Abstract
Background: Consumption of caffeinated beverages such as coffee and tea has been associated with a lower risk of type 2 diabetes (T2D). Paradoxically, short-term metabolic studies have shown that caffeine impairs postprandial glycemic control.
Objective: The objective was to prospectively examine the association of caffeinated compared with caffeine-free beverages, including coffee, tea, sugar-sweetened beverages (SSBs), and carbonated artificially sweetened beverages (ASBs), with T2D risk.
Design: We prospectively observed 74,749 women from the Nurses’ Health Study (NHS, 1984–2008) and 39,059 men from the Health Professionals Follow-Up Study (HPFS, 1986–2008) who were free of diabetes, cardiovascular diseases, and cancer at baseline.
Results: We documented 7370 incident cases of T2D during 24 y of follow-up in the NHS and 2865 new cases during 22 y of follow-up in the HPFS. After major lifestyle and dietary risk factors were controlled for, caffeinated and caffeine-free SSB intake was significantly associated with a higher risk of T2D in the NHS (RR per serving: 13% for caffeinated SSBs, 11% for caffeine-free SSBs; P < 0.05) and in the HPFS (RR per serving: 16% for caffeinated SSBs, 23% for caffeine-free SSBs; P < 0.01). Only caffeine-free ASB intake in NHS participants was associated with a higher risk of T2D (RR: 6% per serving; P < 0.001). Conversely, the consumption of caffeinated and decaffeinated coffee was associated with a lower risk of T2D [RR per serving: 8% for both caffeinated and decaffeinated coffee in the NHS (P < 0.0001) and 4% for caffeinated and 7% for decaffeinated coffee in the HPFS (P < 0.01)]. Only caffeinated tea was associated with a lower T2D risk among NHS participants (RR per serving: 5%; P < 0.0001).
Conclusion: Irrespective of the caffeine content, SSB intake was associated with a higher risk of T2D, and coffee intake was associated with a lower risk of T2D.
INTRODUCTION
Diabetes is a major public health problem with increasing prevalence in the United States and worldwide. An estimated 25.6 million, or 11.3%, of US adults have type 2 diabetes (T2D)4. Another 79 million Americans have prediabetes—a condition that precedes the onset of T2D (1). Given the high burden of disease and the associated costs, prevention through dietary or other approaches is crucial.
Several epidemiologic studies have identified an inverse association between habitual coffee and tea consumption, major sources of caffeine, and T2D (2). Paradoxically, results from short-term metabolic studies have shown that caffeine increases blood glucose concentrations and decreases insulin sensitivity (3–5). Likewise, consumption of carbohydrates along with caffeine or caffeinated coffee was found to impair postprandial blood glucose homeostasis, which suggests a possible synergistic effect between caffeine and carbohydrates on T2D risk (6, 7). Caffeinated sugar-sweetened beverages (SSBs) are important sources of caffeine and carbohydrates. Although there is substantial evidence of an increased risk of T2D with SSB consumption (8), little is known about the difference between the effect of caffeinated and caffeine-free SSB and artificially sweetened beverage (ASB) intake on T2D risk. Therefore, we aimed to prospectively examine the association of caffeinated and caffeine-free forms of SSB and ASB intakes with T2D risk.
We previously reported on the association of caffeinated and decaffeinated coffee and risk of T2D (9). In this updated analysis with longer follow-up, we evaluated the association of both caffeinated and decaffeinated coffee and tea consumption on the risk of T2D. In addition, we investigated the joint effects of caffeine and SSBs and caffeine and coffee on risk of T2D. We also estimated the effects of substituting one serving of caffeinated carbonated beverages with other beverage sources of caffeine on T2D risk.
SUBJECTS AND METHODS
Study population
The Nurses’ Health Study (NHS) was initiated in 1976 as a prospective cohort study of 121,701 female registered nurses aged 30–55 y from 11 US states. The Health Professionals Follow-Up Study (HPFS) is a prospective cohort study of 51,529 male health professionals aged 40–75 y from all 50 states that began in 1986. In both cohorts, participants were followed biennially through validated questionnaires that obtained updated information on their medical history, lifestyle factors, and occurrence of chronic diseases.
For the current investigation, we excluded participants with a baseline history of diabetes, cardiovascular disease, or cancer because these diagnoses may result in changes in diet (10). We excluded women who left ≥10 items blank on the food-frequency questionnaire (FFQ) or who had implausible energy intakes (<500 or >3500 kcal/d). Men who left ≥70 items blank on the FFQ or who reported daily caloric intake outside the plausible range of 800 to 4200 kcal were also excluded. The final analyses included 74,749 women and 39,059 men with complete information. The study was approved by the Human Research Committee of Brigham and Women's Hospital in Boston.
Assessment of beverage intake
In 1984, a 116-item FFQ was administered to NHS participants to obtain information on usual intake of food and beverages. Beginning in 1986, an expanded 131-item FFQ was sent to NHS participants to update their diet every 4 y. By means of the expanded FFQ used in the NHS, usual dietary intakes were collected from HPFS participants every 4 y from 1986 through 2006. In all FFQs, the participants were asked how often on average (never to ≥6 times/d) during the previous year they had consumed caffeinated and decaffeinated coffee (“one cup”), tea (“one cup or glass”), and different types of sugar-sweetened and artificially sweetened carbonated beverages (“one glass, bottle, or can”). Carbonated beverages included caffeinated and caffeine-free colas and carbonated soft drinks. Decaffeinated tea consumption was first ascertained in 1998 in both cohorts. Nutrient and caffeine intakes were computed by multiplying the frequency of consumption of each food or beverage by the nutrient content of the specified portion and summing the contributions from all items. Nutrient intakes were obtained by using the USDA food-composition database supplemented with other sources. We estimated that the caffeine content was 137 mg per cup of coffee, 47 mg per cup of tea, and 46 mg per bottle or can of cola beverage. The validity and reproducibility of the FFQ has been described in detail elsewhere (11–14). In a validation study conducted among a subsample of NHS participants, FFQ assessments of coffee, tea, and caffeinated carbonated beverage intakes were highly correlated with diet record assessments (coffee, r = 0.78; tea, r = 0.93; caffeinated carbonated beverages, r = 0.84), whereas noncaffeinated carbonated beverage intake was moderately correlated (r = 0.36) (13). Similar correlation coefficients were found in a validation study conducted among a subsample of HPFS participants (11).
Assessment of T2D
Participants with self-reported diagnoses of diabetes were mailed a supplementary questionnaire regarding symptoms, diagnostic tests, and hypoglycemic therapy. Cases before 1998 were defined by using the National Diabetes Data Group criteria (15). The American Diabetes Association criteria (16) were used for cases after 1998. The validity of the supplementary questionnaire has been established in 2 previous studies through medical record reviews. In both studies, diagnosis of T2D was confirmed in >98% of the cases (17, 18).
Assessment of covariates
In the biennial follow-up questionnaires, we updated information on a participant's age, weight, smoking status, menopausal status, and use of postmenopausal hormone therapy (for women) and personal history of chronic diseases. Height was ascertained with the 1976 enrollment questionnaire in the NHS and with the 1986 enrollment questionnaire in the HPFS. Among women, the presence or absence of a family history of diabetes (in first-degree relatives) was assessed in 1982 and 1988. Among men, this was assessed in 1987. In both cohorts, adherence to a low-calorie diet was assessed in 1992. Reported 5-y weight change was obtained in 1986 for men. Among women, we calculated weight change between 1980 and 1984 from self-reported weights. An overall measure of diet quality was calculated by using the Alternate Healthy Eating Index (19).
Statistical analysis
We calculated each individual's person-time from the date of return of the baseline questionnaire (1984 for NHS, 1986 for HPFS) to the date of diagnosis of T2D, death, or the end of follow-up (30 June 2008 for NHS, 1 January 2008 for HPFS), whichever came first. We used Cox proportional hazards regression models to examine the association between caffeinated and caffeine-free beverage consumption and risk of T2D. The regression models included age in years as the time scale, stratified by calendar time in 2-y intervals, and allowed for a possible interaction between calendar time and age in the baseline hazards to be accounted for nonparametrically (model 1). In multivariable analysis, we further adjusted for smoking status, postmenopausal hormone use (women only), physical activity, family history of diabetes, a measure of overall diet quality (quintiles of the Alternate Healthy Eating Index), and consumption of other beverages (other than the main exposure, depending on the model), including caffeinated and decaffeinated coffee, caffeinated tea, fruit punch, SSBs, and ASBs (model 2). In this model, we also adjusted for a history of hypertension and hypercholesterolemia, pre-enrollment weight change, and adherence to a low-calorie diet to control for changes to overall health and diabetes risk associated with dieting and also possible changes in beverage intakes. To assess whether BMI and total energy intake are potential mediators or confounders of the association between beverage intake and T2D, we included these variables in our final model (model 3). For each 2-y time period, we updated information on all nondietary covariates to account for changes in risk factors over time. For dietary covariates and the main exposures (beverage intake), we used the cumulative average of food intakes from baseline to the censoring events to best represent long-term diet and minimize within-person variation (10). However, we stopped updating dietary variables on a report of cancer or cardiovascular disease because changes in diet after development of these conditions may confound the relation between diet and diabetes (10). In sensitivity analyses, however, we also examined cumulative average intake that was continually updated even after the development of these outcomes.
To evaluate potential synergistic effects of caffeine and beverage consumption on T2D risk, we cross-classified categories of coffee (<1 cup/d, 1–3 cups/d, >3 cups/d) and SSB (≤1/wk, 2–6/wk, ≥1/d) intake against quintiles of caffeine intake (quintile 1 + quintile 2, quintile 3, quintile 4 + quintile 5). We then evaluated the effect of higher caffeine consumption in each category of coffee and SSB consumption on risk of T2D. The proportional hazards assumption was tested by including an interaction term between the main exposures and months to events (P > 0.05 for all tests). We tested for potential effect modification by smoking status, BMI, and physical activity by including cross-product terms with the exposure variables in our fully adjusted model. To estimate the effect of substituting one serving of caffeinated beverage for another, we included caffeinated coffee, tea, and carbonated beverages as continuous variables in the same multivariable model. We used the difference between the regression coefficients, variances, and covariance to calculate the RRs and the 95% CIs for the substitution effect (10).
Tests for linear trend were conducted by assigning the median value to each quintile or category and treating this as a continuous variable in the regression model. All analyses were performed separately in each cohort because of differences in sex and questionnaires administered. All statistical tests were 2-sided and performed by using SAS version 9.2 for UNIX (SAS Institute Inc).
RESULTS
Baseline characteristics
Characteristics of NHS participants according to consumption of caffeinated and caffeine-free coffee and carbonated beverages are presented in Table 1. Higher caffeinated and caffeine-free carbonated beverage consumption was associated with a lower age, higher BMI, and higher pre-enrollment weight changes. In women, those with a higher consumption of caffeinated, but not caffeine-free, carbonated beverages were more likely to be current smokers. Higher intake of caffeine-free carbonated beverages was associated with a higher alcohol intake and a higher Alternate Healthy Eating Index score. Consumption of caffeinated and decaffeinated coffee was strongly associated with current smoking. Caffeinated coffee consumption was associated with higher alcohol intake and higher high-fat dairy intake.
TABLE 1.
Age-standardized baseline (1984) characteristics according to categories of caffeinated and caffeine-free beverage consumption in 74,749 women1
| Caffeinated carbonated beverage |
Caffeine-free carbonated beverage |
Caffeinated coffee |
Decaffeinated coffee |
|||||||||
| <2/wk | 2–6/wk | ≥1/d | <2/wk | 2–6/wk | ≥1/d | <1/d | 1–3/d | ≥4/d | <1/d | 1–3/d | ≥4/d | |
| Participants (n) | 50,797 | 13,737 | 10,215 | 40,856 | 19,251 | 14,642 | 29,221 | 33,182 | 12,346 | 54,998 | 16,967 | 2784 |
| Age (y) | 51.2 ± 7.12 | 48.7 ± 7.0 | 47.7 ± 6.8 | 51.0 ± 7.2 | 49.7 ± 7.1 | 49.1 ± 7.0 | 50.1 ± 7.4 | 50.6 ± 7.1 | 49.9 ± 6.8 | 50.0 ± 7.2 | 51.3 ± 7.1 | 50.9 ± 6.8 |
| BMI (kg/m2) | 24.5 ± 4.3 | 25.3 ± 4.8 | 26.0 ± 5.2 | 24.3 ± 4.4 | 25.2 ± 4.6 | 25.8 ± 4.9 | 25.1 ± 4.8 | 24.8 ± 4.5 | 24.5 ± 4.2 | 24.9 ± 4.7 | 24.7 ± 4.3 | 24.7 ± 4.2 |
| Pre-enrollment weight gain (kg)3 | 4.9 ± 4.0 | 5.2 ± 4.0 | 5.7 ± 4.2 | 4.8 ± 4.0 | 5.1 ± 4.0 | 5.6 ± 4.1 | 5.1 ± 4.1 | 5.0 ± 4.0 | 5.2 ± 4.0 | 5.1 ± 4.0 | 4.9 ± 4.0 | 5.2 ± 4.0 |
| Pre-enrollment weight loss (kg)3 | 3.7 ± 2.4 | 3.9 ± 2.4 | 4.1 ± 2.4 | 3.6 ± 2.4 | 3.8 ± 2.4 | 4.1 ± 2.4 | 3.8 ± 2.4 | 3.8 ± 2.4 | 3.7 ± 2.4 | 3.8 ± 2.4 | 3.8 ± 2.4 | 3.9 ± 2.4 |
| Current smoker (%) | 23 | 24 | 28 | 27 | 20 | 23 | 16 | 24 | 46 | 25 | 20 | 33 |
| Physical activity (MET-h4/wk) | 14.6 ± 21.6 | 13.4 ± 19.6 | 13.2 ± 18.6 | 13.9 ± 20.9 | 14.3 ± 20.9 | 15.0 ± 20.8 | 14.8 ± 20.4 | 14.3 ± 21.4 | 12.7 ± 20.4 | 13.8 ± 20.6 | 15.3 ± 21.3 | 15.4 ± 22.4 |
| Postmenopausal hormone use (%) | 25 | 24 | 24 | 24 | 25 | 24 | 26 | 24 | 20 | 24 | 25 | 24 |
| Family history of diabetes (%) | 28 | 29 | 29 | 27 | 29 | 30 | 29 | 28 | 28 | 28 | 29 | 30 |
| History of hypertension (%) | 18 | 20 | 21 | 17 | 19 | 22 | 21 | 19 | 13 | 18 | 20 | 18 |
| History of hypercholesterolemia (%) | 7 | 7 | 8 | 7 | 7 | 8 | 8 | 7 | 6 | 7 | 8 | 8 |
| Total energy intake (kcal/d) | 1706 ± 515 | 1812 ± 534 | 1834 ± 567 | 1701 ± 513 | 1789 ± 530 | 1802 ± 561 | 1713 ± 532 | 1752 ± 521 | 1790 ± 541 | 1735 ± 529 | 1765 ± 526 | 1775 ± 548 |
| Caffeine intake (mg/d) | 301 ± 235 | 338 ± 222 | 372 ± 228 | 336 ± 242 | 298 ± 219 | 296 ± 224 | 118 ± 93 | 346 ± 116 | 713 ± 142 | 348 ± 242 | 230 ± 182 | 274 ± 198 |
| Alcohol consumption (g/d) | 7.1 ± 11.4 | 6.9 ± 10.9 | 6.9 ± 11.5 | 6.9 ± 11.4 | 6.7 ± 10.6 | 7.7 ± 12.0 | 5.4 ± 10.1 | 8.2 ± 11.7 | 7.9 ± 12.4 | 7.1 ± 11.6 | 6.8 ± 10.6 | 7.2 ± 11.7 |
| Alternate Healthy Eating Index | 38.5 ± 10.6 | 37.5 ± 10.1 | 36.9 ± 10.2 | 37.5 ± 10.6 | 38.6 ± 10.1 | 39.0 ± 10.4 | 38.2 ± 10.6 | 38.5 ± 10.4 | 36.6 ± 10.1 | 37.6 ± 10.4 | 39.6 ± 10.4 | 38.4 ± 10.4 |
| Low-fat dairy intake (servings/d) | 0.9 ± 1.0 | 0.8 ± 0.9 | 0.8 ± 0.9 | 0.9 ± 0.9 | 0.9 ± 0.9 | 0.9 ± 1.0 | 1.0 ± 1.0 | 0.9 ± 0.9 | 0.8 ± 0.9 | 0.9 ± 0.9 | 1.0 ± 1.0 | 1.0 ± 1.0 |
| High-fat dairy intake (servings/d) | 1.4 ± 1.4 | 1.5 ± 1.4 | 1.5 ± 1.4 | 1.5 ± 1.4 | 1.4 ± 1.3 | 1.4 ± 1.3 | 1.3 ± 1.2 | 1.5 ± 1.4 | 1.7 ± 1.7 | 1.5 ± 1.4 | 1.3 ± 1.2 | 1.4 ± 1.4 |
| Artificially sweetened beverages (servings/d) | 0.3 ± 0.7 | 0.6 ± 0.7 | 1.8 ± 1.7 | 0.2 ± 0.6 | 0.5 ± 0.6 | 1.7 ± 1.5 | 0.6 ± 1.1 | 0.6 ± 1.0 | 0.5 ± 1.0 | 0.6 ± 1.0 | 0.7 ± 1.0 | 0.7 ± 1.1 |
| Sugar-sweetened beverages (servings/d) | 0.1 ± 0.3 | 0.3 ± 0.4 | 0.5 ± 1.0 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.3 ± 0.7 | 0.2 ± 0.6 | 0.2 ± 0.5 | 0.2 ± 0.4 | 0.2 ± 0.5 | 0.2 ± 0.4 | 0.1 ± 0.4 |
| Caffeinated tea (servings/d) | 0.7 ± 1.2 | 0.7 ± 1.1 | 0.7 ± 1.1 | 0.7 ± 1.2 | 0.7 ± 1.1 | 0.6 ± 1.0 | 0.9 ± 1.4 | 0.6 ± 0.9 | 0.5 ± 0.9 | 0.7 ± 1.2 | 0.6 ± 0.9 | 0.5 ± 1.0 |
| Caffeinated coffee (servings/d) | 1.8 ± 1.9 | 2.0 ± 1.8 | 1.9 ± 1.8 | 2.0 ± 1.9 | 1.7 ± 1.8 | 1.6 ± 1.7 | 0.1 ± 0.2 | 2.0 ± 0.7 | 5.3 ± 0.5 | 2.1 ± 1.9 | 1.0 ± 1.3 | 0.9 ± 1.4 |
| Decaffeinated coffee (servings/d) | 0.7 ± 1.3 | 0.6 ± 1.1 | 0.5 ± 1.0 | 0.6 ± 1.2 | 0.7 ± 1.2 | 0.9 ± 1.3 | 1.0 ± 1.5 | 0.5 ± 1.0 | 0.2 ± 0.8 | 0.1 ± 0.2 | 1.8 ± 0.7 | 5.2 ± 0.4 |
| Total coffee (servings/d) | 2.5 ± 2.0 | 2.6 ± 1.9 | 2.4 ± 1.9 | 2.6 ± 2.0 | 2.5 ± 1.9 | 2.4 ± 1.9 | 1.2 ± 1.5 | 2.6 ± 1.1 | 5.5 ± 0.9 | 2.2 ± 1.9 | 2.9 ± 1.5 | 6.1 ± 1.5 |
Data were age-standardized, except for age. One serving is equivalent to a standard 12-oz can (∼355 mL) or cup (∼237 mL).
Mean ± SD (all such values).
Pre-enrollment weight loss and weight gain are defined as the weight change between 1980 and 1984.
MET-h, metabolic equivalent task hours.
Distribution of baseline characteristics of HPFS participants according to consumption of caffeinated and caffeine-free coffee and carbonated beverages are presented in Table 2. Similar to their female counterparts, men with higher caffeinated and caffeine-free carbonated beverage consumption were older, had a higher BMI, and had higher pre-enrollment weight gain. Caffeinated and caffeine-free carbonated beverage consumption was inversely associated with alcohol intake. Men with a higher coffee consumption were more likely to be current smokers. They also had lower intakes of alcohol. Higher caffeinated coffee consumption was associated with higher high-fat dairy intake.
TABLE 2.
Age-standardized baseline (1986) characteristics according to categories of caffeinated and caffeine-free beverage consumption in 39,059 men1
| Caffeinated carbonated beverage |
Caffeine-free carbonated beverage |
Caffeinated coffee |
Decaffeinated coffee |
|||||||||
| <2/wk | 2–6/wk | ≥1/d | <2/wk | 2–6/wk | ≥1/d | <1/d | 1–3/d | ≥4/d | <1/d | 1–3/d | ≥4/d | |
| Participants (n) | 24,235 | 9372 | 5452 | 26,122 | 9147 | 3790 | 20,605 | 14,186 | 4268 | 30,290 | 7495 | 1274 |
| Age (y) | 54.3 ± 9.62 | 50.7 ± 8.8 | 48.2 ± 7.9 | 53.4 ± 9.6 | 51.3 ± 9.1 | 50.1 ± 8.7 | 52.8 ± 9.7 | 52.7 ± 9.4 | 51.5 ± 8.5 | 52.2 ± 9.5 | 54.0 ± 9.4 | 53.0 ± 8.6 |
| BMI (kg/m2) | 24.6 ± 4.8 | 25.1 ± 4.9 | 25.7 ± 5.3 | 24.7 ± 4.8 | 25.1 ± 5.0 | 25.7 ± 5.1 | 24.7 ± 4.9 | 25.0 ± 4.9 | 25.2 ± 5.0 | 24.8 ± 4.9 | 24.9 ± 4.7 | 25.4 ± 5.1 |
| Pre-enrollment weight gain (kg)3 | 1.7 ± 2.6 | 2.0 ± 2.8 | 2.4 ± 3.2 | 1.8 ± 2.7 | 2.0 ± 2.8 | 2.3 ± 3.2 | 1.8 ± 2.7 | 1.9 ± 2.8 | 2.2 ± 3.1 | 1.9 ± 2.8 | 1.9 ± 2.8 | 2.1 ± 3.0 |
| Pre-enrollment weight loss (kg)3 | 0.7 ± 1.7 | 0.6 ± 1.6 | 0.7 ± 1.8 | 0.6 ± 1.7 | 0.6 ± 1.7 | 0.8 ± 1.9 | 0.7 ± 1.7 | 0.6 ± 1.7 | 0.7 ± 1.8 | 0.6 ± 1.7 | 0.8 ± 1.8 | 0.8 ± 2.0 |
| Current smoker (%) | 8 | 10 | 10 | 9 | 7 | 9 | 6 | 10 | 21 | 9 | 8 | 17 |
| Physical activity (MET-h4/wk) | 21.9 ± 29.1 | 21.1 ± 31.2 | 20.8 ± 31.1 | 21.2 ± 29.4 | 22.2 ± 29.6 | 22.1 ± 30.4 | 22.5 ± 30.9 | 21.0 ± 27.8 | 19.1 ± 30.8 | 21.3 ± 29.8 | 22.4 ± 30.0 | 21.4 ± 27.9 |
| Family history of diabetes (%) | 19 | 20 | 20 | 19 | 20 | 20 | 20 | 20 | 21 | 20 | 21 | 19 |
| History of hypertension (%) | 18 | 19 | 22 | 17 | 20 | 24 | 20 | 19 | 15 | 18 | 22 | 20 |
| History of hypercholesterolemia (%) | 10 | 10 | 11 | 10 | 11 | 12 | 11 | 10 | 8 | 10 | 12 | 12 |
| Total energy intake (kcal/d) | 1933 ± 596 | 2060 ± 626 | 2171 ± 679 | 1950 ± 599 | 2080 ± 642 | 2129 ± 678 | 1949 ± 612 | 2027 ± 616 | 2130 ± 658 | 1993 ± 623 | 2005 ± 615 | 2032 ± 621 |
| Caffeine intake (mg/d) | 220 ± 252 | 266 ± 245 | 304 ± 249 | 262 ± 263 | 211 ± 225 | 197 ± 224 | 74 ± 83 | 341 ± 152 | 739 ± 236 | 264 ± 263 | 177 ± 196 | 170 ± 210 |
| Alcohol consumption (g/d) | 11.5 ± 15.5 | 11.1 ± 14.5 | 10.6 ± 15.5 | 11.6 ± 15.4 | 11.0 ± 14.5 | 10.9 ± 16.0 | 9.0 ± 13.5 | 13.7 ± 16.0 | 15.0 ± 18.6 | 11.0 ± 15.3 | 12.2 ± 15.1 | 14.2 ± 16.9 |
| Alternate Healthy Eating Index | 45.2 ± 11.4 | 43.1 ± 10.5 | 42.0 ± 10.9 | 44.1 ± 11.4 | 44.6 ± 10.8 | 43.7 ± 10.7 | 44.9 ± 11.4 | 44.0 ± 10.9 | 41.5 ± 10.4 | 43.9 ± 11.2 | 45.5 ± 10.8 | 43.6 ± 11.2 |
| Low-fat dairy intake (servings/d) | 1.0 ± 1.1 | 0.9 ± 1.0 | 0.9 ± 1.0 | 1.0 ± 1.0 | 1.0 ± 1.0 | 0.9 ± 0.9 | 1.0 ± 1.1 | 0.9 ± 1.0 | 0.9 ± 1.0 | 1.0 ± 1.0 | 1.0 ± 1.0 | 0.9 ± 1.0 |
| High-fat dairy intake (servings/d) | 1.2 ± 1.3 | 1.3 ± 1.3 | 1.4 ± 1.3 | 1.3 ± 1.3 | 1.3 ± 1.2 | 1.2 ± 1.2 | 1.1 ± 1.1 | 1.4 ± 1.3 | 1.7 ± 1.8 | 1.3 ± 1.3 | 1.2 ± 1.2 | 1.3 ± 1.5 |
| Artificially sweetened beverages (servings/d) | 0.2 ± 0.5 | 0.4 ± 0.5 | 1.3 ± 1.4 | 0.2 ± 0.6 | 0.4 ± 0.6 | 1.4 ± 1.4 | 0.4 ± 0.8 | 0.4 ± 0.8 | 0.4 ± 0.9 | 0.3 ± 0.8 | 0.5 ± 0.8 | 0.5 ± 1.0 |
| Sugar-sweetened beverages (servings/d) | 0.1 ± 0.3 | 0.4 ± 0.4 | 0.8 ± 1.0 | 0.2 ± 0.5 | 0.4 ± 0.4 | 0.7 ± 0.9 | 0.3 ± 0.6 | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.2 ± 0.5 | 0.2 ± 0.4 |
| Caffeinated tea (servings/d) | 0.4 ± 0.8 | 0.4 ± 0.8 | 0.4 ± 0.8 | 0.4 ± 0.9 | 0.4 ± 0.8 | 0.4 ± 0.7 | 0.5 ± 0.9 | 0.4 ± 0.8 | 0.3 ± 0.5 | 0.4 ± 0.9 | 0.4 ± 0.8 | 0.3 ± 0.8 |
| Caffeinated coffee (servings/d) | 1.2 ± 1.6 | 1.5 ± 1.6 | 1.4 ± 1.6 | 1.4 ± 1.6 | 1.2 ± 1.5 | 1.0 ± 1.5 | 0.1 ± 0.2 | 2.0 ± 0.7 | 4.9 ± 0.7 | 1.5 ± 1.7 | 0.9 ± 1.2 | 0.8 ± 1.3 |
| Decaffeinated coffee (servings/d) | 0.6 ± 1.1 | 0.5 ± 1.0 | 0.5 ± 1.1 | 0.5 ± 1.1 | 0.7 ± 1.1 | 0.8 ± 1.3 | 0.7 ± 1.2 | 0.5 ± 1.0 | 0.3 ± 0.8 | 0.1 ± 0.2 | 1.8 ± 0.7 | 4.8 ± 0.6 |
| Total coffee (servings/d) | 1.9 ± 1.8 | 2.0 ± 1.8 | 2.0 ± 1.8 | 1.9 ± 1.8 | 1.8 ± 1.7 | 1.9 ± 1.8 | 0.9 ± 1.2 | 2.5 ± 1.2 | 5.1 ± 1.0 | 1.6 ± 1.6 | 2.7 ± 1.4 | 5.7 ± 1.4 |
Data were age-standardized, except for age. One serving is equivalent to a standard 12-oz can (∼355 mL) or cup (∼237 mL).
Mean ± SD (all such values).
Pre-enrollment weight loss and weight gain are defined as the weight change between 1980 and 1984.
MET-h, metabolic equivalent task hours.
Regression analysis
In both cohorts, before adjustment for BMI and total energy intake (model 2), consumption of caffeinated and caffeine-free SSBs and ASBs was associated with a higher risk of T2D. When differences in BMI and total energy intake were accounted for, caffeinated and caffeine-free SSBs remained associated with a higher risk of T2D, whereas the association between caffeinated and caffeine-free ASBs was greatly attenuated and became largely nonsignificant (Table 3).
TABLE 3.
RRs (and 95% CIs) of type 2 diabetes according to categories of caffeinated and caffeine-free carbonated beverage intake1
| Serving category |
||||||
| <1/mo | 1–4/mo | 2–6/wk | ≥1/d | P-trend2 | Per-serving increment3 | |
| NHS | ||||||
| Caffeinated carbonated beverage | ||||||
| Sugar sweetened | ||||||
| Median consumption (servings/d) | 0 | 0.07 | 0.36 | 1.0 | ||
| Cases | 3986 | 2039 | 1045 | 300 | ||
| Person-years | 953,918 | 440,607 | 175,261 | 45,128 | ||
| Model 14 | Ref | 1.06 (1.00, 1.12) | 1.34 (1.25, 1.43) | 1.74 (1.55, 1.96) | <0.0001 | 1.34 (1.27, 1.42) |
| Model 25 | Ref | 1.07 (1.01, 1.14) | 1.27 (1.17, 1.38) | 1.60 (1.41, 1.82) | <0.0001 | 1.28 (1.20, 1.35) |
| Model 36 | Ref | 1.08 (1.01, 1.15) | 1.19 (1.09, 1.28) | 1.29 (1.14, 1.47) | <0.0001 | 1.13 (1.06, 1.20) |
| Artificially sweetened | ||||||
| Median consumption (servings/d) | 0 | 0.07 | 0.29 | 1.0 | ||
| Cases | 2967 | 1739 | 1828 | 836 | ||
| Person-years | 786,192 | 357,216 | 329,918 | 141,588 | ||
| Model 14 | Ref | 1.18 (1.11, 1.26) | 1.30 (1.23, 1.39) | 1.59 (1.47, 1.71) | <0.0001 | 1.23 (1.19, 1.28) |
| Model 25 | Ref | 1.13 (1.06, 1.20) | 1.19 (1.11, 1.27) | 1.35 (1.24, 1.47) | <0.0001 | 1.15 (1.11, 1.20) |
| Model 36 | Ref | 1.02 (0.95, 1.09) | 0.99 (0.92, 1.05) | 1.01 (0.93, 1.10) | 0.99 | 1.01 (0.97, 1.06) |
| Caffeine-free carbonated beverage | ||||||
| Sugar sweetened | ||||||
| Median consumption (servings/d) | 0 | 0.07 | 0.4 | 1.13 | ||
| Cases | 3705 | 2503 | 1008 | 154 | ||
| Person-years | 869,023 | 530,918 | 186,694 | 28,279 | ||
| Model 14 | Ref | 1.02 (0.97, 1.07) | 1.18 (1.10, 1.26) | 1.46 (1.24, 1.71) | <0.0001 | 1.28 (1.17, 1.39) |
| Model 25 | Ref | 0.99 (0.94, 1.05) | 1.02 (0.94, 1.11) | 1.22 (1.04, 1.45) | 0.03 | 1.13 (1.02, 1.25) |
| Model 36 | Ref | 1.01 (0.95, 1.07) | 1.02 (0.95, 1.11) | 1.20 (1.01, 1.42) | 0.05 | 1.11 (1.00, 1.23) |
| Artificially sweetened | ||||||
| Median consumption (servings/d) | 0 | 0.07 | 0.38 | 1.14 | ||
| Cases | 1960 | 1740 | 2422 | 1248 | ||
| Person-years | 564,886 | 393,699 | 444,761 | 211,568 | ||
| Model 14 | Ref | 1.16 (1.09, 1.24) | 1.39 (1.30, 1.47) | 1.76 (1.63, 1.89) | <0.0001 | 1.28 (1.24, 1.31) |
| Model 25 | Ref | 1.18 (1.10, 1.27) | 1.39 (1.29, 1.49) | 1.69 (1.56, 1.83) | <0.0001 | 1.24 (1.20, 1.28) |
| Model 36 | Ref | 1.00 (0.93, 1.07) | 1.02 (0.95, 1.09) | 1.09 (1.00, 1.18) | 0.02 | 1.06 (1.03, 1.10) |
| HPFS | ||||||
| Caffeinated carbonated beverage | ||||||
| Sugar sweetened | ||||||
| Median consumption (servings/d) | 0 | 0.07 | 0.43 | 1.0 | ||
| Cases | 1186 | 897 | 431 | 472 | ||
| Person-years | 328,351 | 236,102 | 145,695 | 32,599 | ||
| Model 14 | Ref | 1.04 (0.95, 1.13) | 1.20 (1.09, 1.32) | 1.49 (1.26, 1.77) | <0.0001 | 1.26 (1.16, 1.38) |
| Model 25 | Ref | 1.07 (0.97, 1.18) | 1.23 (1.09, 1.38) | 1.42 (1.18, 1.70) | <0.0001 | 1.22 (1.11, 1.34) |
| Model 36 | Ref | 1.05 (0.95, 1.16) | 1.19 (1.06, 1.34) | 1.33 (1.10, 1.60) | <0.001 | 1.16 (1.06, 1.27) |
| Artificially sweetened | ||||||
| Median consumption (servings/d) | 0 | 0.07 | 0.43 | 1.29 | ||
| Cases | 1206 | 651 | 677 | 331 | ||
| Person-years | 379,136 | 158,760 | 144,639 | 60,212 | ||
| Model 14 | Ref | 1.22 (1.11, 1.35) | 1.42 (1.29, 1.56) | 1.87 (1.65, 2.12) | <0.0001 | 1.25 (1.19, 1.33) |
| Model 25 | Ref | 1.12 (1.00, 1.25) | 1.13 (1.01, 1.27) | 1.32 (1.15, 1.51) | <0.001 | 1.07 (1.01, 1.14) |
| Model 36 | Ref | 1.03 (0.92, 1.15) | 0.97 (0.87, 1.09) | 1.06 (0.93, 1.22) | 0.55 | 0.98 (0.91, 1.04) |
| Caffeine-free carbonated beverage | ||||||
| Sugar sweetened | ||||||
| Median consumption (servings/d) | 0 | 0.07 | 0.29 | 1.0 | ||
| Cases | 1197 | 1044 | 545 | 79 | ||
| Person-years | 332,802 | 273,819 | 120,909 | 15,208 | ||
| Model 14 | Ref | 1.01 (0.93, 1.10) | 1.22 (1.10, 1.35) | 1.57 (1.25, 1.97) | <0.001 | 1.40 (1.22, 1.60) |
| Model 25 | Ref | 1.03 (0.93, 1.13) | 1.16 (1.03, 1.30) | 1.39 (1.10, 1.76) | <0.001 | 1.27 (1.10, 1.48) |
| Model 36 | Ref | 1.02 (0.93, 1.13) | 1.14 (1.01, 1.29) | 1.37 (1.08, 1.74) | 0.002 | 1.23 (1.06, 1.43) |
| Artificially sweetened | ||||||
| Median consumption (servings/d) | 0 | 0.07 | 0.40 | 1.17 | ||
| Cases | 1076 | 714 | 766 | 309 | ||
| Person-years | 345,770 | 185,617 | 160,632 | 50,728 | ||
| Model 14 | Ref | 1.16 (1.05, 1.27) | 1.40 (1.28, 1.54) | 1.95 (1.71, 2.21) | <0.0001 | 1.37 (1.30, 1.45) |
| Model 25 | Ref | 1.05 (0.94, 1.18) | 1.12 (1.00, 1.26) | 1.32 (1.14, 1.52) | <0.001 | 1.13 (1.06, 1.21) |
| Model 36 | Ref | 1.02 (0.91, 1.14) | 1.04 (0.93, 1.16) | 1.15 (0.99, 1.33) | 0.06 | 1.06 (0.99, 1.14) |
HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; Ref, reference.
Calculated by treating median intake in each category as a continuous variable.
One serving is equivalent to a standard can, glass, or bottle (∼355 mL).
RRs were adjusted for age (in y) and time interval (2-y categories).
RRs were further adjusted for smoking status (never, past, or current 1–14, 15–24, or ≥25 cigarettes/d); alcohol use (0, 0.1–4.9, 5–9.9, 10–14.9, or ≥15 g/d); postmenopausal hormone use (women only; premenopausal, postmenopausal—never, current, or past user); physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic equivalent task hours/wk); family history of diabetes (yes or no); Alternate Healthy Eating Index (quintiles); consumption of other beverages other than the main exposure, depending on the model (total coffee, caffeinated tea, fruit punch, sugar-sweetened beverage, or artificially sweetened beverage); presence of hypertension (yes or no); hypercholesterolemia (yes or no); adherence to a low-calorie diet (yes or no); and reported weight change (between 1980 and 1984 in the NHS and between 1981 and 1986 in the HPFS). The reported weight change represents separate variables for weight gain (0, 0.9–1.8, 2.3–4.1, 4.5–6.4, 6.8–8.6, 9.1–13.2, or ≥13.6 kg) and weight loss (0, 0.9–1.8, 2.3–4.1, 4.5–6.4, or ≥6.8 kg).
RRs were further adjusted for total energy intake (quintiles) and BMI (in kg/m2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40). All statistical tests were conducted by using Cox proportional hazards regression models.
After multivariable adjustment, we observed a modest inverse association between consumption of both caffeinated and decaffeinated coffee and T2D risk (Table 4). In the NHS, there was no difference in the magnitude of association between caffeinated (RR per 1-cup increment: 0.92; 95% CI: 0.91, 0.94) and decaffeinated (RR per 1-cup increment: 0.92; 95% CI: 0.90, 0.95) coffee and the risk of T2D. In the HPFS, caffeinated coffee consumption was associated with a 4% lower risk (RR: 0.96; 95% CI: 0.93, 0.98), and decaffeinated coffee consumption was associated with a 7% lower risk (RR: 0.93; 95% CI: 0.89, 0.97) of T2D. Caffeinated tea consumption was inversely associated with T2D risk in women (RR per 1-cup increment: 0.95; 95% CI: 0.92, 0.97) but not men (RR per 1-cup increment: 0.99; 95% CI: 0.94, 1.04). No significant associations were observed between decaffeinated tea consumption and T2D risk in both cohorts. When we continued updating a participant's diet even after a diagnosis of cancer or cardiovascular disease, risk estimates were similar to those obtained when we stopped updating diet at these diagnoses (data not shown).
TABLE 4.
RRs (and 95% CIs) of type 2 diabetes according to categories of caffeinated and decaffeinated coffee and tea intake1
| Serving category |
||||||||
| Never | <1 cup/d | 1–3 cups/d | >3 cups/d | 4–5 cups/d | >5 cups/d | P-trend2 | Per-cup increment | |
| NHS | ||||||||
| Coffee | ||||||||
| Caffeinated | ||||||||
| Median consumption (cups/d) | — | 0.02 | 1.86 | — | 3.40 | 5.0 | — | — |
| Cases | — | 3096 | 3163 | — | 764 | 347 | — | — |
| Person-years | — | 599,188 | 712,566 | — | 179,688 | 123,472 | — | — |
| Model 13 | — | Ref | 0.84 (0.80, 0.89) | — | 0.73 (0.67, 0.79) | 0.64 (0.57, 0.72) | <0.0001 | 0.91 (0.90, 0.93) |
| Model 24 | — | Ref | 0.89 (0.85, 0.94) | — | 0.73 (0.68, 0.80) | 0.61 (0.54, 0.69) | <0.0001 | 0.91 (0.90, 0.93) |
| Model 35 | — | Ref | 0.91 (0.86, 0.95) | — | 0.78 (0.72, 0.85) | 0.65 (0.58, 0.73) | <0.0001 | 0.92 (0.91, 0.94) |
| Decaffeinated6 | ||||||||
| Median consumption (cups/d) | 0 | 0.22 | 1.42 | 3.53 | — | — | — | — |
| Cases | 2456 | 2855 | 1814 | 245 | — | — | — | — |
| Person-years | 534,342 | 592,505 | 419,846 | 68,221 | — | — | — | — |
| Model 13 | Ref | 0.91 (0.86, 0.96) | 0.85 (0.80, 0.91) | 0.73 (0.64, 0.83) | — | — | <0.0001 | 0.92 (0.90, 0.95) |
| Model 24 | Ref | 0.92 (0.87, 0.98) | 0.83 (0.78, 0.89) | 0.70 (0.61, 0.80) | — | — | <0.0001 | 0.91 (0.88, 0.93) |
| Model 35 | Ref | 0.95 (0.89, 1.00) | 0.86 (0.81, 0.92) | 0.73 (0.64, 0.84) | — | — | <0.0001 | 0.92 (0.90, 0.95) |
| Tea | ||||||||
| Caffeinated | ||||||||
| Median consumption (cups/d) | 0 | 0.19 | 1.31 | 3.75 | — | — | — | — |
| Cases | 1275 | 4240 | 1617 | 238 | — | — | — | — |
| Person-years | 319,525 | 866,451 | 368,836 | 60,102 | — | — | — | — |
| Model 13 | Ref | 1.08 (1.02, 1.16) | 1.03 (0.96, 1.11) | 0.93 (0.81, 1.07) | — | — | 0.05 | 0.98 (0.95, 1.00) |
| Model 24 | Ref | 1.13 (1.06, 1.20) | 1.00 (0.93, 1.08) | 0.85 (0.74, 0.98) | — | — | <0.0001 | 0.95 (0.92, 0.97) |
| Model 35 | Ref | 1.05 (0.98, 1.12) | 0.95 (0.88, 1.02) | 0.81 (0.71, 0.93) | — | — | <0.0001 | 0.95 (0.92, 0.97) |
| Decaffeinated6 | ||||||||
| Median consumption (cups/d) | 0 | 0.14 | 1.19 | 4.5 | — | — | — | — |
| Cases | 1616 | 1333 | 391 | 46 | — | — | — | — |
| Person-years | 263,619 | 223,807 | 66,892 | 6615 | — | — | — | — |
| Model 13 | Ref | 0.96 (0.89, 1.03) | 0.94 (0.84, 1.05) | 1.09 (0.81, 1.46) | — | — | 1.00 | 1.00 (0.95, 1.05) |
| Model 24 | Ref | 1.00 (0.93, 1.09) | 0.99 (0.89, 1.11) | 1.06 (0.79, 1.42) | — | — | 0.82 | 1.01 (0.96, 1.06) |
| Model 35 | Ref | 1.00 (0.93, 1.08) | 1.02 (0.91, 1.15) | 1.03 (0.77, 1.39) | — | — | 0.71 | 1.02 (0.97, 1.07) |
| HPFS | ||||||||
| Coffee | ||||||||
| Caffeinated | ||||||||
| Median consumption (cups/d) | — | 0.02 | 1.75 | — | 3.83 | 6.00 | — | — |
| Cases | — | 1380 | 1092 | — | 336 | 57 | — | — |
| Person-years | — | 365,390 | 378,899 | — | 78,964 | 19,495 | — | — |
| Model 13 | — | Ref | 1.02 (0.94, 1.10) | — | 1.08 (0.96, 1.21) | 0.79 (0.61, 1.03) | 0.98 | 1.00 (0.97, 1.02) |
| Model 24 | — | Ref | 1.01 (0.93, 1.10) | — | 1.01 (0.90, 1.15) | 0.69 (0.53, 0.91) | 0.21 | 0.98 (0.95, 1.01) |
| Model 35 | — | Ref | 0.95 (0.88, 1.03) | — | 0.92 (0.82, 1.05) | 0.65 (0.49, 0.85) | 0.005 | 0.96 (0.93, 0.98) |
| Decaffeinated6 | ||||||||
| Median consumption (cups/d) | 0 | 0.20 | 1.40 | 4.17 | — | — | — | — |
| Cases | 1139 | 1050 | 569 | 107 | — | — | — | — |
| Person-years | 293,265 | 274,230 | 150,088 | 25,164 | — | — | — | — |
| Model 13 | Ref | 0.90 (0.83, 0.98) | 0.89 (0.81, 0.99) | 1.00 (0.82, 1.22) | — | — | 0.60 | 0.97 (0.93, 1.01) |
| Model 24 | Ref | 0.87 (0.80, 0.95) | 0.80 (0.72, 0.89) | 0.86 (0.71, 1.06) | — | — | 0.02 | 0.93 (0.90, 0.97) |
| Model 35 | Ref | 0.92 (0.84, 1.01) | 0.84 (0.75, 0.93) | 0.84 (0.68, 1.03) | — | — | 0.009 | 0.93 (0.89, 0.97) |
| Tea | ||||||||
| Caffeinated | ||||||||
| Median consumption (cups/d) | 0 | 0.14 | 1.25 | 3.83 | — | — | — | — |
| Cases | 874 | 1489 | 444 | 58 | — | — | — | — |
| Person-years | 237,161 | 378,632 | 114,323 | 12,632 | — | — | — | — |
| Model 13 | Ref | 0.98 (0.90, 1.07) | 1.00 (0.89, 1.12) | 1.18 (0.91, 1.55) | — | — | 0.27 | 1.02 (0.98, 1.08) |
| Model 24 | Ref | 0.96 (0.88, 1.05) | 0.96 (0.86, 1.08) | 1.08 (0.82, 1.41) | — | — | 0.79 | 1.00 (0.95, 1.05) |
| Model 35 | Ref | 0.97 (0.89, 1.06) | 0.96 (0.85, 1.08) | 0.99 (0.76, 1.30) | — | — | 0.76 | 0.99 (0.94, 1.04) |
| Decaffeinated6 | ||||||||
| Median consumption (cups/d) | 0 | 0.14 | 1.0 | 4.5 | — | — | — | — |
| Cases | 582 | 274 | 60 | 7 | — | — | — | — |
| Person-years | 119,825 | 63,095 | 12,588 | 1063 | — | — | — | — |
| Model 13 | Ref | 0.90 (0.78, 1.04) | 0.96 (0.74, 1.26) | 1.34 (0.63, 2.81) | — | — | 0.65 | 0.98 (0.86, 1.11) |
| Model 24 | Ref | 0.91 (0.78, 1.06) | 0.99 (0.75, 1.30) | 1.19 (0.56, 2.53) | — | — | 0.72 | 0.98 (0.86, 1.11) |
| Model 35 | Ref | 0.95 (0.81, 1.11) | 1.05 (0.80, 1.38) | 1.08 (0.51, 2.30) | — | — | 0.71 | 0.99 (0.87, 1.13) |
One cup is equivalent to ∼237 mL. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; Ref, reference.
Calculated by treating median intake in each category as a continuous variable.
RRs were adjusted for age (in y) and time interval (2-y categories).
RRs were further adjusted for smoking status (never, past, or current 1–14, 15–24, or ≥25 cigarettes/d); alcohol use (0, 0.1–4.9, 5–9.9, 10–14.9, or ≥15 g/d); postmenopausal hormone use (women only; premenopausal, postmenopausal—never, current, or past user); physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic equivalent task hours/wk); family history of diabetes (yes or no); Alternate Healthy Eating Index (quintiles); consumption of other beverages other than the main exposure, depending on the model (total coffee, caffeinated tea, fruit punch, sugar-sweetened beverage, or artificially sweetened beverage); presence of hypertension (yes or no); hypercholesterolemia (yes or no); adherence to a low-calorie diet (yes or no); and reported weight change (between 1980 and 1984 in the NHS and between 1981 and 1986 in the HPFS). The reported weight change represents separate variables for weight gain (0, 0.9–1.8, 2.3–4.1, 4.5–6.4, 6.8–8.6, 9.1–13.2, or ≥13.6 kg) and weight loss (0, 0.9–1.8, 2.3–4.1, 4.5–6.4, or ≥6.8 kg).
RRs were further adjusted for total energy intake (quintiles) and BMI (in kg/m2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40). All statistical tests were conducted by using Cox proportional hazards regression models.
Follow-up for decaffeinated tea is from 1998 to 2008.
In stratified analyses, there was no evidence that the relation between caffeinated and decaffeinated beverages and T2D was modified by BMI, level of physical activity, or smoking status (P-interaction > 0.05). Caffeine did not modulate the association between coffee and T2D and SSB and T2D (P > 0.05). In both men and women, within each category of total coffee intake (<1 cup/d, 1–3 cups/d, >3 cups/d), risk of T2D was not higher with higher caffeine intake. Higher consumption of SSBs was associated with a higher risk of T2D, but this risk did not change with increased caffeine intake (data not shown).
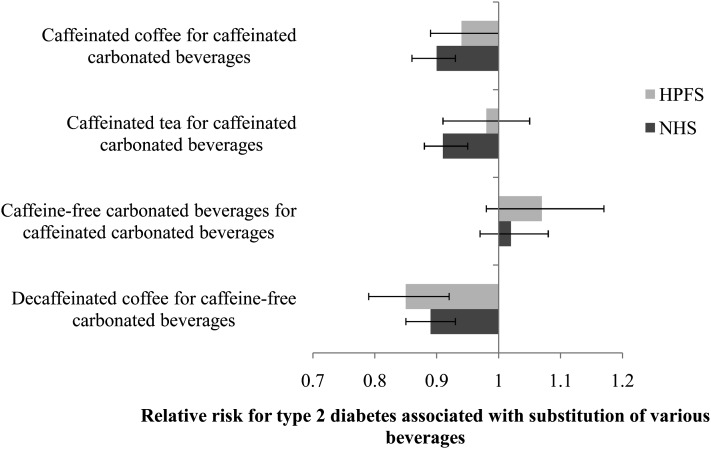
Substituting 1 cup/d of other caffeine sources such as coffee and tea for 1 serving of caffeinated carbonated beverage (SSB and ASB) was associated with a lower risk of T2D in the NHS (RR for 1 cup of coffee: 0.90; 95% CI: 0.86, 0.93; RR for 1 cup of tea: 0.91; 95% CI: 0.88, 0.95). The corresponding RRs (95% CIs) in the HPFS cohort were 0.94 (95% CI: 0.89, 1.00) for coffee and 0.98 (95% CI: 0.91, 1.05) for tea. Replacement of 1 serving of a caffeine-free carbonated beverage (SSB and ASB) with 1 cup of decaffeinated coffee was associated with a lower risk of T2D in both men and women. On the other hand, replacement of 1 serving of a caffeinated carbonated beverage (SSB and ASB) with 1 serving of the caffeine-free form was not associated with a change in T2D risk (Figure 1).
FIGURE 1.
RRs (95% CIs) for type 2 diabetes associated with substitution of caffeinated and caffeine-free beverage sources. Multivariable RRs for type 2 diabetes were adjusted for age (in y); time interval (2-y categories); smoking status (never, past, or current 1–14, 15–24, or ≥25 cigarettes/d); alcohol use (0, 0.1–4.9, 5–9.9, 10–14.9, or ≥15 g/d); postmenopausal hormone use (women only; premenopausal, postmenopausal–never, current, or past user); physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic equivalent hours/wk); family history of diabetes (yes or no); Alternate Healthy Eating Index (quintiles); consumption of beverages other than the main exposure, depending on the model (caffeinated and decaffeinated coffee, caffeinated tea, fruit punch, caffeinated carbonated beverage, or caffeine-free carbonated beverage); hypertension (yes or no); hypercholesterolemia (yes or no); adherence to a low-calorie diet (yes or no); reported weight change (between 1980 and 1984 in the NHS and between 1981 and 1986 in the HPFS); total energy intake (quintiles); and BMI (in kg/m2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40) by using Cox proportional hazards regression models. The 2 beverages for substitution were entered into the model as continuous variables. Error bars represent 95% CIs of substitution estimates. HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
DISCUSSION
In these 2 large cohorts of US men and women, we found that consumption of SSBs, irrespective of the caffeine content, was associated with a higher risk of T2D. We also noted that consumption of coffee, including caffeinated and decaffeinated coffee, was associated with a lower risk of T2D. Caffeinated tea was associated with a lower risk of T2D among women, but this was not observed for decaffeinated tea or among men. Replacement of caffeinated carbonated beverages with other caffeinated beverages such as coffee and tea was associated with a lower risk of T2D. Replacement of decaffeinated carbonated beverages with decaffeinated coffee was also associated with a lower risk of T2D.
Although there are no prospective data that directly compare the effects of caffeinated compared with caffeine-free carbonated beverages on T2D, the association between SSB consumption, irrespective of the caffeine content, and a higher risk of T2D has been reported in several cohort studies. Results from a meta-analysis of 11 prospective cohort studies indicated that a higher consumption of SSBs is associated with weight gain, the metabolic syndrome, and T2D (8). Similar to the findings of Schulze et al (20) and de Koning et al (21), we found that both caffeinated and caffeine-free ASB consumption was associated with a slight nonsignificantly higher risk of T2D after adjustment for BMI and total energy but only among men. Although Nettleton et al (22) did not differentiate between caffeinated and caffeine-free ASBs, we, similar to them, found that higher caffeine-free ASB consumption in women was associated with a higher risk of T2D. Although it remains unclear why such associations were not observed with caffeinated ASB consumption, it may be that caffeine-free ASB consumption is a surrogate for unhealthy lifestyle behaviors that were not accounted for in our analyses. The strong positive associations noted before adjustment for BMI and total energy intake may be attributed to reverse causality and confounding as persons with a higher BMI are more likely to choose ASBs to restrict their energy intake and control their body weight (23). Our results for caffeinated and decaffeinated coffee are largely consistent with those published earlier for the NHS (9), HPFS (9), NHS II (24), and European Prospective Investigation into Cancer and Nutrition–Germany (25) and –Dutch (26) cohorts. The risk reduction of 4% to 8% in incident T2D in our study for every 1-cup increment in coffee consumption is similar to the 5–10% reduction estimated in a meta-analysis of >500,000 individuals (2). In the current updated analysis with longer follow-up in NHS, caffeinated tea, in addition to coffee, was associated with a lower risk of T2D. Our results are consistent with those of a meta-analysis of cohort studies that found that, compared with no tea consumption, consumption of ≥4 cups tea/d was associated with a 20% lower risk of T2D (27). We did not observe this inverse association among HPFS participants, potentially because of the lower consumption of caffeinated tea in this population. Likewise, the nonsignificant associations with decaffeinated tea in both the NHS and HPFS may have been a result of its low consumption and the shorter follow-up period (1998–2008). Our data do not agree with results from short-term metabolic studies in which intake of caffeine alone (3–5) or together with carbohydrate (6, 7) before an oral-glucose-tolerance test or a mixed-meal tolerance test acutely increased postprandial glucose and insulin responses. Although we were unable to examine markers of glycemic control, we found no evidence that caffeine's acute detrimental effects on insulin sensitivity translate into an increased risk of T2D, potentially because other compounds in coffee may modify this effect long term. In support of this hypothesis, we found that caffeine was no longer associated with the risk of T2D after adjustment for coffee intake (data not shown). The similar findings for caffeinated and caffeine-free SSBs and the lack of a significant interaction between caffeine and total SSB consumption implies that the short-term synergy between caffeine and carbohydrate on glucose metabolism may not have a substantial long-term effect beyond that of carbohydrates found in SSB on T2D. Furthermore, tolerance to the effects of caffeine may develop long term (28).
The robust direct positive association between SSBs and T2D, even after control for multiple potential confounders, raises the possibility of direct biological effects. SSBs are sweetened with sucrose or high-fructose corn syrup, 2 rapidly absorbable carbohydrates that increase postprandial blood glucose and insulin concentrations. Fructose can also potentially stimulate lipogenesis (29), which has been suggested to lead to excess weight gain. Furthermore, several studies have shown that liquid calories have a lower satiety compared with solid calories and do not suppress consumption of solid foods. Results from a recent systematic review of the literature support an association between SSB consumption and weight gain (30).
Several biological mechanisms may explain the putative protective effects of coffee and tea on T2D (31). For example, tea contains flavonoids as antioxidants, which may be important because oxidative stress is associated with the pathogenesis of diabetes (32). Coffee is rich in the antioxidant chlorogenic acid, which improved glucose metabolism (33) and inhibited formation of advanced glycation endproducts (34) in rats. In NHS, consumption of both caffeinated and decaffeinated coffee was found to be associated with lower concentrations of serum C-peptide—a marker of insulin secretion (35). In another cross-sectional analysis, caffeinated coffee was associated with better insulin sensitivity, whereas decaffeinated coffee was favorably related to measures of β cell function (36). However, supplementation with coffee, tea, or its individual components in randomized crossover studies in humans has yielded mixed results (28, 37–42), and more trials with longer follow-up are needed to elucidate these mechanisms.
Our results need to be interpreted in the context of several limitations. First, beverage consumption in our study was self-reported; therefore, some misclassification was possible. However, because such misclassification is nondifferential, observed effects will be biased toward the null. Moreover, the FFQs in our study had high validity for intake of most beverages compared with dietary records. Use of repeated measures of diet to calculate cumulative averages reduces random measurement error caused by within-person variation and also accommodates dietary changes over time. As with any observational study, residual confounding remains a possibility. However, we carefully adjusted for several established and potential risk factors for T2D. Our results may not be generalizable to the general population because our study population consisted of health professionals. However, the physiologic effects of the studied beverages are unlikely to be substantially different in groups with other professions. Finally, we were unable to account for the amount or type of sweetener added to coffee and tea. However, given that added sugar is associated with an increased risk of obesity and cardiovascular disease (43), exclusion of this variable in our analysis would lead to negative confounding or an underestimation of the true effect. Likewise, we were unable to assess whether the inverse associations between coffee and risk of T2D extend to blended coffee drinks that typically contain greater amounts of dairy and added sugar and provide a greater number of calories per serving. The strengths of the current study include the large sample size, long duration of follow-up, and use of repeated measures of diet.
Our findings suggest that the association between higher SSB consumption and a higher risk of T2D was potentially a result of the high content of sucrose or high-fructose corn syrup and not to a joint effect of caffeine and sugar. The public health relevance of these findings is significant because substituting caffeinated SSBs with caffeine-free SSBs in the diet is unlikely to decrease the risk of T2D. Instead, our results suggest that replacement of SSB with coffee or tea may lower the risk of T2D. Future research may be needed to evaluate the effects of blended coffee and tea drinks on the risk of T2D. The positive association observed with caffeine-free ASB consumption among women also deserves further study.
Acknowledgments
We are indebted to the participants of the Nurses’ Health Study and the Health Professionals Follow-Up Study for their continuing outstanding support and to the colleagues working in these studies for their valuable help.
The authors’ responsibilities were as follows—SNB: designed and conducted the analysis, interpreted the data, and wrote the manuscript; AP, VSM, JEM, WCW, RMvD, and FBH: assisted in interpreting the data and edited the manuscript; JEM, WCW, and FBH: obtained funding, managed and conducted the Nurses’ Health Study and Health Professionals Follow-Up Study, and critically reviewed the manuscript for important intellectual content; and FBH: had primary responsibility for the final content. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: ASB, artificially sweetened beverage; FFQ, food-frequency questionnaire; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; SSB, sugar-sweetened beverage; T2D, type 2 diabetes.
REFERENCES
- 1.CDC. November 8, 2011. Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf (cited 14 December 2011)
- 2.Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, Grobbee DE, Batty D, Woodward M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med 2009;169:2053–63 [DOI] [PubMed] [Google Scholar]
- 3.Battram DS, Arthur R, Weekes A, Graham TE. The glucose intolerance induced by caffeinated coffee ingestion is less pronounced than that due to alkaloid caffeine in men. J Nutr 2006;136:1276–80 [DOI] [PubMed] [Google Scholar]
- 4.Petrie HJ, Chown SE, Belfie LM, Duncan AM, McLaren DH, Conquer JA, Graham TE. Caffeine ingestion increases the insulin response to an oral-glucose-tolerance test in obese men before and after weight loss. Am J Clin Nutr 2004;80:22–8 [DOI] [PubMed] [Google Scholar]
- 5.Robinson LE, Savani S, Battram DS, McLaren DH, Sathasivam P, Graham TE. Caffeine ingestion before an oral glucose tolerance test impairs blood glucose management in men with type 2 diabetes. J Nutr 2004;134:2528–33 [DOI] [PubMed] [Google Scholar]
- 6.Moisey LL, Kacker S, Bickerton AC, Robinson LE, Graham TE. Caffeinated coffee consumption impairs blood glucose homeostasis in response to high and low glycemic index meals in healthy men. Am J Clin Nutr 2008;87:1254–61 [DOI] [PubMed] [Google Scholar]
- 7.Moisey LL, Robinson LE, Graham TE. Consumption of caffeinated coffee and a high carbohydrate meal affects postprandial metabolism of a subsequent oral glucose tolerance test in young, healthy males. Br J Nutr 2010;103:833–41 [DOI] [PubMed] [Google Scholar]
- 8.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salazar-Martinez E, Willett WC, Ascherio A, Manson JE, Leitzmann MF, Stampfer MJ, Hu FB. Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med 2004;140:1–8 [DOI] [PubMed] [Google Scholar]
- 10.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–40 [DOI] [PubMed] [Google Scholar]
- 11.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6 [DOI] [PubMed] [Google Scholar]
- 12.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26, discussion 27–36 [DOI] [PubMed] [Google Scholar]
- 13.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67 [DOI] [PubMed] [Google Scholar]
- 14.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 15.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–57 [DOI] [PubMed] [Google Scholar]
- 16.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Diabetes Care 1997;20:1183–97 [DOI] [PubMed] [Google Scholar]
- 17.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–8 [DOI] [PubMed] [Google Scholar]
- 18.Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–8 [DOI] [PubMed] [Google Scholar]
- 19.McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutr 2006;9(1A):152–7 [DOI] [PubMed] [Google Scholar]
- 20.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34 [DOI] [PubMed] [Google Scholar]
- 21.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr 2011;93:1321–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR., Jr Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009;32:688–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elfhag K, Tynelius P, Rasmussen F. Sugar-sweetened and artificially sweetened soft drinks in association to restrained, external and emotional eating. Physiol Behav 2007;91:191–5 [DOI] [PubMed] [Google Scholar]
- 24.van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, caffeine, and risk of type 2 diabetes: a prospective cohort study in younger and middle-aged U.S. women. Diabetes Care 2006;29:398–403 [DOI] [PubMed] [Google Scholar]
- 25.Floegel A, Pischon T, Bergmann MM, Teucher B, Kaaks R, Boeing H. Coffee consumption and risk of chronic disease in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Germany study. Am J Clin Nutr 2012;95:901–8 [DOI] [PubMed] [Google Scholar]
- 26.van Dieren S, Uiterwaal CS, van der Schouw YT. van der AD, Boer JM, Spijkerman A, Grobbee DE, Beulens JW. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia 2009;52:2561–9 [DOI] [PubMed] [Google Scholar]
- 27.Jing Y, Han G, Hu Y, Bi Y, Li L, Zhu D. Tea consumption and risk of type 2 diabetes: a meta-analysis of cohort studies. J Gen Intern Med 2009;24:557–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Dam RM, Pasman WJ, Verhoef P. Effects of coffee consumption on fasting blood glucose and insulin concentrations: randomized controlled trials in healthy volunteers. Diabetes Care 2004;27:2990–2 [DOI] [PubMed] [Google Scholar]
- 29.Samuel VT. Fructose induced lipogenesis: from sugar to fat to insulin resistance. Trends Endocrinol Metab 2011;22:60–5 [DOI] [PubMed] [Google Scholar]
- 30.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006;84:274–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dam RM. Coffee and type 2 diabetes: from beans to beta-cells. Nutr Metab Cardiovasc Dis 2006;16:69–77 [DOI] [PubMed] [Google Scholar]
- 32.Pitocco D, Zaccardi F, Di Stasio E, Romitelli F, Santini SA, Zuppi C, Ghirlanda G. Oxidative stress, nitric oxide, and diabetes. Rev Diabet Stud 2010;7:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tunnicliffe JM, Eller LK, Reimer RA, Hittel DS, Shearer J. Chlorogenic acid differentially affects postprandial glucose and glucose-dependent insulinotropic polypeptide response in rats. Appl Physiol Nutr Metab 2011;36:650–9 [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Jeong IH, Kim CS, Lee YM, Kim JM, Kim JS. Chlorogenic acid inhibits the formation of advanced glycation end products and associated protein cross-linking. Arch Pharm Res 2011;34:495–500 [DOI] [PubMed] [Google Scholar]
- 35.Wu T, Willett WC, Hankinson SE, Giovannucci E. Caffeinated coffee, decaffeinated coffee, and caffeine in relation to plasma C-peptide levels, a marker of insulin secretion, in U.S. women. Diabetes Care 2005;28:1390–6 [DOI] [PubMed] [Google Scholar]
- 36.Loopstra-Masters RC, Liese AD, Haffner SM, Wagenknecht LE, Hanley AJ. Associations between the intake of caffeinated and decaffeinated coffee and measures of insulin sensitivity and beta cell function. Diabetologia 2011;54:320–8 [DOI] [PubMed] [Google Scholar]
- 37.Olthof MR, van Dijk AE, Deacon CF, Heine RJ, van Dam RM. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on incretin hormones. Nutr Metab (Lond) 2011;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Dijk AE, Olthof MR, Meeuse JC, Seebus E, Heine RJ, van Dam RM. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 2009;32:1023–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr 2003;78:728–33 [DOI] [PubMed] [Google Scholar]
- 40.Neyestani TR, Shariatzade N, Kalayi A, Gharavi A, Khalaji N, Dadkhah M, Zowghi T, Haidari H, Shab-bidar S. Regular daily intake of black tea improves oxidative stress biomarkers and decreases serum C-reactive protein levels in type 2 diabetic patients. Ann Nutr Metab 2010;57:40–9 [DOI] [PubMed] [Google Scholar]
- 41.Brown AL, Lane J, Coverly J, Stocks J, Jackson S, Stephen A, Bluck L, Coward A, Hendrickx H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br J Nutr 2009;101:886–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wedick NM, Brennan AM, Sun Q, Hu FB, Mantzoros CS, van Dam RM. Effects of caffeinated and decaffeinated coffee on biological risk factors for type 2 diabetes: a randomized controlled trial. Nutr J 2011;10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 2009;120:1011–20 [DOI] [PubMed] [Google Scholar]