Abstract
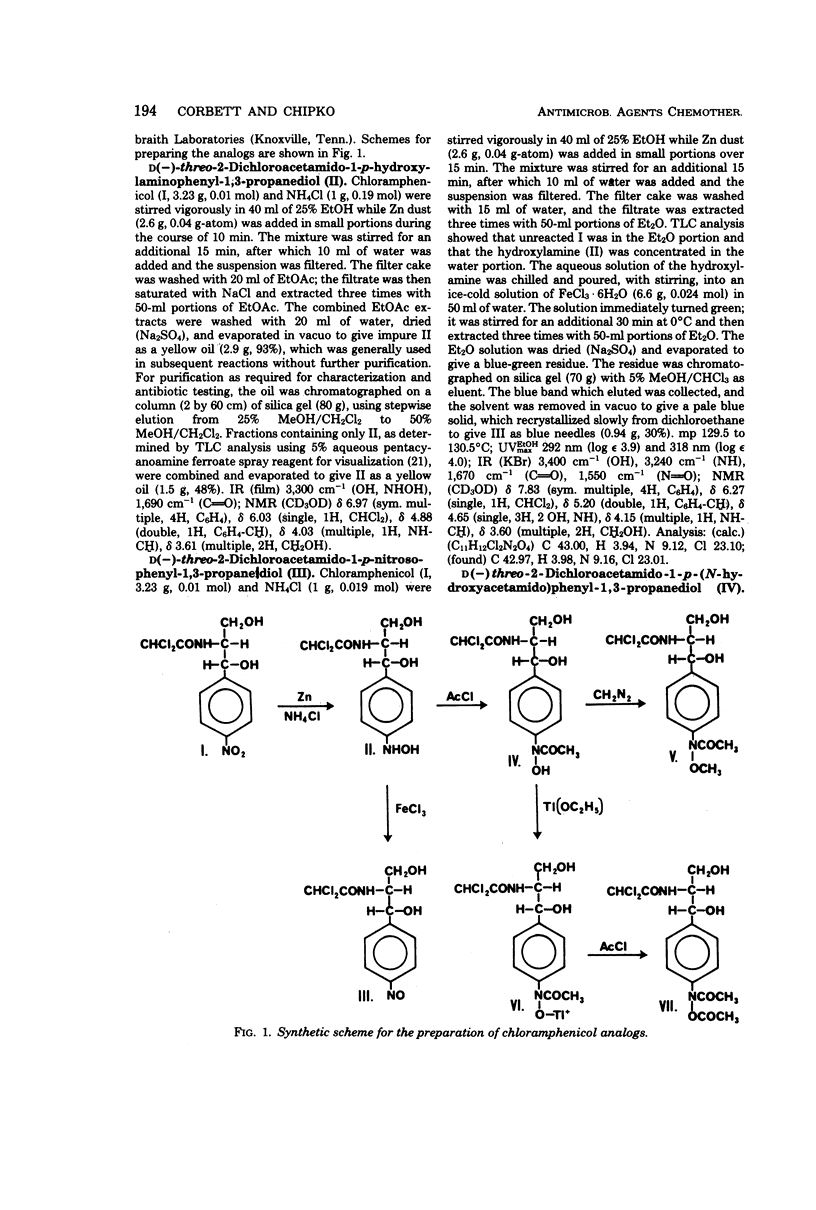
Analogs of chloramphenicol were prepared for the first time in which the nitro group was replaced by hydroxylamine, nitroso, hydroxamic acid, methyl hydroxamate, and O-acetyl hydroxamate functional groups. These compounds were tested for antibiotic activity in order to determine whether the antibiotic activity of chloramphenicol is mediated by one or more of these potential metabolites of chloramphenicol. None of these analogs was as active as chloramphenicol against the four test organisms, and two of the compounds were essentially devoid of activity. The significance of these findings with regard to the importance of the nitro group to the biological activity of chloramphenicol is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corbett M. D., Cahoy J. E., Chipko B. R. Conversion of nitrosobenzene to N-phenylacetohydroxamic acid by yeast pyruvate decarboxylase. J Natl Cancer Inst. 1975 Nov;55(5):1247–1248. doi: 10.1093/jnci/55.5.1247. [DOI] [PubMed] [Google Scholar]
- Corbett M. D., Chipko B. R. N-phenylglycolhydroxamate production by the action of transketolase on nitrosobenzene. Biochem J. 1977 Aug 1;165(2):263–267. doi: 10.1042/bj1650263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman K. B. Action of the N-trifluoroacetyl analogue of D-chloramphenicol. Antimicrob Agents Chemother. 1977 Mar;11(3):563–565. doi: 10.1128/aac.11.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansch C., Nakamoto K., Gorin M., Denisevich P., Garret E. R., Heman-Ackah S. M., Won C. H. Structure-activity relationship of chloramphenicols. J Med Chem. 1973 Aug;16(8):917–922. doi: 10.1021/jm00266a011. [DOI] [PubMed] [Google Scholar]
- Hlavica P., Kehl M. Comparative studies on the N-oxidation of aniline and N,N-dimethylaniline by rabbit liver microsomes. Xenobiotica. 1976 Nov;6(11):679–689. doi: 10.3109/00498257609151680. [DOI] [PubMed] [Google Scholar]
- Kadlubar F. F., Miller J. A., Miller E. C. Microsomal N-oxidation of the hepatocarcinogen N-methyl-4-aminoazobenzene and the reactivity of N-hydroxy-N-methyl-4-aminoazobenzene. Cancer Res. 1976 Mar;36(3):1196–1206. [PubMed] [Google Scholar]
- Kiese M., Taeger K. The fate of phenylhydroxylamine in human red cells. Naunyn Schmiedebergs Arch Pharmacol. 1976;292(1):59–66. doi: 10.1007/BF00506490. [DOI] [PubMed] [Google Scholar]
- King C. M., Traub N. R., Cardona R. A., Howard R. B. Comparative adduct formation of 4-aminobiphenyl and 2-aminofluorene derivatives with macromolecules of isolated liver parenchymal cells. Cancer Res. 1976 Jul;36(7 Pt 1):2374–2381. [PubMed] [Google Scholar]
- Krishna G. Covalent binding of drugs to tissue macromolecules as a biochemical mechanism of drug toxicities with special emphasis on chloramphenicol and thiamphenicol. Postgrad Med J. 1974 Oct;50 (Suppl 5):73–77. [PubMed] [Google Scholar]
- Lotlikar P. D., Miller E. C., Miller J. A., Margreth A. The enzymatic reduction of the N-hydroxy derivatives of 2-acetylaminofluorene and related carcinogens by tissue preparations. Cancer Res. 1965 Nov;25(10):1743–1752. [PubMed] [Google Scholar]
- Manyan D. R., Arimura G. K., Yunis A. A. Comparative metabolic effects of chloramphenicol analogues. Mol Pharmacol. 1975 Sep;11(5):520–527. [PubMed] [Google Scholar]
- Mason R. P., Holtzman J. L. The mechanism of microsomal and mitochondrial nitroreductase. Electron spin resonance evidence for nitroaromatic free radical intermediates. Biochemistry. 1975 Apr 22;14(8):1626–1632. doi: 10.1021/bi00679a013. [DOI] [PubMed] [Google Scholar]
- Prough R. A., Ziegler D. M. The relative participation of liver microsomal amine oxidase and cytochrome P-450 in N-demethylation reactions. Arch Biochem Biophys. 1977 Apr 30;180(2):363–373. doi: 10.1016/0003-9861(77)90050-9. [DOI] [PubMed] [Google Scholar]
- Radomski J. L., Rey A. A., Brill E. Evidence for a glucuronic acid conjugate of N-hydroxy-4-aminobiphenyl in the urine of dogs given 4-aminobiphenyl. Cancer Res. 1973 Jun;33(6):1284–1289. [PubMed] [Google Scholar]
- Reddy B. G., Pohl L. R., Krishna G. The requirement of the gut flora in nitrobenzene-induced methemoglobinemia in rats. Biochem Pharmacol. 1976 May 1;25(9):1119–1122. doi: 10.1016/0006-2952(76)90507-4. [DOI] [PubMed] [Google Scholar]
- Wardman P., Clarke E. D. Oxygen inhibition of nitroreductase: electron transfer from nitro radical-anions to oxygen. Biochem Biophys Res Commun. 1976 Apr 19;69(4):942–949. doi: 10.1016/0006-291x(76)90464-2. [DOI] [PubMed] [Google Scholar]
- Weisburger J. H., Shirasu Y., Grantham P. H., Weisburger E. K. Chloramphenicol, protein synthesis, and the metabolism of the carcinogen N-2-fluorenyldiacetamide in rats. Inhibition by chloramphenicol of carcinogen binding. J Biol Chem. 1967 Feb 10;242(3):372–378. [PubMed] [Google Scholar]
- Weisburger J. H., Weisburger E. K. Biochemical formation and pharmacological, toxicological, and pathological properties of hydroxylamines and hydroxamic acids. Pharmacol Rev. 1973 Mar;25(1):1–66. [PubMed] [Google Scholar]
- Wheeler L. A., Soderberg F. B., Goldman P. The relationship between nitro group reduction and the intestinal microflora. J Pharmacol Exp Ther. 1975 Jul;194(1):135–144. [PubMed] [Google Scholar]
- YUNIS A. A., BLOOMBERG G. R. CHLORAMPHENICOL TOXICITY: CLINICAL FEATURES AND PATHOGENESIS. Prog Hematol. 1964;4:138–159. [PubMed] [Google Scholar]


