Abstract
Netrin and semaphorin axon guidance cues have been found to function in the genesis of several mammalian organs; however, little is known about the underlying molecular mechanisms involved. A genetic approach could help to reveal the underpinnings of these mechanisms. The most anterior ray sensillum (ray 1) in the Caenorhabditis elegans male tail is frequently displaced anterior to its normal position in smp-1/semaphorin-1a and plexin-1/plx-1 mutants. Here we report that UNC-6/netrin and its UNC-40/DCC receptor signal in parallel to SMP-1/semaphorin-1a and its PLX-1/plexin-1 receptor to prevent the anterior displacement of ray 1 and that UNC-6 plus SMP-1 signaling can account entirely for this function. We also report that mab-20/semaphorin-2a mutations, which prevent the separation of neighboring rays and cause ray fusions, suppress the anterior displacements of ray 1 caused by deficiencies in SMP-1 and UNC-6 signaling and this is independent of the ray fusion phenotype, whereas overexpression of UNC-40 and PLX-1 cause ray fusions. This suggests that for ray 1 positioning, a balance is struck between a tendency of SMP-1 and UNC-6 signaling to prevent ray 1 from moving away from ray 2 and a tendency of MAB-20/semaphorin-2a signaling to separate all rays from each other. Additional evidence suggests this balance involves the relative adhesion of the ray 1 structural cell to neighboring SET and hyp 7 hypodermal cells. This finding raises the possibility that changes in ray 1 positioning depend on passive movements caused by attachment to the elongating SET cell in opposition to the morphologically more stable hyp 7 cell. Several lines of evidence indicate that SMP-1 and UNC-6 function permissively in the context of ray 1 positioning.
Keywords: UNC-6/netrin, permissive function, organogenesis, cell and organ positioning
IN recent years several known axon guidance molecules have been discovered to play important roles in the morphogenesis of various vertebrate organs. For example, netrin signaling has been found to profoundly affect organogenesis of lung, kidney, mammary, and cardiovascular structures (Hinck 2004; Baker et al. 2006). Semaphorin signaling also affects development of kidney (Villegas and Tufro 2002; Tufro et al. 2008), heart (Behar et al. 1996; Feiner et al. 2001; Gu et al. 2003; Gitler et al. 2004), bone (Behar et al. 1996; Delorme et al. 2005; Sutton et al. 2008), lung (Ito et al. 2000; Kagoshima and Ito 2001), and cerebrum (Kerjan et al. 2005) in vertebrates. Furthermore, netrins and semaphorins and their receptors also have roles in tumor angiogenesis, which can affect tumor progression and metastasis (Serini et al. 2003; Park et al. 2004; Chedotal et al. 2005; Klagsbrun and Eichmann 2005; Larrivee et al. 2007; Castets and Mehlen 2010). The molecular mechanisms used by these axon guidance cues and their receptors in vertebrate organogenesis are still poorly understood. In most cases, it is not known whether these cues play instructive roles in the formation of specific organs (the way they do for axon guidance), or whether they affect organogenesis permissively, (i.e., noninstructively) (Nasarre et al. 2010).
Following the discovery of semaphorins in Drosophila and vertebrates (Kolodkin et al. 1992, 1993; Luo et al. 1993), analyses of Semaphorin function in Caenorhabditis elegans development were undertaken (e.g., Roy et al. 2000; Fujii et al. 2002). It was found not only that a C. elegans homolog of the secreted MAB-20/semaphorin-2a ligand has a minor role as an axon guidance cue in the nervous system of C. elegans (Roy et al. 2000; Wang et al. 2008), but that it also functions in positioning the processes of 18 groups of three cells comprising the posterior male copulatory sensillae of the male tail called rays (Roy et al. 2000).
The nine rays on each side of the male tail are used to sense the hermaphrodite prior to copulation (Sulston et al. 1980). Each ray sensillum comprises sensory endings from two neurons encircled by the expanded tip of a structural support cell. The support cell and neuron endings are embedded in the fan (a lateral cuticular specialization made by the hypodermis, which is a syncytial epidermis), such that most ray neuron endings protrude through the support cell and the edges of the fan in a characteristic position within a roughly linear A/P oriented array of these sense organs, with ray 1 being most anterior and ray 9 being most posterior on each side (Figure 1A). Other nematode species have variant but reproducible patterns of rays, indicating genetic variation in other species of the mechanisms determining ray positioning (Fitch and Emmons 1995).
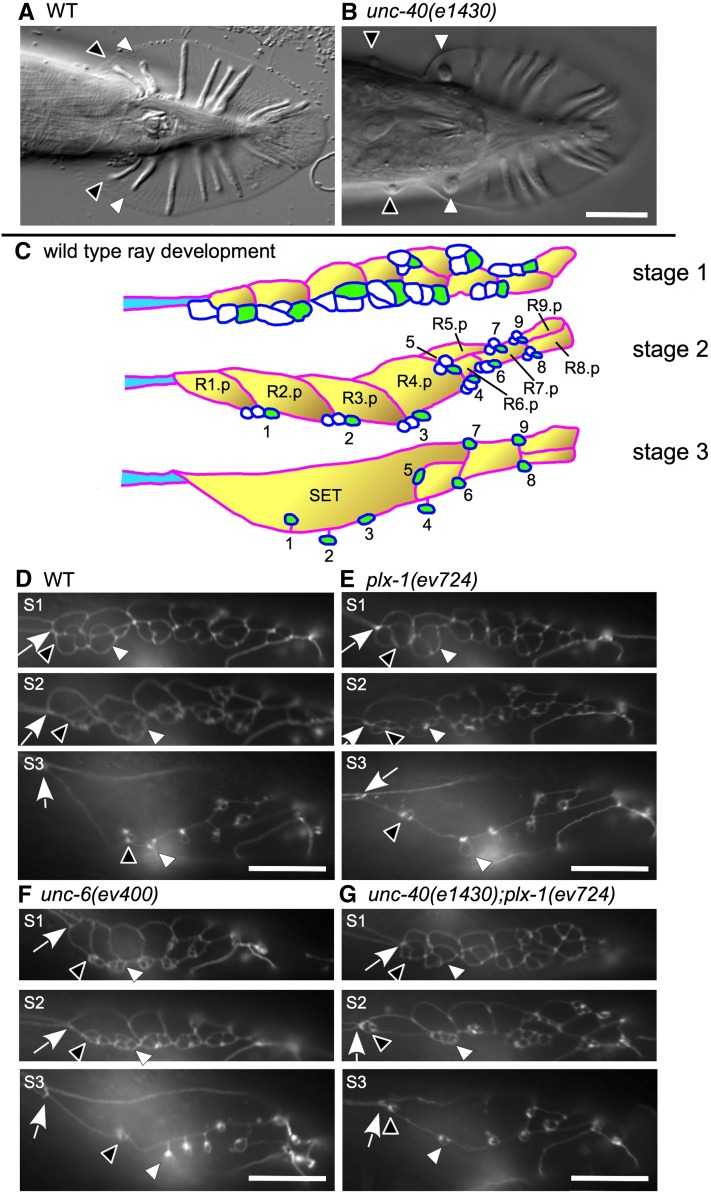
Figure 1 .
Mutants of semaphorin-1a and UNC-6 mediated signaling components share the same ray 1 anterior displacement phenotype. In all panels, anterior is to the left, dorsal is up. (A and B) Ray 1 and 2 process clusters are indicated by black and white arrowheads, respectively, for adult wild-type (A) and unc-40(e1430) (B) mutant males. Note the increased distance between ray 1 and the next adjacent ray (ray 2) in unc-40 mutants. (C) Schematics of stages 1–3 of ray development (stage 4 is shown in Figure 6) (adapted from Emmons 2005). The eff-1 promoter drives expression in Rn.p cells (n = 1–9) (yellow cells with a red outline) at the onset of Rn.p cell fusion (stages 2–4) (Mohler et al. 2002). The lin-32 promoter drives expression in all ray neurons (white cells with blue outline) and in the ray structural cells (green cells) during stages 1 and 2, and diminishes in later stages (Portman and Emmons 2000). The ram-5 promoter drives expression in all ray structural cells starting in stage 1 and onward (Yu et al. 2000). The schematized cells are sandwiched between dorsal and ventral portions of hyp 7 (white background). The body seam is shown in part (blue cell). (D–F) Epifluorescence microscopy images of male ray development in animals carrying the ajm-1::GFP gene reporter. Arrows indicate the seam–SET junction. For each strain, stage 1 (S1), stage 2 (S2), and stage 3 (S3) of male development are shown. No significant differences can be found between wild-type and mutant strains at stage 1 (D–G). However, plx-1(ev724) (E, S2) and unc-6(ev400) (F, S2) single mutants have their ray 1 cell groups displaced anterior to normal at late stage 2 as compared to wild type (D, S2). unc-40(e1430); plx-1(ev724) double mutants show a more severe anterior ray 1 cell group displacement at stages 2 and 3 (G, S2 and S3) and as compared to wild type (D, S2 and S3), plx-1(ev724) (E, S2 and S3), and unc-6(ev400) (F, S2 and S3) single mutants. In this case, the ray 1 cell group either contacts the seam–SET boundary (G, S2) or localizes close to it (G, S3).
Ray formation (Baird et al. 1991) begins with the division of ray precursor cells R1–R9 (Rn cells) in the male tail. The posterior daughter of this division is the Rn.p cell and the anterior daughter is Rn.a (where n denotes the position of the Rn ray precursor along the A/P axis with R1 most anterior and “a” and “p” designate anterior and posterior daughters, respectively, of the progenitor) (Figure 1, C and D). The Rn.a cell then divides twice to produce three cells of each ray sensillum and one cell death. The three cells remain attached to each other as a cluster by what appears to involve specific affinities between cells derived from the same precursor cell. In stage 1 of sensory ray development (as defined in Emmons 2005), each ray cluster contacts neighboring clusters forming a roughly linear arrangement of presumptive ray sensillae along the A/P axis (Figure 1, C and D; stage 1) and these presumptive sensillae are sandwiched between the hyp 7 hypodermal cell and A/P-arranged Rn.p cells. The R1.p–R5.p cells then fuse beginning with R1.p and R2.p and progressing toward the posterior to form the A/P-elongated lateral hypodermal seam cell of the tail (SET), which is aligned with and just posterior to the major lateral hypodermal cell of the body (simply referred to below as the body seam). As Rn.p cells fuse, cell bodies belonging to each ray cell then undergo subhypodermal migration while their corresponding processes remain in the hypodermal layer attached to processes of the same presumptive ray but no longer contacting processes from adjacent ray clusters. At the end of stage 2 (Emmons 2005), the ray cell processes adopt a specific anterior–posterior position at or near the junction of the two most proximal dorsal epidermal/hypodermal cells (Rn.p cells) and the ventral hypodermal cell hyp 7 (Figure 1, C and D; stage 2). For example, the three-cell ray 1 cluster associates with the junction of R1.p, R2.p, and hyp 7, whereas the ray 2 cluster associates with the junction of R2.p, R3.p, and hyp 7. Some dorsal–ventral positioning refinements occur later for the first, second, and fourth clusters, due to engulfment by the lateral SET (e.g., ray 1) or ventral hyp 7 (e.g., rays 2 and 4). Relative ray positions are then maintained even after the ventral edge of the SET retracts dorsally, leaving behind all the rays embedded in hyp 7 (Baird et al. 1991) (see Figures 1C and 6).
Figure 6 .
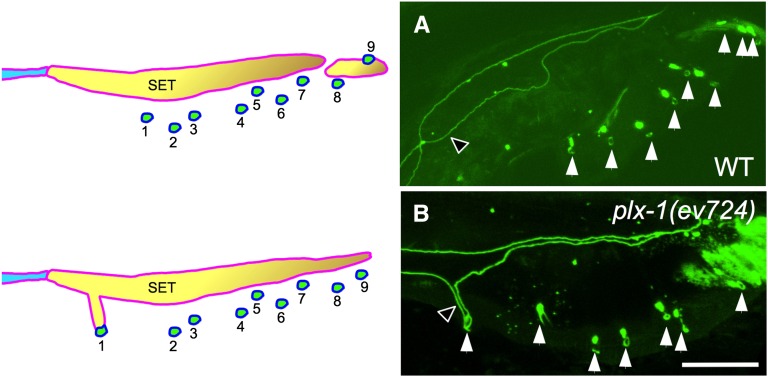
Possible adhesive functions for PLX-1 in ray 1 positioning. The SET remains abnormally attached to ray 1 in young adult plx-1 mutant males (B), but not in comparably staged wild-type males (A) or mutant animals with normally positioned rays (not shown). Cell boundaries are revealed by the ajm-1::gfp reporter for adherens junctions (Mohler et al. 2002). Bar, 16 μm for both panels.
Although male sensory rays are required for copulation, they are not required for viability since C. elegans can reproduce as a self-fertile hermaphrodite. This allows the isolation and propagation of mutations that delete rays or render them nonfunctional. Furthermore, the position of each ray is easy to determine and lends itself to quantitative analysis. Ray positioning is therefore exquisitely amenable to genetic analysis.
Since the discovery that semaphorin-2a affects male ray positioning in C. elegans (Roy et al. 2000), we and others have reported that genes encoding the class 1 transmembrane SMP-1/semaphorin-1a and its PLX-1/plexin-1 receptor, function in preventing an abnormal anterior displacement of the most anterior ray (ray 1) away from its posterior neighbor (ray 2) (Fujii et al. 2002; Ginzburg et al. 2002; Dalpe et al. 2004). We also demonstrated a requirement for Rho family GTPases in ray 1 positioning (Dalpe et al. 2004) including MIG-2, CED-10, and the Rho-GEF UNC-73 (Steven et al. 1998), which presumably function downstream of PLX-1. The original genetic analysis of this phenomenon (Fujii et al. 2002; Dalpe et al. 2004) suggested that at least one other molecular mechanism functions in parallel with the semaphorin-1–plexin-1 pathway to prevent anterior displacement of ray 1. Here we identify the axon guidance cue UNC-6/netrin and its receptors, UNC-40/DCC and UNC-5, as components of a parallel acting ray 1 positioning mechanism that comprises most or all of the semaphorin-1a independent activity involved in preventing the anterior displacement of ray 1. We also show that SMP-1 and UNC-6 signaling defective mutants both require MAB-20/semaphorin-2a (which normally functions to separate neighboring rays) to fully elicit the ray 1 anterior displacement phenotype, suggesting that the final position of ray 1 relative to other rays is influenced by a balance between MAB-20/semaphorin-2a signaling on one hand and SMP-1/semaphorin-1a plus UNC-6/netrin signaling on the other. Finally, we present three kinds of evidence that suggest UNC-6/netrin and SMP-1 function permissively (in which graded extracellular localization is not required) rather than instructively (in which graded extracellular localization is required) to prevent anterior displacement of ray 1. These findings are consistent with the expression patterns of UNC-6 and SMP-1 in all the cells of the ray 1 sensillum as well as with the apparent ability of PLX-1 and UNC-40 expression in the ray structural cells to rescue the ray 1 positioning defects of the corresponding mutants. Our findings reveal a new signaling paradigm for morphogenesis in which axon guidance cues function permissively and in which there is an antagonism between MAB-20/semaphorin-2a function and UNC-6/netrin function as well as between different Semaphorin class members in regulating ray movements and positioning. Possible opposing functions of different Semaphorin classes have been reported in the regulation of vertebrate angiogenesis (Yang et al. 2011; Sakurai et al. 2012), raising the possibility of evolutionary conservation of these mechanisms (also see Discussion).
Materials and Methods
Nematode culture
Standard procedures were used for the culture, maintenance and genetic analysis of C. elegans (Brenner 1974; Wood 1988). Mutant strains used in this study were: Linkage group X (LGX), unc-6(ev400) (Hedgecock et al. 1990); LGI, mab-20(ev574) (Roy et al. 2000), smp-1(ev715) (Ginzburg et al. 2002); unc-40(e1430) (Hedgecock et al. 1990); LGIV, unc-5(e53) (Hedgecock et al. 1990); plx-1(ev724) (Dalpe et al. 2004); and LGV, him-5(e1490) (Hodgkin et al. 1979). Strains not isolated in our laboratory were obtained from the Caenorhabditis elegans Genetics Center, courtesy of T. Stiernagle (University of Minnesota, Minneapolis, MN).
Molecular biology
Standard molecular biology methods (Sambrook et al. 1989) were used unless otherwise noted.
Transgenic constructs
Previously described transgenes used for localization or rescue studies included: (1) unc-6::venus (Asakura et al. 2007) obtained from Dr. Goshima; (2) smp-1p::smp-1::gfp obtained by transgenesis with plasmid pVGS1AGFP (Dalpe et al. 2004); (3) unc-40p::unc-40 and unc-40p::unc-40::gfp minigenes obtained by transgenesis with plasmids pSC11 and pZH22, respectively (Chan et al. 1996); and (4) plx-1p::plx-1 and plx-1p::plx-1::gfp minigenes obtained by transgenesis with plasmids pZH127 and pZH157, respectively (Dalpe et al. 2004).
The following constructs were newly designed and cloned in our lab: (1) pZH282, the full-length smp-1 cDNA fused to gfp (from plasmid pPD95.75) and 638 bp of the unc-54 3′-UTR at its 3′ terminus (Dalpe et al. 2004) all fused downstream to the heat-shock hsp16.41 promoter (from plasmid pPD49.83) (both plasmids are gifts from A. Fire, Stanford University School of Medicine, Palo Alto, CA.); (2) pZH236, a full-length unc-6 cDNA with 550 bp of unc-6 3′-UTR fused downstream to the hsp16.41 promoter (hsp16.41p); (3) pZH267 (ram-5p::plx-1::gfp), the full-length plx-1 cDNA fused to gfp (from plasmid pPD95.75) and 555 bp of plx-1 3′-UTR at its 3′ terminus (Dalpe et al. 2004) all fused downstream to the ram-5 promoter (ram-5p) (Yu et al. 2000); (4) pZH268, the full-length plx-1 cDNA fused to gfp (from plasmid pPD95.75) with 555 bp of plx-1 3′-UTR at its 3′ terminus all fused downstream to the lin-32 promoter (lin-32p) (Portman and Emmons 2000); (5) pZH271, the full-length plx-1 cDNA fused to gfp (from plasmid pPD95.75) with 555 bp of plx-1 3′-UTR at its 3′ terminus all fused downstream to the eff-1 promoter (eff-1p) (Mohler et al. 2002); (6) pZH266, the full-length unc-40 cDNA with 522 bp of unc-40 3′-UTR fused downstream to the ram-5 promoter (ram-5p); (7) pZH269, the full-length unc-40 cDNA with 519 bp of unc-40 3′-UTR fused downstream to the lin-32 promoter (lin-32p); (8) pZH264, the full-length unc-40 cDNA with 522 bp of unc-40 3′-UTR fused downstream to the eff-1 promoter (eff-1p); (9) pSC11(+4.5), the entire pSC11 unc-40 minigene coding region including introns and 5.3 kb of 5′ promoter and 539 bp of unc-40 3′-UTR as reported in (Chan et al. 1996) with an additional 4.5 kb of 5′-regulatory sequence; and (10) pZH22(+4.5), pSC11(+4.5) with an in-frame insertion of gfp in the last exon of unc-40.
Germline transformation
Transgenic strains were as follows: (1) evEx406[hsp16.41p::smp-1] (pZH282 plasmid rescues smp-1 mutants); (2) evEx407[hsp16.41p::unc-6] (pZH236 plasmid rescues unc-6 mutants); (3) evEx408[ram-5p::plx-1::gfp] (pZH267 plasmid rescues plx-1 mutants); (4) evEx409[lin-32p::plx-1::gfp] (pZH268 plasmid rescues plx-1 mutants); (5) evEx410[eff-1p::plx-1::gfp] (pZH271 plasmid does not rescue plx-1 mutants); (6) evEx411[ram-5p::unc-40(cDNA)] (pZH266 plasmid does not rescue unc-40 mutants); (7) evEx412[lin-32p::unc-40(cDNA)] (pZH269 plasmid does not rescue unc-40 mutants); (8) evEx413[eff-1p::unc-40(cDNA)] (pZH264 plasmid does not rescue unc-40 mutants); (9) evEx414[ram-5p::unc-40(cDNA); eff-1p::unc-40(cDNA)] (pZH266 plus pZH264 plasmids combined do not rescue unc-40 mutants); (10) evEx415[lin-32p::unc-40(cDNA); eff-1p::unc-40(cDNA)] (pZH269 plus pZH264 plasmids combined do not rescue unc-40 mutants); (11) evEx416[unc-40p::unc-40(minigene)] (pSC11(+4.5) plasmid rescues unc-40 mutants); (12) evEx419[plx-1p::plx-1::gfp; lin-32p::unc-40(cDNA)] (pZH157 plus pZH269 plasmids induce ray fusions); (13) evEx421[unc-40p::unc-40 minigene::gfp] (pZH22(+4.5)); (14) evIs103 genomically integrated version of pZH22; and (15) evIs139[plx-1p::plx-1] integrated version of pZH127 (Dalpe et al. 2004).
Transgenic strains were generated by co-microinjection of the DNA mix into the distal gonad arms of N2 or him-5(e1490) hermaphrodites (Mello and Fire 1995). DNA mixes consisted of a test construct at a concentration of 50 mg/ml or 30 mg/ml and a co-injection marker sur-5::gfp (Yochem et al. 1998) to create a final DNA concentration of 100 mg/ml.
Heat shock
For heat-shock treatment, synchronized strains were grown on NGM plates coated with OP50 bacteria at 20° until the L2 stage, incubated at 33° for 2 hr (Stringham et al. 1992), then incubated at 20° until they reached adulthood, at which time they were scored for ray 1 displacement defects.
Microscopy
Male tail anterior ray 1 displacement and ray fusion events were scored by mounting 1 mM levamisole-treated animals on 2% agarose pads for observation using DIC optics. All strains carried the him-5(e1490) mutation to increase the frequency of males. Some strains carried the ajm-1::GFP translational reporter (Simske and Hardin 2001) to help assess epidermal cell morphologies and positions with a Leica DMRXA microscope. Confocal microscopy was performed using a Leica DMFLS laser confocal microscope equipped with a 63 PC APO CS lens (1.40–0.60). Confocal images were analyzed by processing confocal z-axis series using Volocity (Quorum Technologies) or ImageJ software (National Center for Biotechnology Information).
Statistics
Standard deviations are for a binomial distribution of the same sample size and the observed proportion as mean for the anterior ray 1 and ray fusion phenotypes. Statistical tests were carried out using a standard (two tailed) comparison of two proportions (Moore and McCabe 1998). All P-values represent the probability that the measured penetrance of the phenotype is significantly different between two strains. A P-value <0.05 is considered significant. All comparisons described as significant in Results were based on this criterion.
Results
UNC-6/netrin signals in parallel to semaphorin-1a to prevent anterior displacement of ray 1 in males
We have found that unc-6/netrin, unc-40/dcc, and unc-5 mutants (much less frequently for the last) share a common adult male ray 1 anterior displacement phenotype similar to the one observed in plx-1/plexin-1 (Figure 1, A and B) and smp-1/semaphorin-1a mutants (Fujii et al. 2002; Ginzburg et al. 2002; Dalpe et al. 2004). Characterization of larvae using the ajm-1::GFP adherens junction reporter (Mohler et al. 2002) to highlight the outline and position of ray cells during male development reveals that all ray cells are present and located at their normal positions up to stage 1 of male ray development (Baird et al. 1991). By contrast, in stage 2, after ray cell bodies begin to migrate subhypodermally and clusters of three ray cell processes embedded in the hypodermis begin to separate from one another, in roughly one- to two-thirds of all plx-1(ev724), unc-6(ev400), and unc-40(e1430) single null mutant males (Figure 1, C–G) [compared to only a few percent in unc-5(e53) null mutant males (not shown)], the ray 1 processes move further anterior than in the wild type as they refine their positions along the anterior–posterior axis of the body wall (Baird et al. 1991).
The ray 1 anterior displacement phenotype in adult males has two levels of severity that can be readily distinguished: a severe anterior position (anterior of the fan) (Dalpe et al. 2004), and a mild one (displaced anterior, but still within the fan area) (Figure 1B). Our description of the anterior ray 1 phenotype will focus on the severe form of this phenotype because this phenotype is distinctly classifiable.
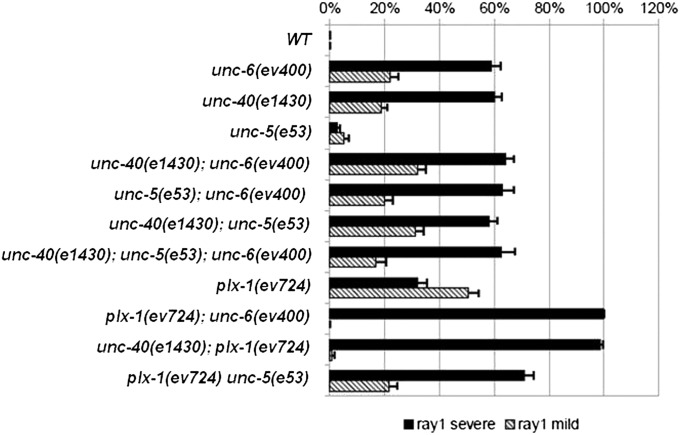
The penetrance of the severe anterior ray 1 displacement phenotype in the plx-1(ev724) single mutant is ∼32%, whereas the penetrance of the severe anterior ray 1 phenotype in unc-6(ev400) and unc-40(e1430) mutants is ∼60% (Figure 2). In comparison, the plx-1(ev724); unc-6(ev400) and unc-40(e1430); plx-1(ev724) double mutants have fully penetrant defects (∼100%) for the severe anterior ray 1 phenotype. In stage 2 male larvae of unc-40(e1430); plx-1(ev724) double mutants, the ray 1 cell cluster is often displaced even more anterior than in either single mutant—as far anterior as the body seam–SET junction (Figure 1G, stage S2). Characterization of the anterior ray 1 cell process cluster in stage 2 larvae and of the adult anterior ray 1 phenotype indicates that the netrin signaling pathway functions in parallel to the semaphorin-1a pathway to prevent anterior displacement of ray 1 and may be the only pathway that acts in parallel with semaphorin-1a in this context.
Figure 2 .
UNC-6, UNC-5, and UNC-40 function in one pathway and plexin-1/PLX-1 functions in a parallel pathway to prevent anterior displacement of ray 1. The frequencies and standard errors for severe (solid bar) and mild (cross-hatched bar) anterior ray 1 displacements are shown for a variety of unc-5, unc-6, unc-40, and plx-1 single, double, and triple null mutants, all in a him-5(1490) (control WT) genetic background. Standard errors for percentages of animals manifesting the anterior ray 1 phenotype were calculated assuming a binomial distribution of the same sample size and the observed proportion as mean.
The unc-40; unc-6, unc-5; unc-6, unc-40; unc-5, and unc-40; unc-5; unc-6 multiple null mutants are not obviously enhanced relative to the most penetrant of the single mutant constituents of each multiple mutant strain. This is consistent with the idea that UNC-6 functions through both UNC-40 and UNC-5 receptors to help position ray 1 as it does in axon guidance. A caveat to this interpretation for UNC-5 is that the penetrance of ray 1 defects in unc-5(e53) null mutants is only 3%, so whether UNC-5 functions as an UNC-6 receptor in this context remains unknown. The penetrance of ray 1 anterior displacement defects in plx-1(ev724); unc-5(e53) double mutants is 71%, which represents a significant enhancement over each plx-1(ev724) and unc-5(e53) single mutant (Figure 2). The increased dependency on UNC-5 signaling in the absence of PLX-1 function suggests a previously unrecognized partial redundancy between PLX-1 and UNC-5 in this context.
Semaphorin-1, UNC-6/netrin, and UNC-40 are expressed in male ray cells
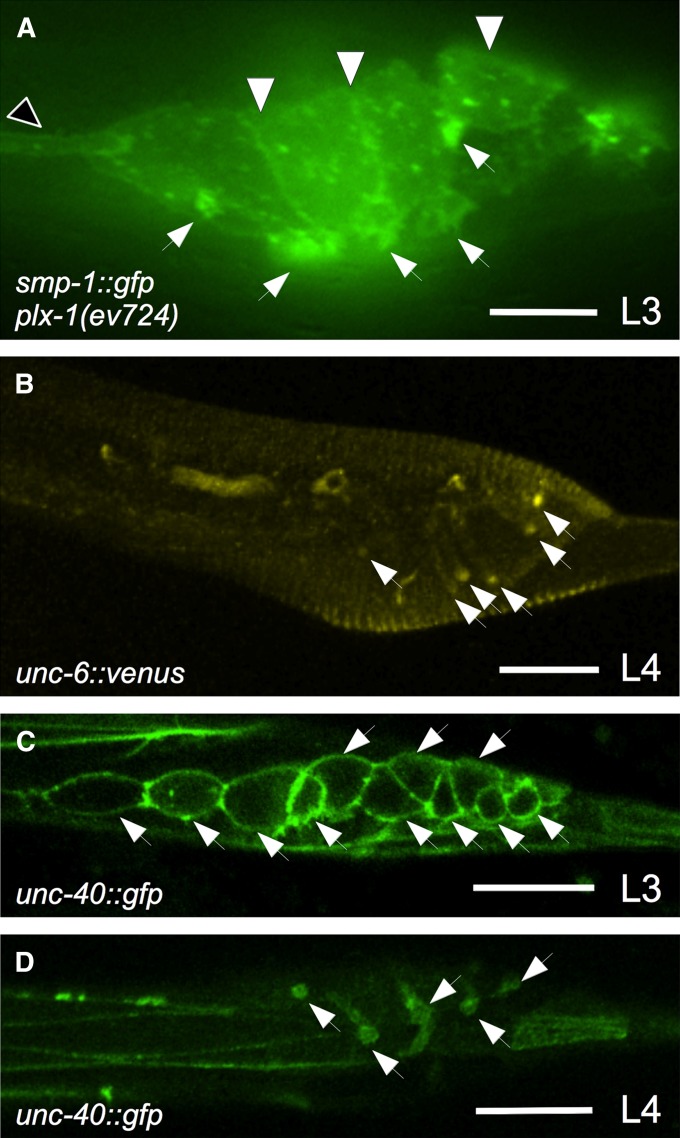
SMP-1 translational reporters were previously undetected in or on ray cells (Ginzburg et al. 2002; Dalpe et al. 2004), however, it is possible that SMP-1 is down-regulated upon binding to its PLX-1 receptor. Consistent with this hypothesis, we found that passing a smp-1p::smp-1::gfp genomic translational reporter gene (tagged at its C terminus) (Dalpe et al. 2004) into a plx-1(ev724) mutant background reveals bright GFP localization at or near the plasma membrane of all ray cell groups and a lesser localization at the plasma membrane of Rn.p cells and the lateral hypodermal body seam (Figure 3). Localization of SMP-1, which has a transmembrane domain, to the surface of ray and Rn.p cells reveals that Rn.p and all rays cells make SMP-1. Furthermore, we found that an unc-6::venus reporter, which rescues the axon guidance defects of unc-6 mutants (Asakura et al. 2007), is localized to all ray structural cells in males by the late L3 and throughout the L4 stage (Figure 3). Secreted, diffusible UNC-6 could be binding to the surface of the structural cells so whether these cells make UNC-6 is questionable. We also found that a functional unc-40p::unc-40::gfp genomic translational reporter gene (Chan et al. 1996) is localized to all dividing ray cells before stage 1 of male development and only in ray structural cells from stage 3 onward (Figure 3), suggesting a function for UNC-40 within ray cell groups. This is consistent with our previous finding that the SMP-1 receptor PLX-1 is also localized to the surface of all ray cells—predominantly in rays 1 and 2 (Dalpe et al. 2004).
Figure 3 .
Semaphorin-1a and UNC-6 signaling components are localized to ray cells. All panels show him-5 males (“wild-type” controls) with anterior to the left and dorsal up. Epifluorescence microscopy (A) and laser confocal microscopy (B–D) images show the localization of smp-1p::smp-1::gfp (in a plx-1(ev724) mutant) (A), unc-6::venus (B), and unc-40p::unc-40::gfp (C and D). (A) plx-1(ev724) male carrying extrachromosomal array evEx170 (pVGS1AGFP), which encodes a smp-1p::smp-1::gfp rescuing construct (Dalpe et al. 2004), is shown. The GFP signal is observed at the cell membrane of all ray cell groups (small white arrowheads), all Rn.p cells (large white arrowheads), and in the body seam hypodermis (black arrowhead). (B) L4 stage males carrying an extrachromosomal array of a genomic unc-6::venus (Asakura et al. 2007) rescuing construct show localization on all ray structural cell processes and their tips (white arrowheads). (C and D) In wild-type males carrying the genomically integrated evIs103 [unc-40p::unc-40::gfp] transgene array, the GFP signal (white arrowheads) is observed at the cell membrane of all dividing ray precursors during third larval (L3) stage (C) and in all ray structural cell processes and their tips at the L4 stage (D). Bars for A and B–D, 25 μm and 16 μm, respectively.
PLX-1 and UNC-40 expression in ray structural cells is sufficient to rescue ray 1 positioning defect of the corresponding mutants
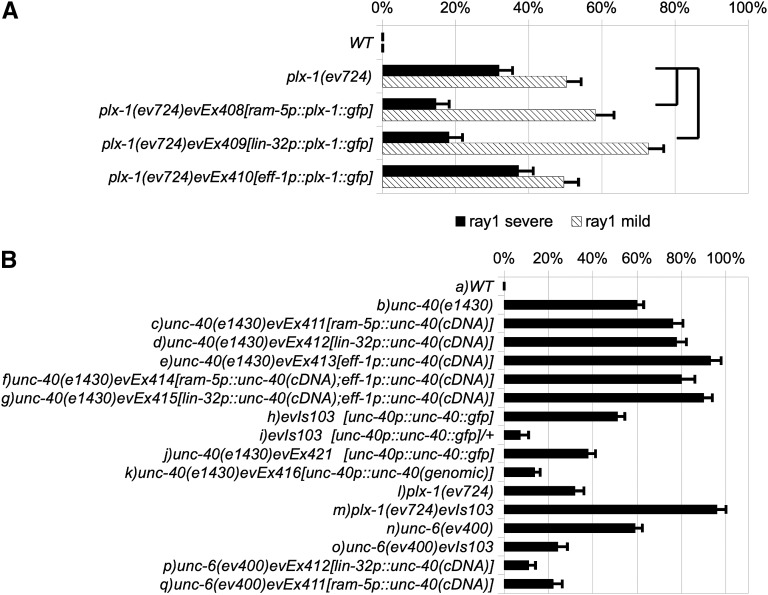
In principle, UNC-6 and SMP-1 could signal through UNC-40 and PLX-1 receptors on one or more ray cell types or even on hypodermal cells that contact the ray cells. To distinguish between these possibilities we examined the ability of cell-type–specific expression of a functional plx-1::gfp translational fusion to rescue the anterior ray 1 phenotype of a plx-1 mutant. Promoters used to drive plx-1::gfp expression included lin-32 (Portman and Emmons 2000), ram-5 (Yu et al. 2000), and eff-1 (Mohler et al. 2002), which drive expression in all ray cells (lin-32p), all ray structural cells (ram-5p), or Rn.p/hypodermal precursor cells (eff-1p), respectively (see Figure 1C for expression pattern schematic). We found that plx-1(ev724) mutant males carrying lin-32p::plx-1::gfp or ram-5p::plx-1::gfp arrays are significantly rescued for the severe anterior ray 1 phenotype (Figure 4A and see Supporting Information, Figure S1 for contructs). In contrast, plx-1(ev724) transgenic males carrying an eff-1p::plx-1::gfp array are not significantly rescued for this phenotype. This suggests that PLX-1 is required in ray cells but more specifically in the ray 1 structural cell for its function in ray 1 positioning.
Figure 4 .
Cell-type–specific rescue of smp-1, unc-6, and plx-1 mutant ray 1 defects. The percentages of severe (solid bar) and mild (cross-hatched bar) anterior ray 1 displacements are shown for a variety of smp-1, unc-6, and plx-1 mutants strains—all in a him-5 (e1490) (control WT) genetic background. (A) plx-1 mutant ray 1defects are rescued by expression of plx-1 in all the rays (driven by lin-32 promoter) or the ray structural cell (driven by the ram-5 promoter), but not by expression in Rn.p cells (driven by the eff-1 promoter). (B) Percentage of wild type (WT = him-5) and unc-40 mutant ray 1 anterior displacement defects are shown (B, a and b) to compare with the same mutant expressing an unc-40cDNA driven by (B, c) the ram-5 promoter (evEx411), or (B, d) the lin-32 promoter (evEx412) or (B, e) the eff-1 promoter (evEx413) or (B, f) both the ram-5 and eff-1 promoter (evEx414) or (B, g) both the lin-32 and eff-1 promoter (evEx415). (B, h) ray 1 anterior displacement defects induced by evIs103 (an unc-40::gfp fusion driven by the unc-40 promoter) in a wild-type background, and (B, i) the effects of halving the dose of the evIs103 transgene on ray 1 defects. (B, j) ray 1 anterior displacement defects in an unc-40 mutant homozygous for the evIs103 transgene array, or (B, k) carrying the evEx416 extrachromosomal array of plasmid pSC11 comprising the entire unc-40 coding sequence plus regulatory sequence previously shown to rescue an unc-40 mutant (Chan et al. 1996). (B, l) plx-1 mutant displacement defects—shown to compare with (B, m) the same mutant carrying evIs103. (B, n) unc-6 mutant ray 1 anterior displacement defects—shown to compare with (B, o) the same mutant homozygous for evIs103 or (B, p) evEx412, or (B, q) evEx411. Standard deviations for percentages of the anterior ray 1 phenotype were calculated assuming a binomial distribution of the same sample size and the observed proportion as mean. Statistical tests were carried out using a standard (two tailed) comparison of two proportions (Moore and McCabe 1998). Joined horizontal lines designate data used for statistical comparisons to determine whether specific transgene arrays rescue unc-40 or unc-6 mutant anterior ray 1 displacement defects. In all cases rescue was significant (P < 0.005).
UNC-40 is expressed in dividing Rn precursors before male stage 1 and in the ray structural cell at stage 3 and onward. We found that unc-40(e1430) mutant males carrying a ram-5p::unc-40, a lin-32p::unc-40, or an eff-1p::unc-40 array do not rescue the ray 1 phenotype (Figure 4B). In fact, these arrays caused an enhancement of the penetrance above the penetrance of unc-40(e1430). We also generated transgenic unc-40(e1430) mutant animals carrying arrays combining the constructs eff-1p::unc-40 with ram-5p::unc-40 or with lin-32p::unc-40, but these also enhanced the ray 1 defect (Figure 4B, a–g).
In a wild-type background, an unc-40p::unc-40::gfp multicopy minigene integrated array (in which UNC-40::GFP expression is regulated by endogenous promoter and introns), also causes ray 1 defects (Figure 4B, h), but these are reduced by halving the dose of the array (Figure 4B, i). However, another array made from the same unc-40p::unc-40::gfp construct partially rescues the unc-40(e1430) null, whereas a genomic unc-40 array, unc-40p::unc-40, more fully rescues (Figure 4B, j and k). Together these results suggest that overexpression of UNC-40 by some arrays interferes with normal ray 1 positioning and halving the dose of the array or depleting endogenous unc-40 activity relieves this interference. This interference hypothesis is further supported by the finding that passing the unc-40p::unc-40::gfp array into a plx-1(ev724) mutant background enhances anterior ray 1 defects just as the unc-40(e1430) null mutation enhances plx-1(ev724) (Figure 4B, l and m, and Figure 2).
We also found that unc-6(ev400) mutant males carrying the unc-40p::unc-40::gfp, the lin-32p::unc-40, or the ram-5p::unc-40 transgene array—each of which is likely to cause UNC-40 overexpression in ray structural cells—are significantly rescued for the anterior ray 1 phenotype (Figure 4B, n–q). The finding that these unc-40 overexpressing arrays rescue unc-6 mutant ray 1 defects but cause these defects in otherwise wild-type animals suggests that UNC-6 activation of UNC-40 increases UNC-40 activity above the hypothetical threshold for interference, and when this activation is eliminated by eliminating UNC-6 function, the below-threshold UNC-40 activity is now able to rescue. This finding also demonstrates that the presence of UNC-40 in the ray structural cells can largely correct the ray 1 defect without any need for UNC-6 signaling and therefore any need for an UNC-6 gradient.
MAB-20/semaphorin-2 is required for the anterior ray 1 displacement phenotype observed in plx-1 and unc-40 mutants
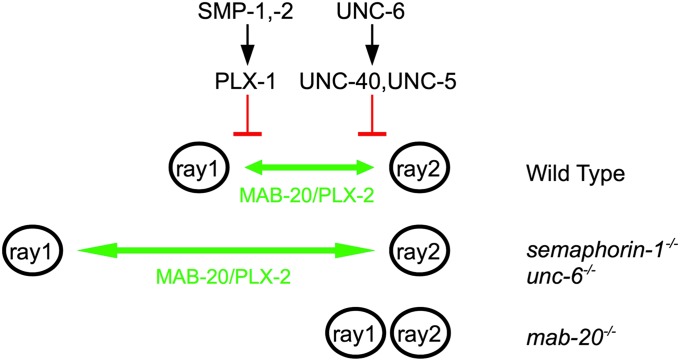
How are UNC-6 and SMP-1 signals able to regulate ray 1 positioning? To shed light on this question, we turned our attention to another member of the C. elegans Semaphorin family—MAB-20/semaphorin-2a. We and others previously found that in mab-20 mutants, ray cell clusters 1–9 on each side often fail to separate between stages 1 and 2 of male development, resulting in an adult ray fusion phenotype (Baird et al. 1991; Roy et al. 2000; Fujii et al. 2002). Interestingly, mab-20 mutant males have a ray fusion phenotype but never display an anterior ray 1 phenotype. This is opposite to smp-1 and unc-6 mutants, which have an anterior ray 1 phenotype but not a ray fusion phenotype. These data suggest that MAB-20 normally signals ray cell groups to separate into distinct ray sensillae, whereas SMP-1 and UNC-6 normally signal to prevent excess separation between ray 1 and ray 2 cell process clusters. Thus MAB-20 and the UNC-6/UNC-40 and SMP-1/PLX-1 signaling pathways appear to act at cross purposes, one keeping rays 1 and 2 together and the other pushing them apart (Figure 5), raising the possibility that ray 1 positioning is determined by a balance between MAB-20 and UNC-6 plus SMP-1 signaling. If this is true, then MAB-20 function could be required for the excessive ray 1 separation from ray 2 that occurs in plx-1 and unc-40 mutants. This appears to be the case for the plx-1 mutant since mab-20(ev574) prevents anterior displacement of ray 1 in plx-1(ev724) males without affecting ray 1 fusions (only 2% of double mutant males have mild anterior ray defects compared to 82% in plx-1 single mutants; see Table 1, C and D combined severe and mild defects). We could not readily test whether this is also true for unc-40 as it is for plx-1 mutants since the unc-40(e1430); mab-20(ev574) and mab-20(ev574); unc-6(ev400) double mutants are lethal. We instead evaluated whether mab-20(ev574) can suppress the severe ray 1 defects caused by the evis103 [unc-40p::unc-40::gfp] array (Chan et al. 1996), which causes apparent interference with the UNC-6 signaling pathway thereby phenocopying an unc-40 mutant (see Results above). Indeed we found that mab-20(ev574) suppresses the anterior ray defects caused by evIs103 (Table 1, F and G). The apparent suppression of ray 1 positioning defects could occur for a trivial reason—it could happen because the fusion of ray 1 with ray 2 caused by the mab-20 deficit prevents ray 1 from moving to a more anterior position. However, in 18% of mab-20; plx-1 double mutant animals, ray 1 is not fused to ray 2. If epistasis by the mab-20 mutation resulted from attachment of ray 1 to more posterior rays, then we would expect 82% [the penetrance of plx-1(ev724)] of these 18% (= 15%) to have an anterior ray 1 defect, but only 2% do (Table 1D), demonstrating that the anterior displacement of ray 1 in a plx-1 mutant depends on MAB-20 even when ray 1 is not fused to another ray. Similar but not statistically significant results were found for the mab-20(ev574); evIs103 double mutant (Table 1G), suggesting that UNC-40 like PLX-1 also inhibits MAB-20 function even when ray fusions are not involved in ray positioning.
Figure 5 .
Model for the role of semaphorin-1a and UNC-6 signaling pathways during ray 1 positioning. SMP-1, SMP-2, and PLX-1 are part of a single pathway that functions in parallel to a pathway comprising UNC-6, UNC-40, and UNC-5. SMP-1 and UNC-6 ligands send a permissive signal to PLX-1 and UNC-40 receptors, required cell autonomously in ray structural cells. In wild-type males, individual ray cell groups form and separate on the anterior–posterior axis. This process is dependent on MAB-20–mediated signaling (green) (as well as the PLX-2 receptor and other unknown components—see Ikegami et al. 2004) since ray cell groups tend to cluster with adjacent cell process groups in mab-20 mutants. This MAB-20/PLX-2–dependent ray separation (green) is normally inhibited by the semaphorin-1a and UNC-6 pathways (red). Loss of function in semaphorin-1a and UNC-6 signaling pathways results in an effective gain of function of MAB-20–mediated signaling in the ray 1 structural cells, which increases separation between ray 1 and ray 2 cell groups.
Table 1 . The anterior ray 1 phenotype of plx-1 and unc-40 mutants requires mab-20.
| Genotypea | R1A-Sb | R1A-M | R1Fc | R2F | R3F | R4F | R5F | R6F | R7F | R8F | R9F | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) WT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 130 |
| (B) mab-20(ev574) | 0 | 0 | 88 | 98 | 99 | 97 | 3 | 61 | 91 | 8 | 90 | 120 |
| (C) plx-1(ev724) | 32 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 157 |
| (D) mab-20(ev574);plx-1(ev724) | 0 | 2 | 82 | 96 | 94 | 98 | 0 | 74 | 37 | 31 | 65 | 89 |
| (E) unc-40(e1430) | 60 | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 290 |
| (F) evIs103[unc-40p::unc-40::gfp]d | 68 | 18 | 3 | 3 | 4 | 5 | 0 | 2 | 0 | 0 | 0 | 133 |
| (G) mab-20(ev574);evIs103[unc-40p::unc-40::gfp] | 4 | 0 | 90 | 99 | 100 | 96 | 9 | 52 | 32 | 44 | 75 | 77 |
| (H) evIs139[plx-1p::plx-1]e | 17 | 39 | 3 | 4 | 7 | 5 | 0 | 1 | 0 | 0 | 0 | 180 |
| (I) evEx419[plx-1p::plx-1::gfp;lin-32p::unc-40(cDNA)]f | 0 | 2 | 12 | 13 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 164 |
(A–D, H, and I) Animals grown at 25° and (E–G) animals grown at 20°.
Percentage of the severe (R1A-S) and mild (R1A-M) anterior ray 1 displacements and of ray fusion events.
(RnF, n = 1–9) fusion to a neighboring ray (left and right sides combined).
evIs103 transgene array causes UNC-40::GFP overexpression.
evIs139 transgene array causes PLX-1 overexpression.
evEx419 transgene array causes PLX-1::GFP overexpression and UNC-40 overexpression in ray cells.
Another prediction of the hypothesis that ray 1 positioning is determined by a balance between MAB-20 and UNC-6 plus SMP-1 signaling is that overexpression of UNC-40 or PLX-1 or both together should cause some ray fusion defects. In fact, overexpression of unc-40 or plx-1 or both together causes a small but significant number of ray fusions (Table 1, F, H, and I). In addition this overexpression of both UNC-40 and PLX-1 also causes suppression of the anterior displacement of ray 1, which is consistent with the proposed ability of UNC-40 and PLX-1 to reduce MAB-20 output. Of note, the two effects of this dual overexpression are unequal—the induction of ray fusions is not nearly as complete as the suppression of the anterior ray 1 displacement phenotype. In the context of UNC-40 plus PLX-1 overexpression, this suggests a greater requirement for MAB-20 in causing anterior displacement of ray 1 than in preventing ray fusions, which is consistent with a function for MAB-20 in separating neighboring rays independently of whether they adhere to one another.
Ray 1 has abnormally perdurant adhesions to the SET in plx-1 mutants that depend on MAB-20
Normally the three cell processes comprising ray 1 at first contact, R1.p, R2.p, and hyp 7, are then surrounded by the SET (after it forms from the fusion of Rn.p cells) and later only by hyp 7 as the boundary between the SET and hyp 7 retracts dorsally during male tail development. However, in SMP-1 and UNC-6 signaling mutants, the ray 1 anterior displacement phenotype (Fujii et al. 2002; Ginzburg et al. 2002; Dalpe et al. 2004) is always associated with an abnormally perdurant attachment of ray 1 to the SET (Figure 6), an association that is virtually never seen when ray 1 is normally positioned in these mutants (see Table 1). This abnormally perdurant adhesion is largely absent in double mutants of mab-20/semaphorin2a and plx-1/plexin (59 of 60 sides examined), indicating that MAB-20 mediates the abnormal ray 1 to SET adhesion in plx-1 mutants, and by inference, in other mutants with anterior ray 1 displacement defects. These results have important implications for the cellular and molecular mechanisms of ray 1 positioning (see Discussion).
SMP-1 and UNC-6 are required permissively to prevent anterior ray 1 displacement
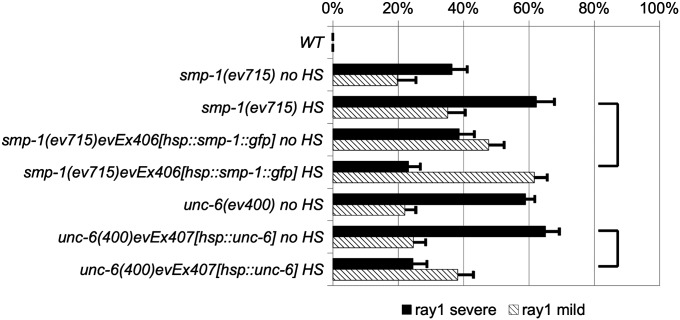
Since the semaphorin-1a and UNC-6/netrin signaling pathways have well-characterized functions in axon guidance and cell migration (Merz et al. 2001; Yu and Bargmann 2001; Pasterkamp and Kolodkin 2003; Tamagnone and Comoglio 2004; Kruger et al. 2005), our initial speculation was that they would also have an instructive role in ray positioning. Instructive signaling by definition would involve the establishment of cellular polarity information by the graded distribution of a guidance cue, which is conveyed to the cell by signaling through appropriate receptors, in this case expressed at the surface of ray structural cells. To begin to address an instructive vs. noninstructive (i.e., permissive) mode of action for SMP-1 and UNC-6 ligands, we conducted smp-1 and unc-6 mutant rescue experiments in which expression of a cDNA for each gene is driven by heterologous promoters. We used the heat shock promoter hsp16.41p to specifically express these genes ubiquitously upon heat shock. We found that heat-shock–induced smp-1(ev715) animals carrying an hsp16.4p::smp-1 extrachromosomal array were significantly rescued for the ray 1 anterior displacement phenotype as compared to controls (Figure 7), suggesting that ubiquitous expression of SMP-1 is sufficient to rescue the smp-1 mutant defect. We also generated unc-6(ev400) mutants carrying an hsp16.41p::unc-6 extrachromosomal array and found that heat-shock–induced animals were also significantly rescued for their anterior ray 1 phenotype as compared to controls (Figure 7). These results are not entirely inconsistent with an instructive function for these cues in ray 1 positioning; however, additional data presented above and discussed below (see Discussion) overwhelmingly favor a permissive function for UNC-6 and SMP-1 in preventing the anterior displacement of ray 1.
Figure 7 .
Heat-shock promoter-driven rescue of smp-1 and unc-6 mutant ray 1 defects. Percentages of severe (solid bar) and mild (cross-hatched bar) anterior ray 1 displacements are shown for smp-1 and unc-6 mutants strains—all in a him-5 (control WT) genetic background. (A) Ray 1 defects of heat-shocked (HS) and nonheat-shocked (no HS) smp-1(ev715) males carrying evEx406 [an extrachromosomal of a wild-type smp-1 gene driven by a heat-shock promoter (hsp16.41p)] are shown. Ray 1 defects of heat-shocked and nonheat-shocked unc-6(ev400) males carrying evEx407 [an extrachromosomal of a wild-type unc-6 gene driven by a heat-shock promoter (hsp16.41p)] are also shown. Statistical analyses were performed as described in the legend to Figure 4. Joined horizontal lines designate data used for statistical comparisons to determine whether specific transgene arrays rescue unc-40 or unc-6 mutant anterior ray 1 displacement defects. In each case, the heat-shock promoter-driven gene (smp-1 or unc-6) causes significant rescue (P < 0.0005) when heat shocked, as compared to heat-shocked mutant controls with no transgene array.
Discussion
In C. elegans males, sensory rays arranged along the anterior–posterior axis of the tail have a reproducible position and morphology resulting from a highly regulated developmental process (Baird et al. 1991; Fitch and Emmons 1995). In our previous study (Dalpe et al. 2004), it was found that ray 1, the most anterior ray sensillum of the nine on each side of the male tail, was displaced anterior to normal in about one-third of plx-1 null mutant animals and plx-1; smp-1 double null animals (see also Fujii et al. 2002), suggesting that at least one other molecular mechanism functions in parallel with semaphorin-1a signaling to position ray 1. Here we report that UNC-6/netrin signaling through its UNC-40/DCC receptor comprises the remaining ray 1 positioning mechanism present in null mutants of semaphorin-1a and plexin-1 signaling. Double mutants predicted to be lacking both UNC-6 and semaphorin-1a signaling mechanisms exhibit almost fully penetrant defects of the severe anterior ray 1 displacement phenotype. This suggests that UNC-6 signaling comprises the major if not only mechanism that functions in parallel with semaphorin-1a signaling to prevent this phenotype.
Cell-type–specific rescue experiments suggest that expression of plx-1 and unc-40 in the ray 1 structural cell is sufficient to rescue ray 1 positioning defects of plx-1 and unc40 mutants, respectively. Thus, signals elicited by UNC-6 and SMP-1 ligand binding to their respective receptors on the ray structural cell are likely to help position ray 1. It is not entirely surprising that ray position is determined by signals to the structural cell, since it is the adhesion of this cell to the hypodermis that cements the final position of each ray sensillum in the male tail (Baird et al. 1991). In principle, the UNC-6 and SMP-1 ligands could regulate this adhesion or the position of the ray 1 structural cell at the time adhesion to the hypodermis occurs or both.
In attempting to determine the ability of cell-type–specific expression of unc-40 to rescue ray 1 defects of an unc-40 mutant, we encountered some complex genetic results. Animals carrying certain multicopy unc-40 transgene arrays enhanced the ray 1 defects of an unc-40 null mutant rather than rescued them. The ray 1 defects induced by the integrated array (evIs103) were ameliorated by halving the dose of this array. Furthermore some unc-40 multicopy arrays could rescue unc-6 and unc-40 mutant ray 1 defects. These results suggest that the ray 1 defect caused by evIs103 occurs by the well-known ability of multicopy arrays to cause overexpression of the genes they carry, which—if signaling above some threshold—would cause interference with normal ray 1 positioning. The ability of the unc-40 arrays (including even those that fail to suppress the ray 1 defects of an unc-40 null) to largely rescue unc-6 null mutant ray 1 defects also suggests that at least part of the hypothetical interfering activity of unc-40 overexpression is dependent on activation of UNC-40 by UNC-6. Furthermore, the cell-type–specific partial rescue of the unc-6 mutant ray 1 defects caused by driving unc-40 expression in the ray 1 structural cell means that polarity information provided by UNC-6 is not necessary for ray 1 positioning so long as enough UNC-40 activity is present in the structural cell. This is most consistent with a permissive function for UNC-6 in this context.
Two pieces of additional evidence support the contention that UNC-6 and SMP-1 normally function permissively to position ray 1. First, heat-shock promoter-driven expression of unc-6 and smp-1, which is predicted to alter or even eliminate a gradient of either cue, partially rescues unc-6 and smp-1 mutant ray 1 defects. Second, data presented above and discussed below strongly suggest that UNC-6 and SMP-1 regulate ray 1 structural cell adhesion to the hypodermis to determine ray 1 position, and since cell adhesion does not require polarity information, UNC-6 and SMP-1 are predicted to function permissively in this context. SMP-1 is also clearly expressed by and localized to the surface of all ray and Rn.p cells during ray positioning, suggesting that it does not function as a graded cue in this context.
The finding that plx-1 and unc-40 genes function in the ray structural cell to position ray 1 does not preclude the possibility that Rn.p cells play a central role in ray 1 positioning. The latter idea largely derives from the highly reproducible positioning of ray 1 precursor cells and processes at the junction of hyp 7 and two Rn.p cells during male tail development (Baird et al. 1991) (also see Figure 1C). Although this Rn.p/hyp 7 adhesion model may help explain ray positioning, it is also clear that an additional mechanism helps position ray 1. mab-20 encodes a secreted semaphorin-2a homolog (Roy et al. 2000). In mab-20 mutant males, ray cell process clusters form abnormal groupings with adjacent clusters between stages 1 and 2 of male ray development and these later appear as fusions of adjacent rays in adult males (Baird et al. 1991; Roy et al. 2000), indicating that MAB-20 normally functions in separating ray cell process clusters as they refine their positions on the anterior–posterior axis. PLX-1 and UNC-40 on the other hand appear to function in the opposite sense in that they normally prevent extra spacing from occurring between ray 1 and ray 2. Consistent with these ideas, mab-20 mutants do not have an anterior ray 1 phenotype, and plx-1 and unc-40 mutants do not show ray fusion phenotypes.
These results raise the possibility that ray 1 positioning is determined by a balance between MAB-20 and opposing semapohorin-1a–PLX-1 and UNC-6–UNC-40 functions (summarized in Figure 5). This hypothesis is supported by a number of findings including (1) the simultaneous timing of the ray fusion and anterior displacement phenotypes; (2) the expression of the MAB-20 receptor PLX-2 in the ray 1 structural cell (Ikegami et al. 2004)—the known focus of UNC-40 and PLX-1 activity; (3) the ability of a MAB-20 deficit to largely rescue the anterior displacement of ray 1 caused by mutation or overexpression of plx-1 or unc-40 (see also Fujii et al. 2002) (indicating that MAB-20 function to separate ray 1 from ray 2 is normally kept in check by PLX-1 and UNC-40); and (4) the ability of unc-40 and plx-1 overexpression to cause ray fusions, including fusions of ray 1 with ray 2 (showing that PLX-1 and UNC-40 at high enough concentrations can inhibit MAB-20 function in ray 1 cells).
In principle, cell positioning can be determined by either or a combination of at least two basic cellular mechanisms. One basic mechanism is active cell migration, the direction and extent of which may be regulated by instructive gradients of one or more guidance cues. Positioning in this case could be akin to the positioning of retinal axons within the vertebrate tectum by gradients of ephrins and Eph receptors (Gebhardt et al. 2012). A second basic mechanism for determining cell position is by passive responses to other moving or morphing cells. For example, differential adhesion to other cells or extracellular matrices (Aufschnaiter et al. 2011) whose shapes are in flux would cause passive movements of attached cells. The final position of any moving cell may depend on either or both active and passive mechanisms to different extents.
If instructive cues position active ray 1 migrations in C. elegans, these cues are unlikely to be UNC-6 or semaphorin-1a because these proteins appear to function permissively in ray 1 positioning; however, UNC-6 and semaphorin-1a could be required permissively for the execution of an instructive mechanism. C. elegans wnts may be candidates for such instructive cues since β-catenin mutants also reportedly have ray 1 defects (Pickett et al. 2007). Since ray 1 positioning appears to be struck by a balance between MAB-20 on one hand and UNC-6–UNC-40 plus SMP-1–PLX-1 signaling on the other, it is also possible that MAB-20 could provide one graded instructive cue for ray 1 positioning that is counterbalanced by UNC-6/netrin and SMP-1/semaphorin-1a signaling. However, its near ubiquitous expression during male development (Roy et al. 2000; Hahn and Emmons 2003) suggests that, like UNC-6 and SMP-1, MAB-20 has a permissive mode of action, although it is possible that undetected local gradients of MAB-20 could guide short-range cell movements.
MAB-20 could also affect ray 1 positioning by regulating differential adhesion of the ray 1 process cluster to cells it normally contacts, one of which is the Rn.p-derived SET cell and the other hyp 7 (Figure 1C). We imagine that as the SET cell elongates toward the anterior it could cause towing of the attached ray 1 cluster toward the anterior and that the extent of towing is limited by the drag on the ray 1 processes caused by their simultaneous, but imperfect adhesion to hyp 7. When UNC-6 or SMP-1 or both signaling functions are reduced, MAB-20 signaling would be enhanced and cause a shift in preferential adhesion of ray 1 processes to the SET cell (and possible additional deadhesion from hyp 7), thereby increasing the tow (and possibly reducing the drag) on the anterior movement of the ray 1 process cluster. The effect of this would be to cause an abnormally anterior displacement of ray 1 in unc-6 and smp-1 mutant males.
At the moment, we cannot distinguish to what extent active migration or passive movement caused by differential adhesion and SET cell elongation are responsible for ray 1 positioning; however, consistent with a role for passive movement dictated by adhesion, every anterior ray 1 (and only anterior ray 1s) in semaphorin-1a and UNC-6 signaling mutants has an abnormally perdurant contact between ray 1 and the SET (Fujii et al. 2002; Ginzburg et al. 2002; Dalpe et al. 2004), which could be explained by a shift in preferential adhesion of the ray 1 structural cell toward the elongating SET in these mutants. Furthermore, the preferential adhesion of ray 1 to the SET in plx-1 mutants (and by inference unc-6 mutants) appears to require mab-20, since these adhesions are largely absent in plx-1mab-20 double mutants. Thus, two semaphorins appear to have opposing functions in adhesion to the SET, with SMP-1 and UNC-6/netrin favoring deadhesion and MAB-20 favoring adhesion. As a result, male ray 1 positioning would be dependent on a balance between these adhesive and deadhesive mechanisms, which in turn would affect the degree of ray 1 towing by the elongating SET opposed by the drag caused by adhesion to hyp 7.
The extent to which adhesive functions underlie the contribution of netrins to vascular, lung, and pancreatic development in mammals remains to be determined; however, an adhesive function for netrin and its receptors in these processes is likely (Hinck 2004; Baker et al. 2006), particularly in the developing mammary ductal network where netrin mediates adhesion between luminal epithelial cells and cap cells (Srinivasan et al. 2003; Strickland et al. 2006). Mammalian semaphorins have also been proposed to have adhesive functions in organogenesis that are unrelated to their axon guidance properties (Casazza et al. 2007). The various proposed roles of netrins and semaphorins in angiogenesis, tumor growth, progression, and metastasis are complex and in some cases controversial (Mehlen and Rama 2007; Gaur et al. 2009; Castets and Mehlen 2010; Klagsbrun and Shimizu 2010), but in many instances they almost certainly involve a permissive, autocrine function (Nasarre et al. 2010) that in this sense at least is similar to the function of UNC-6 and SMP-1 in ray 1 positioning in C. elegans. There is even one instance of a developmental role for netrin-1 that in a general sense parallels the mechanisms we have discovered for ray 1 positioning in C. elegans. In this instance, oligodendrocyte progenitor cells that populate the optic nerve are repelled by one semaphorin (3A) and attracted by another (3F) acting with netrin-1 (Spassky et al. 2002). At the moment, it is difficult to know what to make of these similarities, but the genetic and molecular characterization of UNC-6 signaling mechanisms in a permissive (i.e., nonaxon guidance) context that also involves semaphorin function could shed light on a number of vertebrate processes important to development, regeneration, and cancer prevention.
Summary
The semaphorin-1a and UNC-6/netrin signaling pathways function in parallel to prevent anterior ray 1 positioning defects in C. elegans males, while MAB-20/semaphorin-2a signaling is required for the anterior ray 1 displacement defects of plx-1 and unc-40 mutants to manifest. These and cell-type–specific rescue results indicate that the semaphorin-1a and UNC-6/netrin guidance cues activate PLX-1/plexin-1 and UNC-40/DCC receptors, respectively, in ray structural cells, which, in turn, inhibit MAB-20 mediated signaling—the normal function of which is to separate ray 1 from ray 2. How the abnormally anterior ray 1 displacement occurs in smp-1 and unc-6 mutants is not known, but could involve active migration of ray cells in response to unknown guidance cues that require a permissive function of UNC-6/netrin and semaphorin signaling or it could involve a passive movement of ray 1 cells resulting from a shift caused by unc-6 and semaphorin mutations in the relative adhesion of ray 1 to the differentially elongating hypodermal cells with which it associates. In either case, the permissive function of these proteins in ray 1 positioning represents a new signaling paradigm for these guidance molecules, which if understood in detail, could provide valuable insights into mammalian organogenesis including angiogenesis as well as tumor progression and cancer metastasis, all of which may also involve permissive signaling by vertebrate homologs of these C. elegans axon guidance cues.
Supplementary Material
Acknowledgments
The authors thank Lesley MacNeil for sharing observations, Naomi Levy-Strumpf and Rob Dunn for comments on the manuscript, Y. Goshima (Yokohama City University), and the Caenorhabiditis elegans Genetics Center (supported by the National Institutes of Health Center for Research Resources) for providing strains. This work was supported by grant MT13207 to J.C. from the Canadian Institutes of Health Research.
Footnotes
Communicating editor: M. Sundaram
Literature Cited
- Asakura T., Ogura K., Goshima Y., 2007. UNC-6 expression by the vulval precursor cells of Caenorhabditis elegans is required for the complex axon guidance of the HSN neurons. Dev. Biol. 304: 800–810. [DOI] [PubMed] [Google Scholar]
- Aufschnaiter R., Zamir E. A., Little C. D., Ozbek S., Munder S., et al. , 2011. In vivo imaging of basement membrane movement: ECM patterning shapes Hydra polyps. J. Cell Sci. 124: 4027–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird S. E., Fitch D. H., Kassem I. A., Emmons S. W., 1991. Pattern formation in the nematode epidermis: determination of the arrangement of peripheral sense organs in the C. elegans male tail. Development 113: 515–526. [DOI] [PubMed] [Google Scholar]
- Baker K. A., Moore S. W., Jarjour A. A., Kennedy T. E., 2006. When a diffusible axon guidance cue stops diffusing: roles for netrins in adhesion and morphogenesis. Curr. Opin. Neurobiol. 16: 529–534. [DOI] [PubMed] [Google Scholar]
- Behar O., Golden J. A., Mashimo H., Schoen F. J., Fishman M. C., 1996. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 383: 525–528. [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza A., Fazzari P., Tamagnone L., 2007. Semaphorin signals in cell adhesion and cell migration: functional role and molecular mechanisms. Adv. Exp. Med. Biol. 600: 90–108. [DOI] [PubMed] [Google Scholar]
- Castets M., Mehlen P., 2010. Netrin-1 role in angiogenesis: To be or not to be a pro-angiogenic factor? Cell Cycle 9: 1466–1471. [DOI] [PubMed] [Google Scholar]
- Chan S. S., Zheng H., Su M. W., Wilk R., Killeen M. T., et al. , 1996. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell 87: 187–195. [DOI] [PubMed] [Google Scholar]
- Chedotal A., Kerjan G., Moreau-Fauvarque C., 2005. The brain within the tumor: new roles for axon guidance molecules in cancers. Cell Death Differ. 12: 1044–1056. [DOI] [PubMed] [Google Scholar]
- Dalpe G., Zhang L. W., Zheng H., Culotti J. G., 2004. Conversion of cell movement responses to Semaphorin-1 and Plexin-1 from attraction to repulsion by lowered levels of specific RAC GTPases in C. elegans. Development 131: 2073–2088. [DOI] [PubMed] [Google Scholar]
- Delorme G., Saltel F., Bonnelye E., Jurdic P., Machuca-Gayet I., 2005. Expression and function of semaphorin 7A in bone cells. Biol. Cell 97: 589–597. [DOI] [PubMed] [Google Scholar]
- Emmons S. W., 2005. Male development. WormBook, ed. The C. elegans Research Community, WormBook, pp. 1–22, http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Feiner L., Webber A. L., Brown C. B., Lu M. M., Jia L., et al. , 2001. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development 128: 3061–3070. [DOI] [PubMed] [Google Scholar]
- Fitch D. H., Emmons S. W., 1995. Variable cell positions and cell contacts underlie morphological evolution of the rays in the male tails of nematodes related to Caenorhabditis elegans. Dev. Biol. 170: 564–582. [DOI] [PubMed] [Google Scholar]
- Fujii T., Nakao F., Shibata Y., Shioi G., Kodama E., et al. , 2002. Caenorhabditis elegans PlexinA, PLX-1, interacts with transmembrane semaphorins and regulates epidermal morphogenesis. Development 129: 2053–2063. [DOI] [PubMed] [Google Scholar]
- Gaur P., Bielenberg D. R., Samuel S., Bose D., Zhou Y., et al. , 2009. Role of class 3 semaphorins and their receptors in tumor growth and angiogenesis. Clin. Cancer Res. 15: 6763–6770. [DOI] [PubMed] [Google Scholar]
- Gebhardt C., Bastmeyer M., Weth F., 2012. Balancing of ephrin/Eph forward and reverse signaling as the driving force of adaptive topographic mapping. Development 139: 335–345. [DOI] [PubMed] [Google Scholar]
- Ginzburg V. E., Roy P. J., Culotti J. G., 2002. Semaphorin 1a and semaphorin 1b are required for correct epidermal cell positioning and adhesion during morphogenesis in C. elegans. Development 129: 2065–2078. [DOI] [PubMed] [Google Scholar]
- Gitler A. D., Lu M. M., Epstein J. A., 2004. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev. Cell 7: 107–116. [DOI] [PubMed] [Google Scholar]
- Gu C., Rodriguez E. R., Reimert D. V., Shu T., Fritzsch B., et al. , 2003. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 5: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A. C., Emmons S. W., 2003. The roles of an ephrin and a semaphorin in patterning cell-cell contacts in C. elegans sensory organ development. Dev. Biol. 256: 379–388. [DOI] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Hall D. H., 1990. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4: 61–85. [DOI] [PubMed] [Google Scholar]
- Hinck L., 2004. The versatile roles of “axon guidance” cues in tissue morphogenesis. Dev. Cell 7: 783–793. [DOI] [PubMed] [Google Scholar]
- Hodgkin J., Horvitz H. R., Brenner S., 1979. Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics 91: 67–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami R., Zheng H., Ong S. H., Culotti J., 2004. Integration of semaphorin-2A/MAB-20, ephrin-4, and UNC-129 TGF-beta signaling pathways regulates sorting of distinct sensory rays in C. elegans. Dev. Cell 6: 383–395. [DOI] [PubMed] [Google Scholar]
- Ito T., Kagoshima M., Sasaki Y., Li C., Udaka N., et al. , 2000. Repulsive axon guidance molecule Sema3A inhibits branching morphogenesis of fetal mouse lung. Mech. Dev. 97: 35–45. [DOI] [PubMed] [Google Scholar]
- Kagoshima M., Ito T., 2001. Diverse gene expression and function of semaphorins in developing lung: positive and negative regulatory roles of semaphorins in lung branching morphogenesis. Genes Cells 6: 559–571. [DOI] [PubMed] [Google Scholar]
- Kerjan G., Dolan J., Haumaitre C., Schneider-Maunoury S., Fujisawa H., et al. , 2005. The transmembrane semaphorin Sema6A controls cerebellar granule cell migration. Nat. Neurosci. 8: 1516–1524. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Eichmann A., 2005. A role for axon guidance receptors and ligands in blood vessel development and tumor angiogenesis. Cytokine Growth Factor Rev. 16: 535–548. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Shimizu A., 2010. Semaphorin 3E, an exception to the rule. J. Clin. Invest. 120: 2658–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin A. L., Matthes D. J., O’Connor T. P., Patel N. H., Admon A., et al. , 1992. Fasciclin IV: sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron 9: 831–845. [DOI] [PubMed] [Google Scholar]
- Kolodkin A. L., Matthes D. J., Goodman C. S., 1993. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75: 1389–1399. [DOI] [PubMed] [Google Scholar]
- Kruger R. P., Aurandt J., Guan K. L., 2005. Semaphorins command cells to move. Nat. Rev. Mol. Cell Biol. 6: 789–800. [DOI] [PubMed] [Google Scholar]
- Larrivee B., Freitas C., Trombe M., Lv X., Delafarge B., et al. , 2007. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 21: 2433–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Raible D., Raper J. A., 1993. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 75: 217–227. [DOI] [PubMed] [Google Scholar]
- Mehlen P., Rama N., 2007. [Netrin-1 and axonal guidance: signaling and asymmetrical translation] Med. Sci. (Paris) 23: 311–315. [DOI] [PubMed] [Google Scholar]
- Mello C., Fire A., 1995. DNA transformation. Methods Cell Biol. 48: 451–482. [PubMed] [Google Scholar]
- Merz D. C., Zheng H., Killeen M. T., Krizus A., Culotti J. G., 2001. Multiple signaling mechanisms of the UNC-6/netrin receptors UNC-5 and UNC-40/DCC in vivo. Genetics 158: 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler W. A., Shemer G., del Campo J. J., Valansi C., Opoku-Serebuoh E., et al. , 2002. The type I membrane protein EFF-1 is essential for developmental cell fusion. Dev. Cell 2: 355–362. [DOI] [PubMed] [Google Scholar]
- Moore D. S., McCabe G. P., 1998. Introduction to the Practice of Statistics. W. H. Freeman, New York. [Google Scholar]
- Nasarre P., Potiron V., Drabkin H., Roche J., 2010. Guidance molecules in lung cancer. Cell Adhes. Migr. 4: 130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. W., Crouse D., Lee M., Karnik S. K., Sorensen L. K., et al. , 2004. The axonal attractant Netrin-1 is an angiogenic factor. Proc. Natl. Acad. Sci. USA 101: 16210–16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp R. J., Kolodkin A. L., 2003. Semaphorin junction: making tracks toward neural connectivity. Curr. Opin. Neurobiol. 13: 79–89. [DOI] [PubMed] [Google Scholar]
- Pickett C. L., Breen K. T., Ayer D. E., 2007. A C. elegans Myc-like network cooperates with semaphorin and Wnt signaling pathways to control cell migration. Dev. Biol. 310: 226–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portman D. S., Emmons S. W., 2000. The basic helix-loop-helix transcription factors LIN-32 and HLH-2 function together in multiple steps of a C. elegans neuronal sublineage. Development 127: 5415–5426. [DOI] [PubMed] [Google Scholar]
- Roy P. J., Zheng H., Warren C. E., Culotti J. G., 2000. mab-20 encodes Semaphorin-2a and is required to prevent ectopic cell contacts during epidermal morphogenesis in Caenorhabditis elegans. Development 127: 755–767. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Doci C., Gutkind J. S., 2012. Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer. Cell Res. 22: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fristsch E. F., Maniatis T., 1989. Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Serini G., Valdembri D., Zanivan S., Morterra G., Burkhardt C., et al. , 2003. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424: 391–397. [DOI] [PubMed] [Google Scholar]
- Simske J. S., Hardin J., 2001. Getting into shape: epidermal morphogenesis in Caenorhabditis elegans embryos. Bioessays 23: 12–23. [DOI] [PubMed] [Google Scholar]
- Spassky N., de Castro F., Le Bras B., Heydon K., Queraud-LeSaux F., et al. , 2002. Directional guidance of oligodendroglial migration by class 3 semaphorins and netrin-1. J. Neurosci. 22: 5992–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K., Strickland P., Valdes A., Shin G. C., Hinck L., 2003. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev. Cell 4: 371–382. [DOI] [PubMed] [Google Scholar]
- Steven R., Kubiseski T. J., Zheng H., Kulkarni S., Mancillas J., et al. , 1998. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell 92: 785–795. [DOI] [PubMed] [Google Scholar]
- Strickland P., Shin G. C., Plump A., Tessier-Lavigne M., Hinck L., 2006. Slit2 and netrin 1 act synergistically as adhesive cues to generate tubular bi-layers during ductal morphogenesis. Development 133: 823–832. [DOI] [PubMed] [Google Scholar]
- Stringham E. G., Dixon D. K., Jones D., Candido E. P., 1992. Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol. Biol. Cell 3: 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Albertson D. G., Thomson J. N., 1980. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev. Biol. 78: 542–576. [DOI] [PubMed] [Google Scholar]
- Sutton A. L., Zhang X., Dowd D. R., Kharode Y. P., Komm B. S., et al. , 2008. Semaphorin 3B is a 1,25-Dihydroxyvitamin D3-induced gene in osteoblasts that promotes osteoclastogenesis and induces osteopenia in mice. Mol. Endocrinol. 22: 1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L., Comoglio P. M., 2004. To move or not to move? Semaphorin signaling in cell migration. EMBO Rep. 5: 356–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufro A., Teichman J., Woda C., Villegas G., 2008. Semaphorin3a inhibits ureteric bud branching morphogenesis. Mech. Dev. 125: 558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas G., Tufro A., 2002. Ontogeny of semaphorins 3A and 3F and their receptors neuropilins 1 and 2 in the kidney. Gene Expr. Patterns 2: 151–155. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang W., Cheever T., Schwarz V., Opperman K., et al. , 2008. The C. elegans L1CAM homologue LAD-2 functions as a coreceptor in MAB-20/Sema2 mediated axon guidance. J. Cell Biol. 180: 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B., 1988. The Nematode Caenorhabditis elegans, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Yang Y. H., Zhou H., Binmadi N. O., Proia P., Basile J. R., 2011. Plexin-B1 activates NF-kappaB and IL-8 to promote a pro-angiogenic response in endothelial cells. PLoS ONE 6: e25826. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yochem J., Gu T., Han M., 1998. A new marker for mosaic analysis in Caenorhabditis elegans indicates a fusion between hyp6 and hyp 7, two major components of the hypodermis. Genetics 149: 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R. Y., Nguyen C. Q., Hall D. H., Chow K. L., 2000. Expression of ram-5 in the structural cell is required for sensory ray morphogenesis in Caenorhabditis elegans male tail. EMBO J. 19: 3542–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T. W., Bargmann C. I., 2001. Dynamic regulation of axon guidance. Nat. Neurosci. 4(Suppl): 1169–1176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.