studies. Several factors were considered for determining the dose ranges for DPCPX and CPT that were used. In part, they were based upon doses listed in published behavioral studies involving IP administration in rats (Prediger and Takahashi 2005; Antoniou et al. 2005; Aubel et al. 2007; Maione et al. 2007; Lobato et al. 2008; Karcz-Kubicha et al. 2003; Marston et al. 1998; Justinova et al. 2009; Salamone et al. 2009). Prediger and Takahashi (2005) showed that DPCPX could affect memory in rats at doses of 1.0–3.0 mg/kg IP. However, a previous study showed that the higher dose of DPCPX (3.0 mg/kg IP) actually exacerbated the slowing of maze running speed induced by haloperidol (Mott et al. 2009). CPT was shown to be behaviorally active in the dose range tested (Marston et al. 1998, 3.0 mg/kg; Antoniou et al. 2005; Karcz-Kubicha et al. 2003, 4.8–7.2 mg/kg). In addition, because a pilot study (n=7) indicated that 24.0 mg/kg CPT did not increase FI-240 responding relative to injection of saline vehicle, a lower dose progression (1.5–12.0 mg/kg IP) was used. Doses of CPT higher than 24.0 mg/kg were not studied because such doses would be outside the range of what is normally reported for behavioral studies in the literature.
Experimental procedures
Different groups of rats were used for each experiment. All experiments used a within-group design in which each rat received all IP drug or vehicle treatments in their particular experiment in a randomly varied order (one treatment per week over a 5-week period). Baseline training sessions (i.e., non-drug) were conducted 4 days per week. For each drug, the same dose range was used for both schedules of reinforcement.
Experiment 1—effects of caffeine on FR20 and FI-240 sec lever pressing
Separate groups of animals were used for the FR20 (n=8) and FI-240 sec (n=8) experiments. On the drug treatment day, rats were injected with saline vehicle or 5.0, 10.0, 20.0, or 40.0 mg/kg caffeine 20 min prior to testing.
Experiment 2—effects of theophylline on FR20 and FI-240 sec lever pressing
Different groups of animals were used for the FR20 (n=8) and FI-240 sec (n=7) experiments. Rats were injected with saline vehicle or 5.0, 10.0, 20.0, or 40.0 mg/kg theophylline 20 min prior to testing.
Experiment 3—effects of MSX-3 on FR20 and FI-240 sec lever pressing
Separate groups of rats were used for the FR20 (n=8) and FI-240 sec (n=7) experiments. Rats received injections of saline vehicle or 1.0, 2.0, 4.0, or 8.0 MSX-3 20 min before testing.
Experiment 4—effects of istradefylline (KW6002) on FR20 and FI-240 sec lever pressing
Two groups of animals were used for the FR20 (n=8) and FI-240 sec (n=8) experiments. Rats were injected with either 10:10:80% (DMSO, Tween-80, saline) vehicle or 0.125, 0.25, 0.5, or 1.0 mg/kg istradefylline 20 min prior to testing.
Experiment 5—effects of DPCPX on FR20 and FI-240 sec lever pressing
Different groups of rats were used for the FR20 (n=8) and FI-240 sec (n=8) experiments. Animals were treated with the 10% ethanol vehicle or 0.1875, 0.375, 0.75, or 1.5 mg/kg of DPCPX 30 min before testing.
Experiment 6—effects of CPT on FR20 and FI-240 sec lever pressing
Two separate groups of rats were used for the FR20 (n=6) and FI-240 sec (n=7) experiments. The treatments were saline vehicle or 1.5, 3.0, 6.0, or 12.0 mg/kg CPT solution adminitered 20 min prior to testing.
Statistical analyses
For both schedules of reinforcement, the raw number of lever presses is listed in Table 1. For statistical analyses and graphic presentation, lever pressing data were expressed as a percent of the control rate of responding and were analyzed with repeated measures analysis of variance (ANOVA). Non-orthogonal planned comparisons using the overall ANOVA error term (Keppel 1991) were used to compare each treatment with the vehicle control. Orthogonal analysis of trend also was calculated for each experiment; trend analysis (Keppel 1991) assesses the presence of mathematical functions (e.g., linear, quadratic, cubic) in ANOVA data. For the FI-240 experiments, data within the 240 s FI period were broken down into 12 20-s time bins and analyzed with a time bin × drug treatment factorial ANOVA. For all FI-240 sec experiments, there was a significant effect of time bin, so these ANOVA values were not reported below. However, the time bin × treatment interactions were analyzed to determine if drug treatments altered the “scalloping” pattern (i.e., the increase in responding at the end of the interval) that is characteristic of FI lever pressing; a significant interaction was interpreted to mean that there was a treatment-related alteration in the patterning of responding throughout the 240-s intervals. In addition, the index of curvature (Fry et al. 1960) was calculated for each FI-240 sec experiment to provide another measure of the temporal patterning of responding. The index of curvature is a measure of the proportional distribution of responses across the fixed interval period and has been used in psychopharmacology experiments to provide a marker of patterning of responding within the interval (e.g., Odum and Schaal 2000). For this calculation, we used the following formula (from Odum and Schaal (2000); see also Fry et al. (1960)):
in which R1 is the total number of responses in the first bin, R2 is the total in the first and second bin, R3 is the total in the first three bins, etc., and R12 is the total number of responses occurring in all of the bins.
Table 1.
Mean (± SEM) number of responses for all six FR20 and FI-240 sec experiments
| FR20 | ||||||
| Veh | 5.0 mg/kg | 10.0 mg/kg | 20.0 mg/kg | 40.0 mg/kg | ||
| Caffeine | Mean | 3181.3 | 3259.6 | 2630.3 | 2156.5 | 1493.8 |
| SEM | 249.9 | 204.6 | 162.5 | 181.5 | 267.4 | |
| Veh | 5.0 mg/kg | 10.0 mg/kg | 20.0 mg/kg | 40.0 mg/kg | ||
| Theophylline | Mean | 2867.4 | 3114.6 | 3070.6 | 2540.5 | 1416.5 |
| SEM | 131.2 | 186.4 | 234.0 | 250.8 | 159.1 | |
| Veh | 1.0 mg/kg | 2.0 mg/kg | 4.0 mg/kg | 8.0 mg/kg | ||
| MSX-3 | Mean | 3363.88 | 3271.88 | 3319.75 | 3458.38 | 3384.50 |
| SEM | 182.91 | 128.79 | 90.61 | 114.12 | 97.65 | |
| Veh | 0.125 mg/kg | 0.25 mg/kg | 0.5 mg/kg | 1.0 mg/kg | ||
| Istradefylline | Mean | 3652.6 | 3832.0 | 4197.1 | 3921.4 | 3766.4 |
| SEM | 331.6 | 317.2 | 271.8 | 247.3 | 264.2 | |
| Veh | 0.1875 mg/kg | 0.375 mg/kg | 0.75 mg/kg | 1.5 mg/kg | ||
| DPCPX | Mean | 3263.0 | 3154.0 | 3144.5 | 2687.9 | 2724.6 |
| SEM | 161.3 | 180.7 | 217.2 | 352.7 | 103.9 | |
| Veh | 1.5 mg/kg | 3.0 mg/kg | 6.0 mg/kg | 12.0 mg/kg | ||
| CPT | Mean | 4268.0 | 4404.7 | 3908.3 | 4470.3 | 4366.7 |
| SEM | 333.5 | 244.9 | 264.6 | 205.6 | 281.9 | |
| FI-240 sec | ||||||
| Veh | 5.0 mg/kg | 10.0 mg/kg | 20.0 mg/kg | 40.0 mg/kg | ||
| Caffeine | Mean | 120.6 | 190.9 | 207.6 | 225.1 | 103.6 |
| SEM | 17.8 | 22.8 | 21.8 | 17.7 | 13.9 | |
| Veh | 5.0 mg/kg | 10.0 mg/kg | 20.0 mg/kg | 40.0 mg/kg | ||
| Theophylline | Mean | 97.9 | 130.9 | 179.1 | 186.3 | 179.4 |
| SEM | 12.8 | 10.0 | 17.7 | 20.7 | 28.5 | |
| Veh | 1.0 mg/kg | 2.0 mg/kg | 4.0 mg/kg | 8.0 mg/kg | ||
| MSX-3 | Mean | 82.4 | 93.3 | 162.3 | 129.9 | 69.1 |
| SEM | 15.4 | 21.3 | 26.2 | 18.4 | 11.2 | |
| Veh | 0.125 mg/kg | 0.25 mg/kg | 0.5 mg/kg | 1.0 mg/kg | ||
| Istradefylline | Mean | 80.1 | 101.4 | 134.8 | 150.6 | 113.5 |
| SEM | 13.4 | 17.5 | 26.6 | 28.4 | 14.4 | |
| Veh | 0.1875 mg/kg | 0.375 mg/kg | 0.75 mg/kg | 1.5 mg/kg | ||
| DPCPX | Mean | 127.3 | 97.3 | 120.3 | 101.9 | 95.9 |
| SEM | 34.1 | 24.2 | 25.7 | 23.5 | 18.6 | |
| Veh | 1.5 mg/kg | 3.0 mg/kg | 6.0 mg/kg | 12.0 mg/kg | ||
| CPT | Mean | 82.4 | 63.1 | 83.4 | 69.3 | 95.6 |
| SEM | 16.9 | 16.1 | 14.5 | 12.4 | 11.3 | |
Results
Experiment 1—effects of caffeine on FR20 and FI-240 sec lever pressing
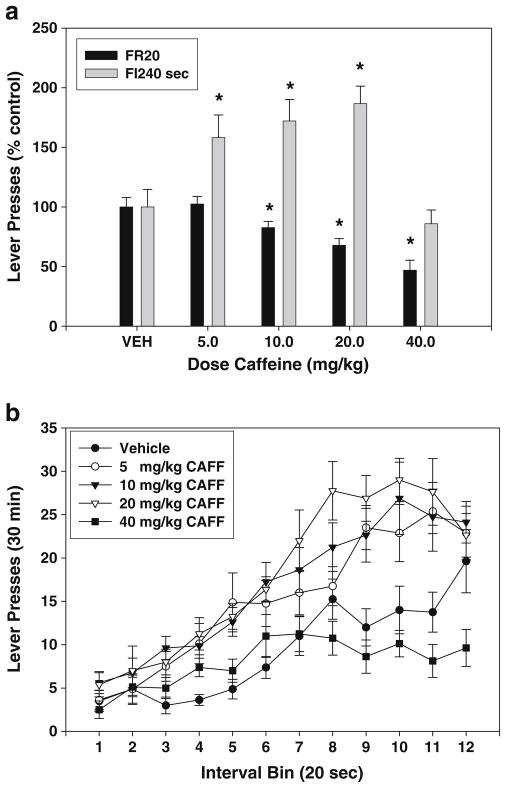
Caffeine produced rate-dependent effects on operant responding (see Table 1 for the data on the total number of responses). In the FR20 experiment, ANOVA showed that the treatment effect of caffeine on FR20 lever pressing was statistically significant (Fig. 1a; F(4,28)=20.962, p<0.001; significant linear trend, p<0.01). Planned comparisons revealed that doses of 10.0, 20.0, and 40.0 mg/kg significantly decreased lever pressing compared to vehicle. ANOVA showed that the treatment effect of caffeine on FI-240 lever pressing was statistically significant (Fig. 1a; F(4,28)=11.12, p<0.001; significant quadratic trend, p<0.01). Planned comparisons revealed that caffeine significantly increased lever pressing compared to vehicle at doses of 5.0, 10.0, and 20.0 mg/kg (p<0.01). The breakdown of responding as a function of caffeine dose and time bin within the interval is shown in Fig. 1b. An analysis of interval bins revealed a significant treatment × interval bin interaction (F(44,308)=3.713, p<0.001). Caffeine also produced a significant effect on the index of curvature, indicating that the drug altered the pattern of responding within the 240-s interval (F(4,28)=5.74, p<0.01; Table 2).
Fig. 1.
Effects of the nonselective adenosine antagonist caffeine on lever pressing during performance on FR20 and FI-240 sec schedules. a Mean (± SEM) number of responses (expressed as percent of the control mean) in rats responding on the FR20 and FI-240 sec schedules after injections of vehicle and various doses of caffeine. Asterisk, planned comparisons, p<0.05, different from vehicle. b Mean (± SEM) number of responses within the 240-s fixed interval period, broken down into 12 20-s interval bins
Table 2.
Mean (± SEM) index of curvature data for all six FI-240 sec experiments
| Vehicle | 5.0 mg/kg | 10.0 mg/kg | 20.0 mg/kg | 40.0 mg/kg | ||
| Caffeinea | Mean | 0.306 | 0.277 | 0.230 | 0.249 | 0.132 |
| SEM | 0.042 | 0.023 | 0.020 | 0.030 | 0.030 | |
| Vehicle | 5.0 mg/kg | 10.0 mg/kg | 20.0 mg/kg | 40.0 mg/kg | ||
| Theophylline | Mean | 0.258 | 0.327 | 0.257 | 0.145 | 0.185 |
| SEM | 0.060 | 0.041 | 0.036 | 0.034 | 0.052 | |
| Vehicle | 0.5 mg/kg | 1.0 mg/kg | 2.0 mg/kg | 4.0 mg/kg | ||
| MSX-3 | Mean | 0.370 | 0.259 | 0.307 | 0.301 | 0.387 |
| SEM | 0.045 | 0.050 | 0.043 | 0.040 | 0.047 | |
| Vehicle | 0.125 mg/kg | 0.25 mg/kg | 0.5 mg/kg | 1.0 mg/kg | ||
| Istradefylline | Mean | 0.414 | 0.260 | 0.311 | 0.321 | 0.289 |
| SEM | 0.041 | 0.047 | 0.018 | 0.026 | 0.053 | |
| Vehicle | 0.1875 mg/kg | 0.375 mg/kg | 0.75 mg/kg | 1.5 mg/kg | ||
| DPCPX | Mean | 0.31 | 0.33 | 0.32 | 0.36 | 0.30 |
| SEM | 0.06 | 0.03 | 0.03 | 0.03 | 0.03 | |
| Vehicle | 1.5 mg/kg | 3.0 mg/kg | 6.0 mg/kg | 12.0 mg/kg | ||
| CPT | Mean | 0.438 | 0.265 | 0.416 | 0.298 | 0.282 |
| SEM | 0.055 | 0.044 | 0.044 | 0.070 | 0.054 |
significant overall effect; 40.0 mg/kg caffeine significantly differed from vehicle.
Experiment 2—effects of theophylline on FR20 and FI-240 sec lever pressing
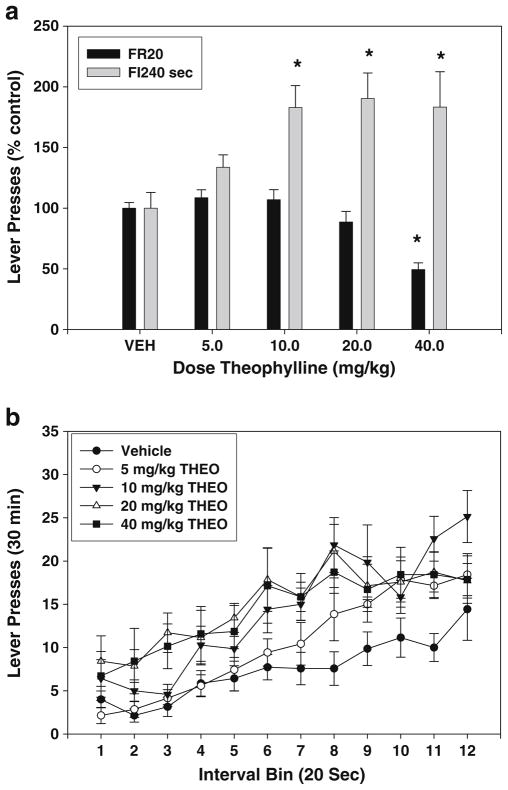
Theophylline also produced rate-dependent effects on operant behavior (see Table 1 for raw data of the number of responses). ANOVA demonstrated that the treatment effect of theophylline on FR20 lever pressing was statistically significant (Fig. 2a; F(4,28)=19.944, p<0.001; significant linear trend, p<0.01). Planned comparisons revealed that a dose of 40.0 mg/kg was capable of significantly decreasing lever pressing compared to vehicle (p<0.01). The overall effect of theophylline treatment in the FI-240 experiment was statistically significant (Fig. 2a; F (4,24)=5.609, p<0.01; significant linear trend, p<0.01). Planned comparisons revealed that theophylline significantly increased lever pressing at doses of 10.0, 20.0, and 40.0 mg/kg (p<0.01). An analysis of interval bins (Fig. 2b) revealed that there was no significant treatment × interval bin interaction (F(44,264)=1.251, n.s.). However, the effect of theophylline on the index of curvature approached significance (F(4,24)=2.733, p=0.053; Table 2).
Fig. 2.
Effects of the nonselective adenosine antagonist theophylline on lever pressing during performance on FR20 and FI-240 sec schedules. a Mean (± SEM) number of responses (expressed as a percent of the control mean) in rats responding on the FR20 and FI-240 sec schedules after injections of vehicle and various doses of theophylline. Asterisk, planned comparisons, p<0.05, different from vehicle. b Mean (± SEM) number of responses within the 240-s fixed interval period, broken down into 12 20-s interval bins
Experiment 3—effects of MSX-3 on FR20 and FI-240 sec lever pressing
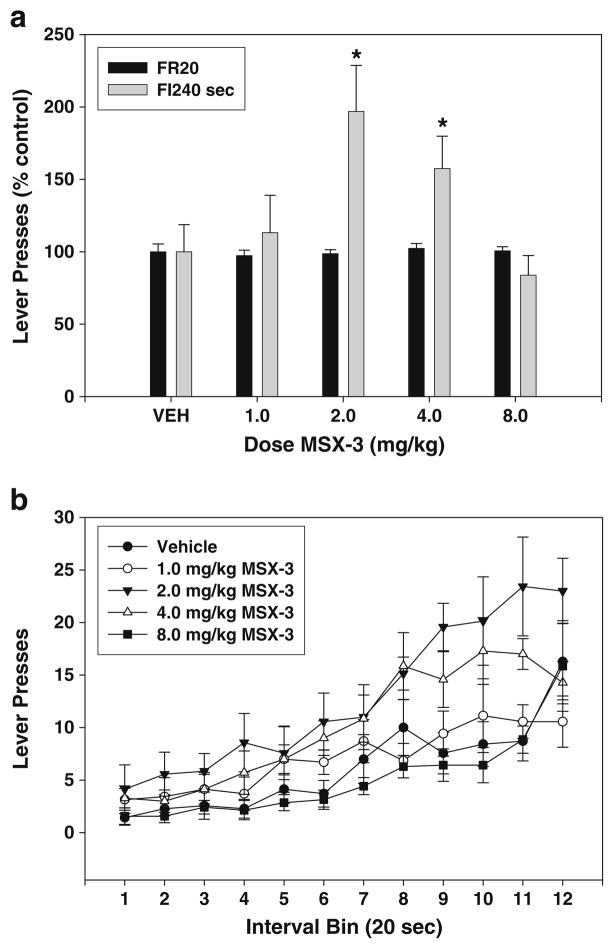
The adenosine A2A antagonist MSX-3 increased the rate of responding on the FI-240 sec schedules of reinforcement but had no significant effect on FR20 responding in the dose range tested (see Table 1). Although an initial study with a lower dose progression (0.5, 1.0, 2.0, and 4.0 mg/kg) showed that 1.0 mg/kg MSX-3 did produce a slight increase in FR20 responding (10% above baseline; data not shown), in the present study there was no significant treatment effect of MSX-3 on FR20 lever pressing (Fig. 3a; F(4,28)=0.516, n.s.; no significant trends). The treatment effect of MSX-3 on FI-240 lever pressing was statistically significant (Fig. 3a; F(4,24)=6.95, p<0.01; significant quadratic trend, p<0.01), and planned comparisons showed that the 2.0 and 4.0 mg/kg doses significantly differed from vehicle (p<0.05). Figure 3b depicts the breakdown of responding as a function of MSX-3 dose and time bin within the interval. There was a significant treatment × interval bin interaction (F(44,264)=2.197, p<0.001), indicating that there was an alteration of the temporal distribution of responses across different treatments. However, the effect of MSX-3 on the index of curvature was not statistically significant (F(4,24)=1.8, n.s.; see Table 2).
Fig. 3.
Effects of the adenosine A2A antagonist MSX-3 on lever pressing during performance on FR20 and FI-240 sec schedules. a Mean (± SEM) number of responses (expressed as percent of the control mean) in rats responding on the FR20 and FI-240 sec schedules after injections of vehicle and various doses of MSX-3. Asterisk, planned comparisons, p<0.05, different from vehicle. b Mean (± SEM) number of responses within the 240-s fixed interval period, broken down into 12 20-s interval bins
Experiment 4—effects of istradefylline (KW6002) on FR20 and FI-240 lever pressing
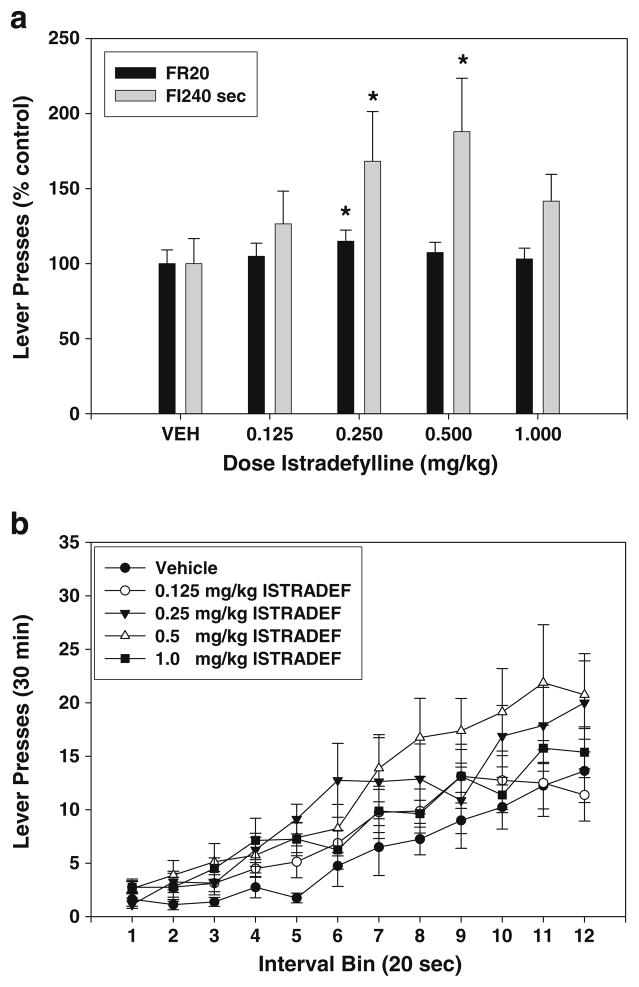
The adenosine A2A antagonist istradefylline increased FI-240 response rate and had a subtle biphasic effect on FR20 lever pressing (Table 1). Istradefylline produced no significant effects on FR20 lever pressing (Fig. 4a). Nevertheless, trend analysis showed that, similar to the low dose progression MSX-3, there was a significant quadratic trend (F(1,7)=9.39, p<0.05), which is consistent with a pattern of increased response rates at intermediate doses. Because of the significant trend effect, additional planned comparisons were performed, and it was revealed that the 0.25-mg/kg dose of istradefylline significantly increased lever pressing relative to vehicle (p<0.05). The effect of istradefylline treatment on FI-240 sec lever pressing also was significant (Fig. 4a; F(4,28)=5.299, p<0.01; significant linear trend, p<0.01). Non-orthogonal planned comparisons revealed that doses of 0.25 and 0.5 mg/kg significantly increased lever pressing over vehicle (p< 0.01). An analysis of interval bins showed that there was a significant treatment × interval bin interaction (F(44,308) =1.581, p<0.05). Nevertheless, the effect of istradefylline on the index of curvature was not statistically significant (F(4,28)=2.402, n.s.; see Table 2).
Fig. 4.
Effects of the adenosine A2A antagonist istradefylline (KW 6002) on lever pressing during performance on FR20 and FI-240 sec schedules. a Mean (± SEM) number of responses (expressed as percent of the control mean) in rats responding on the FR20 and FI-240 sec schedules after injections of vehicle and various doses of istradefylline. Asterisk, planned comparisons, p<0.05, different from vehicle. b Mean (± SEM) number of responses within the 240-s fixed interval period, broken down into 12 20-s interval bins
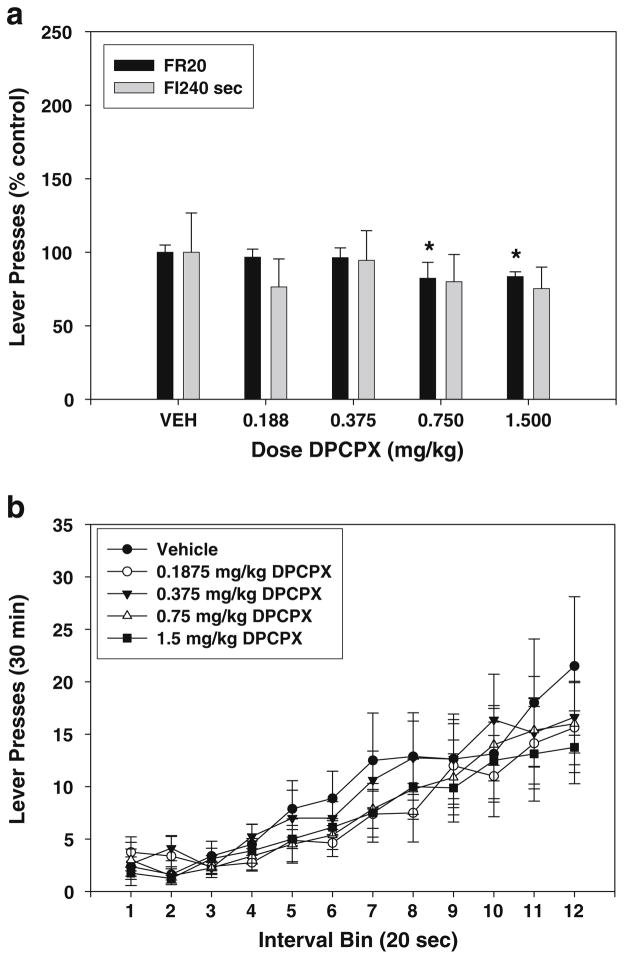
Experiment 5—effects of DPCPX on FR20 and FI-240 lever pressing
The effect of DPCPX treatment on FR20 lever pressing was statistically significant (Fig. 5a; F(4,28)=28.222, p<0.01; significant linear trend, p<0.01). Planned comparisons revealed that doses of 0.75 and 1.5 mg/kg significantly decreased lever pressing compared to vehicle (p<0.05). ANOVA showed that the treatment effect of DPCPX on FI240 s lever pressing was not statistically significant (Fig. 5a; F(4,28)=1.912, n.s.; no significant trends). An analysis of interval bins (Fig. 5b) showed no interval × treatment interactions (F(44,308)=1.044, n.s.), and there was no significant effect on the index of curvature (F(4,28)= 0.471, n.s.; Table 2).
Fig. 5.
Effects of the adenosine A1 antagonist DPCPX on lever pressing during performance on FR20 and FI-240 sec schedules. a Mean (± SEM) number of responses (expressed as percent of the control mean) in rats responding on the FR20 and FI-240 sec schedules after injections of vehicle and various doses of DPCPX. b Mean (± SEM) number of responses within the 240-s fixed interval period, broken down into 12 20-s interval bins
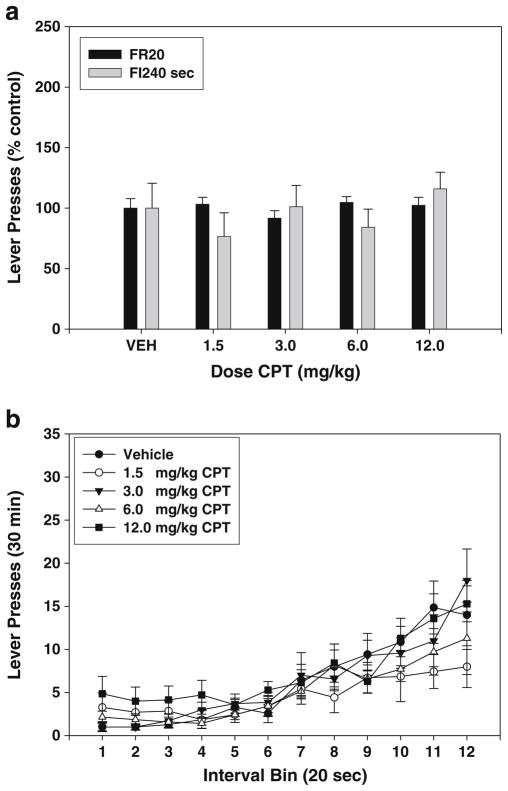
Experiment 6—effects of CPT on FR20 and FI-240 lever pressing
CPT produced no significant effects on FR20 lever pressing (Fig. 6a; F(4,20)=1.187, n.s.; no significant trends) and no significant effect on FI240 s lever pressing (Fig. 6a; F(4,24)=2.213, n.s.; no significant trends). An analysis of interval bins also showed no effects on the pattern of responding within time bins (Fig. 6b; F(44,264)=1.853, n.s.), and there was no significant effect of CPT on the index of curvature (F(4,24)=2.163, n.s.; Table 2).
Fig. 6.
Effects of the adenosine A1 antagonist CPT on lever pressing during performance on FR20 and FI-240 sec schedules. a Mean (± SEM) number of responses (expressed as percent of the control mean) in rats responding on the FR20 and FI-240 sec schedules after injections of vehicle and various doses of CPT. b Mean (± SEM) number of responses within the 240-s fixed interval period, broken down into 12 20-s interval bins
Discussion
One of the classic findings in behavioral pharmacology is that major stimulants such as amphetamines and cocaine can produce rate-dependent effects on operant performance, increasing responding on schedules that generate low rates and decreasing responding on schedules that generate high rates (Dews 1958; Sanger and Blackman 1974; Wenger and Dews 1976). The present experiments demonstrate clearly that the nonselective adenosine antagonists caffeine and theophylline can produce rate-dependent effects on average rates of operant responding. Moreover, the rate-increasing and -decreasing effects of adenosine antagonism appear to depend upon the subtype of adenosine receptor being blocked, with A2A antagonists being effective at increasing response rates and A1 antagonists failing to stimulate increases in responding.
The first two experiments showed that caffeine and theophylline can increase lever pressing rate in rats responding on a FI-240 sec schedule, which generates low baseline rates of responding, and decrease rate of responding on the FR20 schedule, which generates high baseline response rates (Figs. 1 and 2). Thus, these two nonselective adenosine antagonists, which typically are classified as minor stimulants, were able to produce rate-dependent effects on operant responding that are similar to those induced by major stimulants. Previous research with caffeine has yielded mixed results in terms of its ability to produce rate-dependent effects on operant responding. Some research has indicated that caffeine can produce rate-dependent effects on operant lever pressing (Davis et al. 1973; McKim 1980; Katz et al. 1988), while other studies specifically examined the effects of caffeine on FI and FR performance and did not report clear rate-dependent effects (Harris et al. 1978). A number of reports have demonstrated that caffeine can suppress responding on FR schedules that generate high response rates (Davis et al. 1973; Harris et al. 1978; Katz et al. 1988). On the other hand, the rate-increasing effects of caffeine on operant responding appear to vary depending upon the particular conditions being studied (e.g., dose range and schedule). Responding on differential reinforcement of low-rate schedules was shown to be increased by caffeine administration (Ando 1973; Webb and Levine 1978; Sanger 1980; Marek et al. 1993). In addition, caffeine has been reported to produce slight increases in responding on interval schedules in some papers (Mechner and Latranyi 1963; Davis et al. 1973). However, some researchers have failed to identify consistent rate-increasing effects of caffeine on lever pressing on interval schedules (Carney 1982; Glowa 1986; Holtzman and Finn 1988). It is likely that the length of the interval is a critical variable in this regard. For example, some of the studies that did not report increases in interval responding with caffeine administration have used relatively shorter time intervals (e.g., variable interval—30 s, FI-120 s). In contrast, some studies that reported robust increases in responding with caffeine used much longer intervals (i.e., FI-300 s, Logan et al. 1989; FI-600 s, McKim 1980). Goldberg et al. (1985) showed that caffeine produced larger increases in response rate in rats that were lever pressing on a FI-300 s component of a multiple schedule as compared to the FI-120 s component. Pilot studies from our laboratory indicated that caffeine did not consistently increase responding in rats tested on variable interval—30 s or FI-120 s schedules (data not shown). Nevertheless, the FI-240 sec schedule, which generated very low baseline response rates, was sensitive to the rate-increasing effects of adenosine antagonism in the present study. Both caffeine and theophylline were able to produce substantial increases (approximately 80–90% over baseline) with rats tested on the FI-240 sec schedule.
Like the nonselective adenosine blockers, the adenosine A2A antagonists MSX-3 and istradefylline were both able to increase the response rate on the FI-240 sec schedule (Figs. 3a and 4a). The increased response rate induced by adenosine A2A antagonism in rats responding on the FI-240 sec schedule is consistent with previous studies showing that istradefylline could decrease reaction time and increase preparatory responding in rats responding on reaction time tasks (O’Neill and Brown 2006, 2007). MSX-3 produced significant increases in FI-240 sec responding at the 2.0-and 4.0-mg/kg doses, while istradefylline increased response rates at the 0.25- and 0.5-mg/kg doses. This relative potency (e.g., istradefylline > MSX-3) is consistent with previous studies involving the reversal of the effects of DA D2 receptor antagonism (Farrar et al. 2007; Salamone et al. 2009; Nunes et al. 2010). The rate-increasing effects of both drugs were biphasic, with intermediate doses of each drug (2.0–4.0 mg/kg MSX-3; 0.25–0.5 mg/kg istradefylline) producing significant increases in lever pressing but with higher doses (8.0 mg/kg MSX-3; 1.0 mg/kg istradefylline) failing to do so. However, unlike the nonselective adenosine antagonists, neither MSX-3 nor istradefylline significantly decreased FR20 responding in the dose range tested. If anything, istradefylline actually tended to produce a slight increase in FR20 responding at the intermediate dose (0.25 mg/kg istradefylline). Although the highest dose (8.0 mg/kg) of MSX-3 failed to decrease FR20 responding, it is likely that a very high dose (e.g., 30 mg/kg) would suppress lever pressing (Justinova et al. 2009). Nevertheless, the pattern of effects shown by adenosine A2A antagonists appears to be markedly different from that shown by caffeine and theophylline. While the nonselective adenosine antagonists significantly suppressed FR20 lever pressing at doses that also significantly elevated FI-240 sec responding (e.g., 10.0 and 20.0 mg/kg caffeine; 40.0 mg/kg theophylline), the selective adenosine A2A antagonists did not produce rate-increasing and -decreasing effects on the two different schedules at the same doses.
In contrast to the effects of adenosine A2A antagonism, the selective A1 antagonists DPCPX and CPT failed to stimulate responding under any conditions. CPT had no effects on performance of either schedule, while the only significant effect of DPCPX, which is more selective for A1 receptors than CPT (Maemoto et al. 1997), was to decrease responding in rats tested on the FR20 schedule. Recent studies from our laboratory have identified clear differences between the behavioral effects of adenosine A2A and A1 antagonists. Although adenosine A2A antagonists can produce a robust reversal of the effects of DA D2 family antagonists on instrumental behavior (Farrar et al. 2007; Mott et al. 2009; Worden et al. 2009; Salamone et al. 2009; Nunes et al. 2010), locomotion (Collins et al. 2010b) and oral tremor (Correa et al. 2004; Salamone et al. 2008; Betz et al. 2009), A1 antagonism has failed to reverse these effects of D2 antagonism (Mott et al. 2009; Salamone et al. 2009; Nunes et al. 2010; Collins et al. 2010a, b). Moreover, despite the reports indicating that CPT can act as a locomotor stimulant in the dose range tested in the present paper (Marston et al. 1998; Karcz-Kubicha et al. 2003) and was reported to increase lever pressing in monkeys responding on a FI schedule of shock termination (Spealman 1988), this drug did not increase lever pressing rate on the FI-240 sec schedule. Thus, in terms of the ability to increase response rates on food-reinforced FI schedules that generate low baseline rates of responding, this effect was induced by A2A antagonists, but not by A1 antagonists, in the dose range tested. Furthermore, although the moderately selective A1 antagonist CPT did not suppress FR20 responding, the rate-decreasing effects of adenosine antagonism were clearly evident with administration of the highly selective A1 antagonist DPCPX, which is consistent with previous studies demonstrating the motor suppressant effects of high doses of DPCPX in mice (El Yacoubi et al. 2000) and rats (Mott et al. 2009).
Rats responding on the FI-240 sec schedule displayed the characteristic “scalloping” pattern of responding, i.e., they showed low rates of lever pressing shortly after reinforcement delivery but much higher levels of responding as the interval progressed. An analysis of the temporal pattern of responding within the 240-s interval in the FI experiments indicated that the different drugs showed subtly different types of effects. Caffeine significantly altered the pattern of responding during the interval, as marked by the significant interval × drug treatment interaction and the significant reduction in the index of curvature. In contrast, theophylline, MSX-3, and istradefylline produced small or inconsistent effects on response patterning, while DPCPX and CPT did not affect response patterning within the interval. In fact, the effect of caffeine on response patterning did not seem to be directly related to increases in response rate. The doses of caffeine that increased the response rate did not have significant effects on the index of curvature, while the highest dose of caffeine, which did affect the index of curvature and produced an apparent flattening of responding within the interval, did not produce a significant increase in response rate. Instead the increases in response rate induced by 5.0–20.0 mg/kg caffeine were associated with high levels of responding at the terminal end of the 240-s interval (Figs. 1b and 3b); this pattern is similar to that reported by Logan et al. (1989). Overall, these analyses indicate that the doses of caffeine, theophylline, MSX-3, and istradefylline that increased the FI response rate did not produce major alterations in the temporal pattern of responding within the interval because the general scalloping pattern was preserved across these treatments. These observations serve to contrast the effects produced by adenosine antagonists with those produced by major stimulants such as amphetamine and cocaine, which tend to dramatically increase responding early in the interval, but decrease response rates later in the interval, at doses that increase FI response rate (Smith 1964; Kelleher and Morse 1968; McMillan 1968a, b; Heffner et al. 1974; Sanger and Blackman 1974; Robbins et al. 1983; Logan et al. 1989).
It is worthwhile to consider the relation between the present studies on the response-stimulating effects adenosine antagonism and those involving reversal of the effects of DA antagonists. In some ways, there are similarities between the two types of effects. For example, adenosine A2A antagonists induce greater increases in FI-240 sec lever pressing than A1 antagonists, and A2A antagonists also are more effective at reversing the effects of DA antagonists (Salamone et al. 2009; Nunes et al. 2010). In addition, doses of MSX-3 and istradefylline that increase FI-240 sec lever pressing also are effective at reversing the effects of DA D2 family antagonists on tests of instrumental behavior (Farrar et al. 2007; Worden et al. 2009; Salamone et al. 2009; Nunes et al. 2010). Nevertheless, there also are some important differences. For example, caffeine is relatively weak in terms of its ability to reverse the effects of DA antagonism on lever pressing and locomotion (Salamone et al. 2009; Collins et al. 2010b), but this drug nevertheless produces a very robust increase in FI-240 sec responding. Furthermore, adenosine A2A antagonists are much more effective at restoring normal levels of lever pressing in rats treated with D2 antagonists than they are in rats treated with D1 antagonists (Worden et al. 2009; Nunes et al. 2010). This pattern of effects would seem to be unlikely if A2A antagonists were simply producing stimulant effects across a broad range of conditions. Thus, it does not appear that the ability of adenosine A2A antagonists to produce a robust reversal of the effects of D2 antagonists is based solely on the fact that A2A antagonists also have stimulant properties. Instead the results of these reversal experiments appear to reflect a relatively specific interaction between D2 and A2A receptors, which is likely to be based upon the pattern of co-localization of these receptors on the same striatal neurons (Worden et al. 2009; Salamone et al. 2009; Mott et al. 2009; Farrar et al. 2010; Nunes et al. 2010).
In summary, the results of the present experiments demonstrate that methylxanthines such as caffeine and theophylline can produce rate-dependent effects on operant responding. These effects could be due to a general antagonism of adenosine receptors or could be related to other mechanisms of action (Garrett and Holtzman 1995). Importantly, the present studies also showed that adenosine A2A antagonists, but not A1 antagonists, can increase operant response rates, particularly in rats responding on the FI-240 sec schedule. Unlike the nonselective adenosine antagonists, the adenosine A2A antagonists failed to decrease FR20 lever pressing in the same dose range that increased FI-240 sec responding. These results demonstrate that adenosine A2A antagonists have behavioral stimulant properties that can be assessed by using operant schedules that generate low response rates. Nevertheless, these selective drugs also show a different pattern of effects from that produced by the methylxanthines caffeine and theophylline.
Acknowledgments
This work was supported by a grant to J.S. from the National Institute of Mental Health (MH078023) and to M.C. from Conselleria de Empresa, Universitat i Ciència, Generalitat Valenciana (BEST/2009/157). Y.B. and C.E.M. were supported by the BMBF and the European Commission by an ERANET-NEURON grant.
Contributor Information
Patrick A. Randall, Department of Psychology, University of Connecticut, Storrs, CT 06269-1020, USA
Eric J. Nunes, Department of Psychology, University of Connecticut, Storrs, CT 06269-1020, USA
Simone L. Janniere, Department of Psychology, University of Connecticut, Storrs, CT 06269-1020, USA
Colin M. Stopper, Department of Psychology, University of Connecticut, Storrs, CT 06269-1020, USA
Andrew M. Farrar, Department of Psychology, University of Connecticut, Storrs, CT 06269-1020, USA
Thomas N. Sager, Pharmacology Target Research, H. Lundbeck A/S, 9 Ottiliavej, Valby, Copenhagen 2500, Denmark
Younis Baqi, Pharma-Zentrum Bonn, Pharmazeutisches Institut, Pharmazeutische Chemie, Universität Bonn, Bonn, Germany.
Jörg Hockemeyer, Pharma-Zentrum Bonn, Pharmazeutisches Institut, Pharmazeutische Chemie, Universität Bonn, Bonn, Germany.
Christa E. Müller, Pharma-Zentrum Bonn, Pharmazeutisches Institut, Pharmazeutische Chemie, Universität Bonn, Bonn, Germany
John D. Salamone, Email: john.salamone@uconn.edu, Department of Psychology, University of Connecticut, Storrs, CT 06269-1020, USA
References
- Ando K. Development of temporally spaced responding in relation to schedule value in rats. Jpn Psych Res. 1973;15(4):159–163. [Google Scholar]
- Antoniou K, Papadopoulou-Daifoti Z, Hyphantis T, Papathanasiou G, Bekris E, Marselos M, Panlilio L, Müller CE, Goldberg SR, Ferré S. A detailed behavioral analysis of the acute motor effects of caffeine in the rat: involvement of adenosine A1 and A2A receptors. Psychopharmacology. 2005;183(2):154–162. doi: 10.1007/s00213-005-0173-6. [DOI] [PubMed] [Google Scholar]
- Aubel B, Kayser V, Farré A, Hamon M, Bourgoin S. Evidence for adenosine- and serotonin-mediated antihyperalgesic effects of cizolirtine in rats suffering from diabetic neuropathy. Neuropharmacology. 2007;52:487–496. doi: 10.1016/j.neuropharm.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Betz AJ, Vontell R, Valenta J, Worden L, Sink KS, Font L, Correa M, Sager TN, Salamone JD. Effects of the adenosine A 2A antagonist KW 6002 (istradefylline) on pimozide-induced oral tremor and striatal c-Fos expression: comparisons with the muscarinic antagonist tropicamide. Neuroscience. 2009;163(1):97–108. doi: 10.1016/j.neuroscience.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Carney JM. Effects of caffeine, theophylline and theobromine on scheduled controlled responding in rats. Br J Pharmacol. 1982;75 (3):451–454. doi: 10.1111/j.1476-5381.1982.tb09161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta AR, Pinna A, Cauli O, Morelli M. Differential regulation of GAD67, enkephalin and dynorphin mRNAs by chronic-intermittent L-dopa and A2A receptor blockade plus L-dopa in dopamine-denervated rats. Synapse. 2002;44(3):166–174. doi: 10.1002/syn.10066. [DOI] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, Beilstein MA, Hacket E, Fink JS, Low MJ, Ongini E, Schwarzschild MA. The role of the D2 dopamine receptor (D2R) in A2a adenenosine-receptor (A2aR) mediated behavioral and cellular responses as revealed by A2a and D2 receptor knockout mice. Proc Natl Acad Sci. 2001;98:1970–1975. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LE, Galtieri DJ, Brennum LT, Sager TN, Hockemeyer J, Müller CE, Hinman JR, Chrobak JJ, Salamone JD. Oral tremor induced by the muscarinic agonist pilocarpine is suppressed by the adenosine A2A antagonists MSX-3 and SCH58261, but not the adenosine A1 antagonist DPCPX. Pharmacol Biochem Behav. 2010a;94:561–569. doi: 10.1016/j.pbb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Collins LE, Galtieri DJ, Collins P, Jones SK, Port RG, Paul NE, Hockemeyer J, Müller CE, Salamone JD. Interactions between adenosine and dopamine receptor antagonists with different selectivity profiles: effects on locomotor activity. Behav Brain Res. 2010b;211:148–155. doi: 10.1016/j.bbr.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Correa M, Wisniecki A, Betz A, Dobson DR, O’Neill MF, O’Neill MJ, Salamone JD. The adenosine A2A antagonist KF17837 reverses the locomotor suppression and tremulous jaw movements induced by haloperidol in rats: possible relevance to parkinsonism. Behav Brain Res. 2004;148:47–54. doi: 10.1016/s0166-4328(03)00178-5. [DOI] [PubMed] [Google Scholar]
- Daly JW, Fredholm BB. Caffeine—an atypical drug of dependence. Drug Alcohol Depend. 1998;51:199–206. doi: 10.1016/s0376-8716(98)00077-5. [DOI] [PubMed] [Google Scholar]
- Davis TR, Kensler CJ, Dews PB. Comparison of behavioral effects of nicotine, d-amphetamine, caffeine and dimethylheptyl tetrahydrocannabinol in squirrel monkeys. Psychopharmacology. 1973;32:51–65. doi: 10.1007/BF00421707. [DOI] [PubMed] [Google Scholar]
- DeMet EM, Chicz-DeMet A. Localization of adenosine A2A-receptors in rat brain with [3H]ZM-241385. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:478–481. doi: 10.1007/s00210-002-0613-3. [DOI] [PubMed] [Google Scholar]
- Dews PB. Studies on behavior. I. Differential sensitivity to pentobarbital of pecking performance in pigeons depending on the schedule of reward. J Pharmacol Exp Ther. 1955;113:393–401. [PubMed] [Google Scholar]
- Dews PB. Studies on behavior. IV. Stimulant actions of methamphetamine. J Pharmacol Exp Ther. 1958;122:137–147. [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Ménard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar AM, Pereira M, Velasco F, Hockemeyer J, Müller CE, Salamone JD. Adenosine A(2A) receptor antagonism reverses the effects of dopamine receptor antagonism on instrumental output and effort-related choice in the rat: implications for studies of psychomotor slowing. Psychopharmacology. 2007;191:579–586. doi: 10.1007/s00213-006-0554-5. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Segovia KN, Randall PA, Nunes EJ, Collins LE, Stopper CM, Port RG, Hockemeyer J, Müller CE, Correa M, Salamone JD. Nucleus accumbens and effort-related functions: behavioral and neural markers of the interactions between adenosine A2A and dopamine D2 receptors. Neuroscience. 2010;166:1056–1067. doi: 10.1016/j.neuroscience.2009.12.056. [DOI] [PubMed] [Google Scholar]
- Ferré S. Adenosine–dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology. 1997;133:107–120. doi: 10.1007/s002130050380. [DOI] [PubMed] [Google Scholar]
- Ferré S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105:1067–1079. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine–dopamine receptor–receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Popoli P, Giménez-Llort L, Rimondini R, Müller CE, Strömberg I, Ögren SO, Fuxe K. Adenosine/dopamine interaction: implications for the treatment of Parkinson’s disease. Parkinsonism Relat Disord. 2001;7:235–241. doi: 10.1016/s1353-8020(00)00063-8. [DOI] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Canals M, Marcellino D, Burgueno J, Casadó V, Hillion J, Torvinen M, Fanelli F, Benedetti Pd P, Goldberg SR, Bouvier M, Fuxe K, Agnati LF, Lluis C, Franco R, Woods A. Adenosine A2A-dopamine D2 receptor-receptor heteromers. Targets for neuro-psychiatric disorders. Parkinsonism Relat Disord. 2004;10:265–271. doi: 10.1016/j.parkreldis.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Borycz J, Solinas M, Quarta D, Antoniou K, Quiroz C, Justinova Z, Lluis C, Franco R, Goldberg SR. Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Front Biosci. 2008a;13:2391–2399. doi: 10.2741/2852. [DOI] [PubMed] [Google Scholar]
- Ferré S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F, Lluis C, Franco R, Azdad K, Schiffmann SN. An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr Pharm Des. 2008b;14:1468–1474. doi: 10.2174/138161208784480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Fry W, Kelleher RT, Cook L. A mathematical index of performance on fixed-interval schedules of reinforcement. J Exp Anal Behav. 1960;3:193–199. doi: 10.1901/jeab.1960.3-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Jacobsen K, Hillion J, Canals M, Torvinen M, Tinner-Staines B, Staines W, Rosin D, Terasmaa A, Popoli P, Leo G, Vergoni V, Lluis C, Ciruela F, Franco R, Ferré S. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease. Neurology. 2003;61(11 Supp 6):S19–S23. doi: 10.1212/01.wnl.0000095206.44418.5c. [DOI] [PubMed] [Google Scholar]
- Garrett BE, Holtzman SG. D1 and D2 dopamine receptor antagonists block caffeine-induced stimulation of locomotor activity in rats. Pharmacol Biochem Behav. 1994;47:89–94. doi: 10.1016/0091-3057(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Garrett BE, Holtzman SG. Does adenosine receptor blockade mediate caffeine-induced rotational behavior? J Pharmacol Exp Ther. 1995;274:207–214. [PubMed] [Google Scholar]
- Glowa JR. Some effects of d-amphetamine, caffeine, nicotine and cocaine on schedule-controlled responding of the mouse. Neuropsychopharmacology. 1986;25:1127–1135. doi: 10.1016/0028-3908(86)90160-7. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Prada JA, Katz JL. Stereoselective behavioral effects of N6-phenylisopropyl- adenosine and antagonism by caffeine. Psychopharmacol. 1985;87:272–277. doi: 10.1007/BF00432706. [DOI] [PubMed] [Google Scholar]
- Harris RA, Snell D, Loh HH. Effects of d-amphetamine, monomethoxyamphetamines and hallucinogens on schedule-controlled behavior. J Pharmacol Exp Ther. 1978;204:103–117. [PubMed] [Google Scholar]
- Hauber W, Neuscheler P, Nagel J, Müller CE. Catalepsy induced by a blockade of dopamine D1 or D2 receptors was reversed by a concomitant blockade of adenosine A(2A) receptors in the caudate-putamen of rats. Eur J Neurosci. 2001;14:1287–1293. doi: 10.1046/j.0953-816x.2001.01759.x. [DOI] [PubMed] [Google Scholar]
- Heffner TG, Drawbaugh RB, Zigmond MJ. Amphetamine and operant behavior in rats: relationship between drug effect and control response rate. J Comp Physiol Psychol. 1974;86:1031–1043. doi: 10.1037/h0037634. [DOI] [PubMed] [Google Scholar]
- Hettinger BD, Lee A, Linden J, Rosin DL. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol. 2001;431:331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, Canela EI, Zoli M, Agnati LF, Ibanez CF, Lluis C, Franco R, Ferre S, Fuxe K. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- Holtzman SG, Finn IB. Tolerance to behavioral effects of caffeine in rats. Pharmacol Biochem Behav. 1988;29:411–418. doi: 10.1016/0091-3057(88)90179-7. [DOI] [PubMed] [Google Scholar]
- Ishiwari K, Madson LJ, Farrar AM, Mingote SM, Valenta JP, DiGianvittorio MD, Frank LE, Correa M, Hockemeyer J, Müller C, Salamone JD. Injections of the selective adenosine A2A antagonist MSX-3 into the nucleus accumbens core attenuate the locomotor suppression induced by haloperidol in rats. Behav Brain Res. 2007;178:190–199. doi: 10.1016/j.bbr.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Williams M. Direct autoradiographic localization of adenosine A2 receptors in the rat brain using the A2-selective agonist, [3H]CGS 21680. Eur J Pharmacol. 1989;168:243–246. doi: 10.1016/0014-2999(89)90571-2. [DOI] [PubMed] [Google Scholar]
- Jenner P. A novel dopamine agonist for the transdermal treatment of Parkinson’s disease. Neurology. 2005;65(2 suppl 1):S3–S5. doi: 10.1212/wnl.65.2_suppl_1.s3. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferre S, Segal PN, Antoniou K, Solinas M, Pappas LA, Highkin JL, Hockemeyer J, Munzar P, Goldberg SR. Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J Pharmacol Exp Ther. 2003;307:977–986. doi: 10.1124/jpet.103.056762. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferré S, Barnes C, Wertheim CE, Pappas LA, Goldberg SR, Le Foll B. Effects of chronic caffeine exposure on adenosinergic modulation of the discriminative-stimulus effects of nicotine, methamphetamine, and cocaine in rats. Psychopharmacology. 2009;203:355–367. doi: 10.1007/s00213-008-1270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, Pezzola A, Reggio R, Müller CE, Fuxe K, Goldberg SR, Popoli P, Ferré S. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology. 2003;28:1281–1291. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Katz JL, Prada JA, Goldberg SR. Effects of adenosine analogs alone and in combination with caffeine in the squirrel monkey. Pharmacol Biochem Behav. 1988;29:429–432. doi: 10.1016/0091-3057(88)90181-5. [DOI] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol. 1968;60:1–56. doi: 10.1007/BFb0107250. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: a researcher’s handbook. Prentice Hall; Englewood Cliffs: 1991. [Google Scholar]
- Lobato KR, Binfaré RW, Budni J, Rosa AO, Santos AR, Rodrigues AL. Involvement of the adenosine A1 and A2A receptors in the antidepressant-like effect of zinc in the forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:994–999. doi: 10.1016/j.pnpbp.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Logan L, Carney JM, Holloway FA, Seale TW. Effects of caffeine, cocaine and their combination on fixed-interval behavior in rats. Pharmacol Biochem Behav. 1989;33:99–104. doi: 10.1016/0091-3057(89)90436-x. [DOI] [PubMed] [Google Scholar]
- Maemoto T, Finlayson K, Olverman HJ, Akahane A, Horton RW, Butcher SP. Species differences in brain adenosine A1 receptor pharmacology revealed by use of xanthine and pyrazolopyridine based antagonists. Br J Pharmacol. 1997;122:1202–1208. doi: 10.1038/sj.bjp.0701465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione S, de Novellis V, Cappellacci L, Palazzo E, Vita D, Luongo L, Stella L, Franchetti P, Marabese I, Rossi F, Grifantini M. The antinociceptive effect of 2-chloro-2′-C-methyl-N6-cyclopentyladenosine (2′-Me-CCPA), a highly selective adenosine A1 receptor agonist, in the rat. Pain. 2007;131:281–292. doi: 10.1016/j.pain.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Heffner TG, Richards JB, Shaughnessy RA, Li AA, Seiden LS. Effects of caffeine and PD 116,600 on the differential-reinforcement-of-low rate 72-S (DRL 72-S) schedule of reinforcement. Pharmacol Biochem Behav. 1993;45:987–990. doi: 10.1016/0091-3057(93)90153-k. [DOI] [PubMed] [Google Scholar]
- Marston HM, Finlayson K, Maemoto T, Olverman HJ, Akahane A, Sharkey J, Butcher SP. Pharmacological characterization of a simple behavioral response mediated selectively by central adenosine A1 receptors, using in vivo and in vitro techniques. J Pharmacol Exp Ther. 1998;285:1023–1030. [PubMed] [Google Scholar]
- McKim WA. The effect of caffeine, theophylline and amphetamine on operant responding of the mouse. Psychopharmacology. 1980;68:135–138. doi: 10.1007/BF00432130. [DOI] [PubMed] [Google Scholar]
- McMillan DE. The effects of sympathomimetic amines on schedule controlled behavior in the pigeon. J Pharmacol Exp Ther. 1968a;160:315–325. [PubMed] [Google Scholar]
- McMillan DE. Some interactions between sympathomimetic amines and amine-depleting agents on the schedule controlled behavior of the pigeon and the squirrel monkey. J Pharmacol Exp Ther. 1968b;163:172–187. [PubMed] [Google Scholar]
- Mechner F, Latranyi M. Behavioral effects of caffeine, methamphetamine and methylphenidate in the rat. J Exp Anal Behav. 1963;6:331–342. doi: 10.1901/jeab.1963.6-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli M, Wardas J. Adenosine A(2a) receptor antagonists: potential therapeutic and neuroprotective effects in Parkinson’s disease. Neurotox Res. 2001;3:545–556. doi: 10.1007/BF03033210. [DOI] [PubMed] [Google Scholar]
- Mott AM, Nunes EJ, Collins LE, Port RG, Sink KS, Hockemeyer J, Müller CE, Salamone JD. The adenosine A2A antagonist MSX-3 reverses the effects of the dopamine antagonist haloperidol on effort-related decision making in a T-maze cost/benefit procedure. Psychopharmacology. 2009;204:103–112. doi: 10.1007/s00213-008-1441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Santerre JL, Given AB, Sager TN, Correa M, Salamone JD. Differential effects of selective adenosine antagonists on the effort-related impairments induced by dopamine D1 and D2 antagonism. Neuroscience. 2010;170:268–280. doi: 10.1016/j.neuroscience.2010.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL, Schaal DW. The effects of morphine on fixed-interval patterning and temporal discrimination. J Exp Anal Behav. 2000;74:229–243. doi: 10.1901/jeab.2000.74-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill M, Brown VJ. The effect of the adenosine A(2A) antagonist KW-6002 on motor and motivational processes in the rat. Psychopharmacology. 2006;184:46–55. doi: 10.1007/s00213-005-0240-z. [DOI] [PubMed] [Google Scholar]
- O’Neill M, Brown VJ. Amphetamine and the adenosine A (2A) antagonist KW-6002 enhance the effects of conditional temporal probability of a stimulus in rats. Behav Neurosci. 2007;121:535–542. doi: 10.1037/0735-7044.121.3.535. [DOI] [PubMed] [Google Scholar]
- Pinna A. Novel investigational adenosine A2A receptor antagonists for Parkinson’s disease. Expert Opin Investig Drugs. 2010;18:1619–1631. doi: 10.1517/13543780903241615. [DOI] [PubMed] [Google Scholar]
- Popoli P, Reggio R, Pèzzola A, Fuxe K, Ferré S. Adenosine A1 and A2A receptor antagonists stimulate motor activity: evidence for an increased effectiveness in aged rats. Neurosci Lett. 1998;251:201–204. doi: 10.1016/s0304-3940(98)00533-3. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Takahashi RN. Modulation of short-term social memory in rats by adenosine A1 and A(2A) receptors. Neurosci Lett. 2005;376:160–165. doi: 10.1016/j.neulet.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks —a growing problem. Drug Alcohol Depend. 2009;99:1–10. doi: 10.1016/j.drugalcdep.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Roberts DC, Koob GF. Effects of d-amphetamine and apomorphine upon operant behavior and schedule-induced licking in rats with 6-hydroxydopamine-induced lesions of the nucleus accumbens. J Pharmacol Exp Ther. 1983;224:662–673. [PubMed] [Google Scholar]
- Salamone JD. Preladenant, a novel adenosine A(2A) receptor antagonist for the potential treatment of parkinsonism and other disorders. IDrugs. 2010;13:723–731. [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Betz AJ, Ishiwari K, Felsted J, Madson L, Mirante B, Clark K, Font L, Korbey S, Sager TN, Hockemeyer J, Muller CE. Tremorolytic effects of adenosine A2A antagonists: implications for parkinsonism. Front Biosci. 2008;13:3594–3605. doi: 10.2741/2952. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Farrar AM, Font L, Patel V, Schlar DE, Nunes EJ, Collins LE, Sager TN. Differential actions of adenosine A1 and A2A antagonists on the effort-related effects of dopamine D2 antagonism. Behav Brain Res. 2009;201:216–222. doi: 10.1016/j.bbr.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Collins LE. Role of dopamine–adenosine interactions in the brain circuitry regulating effort-related decision making: insights into pathological aspects of motivation. Future Neurol. 2010;5:377–392. [Google Scholar]
- Sanger DJ. The effects of caffeine on timing behaviour in rodents: comparisons with chlordiazepoxide. Psychopharmacology. 1980;68:305–309. doi: 10.1007/BF00428121. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Blackman DE. Rate-dependent effects of drugs: a review of the literature. Pharmacol Biochem Behav. 1974;4:73–83. doi: 10.1016/0091-3057(76)90178-7. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Libert F, Vassart G, Vanderhaeghen JJ. Distribution of adenosine A2 receptor mRNA in the human brain. Neurosci Lett. 1991;130:177–181. doi: 10.1016/0304-3940(91)90391-6. [DOI] [PubMed] [Google Scholar]
- Smith CB. Effects of d-amphetamine upon operant behavior of pigeons: enhancements by reserpine. J Pharmacol Exp Ther. 1964;146:167–174. [PubMed] [Google Scholar]
- Spealman RD. Psychomotor stimulant effects of methylxanthines in squirrel monkeys: relation to adenosine antagonism. Psychopharmacology. 1988;95:19–24. doi: 10.1007/BF00212759. [DOI] [PubMed] [Google Scholar]
- Varty GB, Hodgson RA, Pond AJ, Grzelak ME, Parker EM, Hunter JC. The effects of adenosine A2A receptor antagonists on haloperidol-induced movement disorders in primates. Psychopharmacology. 2008;200:393–401. doi: 10.1007/s00213-008-1214-8. [DOI] [PubMed] [Google Scholar]
- Wardas J, Konieczny J, Lorenc-Koci E. SCH 58261, an A(2A) adenosine receptor antagonist, counteracts parkinsonian-like muscle rigidity in rats. Synapse. 2001;41:160–171. doi: 10.1002/syn.1070. [DOI] [PubMed] [Google Scholar]
- Webb D, Levine TE. Effects of caffeine on DRL performance in the mouse. Pharmacol Biochem Behav. 1978;9:7–10. doi: 10.1016/0091-3057(78)90004-7. [DOI] [PubMed] [Google Scholar]
- Wenger GR, Dews PB. The effects of phencyclidine, ketamine, delta-amphetamine and pentobarbital on schedule-controlled behavior in the mouse. J Pharmacol Exp Ther. 1976;196:616–624. [PubMed] [Google Scholar]
- Worden LT, Shahriari M, Farrar AM, Sink KS, Hockemeyer J, Müller CE, Salamone JD. The adenosine A2A antagonist MSX-3 reverses the effort-related effects of dopamine blockade: differential interaction with D1 and D2 family antagonists. Psychopharmacology. 2009;203:489–499. doi: 10.1007/s00213-008-1396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]