Abstract
Background: Smoking cessation is often followed by weight gain. Eating behaviors and weight change have been linked to the brain response to food, but it is unknown whether smoking influences this response.
Objective: We determined the influence of smoking status (smokers compared with nonsmokers) on the brain response to food in regions associated with weight changes in nonsmokers.
Design: In study 1, we used functional MRI (fMRI) to identify regions of the brain associated with weight change in nonsmokers. BMI and the brain response to a milk shake, which is a palatable and energy-dense food, were measured in a group of 27 nonsmokers (5 men). Sixteen subjects (3 men) returned 1 y later for BMI reassessment. The change in BMI was regressed against the brain response to isolate regions associated with weight change. In study 2, to determine whether smokers showed altered responses in regions associated with weight change, we assessed the brain response to a milk shake in 11 smokers. The brain response to a milk shake compared with a tasteless control solution was assessed in 11 smokers (5 men) in comparison with a group of age-, sex- and body weight–matched nonsmokers selected from the pool of nonsmokers who participated in study 1.
Results: The response in the midbrain, hypothalamus, thalamus, and ventral striatum was positively associated with weight change at the 1-y follow-up in 16 nonsmokers. Compared with nonsmokers, smokers had a greater response to milk shakes in the hypothalamus.
Conclusion: Smokers display an altered brain response to food in the hypothalamus, which is an area associated with long-term weight change in nonsmokers.
INTRODUCTION
Cigarette smoking increases risk of cardiovascular, respiratory, and cancerous illnesses among others (1). Weight gain and the fear of weight gain are well-established obstacles to smoking cessation, especially in women (2–8). Although there is evidence that smoking and, more specifically, nicotine consumption affect body weight through mechanisms such as decreased food intake, decreased metabolic rate, altered nutrient processing, and the release of hypothalamic neuropeptides (2, 9), these effects remain controversial (10–12). In contrast, it is clear that weight gain after smoking cessation is associated with increased caloric intake, and the resumption of smoking is accompanied by a reduction in caloric intake (2, 10, 13–16).
The mechanisms by which smoking influences food intake remain relatively unexplored. Animal studies have shown that self-administered nicotine and the direct injection of nicotine into the hypothalamus decreases food intake and leads to weight loss (17–19), possibly by modulating proopiomelanocortin neurons (20). These findings suggest that one way in which nicotine influences intake is by influencing circuits in the hypothalamus that control eating behavior.
Nicotine may also influence food intake by altering the reinforcing properties of rewards with which it shares neural substrates (21). Consistent with this possibility, neuroimaging studies have shown that an abused reinforcer (eg, cocaine) can change the representation of another nonabused reinforcer (eg, money) (22–24). With respect to nicotine, smokers have shown a reduced brain response to monetary reward (25, 26). It is currently unknown whether smokers also show an altered brain response to palatable foods; however, in rodents, exposure to nicotine or chocolate cues elicits similar c-fos activation patterns, which are a marker of neural activation (27, 28). It has also been shown that abstinent smokers display behavioral patterns that are consistent with an enhanced carbohydrate reward during performance of an operant task (29). Hence, it is possible that nicotine addiction may change the reward value of food and, thereby, influence intake.
Previous studies have shown that long-term weight change is associated with changes in brain structure and the response to food in the mesolimbic pathway including the ventral tegmental area and substantia nigra in addition to the dorsal and ventral striatum (30–33). In the current study, we were interested in testing whether the brain response to milk shakes is modulated by smoking status in regions associated with long-term weight change in nonsmokers. First, functional MRI (fMRI)4 was used to isolate regions in which the brain response to a milk shake was associated with a weight change at 1-y follow-up. The results from this experiment guided a second study in which the response to a milk shake was compared between smokers and nonsmokers. We reasoned that, if smoking influences weight by altering the response to food, smokers should show a differential response in those regions associated with weight change in nonsmokers.
SUBJECTS AND METHODS
Subjects
All subjects gave written informed consent to participate in the study, which was approved by the Yale University Institutional Review Board. Participants were not taking daily medications and had no history of loss of consciousness, psychiatric disorder, chemosensory impairment, or food allergies. Study 1 enrolled 29 nonsmokers (5 men) who reported never smoking. Nonsmokers were part of a larger study that will be reported separately. Two subjects were excluded because of excessive head motion (>2 mm mean displacement) during an fMRI scan. The longitudinal assessment of BMI at 1 y was added several months into the study, and thus, only 16 of the nonsmokers returned 1 y later for BMI reassessment. There was no significant difference between subjects who returned at 1 y and subjects who did not in age (P = 0.87), BMI (P = 0.13), or race (whites, P = 0.34; African Americans, P = 0.34; other, P = 0.22; Asians, P = 0.4; more than one race, P = 0.72). Study 2 enrolled 13 smokers (7 men) who reported currently smoking >5 cigarettes/d for >6 mo. Two smokers were excluded because of excessive head motion, which left 11 smokers (5 men) with analyzable data. A comparison sample of 11 nonsmokers matched for age, BMI, and sex were selected from nonsmokers who participated in study 1. Recruitment for both studies began in November 2005 and continued until May 2009.
Study design and procedures for studies 1 and 2
Subjects participated in one behavioral and fMRI training session and one fMRI scanning session conducted on separate days. All testing sessions occurred between 1100 and 1300. Subjects were instructed to eat 2 h before their session and to refrain from consuming food or drink (except water) until their arrival. We relied on the subjective report of participant satiety state and did not collect food diaries about intakes on the day of the study. To confirm self-reports of smoking status, the carbon monoxide level (in parts per million) of subjects was measured in a sample of expired air by using an electronic gas analyzer (Monoxor II; Bacharach Inc). Heights and weights were measured while subjects were clothed (no shoes or jackets) by using a Detecto 439 Mechanical Scale (Cardinal Scale Manufacturing Co) and calculated by using US CDC standards [BMI (in kg/m2) was calculated as weight divided by the square of height]. For nonsmokers who returned for a second measurement of their BMI after 1 y (n = 16), their change in BMI was calculated as the subtraction of their initial BMI from that measured after 1 y. However, we were not able to obtain the follow-up BMI at the same time of day as at the first time point. At the start and finish of each session, subjects rated feelings of hunger by using a visual analog scale (VAS) that was scaled between −10 and +10 and labeled as follows: left anchor, “I am not hungry at all” midpoint, “neutral” and right anchor, “I have never been more hungry.” Because our goal was to evaluate the brain response to food in smoking-sated subjects, smokers were allowed to smoke as usual on the day of the scan. They were also told that they should not be craving a cigarette during scanning and to inform the experimenter if they would like a smoking break. Need was determined by asking subjects to rate how much they wanted to smoke on a VAS labeled as follows: left anchor, (−10) “no desire to smoke” midpoint, (0) “neutral” and right anchor, (+10) “most intense craving ever experienced” before and after scanning. If a subject rated their desire above neutral, they were sent to smoke.
Next, subjects completed a battery of questionnaires to characterize their eating behavior and personality. Personality was measured by using the Tridimensional Personality Questionnaire (34), impulsivity with the Barratt Impulsiveness Scale Version 11 (35), and the Three-Factor Eating Questionnaire (TFEQ) (36). The severity of nicotine addiction in smokers was measured by using the Fagerström Test for Nicotine Dependence. Smokers also reported their age when they first started to smoke daily, the number of cigarettes smoked each day, and the number of years they had been smoking. The number of pack-years was calculated by dividing the number of cigarettes consumed daily by 20 (the number in a pack) and multiplying that number by the number of years smoking (37).
Stimuli and task
The stimulation and fMRI protocol was based on the procedure we developed to assess anticipatory and consummatory chemosensation in healthy subjects (38). Flavor stimuli included 100 mL Hershey's Cookies ‘n’ Cream Milkshake (The Hershey Company) diluted with 10 mL distilled water and 100 mL Garelick Ultimate Strawberry Milk (Garelick Brothers Farms) plus 5 g sucrose and 1 mL strawberry flavor (Galaxy Flavors). The tasteless baseline solution consisted of 12.5 mmol KCl/L and 1.25 mmol NaHCO3/L in distilled water. Liquids were delivered as 0.5 mL solution over 3 s by using a gustometer system described previously (39). In brief, this system consists of computer-controlled syringe pumps connected to an fMRI-compatible, custom-designed gustatory manifold via a 25-ft length of beverage tubing (Saint-Gobain Performance Plastics). The manifold was mounted on the head coil, and the liquids were dripped from the manifold stylus onto the tongue.
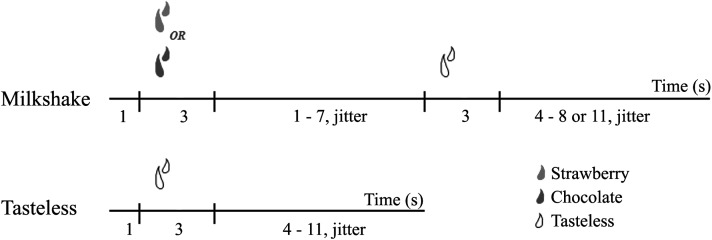
Subjects provided stimulus ratings in the fMRI simulator and actual scanner. The stimulus intensity was measured by using the general Labeled Magnitude Scale (40); a 100-mm vertical line scale with “barely detectable” labeled at the lower anchor point and the label “strongest imaginable sensation” at the upper anchor point was used to measure intensity. Pleasantness was rated by using a 20-mm cross-modal VAS anchored by “most unpleasant sensation ever” at the left anchor point (–10), “neutral” in the center (0), and “most pleasant sensation ever” at the right anchor point (10). Ratings of familiarity, edibility, and wanting to eat were also measured by using a 20-mm VAS with the following respective labels: at the left anchor, “not familiar at all,” “not edible at all,” and “I would never want to eat this” at the middle point, “neutral” and at the right anchor, “very familiar,” “very edible,” and “I would want to eat this more than anything.” After completing the ratings, subjects underwent a single mock run, which served to familiarize them with the procedure. During the mock and real runs, either a milk shake or a tasteless solution (in random order) was presented to the subject. The paradigm is described in detail in Figure 1. Subjects participated in one run during the mock session and 2 runs during the actual fMRI session, each of which lasted 4 min 42 s. These runs yielded a total of 20 presentations of the milk shake and tasteless solution during the actual fMRI session with an equal number of chocolate and strawberry milk shake stimuli received by each subject. Two milk shake flavors were used to minimize sensory adaptation. Subjects provided a second set of ratings after both mock and real scanning.
FIGURE 1.
Timeline of stimulus presentation for milk shakes and tasteless stimuli. A milk shake and a tasteless solution were presented in random order for 3 s. After a 1–7-s variable interval (jitter), the milk shake was followed by a 3-s tasteless rinse and, finally, a 4–8- or 11-s variable interval before the onset of the next event. After delivery of the tasteless solution, the next event would begin between 4 and 11 s later. The tasteless event was not followed by a rinse. Subjects were instructed to hold the solution in their mouths and swallow during the variable interval.
fMRI acquisition
Imaging data were acquired with a Siemens 3 T Trio magnetom scanner (Siemens AG) at the Yale University Magnetic Resonance Research Center by using variables similar to previous studies that identified neural correlates associated with chemosensation of food (38). A high-resolution, T1-weighted, 3-dimensional anatomical image was acquired for each subject (see supplementary methods under “Supplemental data” in the online issue for more details).
fMRI analysis
Image analysis was performed on the data of each subject by using the Oxford Center for Functional MRI of the Brain (FMRIB) Expert Analysis Tool (41; www.fmrib.ox.ac.uk/fsl). The preprocessing of each subject's time series of fMRI volumes encompassed skull extraction by using Brain Extraction Tool, slice time correction, motion correction, spatial smoothing with use of a Gaussian kernel of full width at half maximum of 5 mm, nonlinear high-pass temporal filtering (128 s), and subtraction of the mean of each voxel time course from that time course. Anatomical and functional images were normalized to the standard Montreal Neurological Institute template brain implemented in FMRIB Software Library. The fMRI signal was linearly modeled on a voxel-by-voxel basis by using FMRIB's Improved Linear Model with local autocorrelation correction (42, 43). The 6 vectors for head motion, in addition to the global signal calculated as the average signal intensity of all brain voxels at each volume, were regressed out of the model to correct for head motion and fluctuations in whole-brain signal intensity, respectively (44).
After preprocessing, a design matrix was created for each subject that identified the onset and duration of all events. Event onsets were defined as the start of the 3-s time of taste delivery (when the solution entered the subject's mouth). To measure the neural response during ingestion, event durations were defined as the 3-s taste delivery plus the variable interval that followed. Milk shake and tasteless presentations were modeled as events of interest. The general linear model was used to estimate, at each voxel, condition-specific effects. A canonical hemodynamic response function, which consisted of a double-γ variate function, was used to model the neural response to events. The significance of the model fit to each voxel time series was calculated, which yielded statistical parametric maps for each subject.
Group statistical maps were generated in a second-level random-effects group analysis with the use of FMRIB Local Analysis of Mixed Effects to obtain the main effect of milk shake minus tasteless solution and whole-brain regressions with different covariates of interest. For each resulting cluster of spatially connected voxels that survived the z threshold, a cluster probability threshold of P = 0.05 (family-wise error-rate corrected) was applied to the computed significance of that cluster, which corrected for multiple comparisons (45, 46). A 2-sample unpaired t test was used to compare the response to milk shake minus tasteless solution in smokers with that in matched nonsmokers within clusters shown to correlate with weight change in nonsmokers from study 1. For this analysis, the P value was corrected over the volume of the mask. To correct for behavioral scores and measures that differed between smokers and nonsmokers, we included them separately in the t test as variables of no interest and repeated the test.
Additional statistical analysis
Behavioral data were analyzed by using t tests, regression, repeated-measures ANOVA, and Grubb's test as implemented in Statistica 9 software (StatSoft Inc) with a significance level of P < 0.05.
RESULTS
Subject characteristics
Study 1
The average [mean (±SEM)] age of nonsmokers in study 1 (n = 27) was 25.1 ± 1.1 y, and their average BMI was 27.1 ± 0.9. Sixteen nonsmokers from this group returned 1 y later for BMI reassessment. A Grubb's test identified a single outlier in BMI change, and the subject was excluded from subsequent analyses. No significant change in BMI was observed (0.00 ± 0.27; paired t test; P = 0.97) (see Table S1 under “Supplemental data” in the online issue); however, there was a considerable range of changes (−2.29 to 1.51).
Study 2
The average age and BMI did not differ between the 11 smokers and 11 matched nonsmokers taken from study 1 (unpaired t test; age, P = 0.55; BMI, P = 0.97) (Table 1; see Table S2 under “Supplemental data” in the online issue). Both groups consisted of 5 men and 6 women. Years of education were greater in nonsmokers than in smokers (P = 0.016), and self-reported impulsivity was higher in smokers than in nonsmokers (Barratt Impulsiveness Scale Version 11 total: smokers, 67.1 ± 1.7; nonsmokers, 56.7 ± 2.4; P < 10−3) (Table 1). Compared with nonsmokers, smokers exhibited a trend toward less-disinhibited eating (P = 0.07), and they showed significantly lower scores on the hunger subscale (P = 0.03) of the TFEQ (Table 1). No other differences in eating style were observed. Smoking history is also presented in Table 1.
TABLE 1.
Subject characteristics for the smokers and matched nonsmokers included in study 21
| Smokers | Nonsmokers | |
| Demographics | ||
| Age (y) | 30.6 ± 2.502 | 28.5 ± 2.40 |
| Sex | 5 men, 6 women | 5 men, 6 women |
| BMI (kg/m2) | 27.0 ± 1.63 | 27.1 ± 1.60 |
| Years of education* | 13.7 ± 0.70 | 16.1 ± 0.50 |
| Smoking history | ||
| FTND score | 5.18 ± 0.79 | — |
| Initial smoking age (y) | 15.7 ± 0.83 | — |
| Cigarettes (/d) | 18.5 ± 2.7 | — |
| Smoking years | 11.2 ± 3.17 | |
| Pack-years | 8.87 ± 2.49 | — |
| Questionnaires | ||
| TFEQ-Hunger* | 3.4 ± 0.7 | 6.5 ± 1.2 |
| TFEQ-Disinhibition | 4.1 ± 0.8 | 6.8 ± 1.2 |
| TFEQ-Restraint | 6.5 ± 1.3 | 6.8 ± 1.7 |
| BIS-11* | 67.1 ± 1.7 | 56.7 ± 2.4 |
BIS-11, Barratt Impulsiveness Scale Version 11; FTND, Fagerström Test for Nicotine Dependence; TFEQ, Three-Factor Eating Questionnaire. *Significant difference between smokers and nonsmokers, P < 0.05 (unpaired t test).
Mean ± SEM (all such values).
Behavioral results
Average hunger ratings at the time of scanning were near neutral for both smokers (before scanning: −1.5 ± 1.3; after scanning: −0.4 ± 1.6) and matched those of nonsmokers (before scanning: −0.7 ± 1.41; after scanning: −0.9 ± 1.5) and did not differ between groups (P = 0.56) or as a function of time (before and after scanning; P = 0.20). No group-by-time interaction was observed (P = 0.84). Smokers rated the milk shake intensity significantly higher than nonsmokers did (P = 0.01), and both groups rated the milk shake as more familiar after scanning than before scanning (P = 0.03). There was no main effect of group or time on milk shake wanting, familiarity, edibility, or pleasantness, and there was and only one group-by-time interaction. This interaction arose because pleasantness ratings decreased prescanning to postscanning in smokers and increased prescanning to postscanning in nonsmokers (P = 0.04) (Table 2). The urge to urinate increased after scanning (P < 10−4) similarly for smokers and nonsmokers. Finally, smokers indicated wanting to smoke more postscan than prescan (P = 0.03); however, all ratings were near neutral (−3.8 ± 1.3 compared with 1.7 ± 1.83, with −10 = “no desire to smoke” and 10 = “most intense craving ever experienced”) (Table 2).
TABLE 2.
Behavioral results during scanning for the smokers and matched nonsmokers included in study 21
| Smokers |
Nonsmokers |
|||
| Ratings | Before scanning | After scanning | Before scanning | After scanning |
| Hunger | −1.5 ± 1.3 | −0.4 ± 1.6 | −0.7 ± 1.41 | 0.9 ± 1.5 |
| Fullness | −1.2 ± 1.2 | −0.5 ± 1.7 | −0.7 ± 1.2 | −2.7 ± 1.6 |
| Urge to urinate* | −8.8 ± 0.5 | 1.8 ± 1.9 | −9.1 ± 0.5 | −2.2 ± 2.8 |
| Milk shake intensity† | 5.1 ± 1.0 | 5.4 ± 0.8 | 2.6 ± 0.4 | 2.7 ± 0.5 |
| Milk shake wanting | 5.5 ± 1.0 | 4.5 ± 0.5 | 4.5 ± 1.3 | 4.9 ± 1.1 |
| Milk shake familiarity* | 7.6 ± 0.8 | 8.9 ± 0.4 | 7.3 ± 0.6 | 8.0 ± 0.5 |
| Milk shake edibility | 8.1 ± 0.5 | 8.0 ± 0.6 | 7.2 ± 0.6 | 7.3 ± 0.6 |
| Milk shake pleasantnessDagger | 6.2 ± 0.7 | 5.4 ± 1.0 | 4.3 ± 1.2 | 6.0 ± 0.7 |
| Wanting to smoke* | −3.8 ± 1.3 | 1.7 ± 0.8 | — | — |
All values are means ± SEMs. *Significant change after scanning compared with before scanning, P < 0.05 (repeated-measures ANOVA); †significant difference between smokers and nonsmokers (P < 0.05; repeated-measures ANOVA); ‡significant group (smokers compared with nonsmokers) by time (before scanning compared with after scanning) interaction, P < 0.05 (repeated-measures ANOVA).
Brain imaging results
Study 1: response to milk shakes in nonsmokers
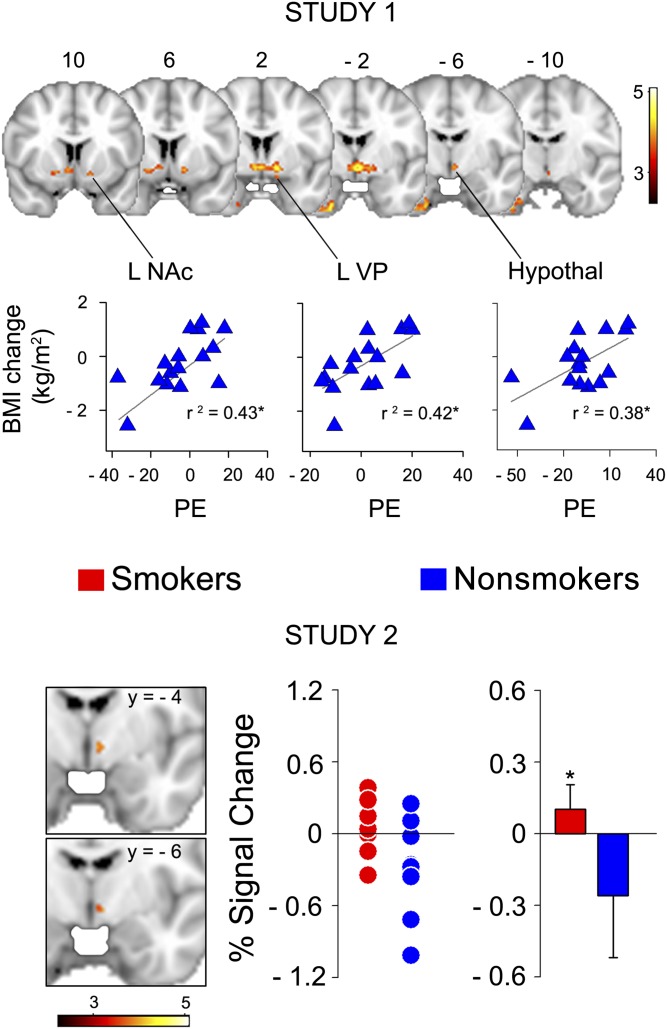
A main effect of the stimulus (milk shake minus tasteless solution; random-effects analysis; n = 27) was observed in the ventromedial prefrontal cortex, dorsomedial prefrontal cortex, anterior cingulate cortex, posterior cingulate cortex, anterior insula, inferior temporal gyrus, amygdala, hippocampus, thalamus, dorsal striatum (caudate and globus pallidus) and ventral striatum [nucleus accumbens (NAc)] in nonsmokers (see Figure 1 and Table S3 under “Supplemental data” in the online issue). We also identified a large cluster of voxels where the response to milk shake minus tasteless solution correlated positively with the change in BMI over a 1-y period. This cluster extended from the midbrain, which likely corresponded to the substantia nigra and ventral tegmental area, into the hypothalamus (bilaterally), right anterior thalamus, left ventral pallidum (VP), and left NAc (Figure 2, upper panel; Table 3). These associations were maintained, after the inclusion of age, sex, and initial BMI, as covariates of no interest. As noted, subjects varied in terms of BMI change, with weight lost in some subjects and weight gained in some subjects. This varied response was important because it suggested that the data described regions where smaller responses were related to weight loss and larger responses to weight gain.
FIGURE 2.
Upper panel: Study 1. Brain activity correlated with the change in BMI at 1 y in nonsmokers (n = 15; random effects; P < 0.05; whole-brain corrected). Scatter plots illustrate the correlation between average PE within all voxels falling in each region encompassed by the significant brain-activity cluster and change in BMI. The activity is depicted in red to yellow, which indicate a positive correlation. Numbers above the slices are y coordinates in millimeters of standard space. Lower panel: Study 2. Smokers had an increased brain response to milk shakes in the hypothalamus. The brain activity for milk shake minus tasteless solution (n = 11 per group; unpaired t test; random effects, P < 0.05 corrected over all voxels within the mask from study 1) in smokers compared with nonsmokers. Coronal sections depict a significant increase in activity for smokers compared with nonsmokers in the hypothalamus. The plots show the individual (left) and averaged (right) percentage signal changes for smokers (red) and nonsmokers (blue) within the hypothalamus (mean ± SEM: smokers: 4.05 ± 4.65; nonsmokers: −14.04 ± 5.23). *Statistical significance, P < 0.05. Hypothal, hypothalamus; L, left; NAc, nucleus accumbens; PE, parameter estimates; VP, ventral pallidum.
TABLE 3.
Brain activity for the main effects of a milk shake compared with a tasteless solution correlated with BMI change at 1 y in nonsmokers1
| MNI coordinates |
|||||
| Region: areas positively correlated to BMI change | x | y | z | z | Cluster |
| Right NAc | 6 | 8 | −6 | 3.00 | 1 |
| Left ventral pallidum | −14 | 2 | −8 | 3.95 | 1 |
| Right hypothalamus | −4 | −2 | −6 | 4.24 | 1 |
| Left anterior thalamus | −4 | −4 | −2 | 2.99 | 1 |
| Midbrain | −2 | −10 | −8 | 2.32 | 1 |
| Right entorhinal cortex | 32 | −10 | −40 | 3.46 | 2 |
Regression map contained the following 2 clusters (n = 15 nonsmokers): cluster 1 had 293 voxels (P = 0.0241), and cluster 2 had 294 voxels (P = 0.0236). MNI, Montreal Neurological Institute; NAc, nucleus accumbens.
Study 2: smokers compared with nonsmokers
To test our hypothesis that smoking is associated with altered the brain response in regions associated with weight gain in nonsmokers we compared the response to milk shake minus tasteless solution between smokers and nonsmokers within regions where the response correlated with the change in BMI in study 1. This was done by using the regression map from study 1 as a prethresholding mask in FMRIB Expert Analysis Tool. Compared with nonsmokers, smokers had a greater response in the hypothalamus (peak z = 3.03; at coordinates x = −6, y = −6, and z = −4; corrected P = 0.03; Figure 2, lower panel). The increased hypothalamic response in smokers did not change after the covarying out of potentially confounding factors revealed by the behavioral analyses (years of education, impulsivity scores, TFEQ hunger subscale scores, and milk shake intensity ratings). Because nonsmokers, but not smokers, rated the milk shake as more pleasant after scanning than before scanning, we tested for the presence of an association between this change and the differential response in the hypothalamus. There was no significant correlation between the difference in hypothalamic response and the difference in ratings of milk shake pleasantness. We also investigated the presence of any differential brain response to milk shakes compared with the tasteless solution in smokers compared with nonsmokers with a whole-brain analysis (ie, without any masking). No significant cluster survived the threshold.
Published data reported global differences in brain activity in smokers compared with nonsmokers (47). To determine whether global differences were present in our sample, we compared the global signal (44) between smokers and matched nonsmokers. No significant effect of group was observed (P = 0.17; repeated-measures ANOVA). A regression with carbon monoxide levels for smokers also revealed no significant association with whole-brain activity.
DISCUSSION
The aim of this study was to investigate whether smoking status modulates brain activity in response to milk shakes in brain areas associated with long-term weight change. We showed that the response in the midbrain, hypothalamus, thalamus, and ventral striatum (NAc and VP) was positively correlated with weight change (Figure 2, upper panel). We also showed that smokers had a significantly greater response to milk shakes in the hypothalamus (Figure 2, lower panel). This effect of smoking status was still present after accounting for differences in subject characteristics or behavioral measures. Thus, our findings were consistent with our prediction that the brain response to a palatable and energy-dense food differs in smokers than in nonsmokers.
The hypothalamus is an intricate hub of neuroendocrine, homeostatic, and appetite regulation (48). Human fMRI studies have shown that brain activity in the hypothalamus is attenuated by feeding (49, 50) and the administration of sibutramine, which is a drug used to reduce weight in obese individuals (51). The response to food pictures in the hypothalamus is also positively associated with immediate food intake in a nonsated state (52). Thus, the relation between feeding and the blood oxygen level–dependent hypothalamic response has been established. Consistent with these previous observations, we showed a positive association between the hypothalamic response to milk shakes and weight change. This suggested that heightened hypothalamic responses to food in individuals in a nonsated state may be associated with weight-gain susceptibility.
We also identified positive associations with weight change in the midbrain, ventral striatum (NAc and VP), and thalamus. Therefore, a possibility is that the hypothalamic association with weight change reflects the ability of these reward circuits to impact hypothalamic processing and, thereby, influence intake. Both the NAc and VP are reciprocally connected to the midbrain and project to the hypothalamus (53). The electrical or chemical stimulation of particular locations within the VP enhances incentive motivation or palatability (54–56). Similarly, the thalamus and midbrain have been shown to be critical in enabling conditioned cues that predict rewards like food to influence action (57–60), with the latter serving a more general role in action initiation on the basis of reward probability (61) and the former allocating attention toward predictive cues such as food odors (38). In addition, altered responses in this circuit have been associated with obesity and eating disorders. Obese women show a heightened brain response to food cues in the dorsal and ventral striatum (62, 63), and patients with eating disorders have altered gray-matter density in the hypothalamus (64) and altered brain responses in the hypothalamus (65) and dorsal striatum (66).
Previous studies have also reported an association between weight change and the response to food receipt or food cues. Murdaugh et al (31) reported that the NAc response to food cues predicted weight change at 3 mo, whereas the midbrain response to food cues predicted weight change at 9 mo. Yokum et al (30) and Stice et al (32, 33, 67) reported an inverse relation between the response in and structure of the dorsal striatum and BMI. However, to our knowledge, no associations have been reported between the brain response to food receipt and weight change in any of the regions identified in the current study. There are several possible reasons for the discrepant results. First, Murdaugh et al (31) examined the brain response to food cues but not to food receipt. This distinction may be critical because separable circuits have been shown to respond to food cues compared with food receipt (38). Second, the inverse relation between weight gain and dorsal striatal response to food receipt may be dependent on whether subjects possess a copy at the A1 allele of the DRD2/ANKK1 gene locus (32, 67). In addition, although our subjects were not hungry or full, the earlier studies were performed on fasted individuals. Because the hypothalamus and reward circuits identified in the current study are influenced by satiety, it is possible that ceiling effects accounted for the lack of association in fasted subjects.
After we identified regions where the response to milk shakes was associated with weight change, we asked whether smoking status moderated these responses. As predicted, we showed that smoking status was associated with a differential response in the hypothalamus, which is a region that is significantly correlated with weight change in nonsmokers. More specifically, the response to milk shake minus tasteless solution was greater in smokers than in nonsmokers. These results were consistent with work that indicated a critical role for hypothalamic circuits in food reward and weight gain (17, 68). Nicotine binds to specific cholinergic receptors on proopiomelanocortin neurons of the arcuate nucleus, which then project to the paraventricular nucleus where they activate the melanocortin-4 receptors that are critical for food intake and energy expenditure. Knocking down either the acetylcholine receptor on the proopiomelanocortin neurons or melanocortin-4 receptors in the paraventricular nucleus disrupts nicotine effects on feeding (20). Thus, our results are in agreement with other work that indicated that smoking influences the hypothalamic response to food.
What remains unclear is how to interpret the directions of the effects observed in our experiments. Because the response in the hypothalamus is positively associated with weight change in nonsmokers, and nicotine has an anorectic effect, it would be more intuitive if smokers showed a reduced response in the hypothalamus. Instead, we observed an increased response. One possible interpretation of this pattern of results is that hypothalamic responses from studies 1 and 2 reflected different populations of neurons, with the response related to weight gain in nonsmokers reflecting activity of a circuit promoting consumption and the response difference between smokers and nonsmokers reflecting a circuit that inhibits food consumption. An alternative possibility is that the responses reflected the activity of the same circuit. Indeed, previous work has shown that acute or chronic nicotine exposure enhances responses to food cues in animal models (21, 69–72). If this effect is true, an important concern to address in future research is how the ability of nicotine to enhance responses to food is associated with the anorectic effects of this substance.
One caveat is that, because there is clear evidence that the brain response to food varies as a function of BMI (73, 74), and our sample of nonsmokers was primarily overweight, we could not rule out the possibility that responses associated with BMI change might differ in a homogeneous sample of lean, overweight, or obese individuals. However, the average BMI of our sample was reflective of the general population (75). In addition, effects may have been related to weight loss as well as weight gain because our sample consisted of a distribution of people who lost or gained weight. Also, some participants had a very small change in BMI after 1 y, which could have reflected day-to-day variations in weight or that follow-up measurements at 1 y were not necessarily performed at the same time of the day as at time point one. However, the variation of weight change that we observed agrees with previously published longitudinal naturalistic studies of BMI change (31–33, 76, 77).
In conclusion, to our knowledge, we provide the first evidence in humans that smokers have a differential brain response to a palatable and energy-dense food. Compared with nonsmokers, smokers, who were not hungry or full and who had recently smoked, had an increased response in the hypothalamus during the ingestion of a milk shake. This result accords with other recent work in suggesting that it is the influence of nicotine on hypothalamic circuits that may be associated with the link between smoking and body weight.
Supplementary Material
Acknowledgments
David Kessler played no role in the design, conduct, analysis, or interpretation of the study. However, he developed a questionnaire, which we administered to our subjects.
The authors’ responsibilities were as follows—PYG: helped analyze and interpret data and draft the manuscript; KA and JF: helped design the study and collect data; SSO: helped design the study and critically revise the manuscript; and DMS: helped design the study, analyze and interpret data, and draft the manuscript. SSO is a member of the American College of Neuropsychopharmacogy workgroup, the Alcohol Clinical Trial Initiative, sponsored by Alkermes, Abbott Laboratories, Eli Lilly & Company, GlaxoSmithKline, Johnson & Johnson Pharmaceuticals, Lundbeck, Pfizer, and Schering Plough; is a partner at Applied Behavioral Research; has received medication supplies from Pfizer for an investigator-initiated study; has received a contract as principal investigator on a multisite study sponsored by NABI Biopharmaceuticals; is on the advisory board of Gilead Pharmaceuticals; is a consultant at Pfizer, Alkermes, GlaxoSmithKline, Brown University, and the University of Chicago; and is on the scientific panel of advisors of the Hazelden Foundation. PYG, KA, JF, and DMS had no conflicts of interest.
Footnotes
Abbreviations used: fMRI, functional MRI; FMRIB, Oxford Center for functional MRI of the brain; NAc, nucleus accumbens; TFEQ, Three-Factor Eating Questionnaire; VAS, visual analog scale; VP, ventral pallidum.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC) Smoking-attributable mortality, years of potential life lost, and productivity losses–United States, 2000-2004. MMWR Morb Mortal Wkly Rep 2008;57:1226–8 [PubMed] [Google Scholar]
- 2.Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obes Rev 2004;5:95–103 [DOI] [PubMed] [Google Scholar]
- 3.Klesges RC, Meyers AW, Klesges LM, La Vasque ME. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychol Bull 1989;106:204–30 [DOI] [PubMed] [Google Scholar]
- 4.Lopez EN, Drobes DJ, Thompson JK, Brandon TH. Effects of a body image challenge on smoking motivation among college females. Health Psychol 2008;27:S243–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pomerleau CS, Kurth CL. Willingness of female smokers to tolerate postcessation weight gain. J Subst Abuse 1996;8:371–8 [DOI] [PubMed] [Google Scholar]
- 6.Rahmanian SD, Diaz PT, Wewers ME. Tobacco use and cessation among women: research and treatment-related issues. J Womens Health (Larchmt) 2011;20:349–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swan GE, Carmelli D. Characteristics associated with excessive weight gain after smoking cessation in men. Am J Public Health 1995;85:73–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward KD, Klesges RC, Zbikowski SM, Bliss RE, Garvey AJ. Gender differences in the outcome of an unaided smoking cessation attempt. Addict Behav 1997;22:521–33 [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Hansen MJ, Jones JE, Vlahos R, Bozinovski S, Anderson GP, Morris MJ. Cigarette smoke exposure reprograms the hypothalamic neuropeptide Y axis to promote weight loss. Am J Respir Crit Care Med 2006;173:1248–54 [DOI] [PubMed] [Google Scholar]
- 10.Bellinger LL, Wellman PJ, Harris RB, Kelso EW, Kramer PR. The effects of chronic nicotine on meal patterns, food intake, metabolism and body weight of male rats. Pharmacol Biochem Behav 2010;95:92–9 [DOI] [PubMed] [Google Scholar]
- 11.Miyata G, Meguid MM, Varma M, Fetissov SO, Kim HJ. Nicotine alters the usual reciprocity between meal size and meal number in female rat. Physiol Behav 2001;74:169–76 [DOI] [PubMed] [Google Scholar]
- 12.Perkins KA. Effects of tobacco smoking on caloric intake. Br J Addict 1992;87:193–205 [DOI] [PubMed] [Google Scholar]
- 13.Grunberg NE. The effects of nicotine and cigarette smoking on food consumption and taste preferences. Addict Behav 1982;7:317–31 [DOI] [PubMed] [Google Scholar]
- 14.Miyata G, Meguid MM, Fetissov SO, Torelli GF, Kim HJ. Nicotine's effect on hypothalamic neurotransmitters and appetite regulation. Surgery 1999;126:255–63 [PubMed] [Google Scholar]
- 15.Perkins KA, Epstein LH, Stiller RL, Fernstrom MH, Sexton JE, Jacob RG. Perception and hedonics of sweet and fat taste in smokers and nonsmokers following nicotine intake. Pharmacol Biochem Behav 1990;35:671–6 [DOI] [PubMed] [Google Scholar]
- 16.Stamford BA, Matter S, Fell RD, Papanek P. Effects of smoking cessation on weight gain, metabolic rate, caloric consumption, and blood lipids. Am J Clin Nutr 1986;43:486–94 [DOI] [PubMed] [Google Scholar]
- 17.Kramer PR, Guan G, Wellman PJ, Bellinger LL. Nicotine's attenuation of body weight involves the perifornical hypothalamus. Life Sci 2007;81:500–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li MD, Kane JK, Parker SL, McAllen K, Matta SG, Sharp BM. Nicotine administration enhances NPY expression in the rat hypothalamus. Brain Res 2000;867:157–64 [DOI] [PubMed] [Google Scholar]
- 19.Yu G, Chen H, Zhao W, Matta SG, Sharp BM. Nicotine self-administration differentially regulates hypothalamic corticotropin-releasing factor and arginine vasopressin mRNAs and facilitates stress-induced neuronal activation. J Neurosci 2008;28:2773–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gundisch D, Diano S, De BM, Horvath TL, Gao XB, et al. Nicotine decreases food intake through activation of POMC neurons. Science 2011;332:1330–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donny EC, Caggiula AR, Weaver MT, Levin ME, Sved AF. The reinforcement-enhancing effects of nicotine: implications for the relationship between smoking, eating and weight. Physiol Behav 2011;104:143–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 2000;157:1789–98 [DOI] [PubMed] [Google Scholar]
- 23.Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry 2007;164:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage 2007;35:787–94 [DOI] [PubMed] [Google Scholar]
- 25.Martin-Soelch C, Missimer J, Leenders KL, Schultz W. Neural activity related to the processing of increasing monetary reward in smokers and nonsmokers. Eur J Neurosci 2003;18:680–8 [DOI] [PubMed] [Google Scholar]
- 26.Martin-Sölch C, Magyar S, Kunig G, Missimer J, Schultz W, Leenders KL. Changes in brain activation associated with reward processing in smokers and nonsmokers. A positron emission tomography study. Exp Brain Res 2001;139:278–86 [DOI] [PubMed] [Google Scholar]
- 27.Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: studies of gene activation in corticolimbic regions. Physiol Behav 2005;86:11–4 [DOI] [PubMed] [Google Scholar]
- 28.Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience 2001;105:535–45 [DOI] [PubMed] [Google Scholar]
- 29.Spring B, Pagoto S, McChargue D, Hedeker D, Werth J. Altered reward value of carbohydrate snacks for female smokers withdrawn from nicotine. Pharmacol Biochem Behav 2003;76:351–60 [DOI] [PubMed] [Google Scholar]
- 30.Yokum S, Ng J, Stice E. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. Int J Obes (Lond) 2012;36:656–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murdaugh DL, Cox JE, Cook EW, III, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage 2012;59:2709–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 2008;322:449–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci 2010;30:13105–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cloninger CR, Bayon C, Svrakic DM. Measurement of temperament and character in mood disorders: a model of fundamental states as personality types. J Affect Disord 1998;51:21–32 [DOI] [PubMed] [Google Scholar]
- 35.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol 1995;51:768–74 [DOI] [PubMed] [Google Scholar]
- 36.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985;29:71–83 [DOI] [PubMed] [Google Scholar]
- 37.Bernaards CM, Twisk JW, Snel J, Van MW, Kemper HC. Is calculating pack-years retrospectively a valid method to estimate life-time tobacco smoking? A comparison between prospectively calculated pack-years and retrospectively calculated pack-years. Addiction 2001;96:1653–61 [DOI] [PubMed] [Google Scholar]
- 38.Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron 2008;57:786–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veldhuizen MG, Bender G, Constable RT, Small DM. Trying to detect taste in a tasteless solution: modulation of early gustatory cortex by attention to taste. Chem Senses 2007;32:569–81 [DOI] [PubMed] [Google Scholar]
- 40.Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses 1996;21:323–34 [DOI] [PubMed] [Google Scholar]
- 41.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister P, De Luca CJ, Drobnjak I, Flitney DE. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(suppl 1):208–19 [DOI] [PubMed] [Google Scholar]
- 42.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 2001;14:1370–86 [DOI] [PubMed] [Google Scholar]
- 43.Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 2004;21:1732–47 [DOI] [PubMed] [Google Scholar]
- 44.Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 2009;101:3270–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RS. Statistic parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 1994;2:189–210 [Google Scholar]
- 46.Worsley KJ. Oxford University Press, 2001:251–70 [Google Scholar]
- 47.Domino EF, Minoshima S, Guthrie S, Ohl L, Ni L, Koeppe RA, Zubieta JK. Nicotine effects on regional cerebral blood flow in awake, resting tobacco smokers. Synapse 2000;38:313–21 [DOI] [PubMed] [Google Scholar]
- 48.Kalra SP, Kalra PS. Neuroendocrine control of energy homeostasis: update on new insights. Prog Brain Res 2010;181:17–33 [DOI] [PubMed] [Google Scholar]
- 49.Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J, DeFronzo RA, Fox PT, Gao JH. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 1999;48:1801–6 [DOI] [PubMed] [Google Scholar]
- 50.Smeets PA, Vidarsdottir S, de Graaf C, Stafleu A, van Osch MJ, Viergever MA, Pijl H, van der Grond J. Oral glucose intake inhibits hypothalamic neuronal activity more effectively than glucose infusion. Am J Physiol Endocrinol Metab 2007;293:E754–8 [DOI] [PubMed] [Google Scholar]
- 51.Fletcher PC, Napolitano A, Skeggs A, Miller SR, Delafont B, Cambridge VC, de Wit S, Nathan PJ, Brooke A, O'Rahilly S, et al Distinct modulatory effects of satiety and sibutramine on brain responses to food images in humans: a double dissociation across hypothalamus, amygdala, and ventral striatum. J Neurosci 2010;30:14346–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 2007;450:106–9 [DOI] [PubMed] [Google Scholar]
- 53.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010;35:4–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci 2005;25:8637–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res 2009;196:155–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taha SA, Katsuura Y, Noorvash D, Seroussi A, Fields HL. Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neuroscience 2009;161:718–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corbit LH, Muir JL, Balleine BW. Lesions of mediodorsal thalamus and anterior thalamic nuclei produce dissociable effects on instrumental conditioning in rats. Eur J Neurosci 2003;18:1286–94 [DOI] [PubMed] [Google Scholar]
- 58.Corbit LH, Janak PH, Balleine BW. General and outcome-specific forms of Pavlovian-instrumental transfer: the effect of shifts in motivational state and inactivation of the ventral tegmental area. Eur J Neurosci 2007;26:3141–9 [DOI] [PubMed] [Google Scholar]
- 59.Murschall A, Hauber W. Inactivation of the ventral tegmental area abolished the general excitatory influence of Pavlovian cues on instrumental performance. Learn Mem 2006;13:123–6 [DOI] [PubMed] [Google Scholar]
- 60.Ostlund SB, Balleine BW. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J Neurosci 2008;28:4398–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct 2010;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 2007;37:410–21 [DOI] [PubMed] [Google Scholar]
- 63.Stoeckel LE, Weller RE, Cook EW, III, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008;41:636–47 [DOI] [PubMed] [Google Scholar]
- 64.Boghi A, Sterpone S, Sales S, D'Agata F, Bradac GB, Zullo G, Munno D. In vivo evidence of global and focal brain alterations in anorexia nervosa. Psychiatry Res 2011;192:154–9 [DOI] [PubMed] [Google Scholar]
- 65.Lock J, Garrett A, Beenhakker J, Reiss AL. Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. Am J Psychiatry 2011;168:55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brooks SJ, O'Daly OG, Uher R, Friederich HC, Giampietro V, Brammer M, Williams SC, Schioth HB, Treasure J, Campbell IC. Differential neural responses to food images in women with bulimia versus anorexia nervosa. PLoS ONE 2011;6:e22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage 2010;50:1618–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jo YH, Talmage DA, Role LW. Nicotinic receptor-mediated effects on appetite and food intake. J Neurobiol 2002;53:618–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76 [DOI] [PubMed] [Google Scholar]
- 70.Perkins KA, Epstein LH, Sexton JE, Solberg-Kassel R, Stiller RL, Jacob RG. Effects of nicotine on hunger and eating in male and female smokers. Psychopharmacology (Berl) 1992;106:53–9 [DOI] [PubMed] [Google Scholar]
- 71.Popke EJ, Mayorga AJ, Fogle CM, Paule MG. Effects of acute nicotine on several operant behaviors in rats. Pharmacol Biochem Behav 2000;65:247–54 [DOI] [PubMed] [Google Scholar]
- 72.Raiff BR, Dallery J. Effects of acute and chronic nicotine on responses maintained by primary and conditioned reinforcers in rats. Exp Clin Psychopharmacol 2006;14:296–305 [DOI] [PubMed] [Google Scholar]
- 73.Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 2008;42:1537–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet 2001;357:354–7 [DOI] [PubMed] [Google Scholar]
- 75.Ford ES, Li C, Zhao G, Tsai J. Trends in obesity and abdominal obesity among adults in the United States from 1999-2008. Int J Obes (Lond) 2011;35:736–43 [DOI] [PubMed] [Google Scholar]
- 76.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011;377:557–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johansson I, Nilsson L, Stegmayr B, Boman K, Hallmans G, Winkvist A. Associations among 25-year trends in diet, cholesterol and BMI from 140,000 observations in men and women in Northern Sweden. Nutr J 2012;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.