Abstract
Recent research implicates the COMT Val108/158Met polymorphism in stress-sensitivity, via modulation of hypothalamic-pituitary-adrenal (HPA) function. In healthy samples, Met-homozygosity has been associated with greater HPA activity (i.e., cortisol) and stress sensitivity, though findings are mixed among clinical samples. To date, there are no reports examining baseline or longitudinal changes in HPA activity as a function of COMT genotype in youth. This study examined the association of COMT with salivary cortisol across a one-year period in healthy and at-risk adolescents with DSM-IV-TR Axis II diagnoses. Results indicated higher cortisol levels for Met-homozygotes (compared to heterozygotes and Val homozygotes) at the one-year follow-up, and increased mean cortisol levels across a one-year period among Met-carriers, suggesting that COMT associates with differences in cortisol secretion during adolescence. Findings are discussed with respect to COMT genotype as a potential genetic indicator of psychiatric risk that modulates developmental changes in HPA activity.
Keywords: genetic, COMT, catechol-o-methyltransferase, psychosis, schizophrenia, stress, risk, cortisol, hypothalamic-pituitary-adrenal axis, HPA
Introduction
Research is increasingly aimed at identifying genetic determinants of stress vulnerability as risk factors for mental disorders. It has been proposed that dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, a key system in the biological stress response, is a component of vulnerability for psychiatric disorders (Walker and Diforio, 1997). Genetic factors account for approximately 50% of variability in cortisol secretion (Linkowski et al., 1993), a measure of HPA activity. Thus, it appears that some genes, in concert with other genetic and environmental factors (van Os et al., 2003), determine individual differences in HPA activity.
Dopamine has also been implicated in the stress response (Laruelle, 2000), and one of the genes responsible for dopamine metabolism, Catechol-O-Methyltransferase (COMT) is among the genes theorized to impact stress-sensitivity (Zubieta et al., 2003). COMT Val108/158Met is a functional polymorphism (i.e., a single nucleotide involving an amino acid change from Valine (Val) to Methionine (Met) at codon 158) affecting enzyme activity. Val homozygosity is associated with 3-4 fold higher enzyme activity than Met homozygosity (Mannisto and Kaakkola, 1999; Lachman et al., 1996), and because the alleles are codominant, heterozygotes are intermediate (Männisto and Kaakkola, 1999; Lachman et al., 1996). Accordingly, Val/Val, Val/Met and Met/Met genotypes correspond, in theory, to highest, intermediary, and lowest dopamine degradation, respectively.
Two mechanisms have been suggested for COMT allelic variants to alter HPA function (Oswald et al., 2004). First, because low-activity COMT (Met variant) is associated with higher catecholinergic activity, it would be expected to be linked with greater hypothalamic corticotropin-releasing hormone (CRH) release. Second, more pronounced μ-opioid receptor binding potential has been observed in response to stress in humans who are COMT Met/Met homozygous (Zubieta et al., 2003), suggesting that the low-activity form of COMT could alter HPA activity indirectly through influencing DA-mediated regulation of opioid neurotransmission.
Several studies implicate COMT Val108/158Met in stress-sensitivity, including COMT modulation of HPA function. Among healthy individuals, greater stress-sensitivity among COMT Met homozygotes compared to Val-carriers is suggested by 1) higher plasma ACTH and cortisol response to psychosocial stress (Jabbi, 2007), 2) greater cortisol response to naloxone, an opioid antagonist (Oswald et al., 2004), 3) higher sensory and affective responses to pain, and more negative internal affective state, (Zubieta et al., 2003), and increased limbic and prefrontal activation in response to unpleasant stimuli, with a greater propensity to negative mood (Smolka et al., 2004).
Data from clinical populations are mixed. To date, we know of only one study examining COMT genotype and HPA activity in a clinical risk sample (N=25, Jabbi, 2007), and the findings indicate that, among individuals at familial high-risk for depression, COMT genotype was not associated with cortisol response. However, a study of self-reported symptoms found that Met homozygote psychotic patients reported more negative affect and psychotic symptoms in response to daily stress compared to other genotypes (van Winkel et al., 2008). In contrast, another study examining normal military inductees (19-24 years; N=2243) found Val-carriers (compared to Met homozygotes) to be more sensitive to army induction training stress as reflected by higher psychosis symptom ratings (Stefanis et al., 2007).
Adolescence is a critical period for the onset of mental disorders, and it is also characterized by heightened stress sensitivity, including a marked increase in cortisol secretion (Walker, 2002). Increased cortisol secretion during adolescence has been demonstrated in cross-sectional (Kenny et al., 1966; Kiess et al 1995; Lupien et al., 2002) and longitudinal (Walker et al., 2001; Walker et al., 2002; Wajs-Kuto et al., 1999) studies, and has been implicated in the escalating risk of mental disorders during this period. Increases in cortisol levels occur with each year of age, beginning around 13 and may extend into the early twenties. To date, no studies have explored the modulating role of COMT on cortisol during adolescence.
The present study tested the hypothesis that COMT genotype would be associated with cortisol secretion in normal and at-risk adolescents: specifically, that COMT genotype would be linked in a dose-response manner, such that Met homozygotes would have the highest salivary cortisol levels, followed by heterozygotes, then Val homozygotes. In addition, the relation of COMT genotype with longitudinal changes in cortisol was examined.
Methods
The present sample is a subgroup of participants from the Emory University adolescent development project, a longitudinal study of youth at-risk for Axis I mental disorders. Data on COMT genotype and salivary cortisol (baseline and two follow-ups) were available for 63 adolescents, ages 12 to 18 years (mean=14.52, SD=1.87). Follow-up endocrine assessments were conducted at 7-10 months and one year later. (See Trotman et al. (2006) for a description of the sample.) Recruitment focused on adolescents with DSM-IV-TR schizotypal personality disorder (SPD) (n = 11 males and 6 females) and other Axis II syndromes (n=16 males and 13 females) linked with risk for developing Axis I disorders, especially psychosis; together, this constituted the high-risk group. Healthy adolescents (n=7 males and 10 females) did not meet criteria for any DSM-IV-TR disorder. No participant met criteria for an Axis I disorder at baseline. There were no diagnostic group differences in sex [χ2(2)=1.926, p=0.382], age [F(2)=.148, p=0.862], or ethnicity [χ2(6)=6.819, p=0.338]. Assent and written consent was obtained from all participants and a parent, in accordance with guidelines of the Emory University Institutional Review Board.
The Structured Interview of DSM-IV Personality Disorders (SIDP-IV; Pfohl et al., 1997) and the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID-I/P; First et al., 1998) were administered. Diagnosticians demonstrated high inter-rater reliabilities (Kappa > 0.80).
A detailed description of methods for salivary collection and assay of cortisol can be found in Mittal et al. (2007). The current analyses used the first three cortisol samples, which were collected in the morning before lunch. The measure of cortisol at baseline (upon the initial assessment) and each of the two follow-ups index an average of the individual’s cortisol level over an approximate 1-2 hour period in the morning, when levels are at a relative peak in accord with the natural diurnal rhythm. Participants were instructed not to ingest products containing caffeine or engage in vigorous physical activity or exercise prior to sample collection.
Participants provided saliva samples in the Oragene DNA Self-Collection kit (DNA Genotek Inc., Ottawa, Canada, Rylander-Rudqvist et al., 2006). DNA was extracted from saliva by using the Qiagen M48 automated extraction system. COMT Val-Met (rs4680) genotypes were determined using the 5’-exonuclease (TaqMan®) method. The assay kits and genotyping reagents were ordered from Applied Biosystems (Foster City, CA). Genotyping was performed on an ABI 7900HT system, with samples arrayed in 384-well plates. For the total sample, genotype frequencies were Met/Met = 11, Val/Met = 30 and Val/Val = 22; there was no deviation from Hardy-Weinberg Equilibrium (chisq, 2 d.f. = .02, p = .88). There were no significant differences in distribution of allelic frequencies by diagnostic group [χ2(4)=3.879, p=0.423] or racial/ethnic group [χ2(6)=9.273, p=0.159].
Results
Consistent with previous reports, the present sample showed a developmental increase in cortisol secretion, however, the magnitude of the increase varied by genotype. Mean cortisol by time and genotype is illustrated in Figure 1. In the Met/Met group, mean cortisol was increased significantly at Follow-up 1 [t(8)=-2.434, p=.041] and Follow-up 2 [t(10)=-3.310, p=.008], compared to Baseline, with no significant difference between Follow-ups 1 and 2 [t(8)=.840; p=.425]. Among Val/Met, mean cortisol was increased at Follow-up 1 compared to Baseline at the trend level [t(26)=-1.914, p=.067l), but the difference between Baseline and Follow-up 2 [t(25)=-1.394, p=.176] and between Follow-ups 1 and 2 [t(23)=.024, p=.981] did not approach significance. Among Val/Val, there were no significant changes over time; Baseline vs. Follow-up 1 [t(19)=-.800, p=.434], Baseline vs. Follow-up 2 [t(21)=-.767, p=.452], and Follow-up 1 vs. 2 [t(19)=.122, p=.904]. In summary, Met/Met homozygotes demonstrated the greatest increase in mean cortisol level over time, with the most marked increase between Baseline and one year later (Follow-up 2). Given the small sample size, the aforementioned analyses were repeated after combining all Met-carriers into one group (that is, Met/Met and Val/Met). Accordingly, among Met-carriers, mean cortisol was increased significantly at Follow-up 1 [t(35)=-2.688, p=.011] and Follow-up 2 [t(36)= -2.529, p=.016], compared to Baseline, with no significant difference between Follow-ups 1 and 2 [t(32)=-.348; p=.730].
Figure 1.
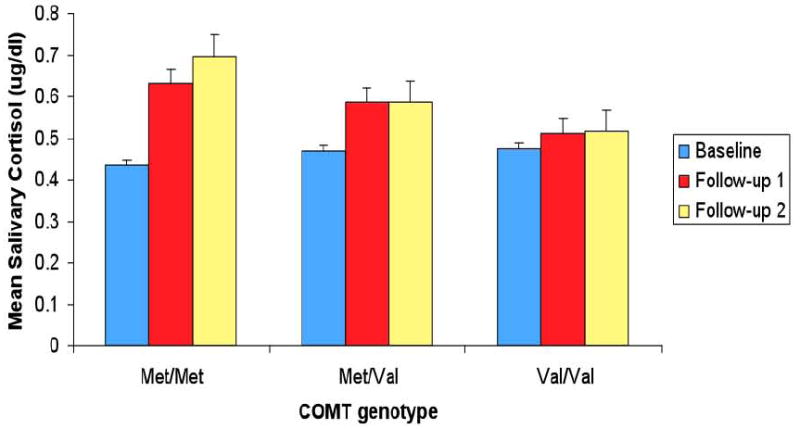
Mean Salivary Cortisol Across a One-Year Period by COMT (rs4680) Genotype for the Total Sample
There were no significant differences in cortisol levels as a function of genotype at Baseline. However, at Follow-up 1, there was a trend toward higher mean cortisol for Met/Met than Val/Val at the trend level [t(25)=1.504, p=.073].
At Follow-up 2, mean cortisol was significantly greater for Met/Met compared to Val/Val [t(31)=1.831, p=.039]. Comparisons by sample showed that cortisol sample #1 was significantly greater for Met/Met compared to Val/Met [t(34)=3.997, p=.000] and Val/Val [t(31)=2.064,p=.024], and at the trend level greater for Val/Met compared to Val/Val [t(45)=-1.437, p=.079]; cortisol #3 was greater for Met/Met [t(21)=1.567, p=.066] and Val/Met [t(36)=1.528, p=.068] compared to Val/Val at the trend level.
Group differences in cortisol as a function of genotype were also examined using the average of the mean cortisol values across the 3 assessments (Baseline and Follow-ups 1 and 2). Accordingly, there were no significant differences across Met homozygotes (mean=.5812, SD=.1547), Met heterozygotes (mean=.5505, SD=.2364), and Val homozygotes (mean=.5018, SD=.1647) [F(2)=.666, p>.10)].
Percentage change in cortisol levels at each of the follow-ups relative to baseline was also examined, as another method towards illustrating the pattern of longitudinal change in cortisol by gentoype. Percentage change values were calculated by dividing mean cortisol at each of the follow-ups by mean cortisol at baseline, respectively, and then multiplying by 100. Results indicated no significant genetic group differences (Met/Met, Met/Val, Val/Val) in percentage change in cortisol from Baseline to Follow-up 1 [F(2)=.882, p>.10)], or from Baseline to Follow-up 2 [F(2)=1.844, p>.10]. Analyses comparing Met-carriers to Val homozygotes revealed no significant genetic group differences in percentage change in cortisol from Baseline to Follow-up 1 [F(1)=1.725, p>.10], though there was a greater percentage change from Baseline to Follow-up 2 [F(1)=2.887, p<.10] among Met-carriers compared to Val homozygotes.
Consistent with previous reports, Met/Met genotype was the least frequent, with only 3 such individuals in the healthy control group. Thus, for within-diagnostic group comparisons, Met heterozygotes (Val/Met) and homozygotes (Met/Met) were combined. Further, due to the small number of subjects when participants were divided by both genotype and diagnostic group, the psychiatric diagnostic groups were combined into one high-risk group for the initial comparison. This yielded 32 at-risk Met-carriers, 14 at-risk Val homozygotes, 9 healthy control Met-carriers, and 8 healthy control Val homozygotes.
Within group comparisons revealed that, among healthy controls, Met-carriers showed significant increases in mean cortisol from baseline to Follow-up 1 [t(7)=-3.124, p=.017] and a trend at Follow-up 2 [t(6)=-2.297, p=.061]. Among Val/Val healthy controls, there were no significant increases.
Among high-risk adolescents, Met-carriers showed a trend level increase in mean cortisol at Follow-up 2 compared to baseline [t(29)=-1.920, p=.065]. Among Val/Val high-risk adolescents, there was a trend toward greater mean cortisol at Follow-up 1 compared to baseline [t(11)=-1.839, p=.093]. No other changes approached significance.
Comparisons across genotypes within diagnostic groups revealed no significant differences in mean cortisol by genotype for the healthy controls, although analyses of the individual cortisol samples revealed that at Follow-up 1, cortisol 1 was greater for Met-carriers (mean=.8235; SD=.5260) than Val homozygotes (mean=.3979; SD=.3596) at the trend level [t(14)=1.889, p=.080]. No other results were significant for the healthy control group or the high-risk group, and this likely reflects the reduction in power due to the smaller number of subjects.
Discussion
In summary, consistent with previous reports, this study revealed higher cortisol levels in COMT Met homozygotes compared to Val homozygotes and Met heterozygotes. However, the genotype-associated differences in cortisol were observed at Follow-up 2 only, not Baseline or Follow-up 1. The absence of a relation at Baseline and Follow-up 1, together with our finding of longitudinal increases in cortisol levels (that are stronger for Met homozygotes alone than all Met-carriers at Follow-up 2, and weaker for Met homozygotes alone than all Met-carriers at Follow-up 1), suggests the possibility that COMT Val/Met genotype modulates longitudinal changes in cortisol during adolescent maturation, a period marked by a variety of neurodevelopmental changes and increasing risk for the onset of mental disorder (Sowell et al., 2007; Walker, 2002). We hypothesize that such an association reflects lower COMT activity in Met carriers, which in turn alters regulation of HPA function by dopamine, and possibly, norepinephrine. This study is unique in that it is the first to consider the modulating role of genetic factors, namely COMT, in regulating cortisol secretion specifically among youth at heightened risk for psychosis. Findings have implications for understanding the neurodevelopment of psychiatric disorders, particularly psychosis, and the advancement of novel treatment strategies and routes for prevention of mental disorders. One study limitation is the small sample size, which could result in false positives. Thus, replication of findings using a larger sample is warranted. Large-scale longitudinal studies examining the relation of genotype with neurohormonal development in adolescents may prove fruitful in elucidating heritable factors influencing the adolescent maturational process involved in the onset of Axis I disorders.
Acknowledgments
This research was supported in part by grant # RO1 MH4062066 awarded to Dr. Walker by the National Institute of Mental Health.
References
- First MB, Spitzer RL, Gibbon M, Williams BW. Structured Clinical Interview for the DSM-IV Axis I Disorders, Patient Edition (SCID I) Washington, D.C: American Psychiatric Press, Inc.; 1998. [Google Scholar]
- Jabbi M, Kema IP, van der Pompe G, te Meerman GJ, Ormel J, den Boer JA. Catechol-o-methyltransferase polymorphism and susceptibility to major depressive disorder modulates psychological stress response. Psychiatr Genet. 2007;17:183–193. doi: 10.1097/YPG.0b013e32808374df. [DOI] [PubMed] [Google Scholar]
- Kenny FM, Preeyasombat C, Migeon CJ. Cortisol production rate. II. Normal infants, children, and adults. Pediatrics. 1966;37:34–42. [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendorfer RA, Schriever K, Kessler U, Konig A, Schwarz HP, Strasburger CJ. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res. 1995;37(4 Pt 1):502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Laruelle M. The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies. Brain Res Brain Res Rev. 2000;31:371–384. doi: 10.1016/s0165-0173(99)00054-5. [DOI] [PubMed] [Google Scholar]
- Linkowski P, Van Onderbergen A, Kerkhofs M, Bosson D, Mendlewicz J, Van Cauter E. Twin study of the 24-h cortisol profile: evidence for genetic control of the human circadian clock. Am J Physiol. 1993;264:173–181. doi: 10.1152/ajpendo.1993.264.2.E173. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Wilkinson CW, Briere S, Menard C, Ng Ying Kin NM, Nair NP. The modulatory effects of corticosteroids on cognition: Studies in young human populations. Psychoneuroendocrinology. 2002;27:401–416. doi: 10.1016/s0306-4530(01)00061-0. [DOI] [PubMed] [Google Scholar]
- Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- Mittal VA, Dhruv S, Tessner KD, Walder DJ, Walker EF. The relations among putative biorisk markers in schizotypal adolescents: Minor physical anomalies, movement abnormalities, and salivary cortisol. Biol Psychiatry. 2007;61:1179–1186. doi: 10.1016/j.biopsych.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Oswald LM, McCaul M, Choi L, Yang X, Wand GS. Catechol-O-methyltransferase polymorphism alters hypothalamic-pituitary-adrenal axis responses to naloxone: a preliminary report. Biol Psychiatry. 2004;55:102–105. doi: 10.1016/j.biopsych.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Pfhol B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality: SIDP-IV. Washington, DC: American Psychiatric Press, Inc.; 1997. [Google Scholar]
- Rylander-Rudqvist T, Hakansson N, Tybring G, Wolk A. Quality and quantity of saliva DNA obtained from the self-administrated oragene method--a pilot study on the cohort of Swedish men. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1742–1745. doi: 10.1158/1055-9965.EPI-05-0706. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, Braus DF, Goldman D, Buchel C, Heinz A. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25(4):836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping adolescent brain maturation using structural magnetic resonance imaging. In: Romer D, Walker EF, editors. Adolescent psychopathology and the developing brain: Integrating brain and prevention science. New York, NY: Oxford University Press; 2007. pp. 55–84. [Google Scholar]
- Stefanis NC, Henquet C, Avramopoulos D, Smyrnis N, Evdokimidis I, Myin-Germeys I, Stefanis CN, Van Os J. COMT Val158Met moderation of stress-induced psychosis. Psychol Med. 2007;37:1651–1656. doi: 10.1017/S0033291707001080. [DOI] [PubMed] [Google Scholar]
- Trotman H, McMillan A, Walker E. Cognitive function and symptoms in adolescents with schizotypal personality disorder. Schizophr Bull. 2006;32:489–497. doi: 10.1093/schbul/sbj069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Hanssen M, Bak M, Bijl RV, Vollebergh W. Do urbanicity and familial liability coparticipate in causing psychosis? Am J Psychiatry. 2003;160:477–482. doi: 10.1176/appi.ajp.160.3.477. [DOI] [PubMed] [Google Scholar]
- van Winkel R, Henquet C, Rosa A, Papiol S, Fananas L, De Hert M, Peuskens J, van Os J, Myin-Germeys I. Evidence that the COMT(Val158Met) polymorphism moderates sensitivity to stress in psychosis: an experience-sampling study. Am J Med Genet Part B: Neuropsychiatr Genet. 2008;147:10–17. doi: 10.1002/ajmg.b.30559. [DOI] [PubMed] [Google Scholar]
- Wajs-Kuto E, De Beeck LO, Rooman RP, Caju MV. Hormonal changes during the first year of oestrogen treatment in constitutionally tall girls. Eur J Endocrinol. 1999;141:579–584. doi: 10.1530/eje.0.1410579. [DOI] [PubMed] [Google Scholar]
- Walker EF. Adolescent neurodevelopment and psychopathology. Curr Dir in Psychol Sci. 2002;11:24–28. [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: A neural diathesis-stress model. Psychol Rev. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Dev Psychopathol. 2001;13:721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Zubieta J-K, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]


