Abstract
Background: The effects of vitamin D supplementation in healthy prepubertal children on physiologic outcomes have not been investigated.
Objective: The objective was to evaluate the effects of supplementation with 1000 IU vitamin D3/d on calcium absorption.
Design: In a double-blind, placebo-controlled trial, we randomly assigned 64 children to 1000 IU vitamin D3/d (n = 32) or placebo (n = 32) for 8 wk. Stable isotopes were used to assess calcium absorption. The main outcome measure was calcium absorption before and after supplementation.
Results: All of the data are shown as means ± SDs. At baseline, vitamin D intake was 221 ± 79 IU/d and calcium intake was 830 ± 197 mg/d. Baseline serum 25-hydroxyvitamin D [25(OH)D] was not significantly correlated with fractional or total calcium absorption. After 8 wk, with baseline values used as a covariate, no differences were seen in fractional or total calcium absorption based on supplementation group (P = 0.75 and 0.36, respectively). Supplemented children had a significant increase in 25(OH)D concentrations (from 27.7 ± 7.4 to 36.0 ± 10.3 ng/mL; P < 0.0001) and a decrease in parathyroid hormone (from 21.4 ± 10.4 to 12.9 ± 7.1 pg/mL; P < 0.001); no significant changes in the placebo group were observed. No adverse side effects were noted in either group.
Conclusions: Vitamin D3 supplementation at 1000 IU/d increases 25(OH)D and decreases parathyroid hormone in children with average vitamin D intakes below the dietary recommendations of the Institute of Medicine. However, no significant effects of this change on calcium absorption occurred. This trial was registered at clinicaltrials.gov as NCT 00868738.
INTRODUCTION
Recently, the Institute of Medicine (IOM)5 revised its guidelines for vitamin D intake in children and adults in the United States and Canada (1). A Recommended Dietary Allowance (RDA) of 600 IU/d was set for children older than 12 mo with the goal of achieving a serum 25-hydroxyvitamin D [25(OH)D] concentration of ≥20 ng/mL. Although others have targeted a higher serum 25(OH)D concentration (2–4), the IOM committee has stood by its perspective indicating that there was inadequate evidence to support higher concentrations of serum 25(OH)D in a healthy population (5). However, the IOM noted that there are few data related to children <6 y of age, and, in general, there are few outcome data related to vitamin D intakes in prepubertal children in the United States (1).
In pubertal children and older adolescents, recent reports have generally failed to find a close relation between calcium absorption and serum 25(OH)D across a broad range of 25(OH)D concentrations, although few subjects with 25(OH)D <12 ng/mL have been studied (6, 7). Except for one very small study in adolescents, virtually all pediatric data relating vitamin D intake and calcium absorption are cross-sectional in nature (8).
Achieving serum 25(OH)D concentrations >20 or 30 ng/mL in all children would clearly be difficult without either a comprehensive food-fortification strategy exceeding the current one or the widespread use of vitamin D supplements (1). Because these strategies would not be simple or inexpensive to implement, continuing evaluation of the basis for these recommendations is necessary.
Changes in calcium absorption during adolescence, such as those during pregnancy, are likely mediated primarily by hormonal factors and less by changes in vitamin D status, excluding adolescents who are severely vitamin D deficient. As such, evaluating the effects of vitamin D directly on calcium absorption in children may best be done in those who are prepubertal. Although it would be ideal to evaluate a range of intakes and vitamin D status in each child studied, this is impractical. Therefore, we chose to evaluate a single dose, 1000 IU/d, that reflects a supplement amount that is easily obtainable in the marketplace and commonly recommended.
We hypothesized that consumption of a 1000-IU/d supplement of vitamin D for 8 wk would significantly increase serum 25(OH)D without increasing calcium absorption in a population of healthy 4–8-y-old children not identified as having a high risk of vitamin D insufficiency. We conducted a randomized, double-blind, placebo-controlled trial to ensure that changes over time in a supplemented group could be compared with those in a nonintervention group.
SUBJECTS AND METHODS
Subjects and research studies
The subjects for the study were selected to approximately match the ethnic distribution of the greater Houston, Texas, area. To be enrolled, subjects had to be healthy, to not be using any medications or multivitamins/minerals, and to have a usual dietary calcium intake of 600 to 1200 mg/d. Written informed consent was obtained from a parent or legal guardian for each subject. The Institutional Review Board of Baylor College of Medicine and Affiliated Hospitals approved the protocol.
The subjects were 4.0–8.9 y of age at the time they started the study. We targeted an enrollment of 32 subjects for both the placebo and supplement groups to ensure that 30 subjects completed the study. No meaningful changes were made to the protocol once the study commenced. The primary outcome was fractional and total calcium absorption and 25(OH)D concentrations before and after an 8-wk supplementation period with either 1000 IU vitamin D3 or placebo measured by using a dual-stable-isotope technique.
Study procedures
This randomized, double-blind, controlled trial included 3 visits to the General Clinical Research Center of Texas Children's Hospital in Houston, Texas. The first was an outpatient screening visit to confirm eligibility, the second an inpatient baseline study visit to determine calcium absorption, and the third an inpatient follow-up study visit after intervention to determine calcium absorption. To confirm eligibility, usual dietary nutrient intake was determined from two 24-h telephone dietary recalls and a 3-d weighed dietary record at home that subjects performed immediately after the screening visit.
Vitamin D concentrations [25(OH)D and 1,25-dihydroxyvitamin D (1,25(OH)2D)], parathyroid hormone (PTH) concentrations, and alkaline phosphatase activity were measured at the time of each calcium absorption study visit. Serum 25(OH)D samples were sent to ARUP Laboratories for analysis by using a Diasorin quantitative chemiluminescent immunoassay with an analytic sensitivity of 4 ng/mL (LIAISON; DiaSorin Inc) and a CV of 3% to 4%. This laboratory participates in the NIH Office of Dietary Supplements Vitamin D standardization program. The analyses included both 25(OH)D3 and 25(OH)D2. Serum 1,25(OH)2D was measured by LabCorp with the use of a radioimmunoassay with a lower limit of detection of 2 pg/mL and a CV of 5% to 10%. Intact PTH was measured by Quest Diagnostics by using an immunochemiluminometric assay with a lower limit of detection of 3 pg/mL and a CV of 2%. Alkaline phosphatase activity was quantitatively measured by using the VITROS ALKP slide method by Texas Children's Hospital with a CV of 2% to 3%.
Baseline studies of total-body bone mineral content (BMC) were performed by dual-energy X-ray absorptiometry with a Hologic Delphi Model (Hologic Inc). The dual-energy X-ray absorptiometry instrument undergoes regularly scheduled quality-control testing for phantom reproducibility and signal uniformity. Subject scanning does not take place unless all quality control results fall within acceptable limits. Values were compared with age-, sex-, and race-matched normal values for our institution (http://www.bcm.edu/bodycomplab).
Once the results from the baseline blood tests were available, the study dietitian reported the baseline 25(OH)D concentration to the Investigational Pharmacy of Texas Children's Hospital, who assigned participants to the intervention groups. The subjects were randomly assigned by baseline 25(OH)D concentrations to receive 8 wk of 1000 IU vitamin D3/d supplementation or placebo. The randomization scheme was generated by using the website randomization.com (http://www.randomization.com). The subjects were stratified by their baseline 25(OH)D concentrations as low (<20 ng/mL), middle (20–32 ng/mL), or high (>32 ng/mL). On the basis of the subject's baseline vitamin D concentration, the pharmacist referred to the appropriate randomization table (low, medium, or high) and assigned the next treatment assignment on that table, either vitamin D supplement or placebo. Randomization tables were generated in blocks of 2 (vitamin D3 and placebo) so that if one serum vitamin D concentration group enrolled significantly more than the others, the treatment assignments would still come out as 1:1. The subjects were assigned ∼1 wk after the first inpatient visit once laboratory results were finalized so that baseline serum 25(OH)D concentrations could be reported to the Investigational Pharmacy. The principal investigator, study dietitian, study staff, nurses, and participants were all blinded throughout the study. Only the Investigational Pharmacists who were not involved in the data collection or analysis were aware of study group assignment.
Dietary methods
At the screening visit, the study dietitian asked subjects what they usually ate on a normal day with the use of two 24-h dietary recalls, and food preferences were obtained. Inpatient menus for the overnight study visit were based on the usual daily calcium intake. All foods and beverages during the inpatient and outpatient visits were weighed before and after consumption to accurately determine intake. The subjects were provided food scales and were instructed to keep weighed food records for 3 d at home after the first inpatient calcium-absorption stable-isotope test and for 3 d at home before the second inpatient calcium-absorption stable-isotope test. To reflect the marketplace changes in dietary food contents during the study, dietary intake data were collected by using Nutrition Data System for Research software developed by the Nutrition Coordinating Center (University of Minnesota).
Supplement
Vitamin D3 (1000 IU/mL) was added to almond oil (sweetened with 0.1% saccharin powder) and flavored with orange oil. The placebo solution was prepared the same way without the vitamin D. The 2 study solutions were provided by Greenpark Compounding Pharmacy. The amount of vitamin D in the supplements was measured by an independent laboratory (Professional Compounding Centers of America) to confirm that the solution contained 1.0 million–1.3 million IU/g by using quality-control procedures compliant with current Good Manufacturing Practices and Regulations. The subjects were provided with calendars and instructions for marking each day that they remembered to take the supplement solution to monitor day-to-day compliance. In addition, supplement solution bottles were returned at the end of the study to calculate the amount of liquid consumed over the 8-wk period.
Calcium absorption and analytic methods
Calcium absorption efficiency (fractional absorption of calcium, commonly expressed as the percentage of absorption) was measured by using a dual-tracer stable-isotope technique that we described in detail previously (6, 9). A stable isotope of calcium (1.2 mg 42Ca) was administered intravenously over ∼2 min. Subsequently, the children were given a standard breakfast. Toward the end of breakfast, the subjects were given a stable isotope of calcium (20 μg 46Ca) that had been mixed with 120 mL Ca and vitamin D–fortified orange juice. Meals were identical for each subject at their 2 visits with a calcium load reflecting one-third of their usual dietary intake.
Beginning with breakfast, a complete 24-h urine collection was obtained. Urine samples were prepared for thermal ionization mass spectrometric analysis as previously described by using an oxalate precipitation technique (9). The urine samples obtained were analyzed to determine their calcium isotope ratios by using a Finnigan MAT 261 (Thermoquest) magnetic sector thermal ionization mass spectrometer.
Fractional absorption of calcium was calculated as the relative recovered oral compared with intravenous isotope in a 24-h urine specimen collected at the time of the tracer administration as previously described (10, 11). Total calcium absorption was calculated as the product of calcium intake and fractional calcium absorption.
Sample size determination
Sample size determination was based on considering a difference in total calcium absorption (fractional absorption × dietary intake) of ∼50 mg/d as being biologically significant, because this is the difference that might be seen with a typical calcium supplement in this age group. We hypothesized a mean calcium absorption efficiency of 30% and an SD of 30% of the mean (∼9%), which resulted in total absorbed calcium of 240 ± 72 mg/d (10, 12). Assuming the smallest clinically significant difference in calcium absorption to be 50 mg/d (equivalent to the increment in calcium absorption from typical calcium supplements), a sample size of 30 in each group would have a power of >95% to detect such a difference at P < 0.05. This comparison was based on a change from baseline in the supplement group, assuming no change in the placebo group. We also preplanned to compare the changes between the start and end of the study in the 2 groups and to evaluate the relative changes in the 2 groups in serum 25(OH)D, 1,25(OH)2D, and PTH.
Statistical methods
The primary outcome was the effect of the intervention on calcium absorption variables over the 8 wk of the study. This was analyzed by general linear modeling with the use of the intervention as a group characteristic and baseline values to determine the effects of treatment group on outcomes. The relation between percentage and total calcium absorption and 25(OH)D was evaluated by linear regression analysis. Baseline differences in values between supplement and placebo groups were determined by unpaired t tests. Within-group endpoint differences were determined by paired t tests. All data were analyzed by using SPSS (version 16.0; SPSS Inc). Study data are reported as means ± SDs, except as specifically noted.
RESULTS
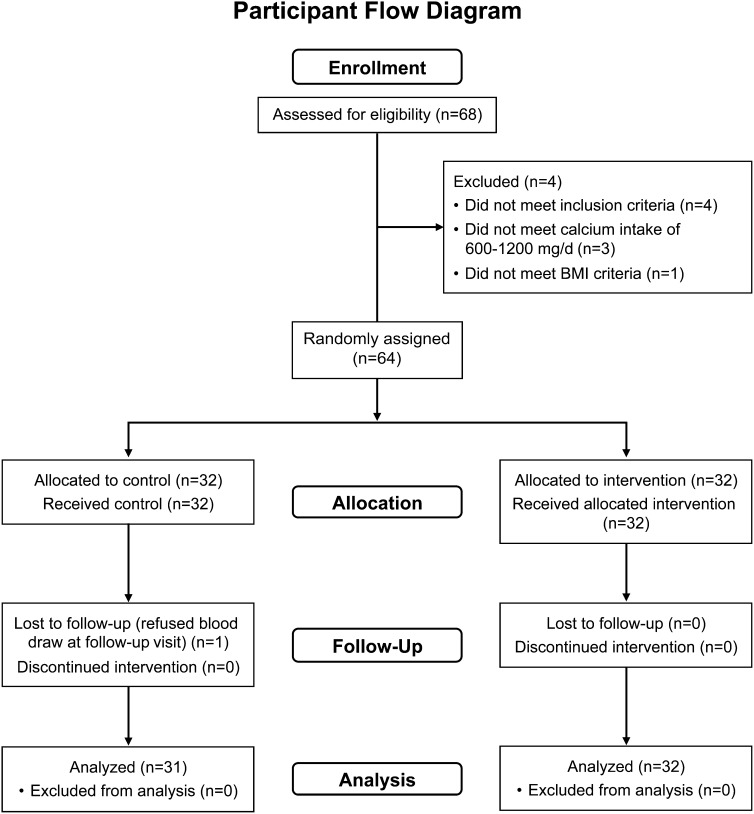
Baseline findings for the study population are shown in Table 1. There were 27 white, 12 African American, 23 Hispanic, and 1 Asian subject, which closely represented the racial and ethnic distribution of the greater Houston area. One subject (supplement group) did not return for follow-up; therefore, the final number of enrolled subjects was 31 in the placebo group and 32 in the supplement group (Figure 1). Whereas intention-to-treat analysis using the last value carried forward might have been considered, it was not appropriate for this study because the subject who dropped out was in the vitamin D supplement group. Therefore, that subject was excluded from the final analysis. Subjects were recruited throughout the year. No significant difference in season of study was found between the placebo and supplement groups. Furthermore, serum 25(OH)D was not significantly associated with the season of measurement at baseline (P = 0.9); therefore, the season of measurement was not considered further in the analysis. Enrollment occurred continuously from July 2009 to August 2011. No adverse side effects were noted in either group. On the basis of the before and after weights of the supplement bottles as a marker of compliance, 86% (n = 50) of the subjects consumed the supplement ≥75% of the days during the 8-wk period. Six subjects (10%) did not return their supplement bottles at the end of the study. The percentage compliance was not related to study outcome. The trial ended after all study visits were completed.
TABLE 1.
Baseline characteristics of the subjects who completed a controlled trial evaluating the effects of 1000 IU vitamin D3/d compared with placebo1
| Characteristic | Placebo group(n = 31) | Supplement group, 1000 IU/d (n = 32) | P-difference2 |
| Age (y) | 6.4 ± 1.4 | 6.7 ± 1.4 | 0.96 |
| Weight (kg) | 21.7 ± 3.8 | 23.3 ± 4.6 | 0.37 |
| BMI z score | −0.1 ± 0.8 | 0.0 ± 0.7 | 0.9 |
| Dietary calcium intake (mg/d) | 793 ± 185 | 864 ± 206 | 0.21 |
| Dietary vitamin D intake (IU/d) | 216 ± 80 | 224 ± 80 | 0.91 |
| Serum 25(OH)D (ng/dL) | 27.6 ± 7.3 | 27.7 ± 7.4 | 0.68 |
| Serum calcium (mg/dL) | 9.8 ± 0.5 | 9.6 ± 0.4 | 0.19 |
| Serum 1,25(OH)2D (pg/mL) | 58.1 ± 16.3 | 53.7 ± 11.1 | 0.64 |
| Total-body bone mineral content z score3 | 0.13 ± 0.80 | 0.15 ± 0.85 | 0.49 |
All values are means ± SDs. 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
Determined by unpaired t tests.
Compared with internal age-, sex-, and race-matched population (http://www.bcm.edu/bodycomplab/Flashapps/AllDXArefsChartpage.html).
FIGURE 1.
Consolidated Standards of Reporting Trials flow diagram for enrollment in controlled trial of vitamin D supplementation in 4–8-y-old children.
Eight of the 63 subjects had a baseline 25(OH)D value <20 ng/mL, and 1 subject had a concentration <10 ng/mL (at 9 ng/mL). No subject had a concentration >50 ng/mL at baseline, and the highest concentration was 44 ng/mL. None of the baseline PTH values were >50 pg/mL, and 8 were >30 pg/mL. Dual-energy X-ray absorptiometry scans showed that no subject had a total-body BMC z score below −1.5, and 2 subjects had BMC z scores above +2.0.
Calcium absorption
Results for calcium intake, absorption, and urinary excretion are shown in Table 2. Although baseline values for both calcium intake and fractional absorption were not significantly different between the placebo and supplement groups, both were slightly higher in the supplement group. The product of the two, total calcium absorption, was significantly higher at baseline in the supplement group. The results of the analysis shown in Table 2 indicate that there were no significant differences in end of study fractional or total calcium absorption based on study group assignment (supplement or placebo) by using baseline values as covariates.
TABLE 2.
Calcium absorption and urinary calcium excretion before the intervention in subjects who completed a controlled trial of 1000 IU vitamin D3/d compared with placebo1
| Placebo group (n = 31) |
Supplement group (n = 32) |
P-difference |
||||||
| Preintervention study | Postintervention study | P-difference2 | Preintervention study | Postintervention study | Supplement group(preintervention vs placebo group preintervention)2 | Supplement group (pre- and postintervention)2 | Supplement group (postintervention vs placebo group postintervention)3 | |
| Calcium intake (mg/d) | 793 ± 185 | 731 ± 182 | 0.11 | 864 ± 206 | 812 ± 208 | 0.15 | 0.13 | 0.28 |
| Calcium absorption efficiency (%) | 29.9 ± 7.8 | 31.7 ± 9.5 | 0.30 | 33.5 ± 9.8 | 34.6 ± 12.3 | 0.11 | 0.58 | 0.75 |
| Total calcium absorption (mg/d) | 236 ± 78 | 228 ± 77 | 0.65 | 285 ± 98 | 277 ± 119 | 0.030 | 0.62 | 0.36 |
| Urinary excretion (mg/d) | 53 ± 54 | 53 ± 46 | 0.92 | 65 ± 40 | 72 ± 40 | 0.34 | 0.34 | 0.17 |
All values are means ± SDs.
Determined by unpaired t tests.
Group differences were analyzed by using a general linear model in which group assignment and baseline value were used as covariates in the analysis.
Of note, a significant correlation in both the placebo and supplement groups was found between the fractional absorption of calcium at the start and end of the study: whole group (n = 63; r = 0.46, P < 0.001), supplement group (n = 32; r = 0.50, P = 0.004), and placebo group (n = 31; r = 0.36, P = 0.046).
Vitamin D and parathyroid hormone
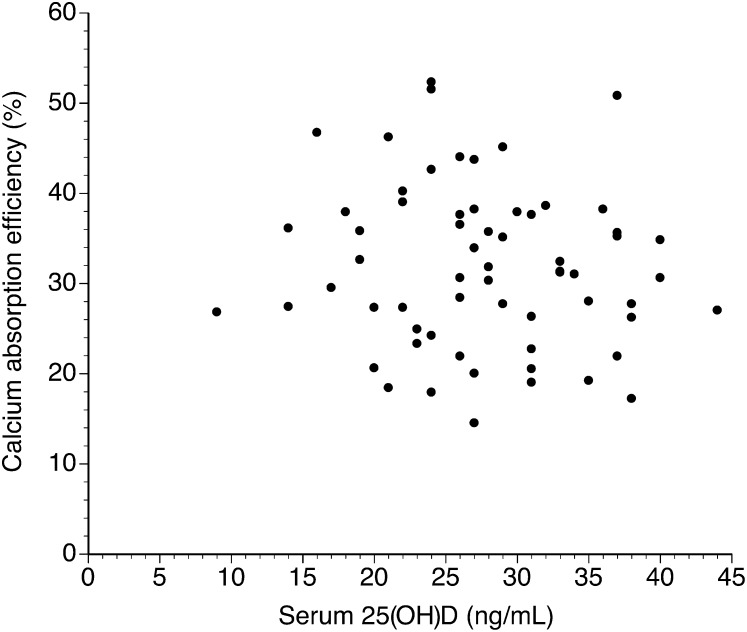
No significant relation was found between baseline fractional or total calcium absorption and serum 25(OH)D or 1,25(OH)2D concentrations. The relation between fractional absorption and serum 25(OH)D at baseline is shown in Figure 2. The results were similar for the correlations between fractional absorption and 1,25(OH)2D (r < 0.01, P = 0.83). Values for total calcium absorption also showed no significant relation with either 25(OH)D (r = 0.03, P = 0.80) or 1,25(OH)2D (r = 0.09, P = 0.48).
FIGURE 2.
Relation between 25(OH)D and calcium absorption efficiency in all subjects at baseline determined by linear regression analysis (n = 63; r = 0.10, P = 0.41). 25(OH)D, 25-hydroxyvitamin D.
At the end of the 8-wk study, there was no relation overall between serum 25(OH)D and fractional calcium absorption (r = 0.17, P = 0.18), nor was there a difference between subjects in the placebo group (r = 0.03, P = 0.52) or the supplement group (r = −0.31, P = 0.08), although a trend toward a negative relation was noted for the supplement group. Similar results were seen for the relation with total calcium absorption.
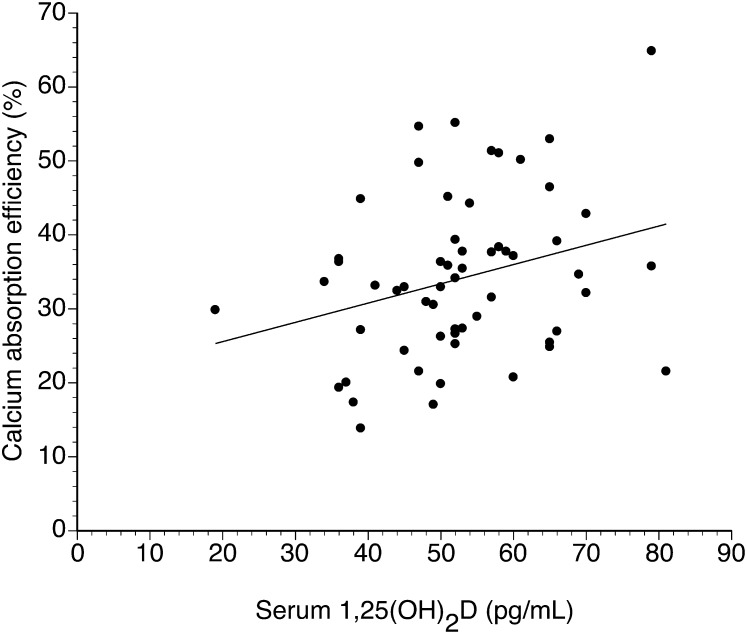
For 1,25(OH)2D, although there was no significant relation with fractional calcium absorption at baseline, they were significantly related at the end of the study (r = 0.29, P = 0.029) (Figure 3). Serum PTH was not significantly related to calcium absorption efficiency at the start (r = 0.09, P = 0.48) or end (r = 0.16, P = 0.22) of the study.
FIGURE 3.
Relation between 1,25(OH)2D and calcium absorption efficiency in all subjects at the end of the study period determined by linear regression analysis (n = 63; r = 0.29, P = 0.029, SE of slope = 0.12). y = 0.26x + 20.41. 25(OH)2D, 1,25-dihydroxyvitamin D.
The changes in serum 25(OH)D and PTH during the study are shown in Table 3. A significant increase (P < 0.001) in 25(OH)D was associated with supplementation and with a significantly higher final 25(OH)D concentration in the supplement group than in the placebo group. Of note was the finding of significant suppression of PTH in the supplement group but not in the placebo group. The differences at the end of the study between the placebo and supplement group were significantly different (P < 0.001).
TABLE 3.
Changes in vitamin D and PTH concentrations in subjects who completed a controlled trial of 1000 IU vitamin D3/d compared with placebo1
| Placebo group (n = 31) |
Supplement group (n = 32) |
P-difference |
|||||
| Preintervention study | Postintervention study | P-difference2 | Preintervention study | Postintervention study | Supplement group (pre- and postintervention)2 | Supplement group (postintervention vs placebo group postintervention)3 | |
| Serum 25(OH)D (ng/mL) | 27.6 ± 7.3 | 29.9 ± 12.4 | 0.25 | 27.7 ± 7.4 | 36.0 ± 10.3 | <0.001 | 0.012 |
| Serum PTH (pg/mL) | 18.4 ± 10.5 | 17.6 ± 10.9 | 0.67 | 21.4 ± 10.4 | 12.9 ± 7.1 | <0.001 | <0.001 |
| Serum 1,25(OH)2D (ng/mL) | 58.7 ± 16.8 (24) | 52.2 ± 11.6 (24) | 0.12 | 53.3 ± 11.1 | 53.9 ± 13.1 | 0.85 | 0.77 |
All values are means ± SDs. n in parentheses. At baseline, serum 25(OH)D was similar between groups (P = 0.98), serum PTH was similar between groups (P = 0.27), and serum 1,25(OH)2D was similar between groups (P = 0.23). PTH, parathyroid hormone; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
Determined by using paired t tests.
Group differences were analyzed by using a general linear model in which group assignment and baseline value were used as covariates in the analysis.
At the end of the study, 3 subjects had serum 25(OH)D values >50 ng/mL, 2 of which were in the supplement group (53 and 60 ng/mL) and 1 of which was the highest value and occurred in the placebo group (86 ng/mL). That subject was a 7-y-old Hispanic boy who was studied during early and late summer; his baseline 25(OH)D concentration was 35 ng/mL.
The relation between total vitamin D intake from the diet and supplement and serum 25(OH)D was evaluated by using the method from the IOM (1), in which the serum 25(OH)D concentration was divided by the natural log intake to identify a conversion constant and the range of that constant measured. For the entire 126 measurements, the mean (±SD) constant was 5.3 ± 1.7, or serum 25(OH)D = 5.3 × ln (total dietary vitamin D intake). Evaluation of the only subgroups led to little change in this value. For example, looking only at the follow-up postintervention measurements, the mean (±SD) constant was 5.4 ± 1.9.
DISCUSSION
Our study was the first of which we are aware to directly assess the effects in prepubertal children of a biologically meaningful dose of vitamin D3 on calcium absorption by using a randomized, double-blind, parallel, placebo-controlled trial. We found no increase in calcium absorption fraction with this intervention over 8 wk but also found a significant increase in serum 25(OH)D concentration to a mean >32 ng/mL and a statistically significant decrease in serum PTH with intervention. Neither of these changes in serum 25(OH)D or PTH occurred in the placebo group.
These findings suggest that small children have responses (eg, changes in PTH concentration) to vitamin D intake appropriate and similar to those in older children and adults. They do not, however, suggest any specific benefit in our population of children from the southern part of the United States to supplementation with 1000 IU vitamin D/d related to calcium metabolism or to achieving a serum 25(OH)D concentration >32 ng/mL.
Most of the scientific and medical literature related to vitamin D and bone health in children is from either infants or toddlers who are at high risk of rickets because of the very low content of vitamin D in breast milk and relatively low calcium concentration of human milk for older infants or in adolescents. Prepubertal children, however, are also an important group. Traumatic fractures occur frequently in this age group, and dietary patterns are established for adolescents and adulthood during this time period.
The estimated average requirement of 400 IU/d and the RDA of 600 IU/d were established for children largely based on data from adolescents and adults. Limited studies suggest that the response rate of 25(OH)D to supplementation in children older than the age of ∼6 y was similar to that of adolescents and adults (1). The 8-ng/mL increase in 25(OH)D in the supplement group (1000 IU/d) in this study was very similar to the 1-nmol/L increase per 40-IU/d (25 nmol/L, or 10 ng/mL per 1000 IU) supplement described by the IOM as being typical, recognizing a considerable amount of variability based on baseline concentration (1). However, for the ratio of serum 25(OH)D divided to the ln (total vitamin D intake), the mean value of our subjects (∼5.4) fell below the value of 9 to 10 in the IOM report. We identified little seasonal effect on 25(OH)D concentrations. This is expected in an area where there is solar conversion to vitamin D year-round and many children spend the summers indoors because of the heat. However, in a few children, very high concentrations of 25(OH)D are sometimes seen in the summer in children with a large amount of outdoor time (10). Overall, these data suggest that the effects of a 1000-IU/d supplement in a southern climate will be at or somewhat below that expected in other populations.
In this study, we found no significant relation between serum 25(OH)D and calcium absorption fraction (efficiency of utilization of dietary calcium) or total calcium absorbed. This finding is similar to that shown in several cross-sectional studies (6, 7, 10). Absorption efficiency of 30% to 35% in this study is within the range found in our earlier study of 7–8-y-old girls, although in that study dietary calcium intakes averaged ∼1100 mg/d, which is somewhat higher than in this study (10). Furthermore, we found no significant increase in urinary calcium excretion with vitamin D supplementation. We note that our current data, similar to those reported previously, did not include virtually any children with serum 25(OH)D values <12–14 ng/mL, and we do not suggest that these results can be applied to children with such low values for serum 25(OH)D. Our data do not allow a determination of the lowest value of 25(OH)D that is not associated with decreased calcium absorption efficiency.
Of interest was the finding that after 8 wk of supplementation, we found a significant relation between 1,25(OH)2D concentration and calcium absorption. Although this may be a chance finding or one without important physiologic meaning, we previously found a similar relation between calcium absorption and 1,25(OH)2D but not 25(OH)D (6). This relation does not, however, provide direct evidence of any given level of vitamin D intake or target for 1,25(OH)2D.
The suppression of PTH with vitamin D supplementation is also of interest. This was expected based on cross-sectional (13) studies. However, because PTH concentrations were not elevated at the start of our study, it is uncertain what the functional consequences of this suppression might be in healthy children. It would be of interest to know whether lower PTH concentrations sustained over a longer period would lead to beneficial or harmful effects on calcium absorption and bone mineral accretion. No data are currently available to specifically answer these questions and long-term studies would be needed to evaluate these relationships in a meaningful manner.
Our data do not provide evidence for any specific dietary requirement for vitamin D, especially for a vitamin D–sufficient population. We selected a supplement of 1000 IU/d because this is an amount that has widely been recommended for all age groups, including children (14). Since then, a large range of vitamin D intakes have been recommended, from the RDA of 600 IU/d to much higher intakes (1–4).
Our study was limited by enrolling relatively healthy children who were not subject to very low 25(OH)D concentrations despite relatively low intakes. By design, our population was a diverse one in ethnicity and race, but did not include those who were obese or taking chronic medications. Still, our data provide no support for providing 1000 IU vitamin D/d to healthy children or specifically targeting 25(OH)D concentrations >30 ng/mL, at least as related to calcium absorption. Although it has been suggested that factors other than vitamin D primarily drive higher calcium absorption during puberty (15), these data indicate that higher serum 25(OH)D concentrations, at least those above the presumed target of 30 ng/mL, do not lead to increased calcium absorption in children from 4 to 8 y of age.
Acknowledgments
We acknowledge the support and contributions of Texas Children's Hospital Investigational Pharmacists Jennifer Lynds and Tara McCartney (Texas Children's Hospital General Clinical Research Center Staff) and students Melissa Mohammed, Michelle Taub, and Jennifer Haden.
The authors’ responsibilities were as follows—SAA: designed the research protocol, analyzed the data, wrote the manuscript, and had primary responsibility for the final content of the manuscript; KMH: assisted with the design of the research protocol, conducted the research, recorded and analyzed the data, assisted in writing the manuscript, and had secondary responsibility for the final content of manuscript; and ZC: analyzed all isotope recovery samples on the mass spectrometer and assisted with the manuscript preparation. The authors did not have any conflicts of interest to disclose.
Footnotes
Abbreviations: BMC, bone mineral content; IOM, Institute of Medicine; PTH, parathyroid hormone; RDA, Recommended Dietary Allowance; 25(OH)D, serum 25-hydroxyvitamin D; 1,25(OH)2D, serum 1,25-dihydroxyvitamin D.
REFERENCES
- 1.Institute of Medicine Dietary Reference Intakes for calcium and vitamin D. Washington, DC: National Academies Press, 2011 [PubMed] [Google Scholar]
- 2.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30 [DOI] [PubMed] [Google Scholar]
- 3.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007;85:649–50 [DOI] [PubMed] [Google Scholar]
- 4.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr 2005;135:317–22 [DOI] [PubMed] [Google Scholar]
- 5.Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, et al. IOM committee members respond to endocrine society vitamin D guideline. J Clin Endocrinol Metab 2012;97:1146–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abrams SA, Griffin IJ, Hawthorne KM, Gunn SK, Gundberg CM, Carpenter TO. Relationships among vitamin D levels, PTH, and calcium absorption in young adolescents. J Clin Endocrinol Metab 2005;90:5576–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weaver CM, McCabe LD, McCabe GP, Braun M, Martin BR, Dimeglio LA, Peacock M. Vitamin D status and calcium metabolism in adolescent black and white girls on a range of controlled calcium intakes. J Clin Endocrinol Metab 2008;93:3907–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park CY, Hill KM, Elble AE, Martin BR, DiMeglio LA, Peacock M, McCabe GP, Weaver CM. Daily supplementation with 25 μg cholecalciferol does not increase calcium absorption or skeletal retention in adolescent girls with low serum 25-hydroxyvitamin D. J Nutr 2010;140:2139–44 [DOI] [PubMed] [Google Scholar]
- 9.Abrams SA. Using stable isotopes to assess mineral absorption and utilization by children. Am J Clin Nutr 1999;70:955–64 [DOI] [PubMed] [Google Scholar]
- 10.Abrams SA, Copeland KC, Gunn SK, Stuff JE, Clarke LL, Ellis KJ. Calcium absorption and kinetics are similar in 7- and 8-year-old Mexican-American and Caucasian girls despite hormonal differences. J Nutr 1999;129:666–71 [DOI] [PubMed] [Google Scholar]
- 11.Abrams SA, Copeland KC, Gunn SK, Gundberg CM, Klein KO, Ellis KJ. Calcium absorption, bone accretion and kinetics increase during early pubertal development in girls. J Clin Endocrinol Metab 2000;85:1805–9 [DOI] [PubMed] [Google Scholar]
- 12.Ames SK, Ellis KJ, Gunn SK, Copeland KC, Abrams SA. Vitamin D receptor gene Fok1 polymorphism predicts calcium absorption and bone mineral density in children. J Bone Miner Res 1999;14:740–6 [DOI] [PubMed] [Google Scholar]
- 13.Hill KM, McCabe GP, McCabe LD, Gordon CM, Abrams SA, Weaver CM. An inflection point of serum 25-hydroxyvitamin D for maximal suppression of parathyroid hormone is not evident from multi-site pooled data in children and adolescents. J Nutr 2010;140:1983–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holick MF. The vitamin D epidemic and its health consequences. J Nutr 2005;135:2739S–48S [DOI] [PubMed] [Google Scholar]
- 15.Abrams SA, Strewler GJ. Adolescence: how do we increase intestinal calcium absorption to allow for bone mineral mass accumulation? Bonekey Osteovision 2007;4:147–57 [Google Scholar]