Abstract
Raman spectroscopy is being adopted as a nondestructive instrumentation for the robotic exploration of Mars to search for traces of life in the geological record. Here, miniaturized Raman spectrometers of two different types equipped with 532 and 785 nm lasers for excitation, respectively, were compared for the detection of microbial biomarkers in natural halite from the hyperarid region of the Atacama Desert. Measurements were performed directly on the rock as well as on the homogenized, powdered samples prepared from this material—the effects of this sample preparation and the excitation wavelength employed in the analysis are compared and discussed. From these results, 532 nm excitation was found to be superior for the analysis of powdered specimens due to its high sensitivity toward carotenoids and hence a higher capability for their detection at relatively low concentration in bulk powdered specimens. For the same reason, this wavelength was a better choice for the detection of carotenoids in direct measurements made on the rock samples. The 785 nm excitation wavelength, in contrast, proved to be more sensitive toward the detection of scytonemin. Key Words: Miniaturized portable Raman—Atacama—Mars—Biomarker detection. Astrobiology 12, 1095–1099.
1. Introduction
Raman spectroscopy is one of the analytical techniques to be employed in future missions designed to search for life on Mars, for example, the ExoMars mission. It has already been demonstrated that benchtop instrumentation is able to detect biomolecules associated with extremophilic colonization of rocks (Russell et al., 1998; Wynn-Williams and Edwards, 2000a, 2000b; Edwards et al., 2005; Villar et al., 2005; Marshall et al., 2006; Fendrihan et al., 2009), which provide model terrestrial organisms as analogues for those that may have been present on Mars. A specially constructed miniaturized Raman system, however, is an essential requirement for implementation of these in situ robotic measurements on Mars. It is therefore strategically important and relevant to future space missions to evaluate miniaturized Raman spectrometers for comparison with both laboratory instrumentation and flightlike experimental prototypes.
Investigators have previously identified pure biomolecules at low temperatures and high altitudes in a mountainous area and demonstrated the potential of the technique to work under more extreme conditions terrestrially (see Jehlička et al., 2009). However, a major question arises that, to date, remains essentially unanswered with only limited response in the current literature: What is the potential of a miniaturized Raman system to detect biomolecular life signatures dispersed in rocks? Dickensheets et al. (2000) presented some preliminary results that were obtained with a miniaturized Raman system with 852 nm excitation for the detection of biomolecules associated with the yellow Antarctic lichen Acarospora chlorophana. Vítek et al. (2012) applied 1064 nm excitation in a miniaturized system for the detection of biosignatures of the same epilithic lichen species and for a preliminary survey of endolithic microbial communities in halite pinnacles from the Atacama Desert. The present study reports the detection of scytonemin and carotenoid pigments associated with the endolithic cyanobacteria in this context, with a more systematic and novel approach. Performance of the two different spectrometers was tested on the three different zones of the rock with varying composition of the biomolecular targets. Analysis performed directly on the rock (without any pretreatments) was compared with measurements performed on the powdered and homogenized material.
Comprehensive evaluation of the analytical protocol is relevant within the frame of analytical testing related to future astrobiologically focused missions. We present our first results and show the application of the miniaturized Raman systems for the identification of biomarkers in native salt crusts from the hyperarid core of the Atacama Desert. This environment represents the driest area on Earth, with a mean annual rainfall of less than 2 mm (McKay et al., 2003), and is considered an important Mars analog site. Halite crusts from the Yungay area were studied, with known endolithic colonization dominated by cyanobacteria (Wierzchos et al., 2006; de los Ríos et al., 2010), which has already been the subject of Raman microspectrometric examination with large-scale laboratory equipment (Vítek et al., 2010). Here, two miniature spectrometers were compared by using different excitation wavelengths, namely, 532 nm, which is that adopted for the laser used in the ExoMars RLS Raman spectrometer, and 785 nm, which is normally considered to be a universal laboratory source for the identification of a variety of organic molecules with good performance for identification of inorganic mineral phases as well.
2. Materials and Methods
Halite samples colonized by endolithic cyanobacteria were collected in the Yungay hyperarid zone of the Atacama Desert in May 2011. One of the halite samples that represents the upper part of the halite crust with relatively rich biological colonization that is found in this part of the desert was selected for further assessment of miniaturized Raman spectrometers. Prior to the analysis, the sample was stored at room temperature in the dark as a whole pinnacle to maintain the stable conditions inside the sample.
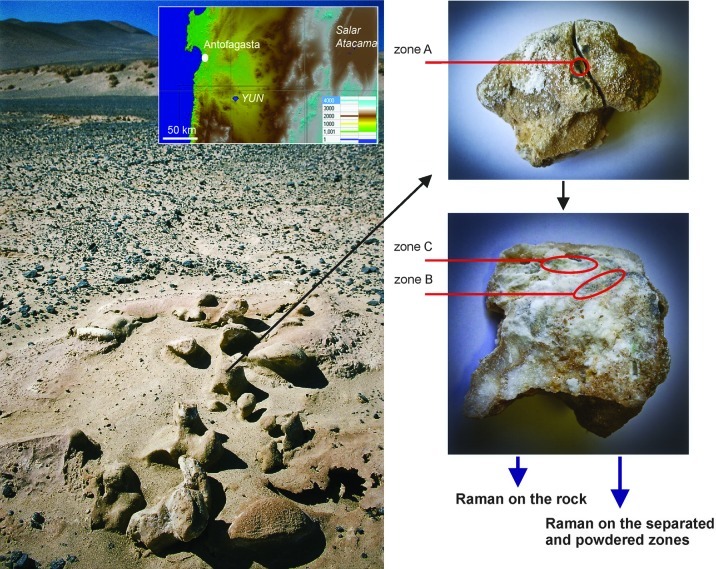
The typical landscape of the salar from which the halite was sampled is depicted within Fig. 1, as well as the individual sampling zones that were the subjects of this study. The halite pinnacle was broken to reveal the interior endolithic colonization zones. The sample contains three major zones of interest: the first is the sample surface and shallow subsurface, with intensively colored black surface stripes and the gray interior colonization as well (zone A); the second is a gray interior zone of moderate pigment intensity (zone B). The gray to black coloration suggests that the scytonemin pigment is present (Vítek et al., 2010). The third is a green interior zone (zone C) (see Fig. 1).
FIG. 1.
Sampling site and the sample of the halite crust from the Atacama Desert (Yungay, hyperarid region) studied with portable Raman instrumentation. Three different zones of interest were examined: A=surface zone, rich in scytonemin pigmentation; B=interior gray colonization zone; C=interior green colonization zone.
First, these three zones were analyzed directly on the sample, without any further pretreatment. Second, the parts of the stone that contained the cyanobacterial colonization zone were separated. The separated parts were crushed, powdered, and homogenized in agate mortar. Then, the flattened surface of the powder was measured at randomly selected areas in three replications. The sample crushing and powdering is a part of the sampling protocol that is going to be applied within the ExoMars mission, where the Raman spectroscopic analysis will be a first-pass interrogation of powdered rock or drill core samples.
2.1. Raman instrumentation
The portable Raman spectrometers, Advantage and Rock Hound by DeltaNu (Laramie, WY, USA), were employed. The Rock Hound is a handheld portable instrument (1.9 kg) equipped with a 785 nm diode laser for excitation, with a maximum output power of 120 mW and a thermoelectrically cooled charge-coupled device detector, with an operational wavenumber range of 200–2000 cm−1 and spectral resolution 8 cm−1.
The Advantage 532 is a compact Raman spectrometer that measures 12″×8″×4″ (LWH) in size and weighs 9 kg. Though miniaturized, the Advantage 532 is not a handheld unit as is the 785 nm system. It is equipped with a 532 nm frequency doubled Nd:YAG laser that has a maximum laser power of 100 mW and a thermoelectrically cooled charge-coupled device detector, with an operational wavenumber range of 200–3400 cm−1. The spectral resolution of the instrument is 10 cm−1, which is slightly lower compared to the planned ExoMars Raman instrument (6–8 cm−1).
Both instruments are equipped with NuSpec software, which permits control of the spectrometer functions, namely, a five-step setting of laser power, exposure time, the number of spectral accumulations acquired, and the spectral resolution. The typical exposure time was 10 s (one scan).
All spectra are baseline corrected. No other spectral treatments were undertaken.
3. Results
3.1. Direct analysis on the rock
The distribution of microbes within the halite crust, whether they are alive or dead, is found to be highly heterogeneous at the microscale. Hence, the laser spotlight diameter is a highly important parameter, and the successful outcome of the search for biomolecular signatures is dependent on careful and specific instrument operations. This proved to be important especially for zones A and B, where biosignatures were recorded only after several minutes of positioning of the spectrometer footprint image within the particular zone selected for study (Table 1). Scytonemin, a well-known UV protective molecule, is responsible for the dark coloration of these zones (see Vítek et al., 2010).
Table 1.
Raman Signal Obtained on Different Zones within Halite from the Atacama Desert
| Halite zones of interest | 532 nm | 785 nm |
|---|---|---|
| A - rock | weak S, after positioning | S,after positioning |
| B - rock | C+very weak S, after positioning | S,after positioning |
| C - rock | C, stable | N/A |
| A - powder | C, stable | N/A |
| B - powder | C, stable | N/A |
| C - powder | C, stable | N/A |
S=scytonemin, C=carotenoid, N/A=no organic signal obtained.
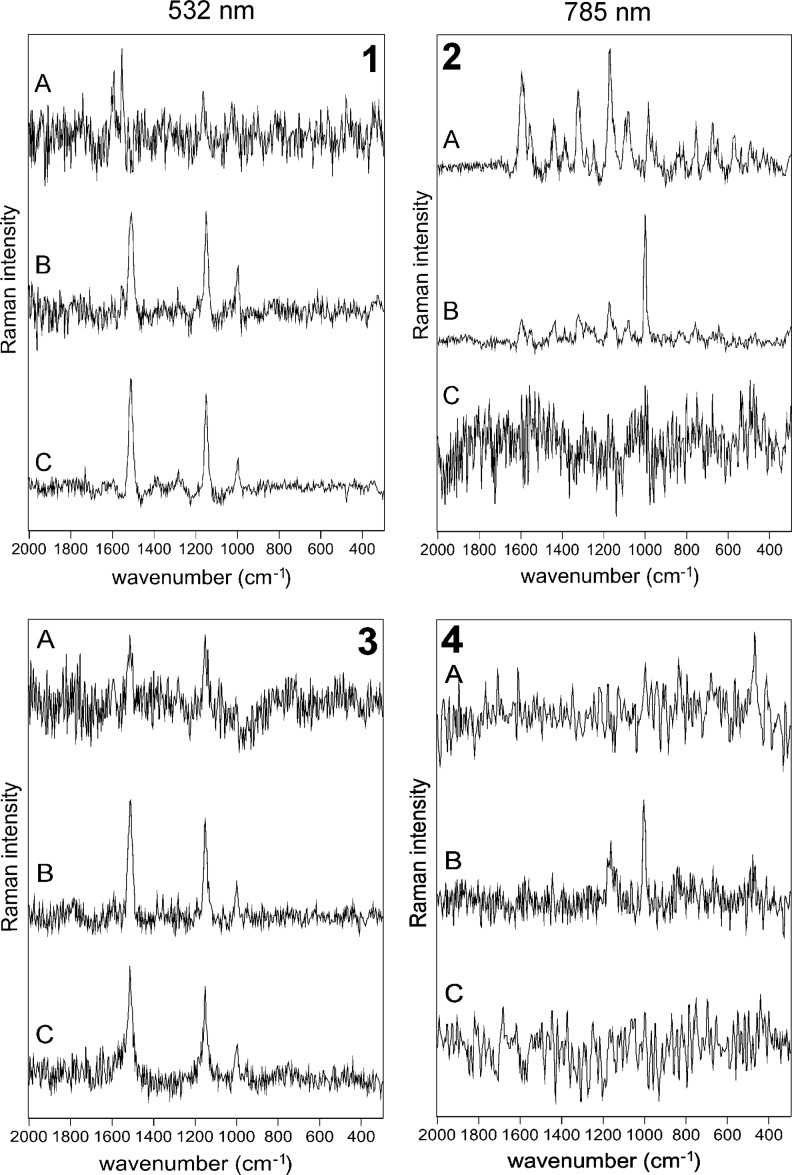
Significant differences in biomolecular detection capability were observed when using the two different laser excitation sources. In the case of the 532 nm laser excitation, only weak or very weak scytonemin signals were recognized within zones A and B, respectively. Instead, it was observed that carotenoid signals dominate the spectra from zone B (Fig. 2-1). This compound is evidenced by the presence of corroborative Raman bands at 1518, 1155, and 1000 cm−1. These Raman bands appear due to in-phase ν(C=C) and ν(C–C) stretching vibrations of the polyene chain and the very weak corroborative band around 1000 cm−1, which correspond to the in-plane rocking modes of the CH3 groups.
FIG. 2.
Raman spectra as obtained by portable Raman spectrometers within the three zones directly on the rock (1, 2) and on the powders (3, 4).
When the 785 nm wavelength was employed for excitation, clearly scytonemin features dominate the spectra from zones A and B with stronger signals detected from zone A, which indicates that a higher concentration of scytonemin occurs here (Fig. 2-2). The pigment was identified based on its characteristic Raman bands at 1595, 1554 due to ν(CCH) modes, and 1173 cm−1 due to the stretching vibration of the ν(C=C–C=C) conjugated system (Edwards et al., 2000). Other scytonemin bands of medium to weak intensity are also present in the spectrum. Signals due to photosynthetic pigments were not recognized in the spectra recorded when using 785 nm excitation.
This observation reflects the sensitivity of the individual excitation sources toward these two key biomolecular pigments, with the 532 nm wavelength coincidence with carotenoid absorption resulting in a resonance Raman enhancement, which leads to the appearance of stronger carotenoid Raman signals (see Merlin 1985; Marshall et al., 2007). On the other hand, lower sensitivity toward scytonemin compared to the 785 nm laser wavelength was observed in the case of the 532 nm excitation.
Further Raman signals in zone C were observed when analyzed by 532 nm laser excitation (see below). The zone visually appears green, and results of benchtop Raman analysis (Vítek et al., 2010) as well as the current results from the portable systems point to the relative absence of scytonemin. The stronger signal compared with the first two zones was registered when using the 532 nm excitation source due to carotenoids benefiting from the resonance Raman effect. No appropriate signal was returned when using a 785 nm excitation, which in this case points to the importance of the difference in sensitivity toward carotenoids between these two excitation wavelengths.
3.2. Analysis of powdered rock
The analysis of powdered geological material aboard the rover vehicle is an important part of the analytical protocol envisaged for the ExoMars mission.
When using 532 nm excitation, analysis of all three powders from the three different zones described above returned clear and reproducible signals indicative of carotenoids (Fig. 2–3). Although scytonemin was determined to be present within the powdered samples from zones A and B in the rocks when using 785 nm excitation, its Raman signature was not recorded when the 532 nm excitation wavelength was used on the powders. This is attributed to the low concentration of scytonemin in the mineral matrix to be detected in bulk at visible wavelengths, but in contrast there is a sufficient concentration of carotenoids for which detection at 532 nm with resonant enhancement is more sensitive.
When using the 785 nm laser wavelength for excitation, no Raman vibrational modes due to organic compounds were recorded for powdered specimens from all three zones (Fig. 2–4). This result is ascribed to a content of expected pigments that was too low, along with a lower sensitivity at 785 nm toward carotenoids when using this excitation wavelength compared with the 532 nm laser.
4. Implications and Conclusions
4.1. Positive factors toward detection of traces of life
Molecular signatures of life (biomarkers) were detected by miniaturized Raman spectrometers in the rock specimens from one of the most challenging environments for life on Earth.
The substrate (halite) and the environment itself (salt pan) are relevant scenarios for particular areas recognized on Mars.
Very good reproducibility of the results was achieved on powdered samples when using 532 nm excitation, and the organic signatures were reproducible for at least three replications from each of the zones.
4.2. Instrumental implications
532 nm excitation is preferential to 785 nm excitation for bulk analysis for organic spectral signatures due to its exceptional sensitivity toward carotenoids, which can be detected at very low concentration levels (down to about ∼100 wt ppb, see Vítek et al., 2009) compared to other organic biomolecules.
785 nm excitation is better for detection of scytonemin directly on the rock without any pretreatment, but it requires careful positioning to focus particular aggregates of cyanobacterial cells or their remnants.
A critical parameter is the analyzed sample volume (laser spot-size) and the homogeneity/heterogeneity of the measured sample. As documented by the spectroscopic measurements performed directly on the rock, the precise focusing on the particular cell aggregates became a key requirement for obtaining any signal in that case, and was crucial especially for the 785 nm excitation, as the content of the pigments in bulk after sample homogenization was too low to be detected with this wavelength.
In the case of powdered samples, the spatial information about the distribution of organics in rock is lost, but the appropriate homogenization of the rock sample may be advantageous in cases where the concentration of biomarker in bulk is above the limit of detection of Raman spectroscopy. In that case, the detection of biomarker is reproducible and possible without any handling as documented on the example of carotenoid signal obtained from the powdered samples with 532 nm for excitation. For more comprehensive discussion about the detection limits in Raman spectroscopical analysis of biomarkers in rocks see the work of Vandenabeele et al. (2012).
4.3. Tasks for further steps
The samples that were measured contained endolithic colonies, which could be located visually and then separated for powder preparation; the martian exploration scenario will be different in that unknown, remotely selected samples will be analyzed.
A relatively recent geobiological system was studied here. What will be the capability of the instrumentation to detect biomarker signatures in fossil microbes or relict biomarkers derived from decayed and extinct cells?
Halite is an important mineral substrate for astrobiological prospecting of Mars; however, different relevant materials should be evaluated in a similar way.
Although the employed Raman instrumentation represents miniaturized spectrometers (handheld in the case of the 785 nm system) and its application in this context is an important step, the exact prototype for ExoMars or any other mission should be tested in a similar manner in the future.
Acknowledgments
This work has been supported by the Czech Science Foundation (Project 210/10/0467 and P210/12/P330) and a grant MSM0021620855 from the Ministry of Education of the Czech Republic. C.A. and J.W. were supported by grant CGL2010-16004 from the Spanish Ministry of Economy and Competitiveness.
Author Disclosure Statement
No competing financial interests exist.
References
- de los Ríos A. Valea S. Ascaso C. Davila A. Kaštovský J. McKay C.P. Gómez-Silva B. Wierzchos J. Comparative analysis of the microbial communities inhabiting halite evaporites of the Atacama Desert. Int Microbiol. 2010;13:79–89. doi: 10.2436/20.1501.01.113. [DOI] [PubMed] [Google Scholar]
- Dickensheets D.L. Wynn-Williams D.D. Edwards H.G.M. Schoen C. Crowder C. Newton E.M. A novel miniature confocal microscope/Raman spectrometer system for biomolecular analysis on future Mars missions after Antarctic trials. J Raman Spectrosc. 2000;31:633–635. [Google Scholar]
- Edwards H.G.M. Garcia-Pichel F. Newton E.M. Wynn-Williams D.D. Vibrational Raman spectroscopic study of scytonemin, the UV-protective cyanobacterial pigment. Spectrochim Acta A Mol Biomol Spectrosc. 2000;56:193–200. doi: 10.1016/s1386-1425(99)00218-8. [DOI] [PubMed] [Google Scholar]
- Edwards H.G.M. Moody C.D. Villar S.E.J. Wynn-Williams D.D. Raman spectroscopic detection of key biomarkers of cyanobacteria and lichen symbiosis in extreme Antarctic habitats: evaluation for Mars lander missions. Icarus. 2005;174:560–571. [Google Scholar]
- Fendrihan S. Musso M. Stan-Lotter H. Raman spectroscopy as a potential method for the detection of extremely halophilic archaea embedded in halite in terrestrial and possibly extraterrestrial samples. J Raman Spectrosc. 2009;40:1996–2003. doi: 10.1002/jrs.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehlička J. Vítek P. Edwards H.G.M. Raman spectra of organic acids obtained using a portable instrument at −5°C in a mountain area at 2000 m above sea level. J Raman Spectrosc. 2009;41:440–444. [Google Scholar]
- Marshall C.P. Carter E.A. Leuko S. Javaux E.J. Vibrational spectroscopy of extant and fossil microbes: relevance for the astrobiological exploration of Mars. Vib Spectrosc. 2006;41:182–189. [Google Scholar]
- Marshall C.P. Leuko S. Coyle C.M. Walter M.R. Burns B.P. Neilan B.A. Carotenoid analysis of halophilic archaea by resonance Raman spectroscopy. Astrobiology. 2007;7:631–643. doi: 10.1089/ast.2006.0097. [DOI] [PubMed] [Google Scholar]
- McKay C.P. Friedmann E.I. Gómez-Silva B. Cáceres-Villanueva L. Andersen D.T. Temperature and moisture conditions for life in the extreme arid region of the Atacama Desert: four years of observations including the El Niño of 1997–1998. Astrobiology. 2003;3:393–406. doi: 10.1089/153110703769016460. [DOI] [PubMed] [Google Scholar]
- Merlin J.C. Resonance Raman spectroscopy of carotenoids and carotenoid-containing systems. Pure Appl Chem. 1985;57:785–792. [Google Scholar]
- Russell N.C. Edwards H.G.M. Wynn-Williams D.D. FT-Raman spectroscopic analysis of endolithic microbial communities from Beacon sandstone in Victoria Land, Antarctica. Antarct Sci. 1998;10:63–74. [Google Scholar]
- Vandenabeele P. Jehlička J. Vítek P. Edwards H.G.M. On the Raman spectroscopic detection limits for the analysis of biomarkers in solid matrices. Planet Space Sci. 2012;62:48–54. [Google Scholar]
- Villar S.E.J. Edwards H.G.M. Cockell C.S. Raman spectroscopy of endoliths from Antarctic cold desert environments. Analyst. 2005;130:156–162. doi: 10.1039/b410854j. [DOI] [PubMed] [Google Scholar]
- Vítek P. Jehlička J. Edwards H.G.M. Osterrothová K. Identification of β-carotene in an evaporitic matrix—evaluation of Raman spectroscopic analysis for astrobiological research on Mars. Anal Bioanal Chem. 2009;393:1967–1975. doi: 10.1007/s00216-009-2677-0. [DOI] [PubMed] [Google Scholar]
- Vítek P. Edwards H.G.M. Jehlička J. Ascaso C. de los Ríos A. Valea S. Villar S.E.J. Davila A.F. Wierzchos J. Microbial colonization of halite from the hyper-arid Atacama Desert studied by Raman spectroscopy. Philos Transact A Math Phys Eng Sci. 2010;368:3205–3221. doi: 10.1098/rsta.2010.0059. [DOI] [PubMed] [Google Scholar]
- Vítek P. Ali E.M.A. Edwards H.G.M. Jehlička J. Cox R. Page K. Evaluation of portable Raman spectrometer with 1064 nm excitation for geological and forensic applications. Spectrochim Acta A Mol Biomol Spectrosc. 2012;86:320–327. doi: 10.1016/j.saa.2011.10.043. [DOI] [PubMed] [Google Scholar]
- Wierzchos J. Ascaso C. McKay C.P. Endolithic cyanobacteria in halite rocks from the hyperarid core of the Atacama Desert. Astrobiology. 2006;6:415–422. doi: 10.1089/ast.2006.6.415. [DOI] [PubMed] [Google Scholar]
- Wynn-Williams D.D. Edwards H.G.M. Proximal analysis of regolith habitats and protective biomolecules in situ by laser Raman spectroscopy: overview of terrestrial Antarctic habitats and Mars analogs. Icarus. 2000a;144:486–503. [Google Scholar]
- Wynn-Williams D.D. Edwards H.G.M. Antarctic ecosystems as models for extraterrestrial surface habitats. Planet Space Sci. 2000b;48:1065–1075. [Google Scholar]