Abstract
Drugs that interfere with cannabinoid CB1 transmission suppress food-motivated behaviors, and may be useful clinically as appetite suppressants. However, there may also be undesirable side effects (e.g., nausea, malaise, anxiety, depression) that are produced by the current generation of CB1 inverse agonists such as rimonabant and taranabant. For that reason, it is important to continue research on novel cannabinoid antagonists. The present studies examined the effects of the novel compound AM6545, which is a neutral antagonist of CB1 receptors that is thought to have relatively poor penetrability into the central nervous system. Intraperitoneal administration of AM6545 significantly reduced food-reinforced operant responding at doses of 4.0, 8.0 and 16.0 mg/kg. AM6545 also produced a strong suppression of the intake of high carbohydrate and high fat diets in the same dose range, but only produced a mild suppression of lab chow intake at the highest dose (16.0 mg/kg). Although AM6545 did not affect food handling, it did reduce time spent feeding and feeding rate. Taken together, these results suggest that AM6545 is a compound that warrants further study as a potential appetite suppressant drug.
Keywords: appetite, motivation, operant, feeding, THC, rimonabant
1. Introduction
Cannabinoid systems are involved in the regulation of feeding and food-motivated behaviors. CB1 receptor agonists such as delta-9 tetrahydrocannabinol, anandamide and 2-AG have been shown to elevate levels of food intake (Jamshidi and Taylor, 2001; Kirkham et al, 2002; Williams and Kirkham 1999). Conversely, food intake is impaired by CB1 receptor inverse agonists such as rimonabant (SR141716A), AM251 and AM1387 (Arnone et al, 1997; Colombo et al, 1998; Simiand et al, 1998; Williams and Kirkham 1999; Cooper 2004; McLaughlin et al, 2003, 2005, 2006; Salamone et al, 2007), and by CB1 antagonists including AM4113 and O-2050 (Gardner and Mallet 2006; Salamone et al, 2007; Chambers et al. 2007; Sink et al, 2008a, 2008b). Drugs that interfere with CB1 receptor transmission also have been shown to impair food-reinforced behavior (Freedland et al, 2000; McLaughlin et al, 2003, 2006; Ward and Dyskstra 2005; Thornton-Jones et al. 2005; Salamone et al, 2007; Sink et al, 2008a, 2008b, 2009a). Based upon these actions on food intake and food-reinforced behavior, as well as other metabolic effects, it has been suggested that drugs that interfere with cannabinoid CB1 transmission could be useful as treatments for obesity (Pi-Sunyer et al, 2006; Van Gaal et al, 2005). Indeed, clinical trials assessing the CB1 inverse agonists rimonabant and taranabant have shown that these drug can be effective at reducing body weight (Curioni and Andre, 2006; Despres et al., 2005; Pi-Sunyer et al., 2006; Van Gaal et al., 2005; Addy et al., 2008).
Despite these promising reports, there also are problems associated with the use of CB1 receptor inverse agonists in humans. For example, rimonabant produced nausea and sickness in a number of participants in clinical research (Pi-Sunyer et al., 2006). In addition, clinical studies with rimonabant and taranabant have reported evidence of serious psychiatric side effects including depression and anxiety (US Food and Drug Administration Advisory Committee, 2007; Addy et al., 2008). Animal research also indicates that CB1 receptor inverse agonists can produce anxiogenic effects (Rodgers et al. 2005; Sink et al. 2010a), and can affect retention of conditioned fear (Sink et al. 2010b). Based upon the complications associated with the use of CB1 receptor inverse agonists, the Food and Drug Administration denied approval of rimonabant for the treatment of obesity in the United States (US Food and Drug Administration Advisory Committee, 2007). Nevertheless, there are important reasons to continue research on the appetite-related effects of drugs that act upon CB1 receptors (Salamone et al. 2007; Sink et al. 2008a, 2009b; Le Foll et al. 2009). Although some of the side effects of rimonabant are related to central nervous system actions, it is possible that a CB1 receptor antagonist with poor penetration into the brain could be useful as an appetite suppressant (Gomez et al. 2002; Sink et al. 2010). Another important line of research is the assessment of the appetite suppressant effects of CB1 receptor neutral antagonists such as AM4113. Recent studies with AM4113 showed that this drug can suppress food intake and food-reinforced behavior at doses that do not induce signs of nausea, conditioned food avoidance, or anxiety (Chambers et al. 2007; Sink et al. 2008a,b, 2009b, 2010a).
For these reasons, it is important to assess the food-related actions of novel cannabinoid antagonists. The present studies assessed the effects of the recently developed compound, AM6545. This compound binds to CB1 receptors, and has the biochemical characteristics of a neutral CB1 receptor neutral antagonist (Cluny et al. in press). AM6545 binds to CB1 receptors with a 300-fold selectivity over CB2 receptors (CB1 receptor Ki = 1.7 nM). Assays of c-AMP production in transfected cells indicate that AM6545 has no significant intrinsic activity at CB1 receptors compared to the known CB1 inverse agonist AM251 (Cluny et al., in press). In addition, AM6545 has very low penetrability into the brain relative to AM4113, as measured by brain-to-plasma levels (i.e. 7-20 fold lower penetrability; Cluny et al. in press). A CB1 receptor neutral antagonist could be a very useful compound, because it may be able to suppress food intake due to peripheral actions, but minimize side effects that depend upon central nervous system effects (Gomez et al. 2002; Sink et al. 2009b). The present studies examine the effects of AM6545 on food-reinforced behavior and feeding paradigms that have been used previously to assess the effects of a variety of drugs that affect CB1 transmission, including rimonabant, AM251, AM1387, AM6527, and AM4113 (McLaughlin et al. 2003, 2005, 2006; Sink et al. 2008a, 2009a). These tests included food-reinforced lever pressing on a fixed ratio (FR) 5 schedule (McLaughlin et al. 2003, 2006; Sink et al. 2008), intake of three different types of food (high carbohydrate diet, high fat diet, laboratory chow; see McLaughlin et al. 2003, 2006; Sink et al. 2008) and detailed observations of food intake parameters (Salamone et al. 1990, 1993; McLaughlin et al. 2005). The analysis of specific feeding parameters was conducted in order to determine if AM6545 altered forepaw usage or food handling in a way that is similar to the motoric effects of ventrolateral neostriatal dopamine depletions (Salamone et al. 1993), and also to characterize the temporal organization of feeding behavior in rats treated with AM6545.
2. Materials and Methods
2.1. Animals
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed in a colony room on a 12-h light-dark cycle (lights on during 0700-1900). Different groups of rats were used for each experiment (total n = 45). All experiments were conducted during the light part of the cycle. For experiment 1, rats were food deprived to 85% of their free-feeding body weight and weighed daily. All animals protocols were approved by the Institution for Animal Care and Use Committee and the methods were in accordance with the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animals Resources, 1996).
2.2. Drugs
AM6545 (5-aryl-1-(2,4-dichlorophenyl)-4-methyl-N-(cycloalkyl)-1H-pyrazole-3-carboxamide) was synthesized in the laboratory of A. Makriyannis at the Center for Drug Discovery, Northeastern University. AM6545 was suspended in dimethylsulfoxide (DMSO), Tween 80, and 0.9% Saline, with a ratio of 1:1:8. This combination also served as the vehicle control solution. Drug or vehicle was administered IP (see descriptions of individual experiments for drug administration schedules). Doses of AM6545 used were 2.0, 4.0, 8.0, and 16.0 mg/kg, IP; this progression was based upon extensive pilot work, and on comparisons with the effects of other drugs such as AM4113 and AM251.
2.3. Experimental Procedures
Experiment 1 – OPERANT LEVER PRESSING ON FR5 SCHEDULE
Behavioral sessions were conducted in operant chambers (28 × 23 × 23 cm, Med Associates, Georgia, VT). Animals were trained in 30 min sessions, 5 days per week. In the first week of training, all rats (n = 8) were trained to lever press for 45 mg pellets (Research Diets, Inc., New Brunswick, NJ, USA) on a continuous (FR1) schedule. In the second week, animals were shifted to an FR5 schedule (i.e., 5 lever presses were required for delivery of each food pellet), which was maintained for 6 weeks to allow rats to achieve a steady baseline (approx. 1600 responses per session). To assess the effects of AM6545, rats were injected once a week and tested on Fridays; baseline behavioral data were collected on Monday-Thursday. Operant conditioning test sessions were controlled by Med-PC software (Med Associates, St. Albans, VT), which also gathered the data. Rats in experiment 1 received IP injections of 1.0 ml/kg of the vehicle solution or 2.0, 4.0, 8.0, and 16.0 mg/kg doses of AM6545 30 min before the operant test session. Over the course of the experiment all rats received all treatments, one treatment per week, in a randomized order using a repeated measures design.
Experiment 2 – EFFECT OF AM6545 ON CONSUMPTION OF STANDARD CHOW, HIGH-FAT OR HIGH-CARBOHYDRATE DIETS
Animals were assigned to three different diet conditions (n = 10:9:10, respectively). One group was assigned to a high-fat diet (HF; Diet #D12451, Research Diets, New Brunswick, NJ, 20% protein, 45% fat, 35% carbohydrate). A second group was given a high-carbohydrate diet (HC; Diet #D12450B, Research Diets, New Brunswick, NJ, 20% protein, 10% fat, 70% carbohydrate). The remaining group was fed a standard chow diet (LC, 5P00 Prolab RMH 3000, PMI Nutrition International, St Louis, MO; 26% protein, 14% fat, 60% carbohydrate). Food blocks from each type of diet were nutritionally complete and similar in appearance and weight. Rats were given free access to lab chow in their home cages until the beginning of a 5-day habituation period. On the first day of habituation, rats were assigned to their respective dietary groups and moved into suspended wire mesh test cages containing their assigned food type. After spending 30 min in the test cage, they were returned to their home cages. After this initial habituation period, rats were given free access to lab chow in their home cages every Thursday afternoon through Monday afternoon. Each Tuesday and Wednesday, rats spent 30 min in the test cages with their assigned diets. On Thursdays, animals were injected and placed in the test cages with a pre-weighed amount of assigned food. A piece of cardboard was placed underneath the chamber to catch spillage. Following each session, all remaining food plus any spillage was collected and weighed. The difference between pre- and post-session food weights was considered to be the amount of intake. These are the same procedures that were used in previous studies of rimonabant, AM251, AM1387 and AM4113 (McLaughlin et al. 2003, 2006; Sink et al. 2008a). Rats in experiment 2 received IP injections of 1.0 ml/kg vehicle or 4.0, 8.0, and 16.0 mg/kg doses of AM6545 30 min before the test session, and their treatments were administered in a randomized order over successive order using a repeated measures design.
Experiment 3 – EFFECTS OF AM6545ON FOOD INTAKE PARAMETERS
For this experiment, the same procedure was used for training animals (n=8) as in experiment 2, with the exception that only the high carbohydrate diet was used. Additionally, following the 5 day habituation period, animals were given 3 days (Tuesday-Thursday) of habituation with experimenter present in testing room, one animal at a time, mimicking the actual observation session. On test days, rats received IP injections of vehicle, 4.0, 8.0, and 16.0 mg/kg of AM6545 or vehicle, 30 min prior to the behavioral session. During the feeding session, an experimenter blind to the treatment condition observed each rat and recorded time spent feeding with zero, one, or two paws by pressing one of three levers on a panel connected to an interface (Med Associates). The interface was connected to a computer that recorded total amount of time in seconds for each lever (one lever press started a timer, a second press stopped it) using custom-written software (Med-Pc, v1.2). Feeding was operationally defined to include only gnawing on food pellets and subsequent chewing that was initiated with pellet contact. Following each session, remaining food and spillage was weighed and food intake was defined as the difference between pre-session and post-session food weights. Feeding rate or efficiency (g/min) was defined as intake for each session divided by total time spent feeding during the session. Over successive weeks, each rat received all treatments in a randomly varied order, once per week, in a repeated measures design.
2.4. Statistical Analyses
Data were analyzed using SPSS v.17. Data from experiment 1 and 3 were analyzed using repeated measures analysis of variance (ANOVA). Experiment 2 was first analyzed by a mixed design factorial ANOVA (3 food types × 4 treatments), and then subsequently data for each food type were analyzed separately. In addition, nonorthogonal planned comparisons were performed to compare each dose of drug to vehicle; thus the number of comparisons was restricted to the number of conditions minus one (Keppel, 1982). The overall error term was used for each comparison.
3. Results
3.1. Experiment 1 – OPERANT LEVER PRESSING
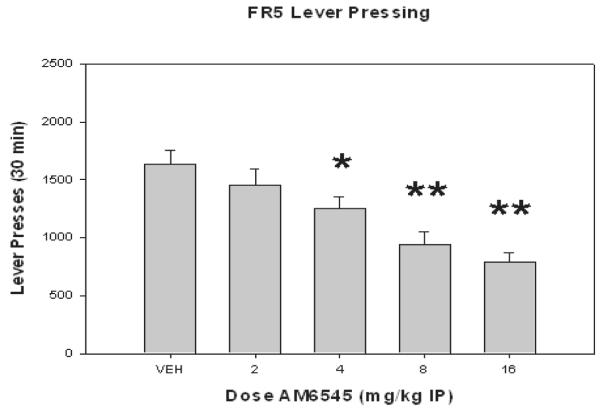
AM6545 decreased food-reinforced lever pressing on a FR5 schedule in a dose-related manner. ANOVA showed that there was a significant overall effect of treatment [F(4, 28) = 10.638, p<0.001; Figure 1]. Planned comparisons show that AM6545 significantly decreased lever pressing rate relative to vehicle at doses of 4.0, 8.0, and 16.0 mg/kg (p<0.05).
Figure 1.
The effects of IP administration of AM6545 on FR5 lever pressing. Mean (± SEM) number of lever presses (FR5 schedule) during the 30-min session for rats that received treatment with vehicle or 4.0, 8.0 or 16.0 mg/kg AM6545. (*p<0.05, **p<0.01, different from vehicle)
3.2. Experiment 2 – CONSUMPTION OF STANDARD LABORATORY CHOW, HIGH-FAT, AND HIGH-CARBOHYDRATE DIETS
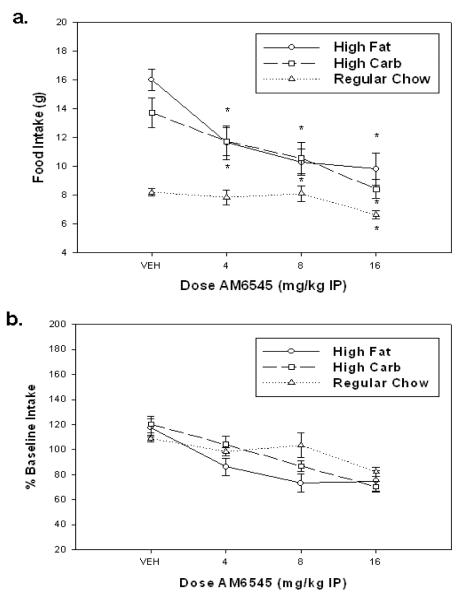
Figure 2 shows the effects of AM6545 on food consumption. AM6545 significantly suppressed gram quantity of food intake (Figure 2A) over vehicle across all diet groups [F(3,78) = 18.243, p < 0.001]. There was also a significant effect of diet [F(2,26) = 19.170, p < 0.001] and a significant treatment by diet interaction [F(6,78) = 17.032, p < 0.001]. Planned comparisons show that every dose of AM6545 significantly decreased feeding compared to vehicle with rats tested on both the high-fat (p<0.01) and high-carbohydrate (p<0.05) diets. In contrast, only the highest dose (16.0 mg/kg) was capable of significantly decreasing intake over vehicle on the standard chow diet (p<0.05). In order to correct for differences in the baseline level of intake for the three different diets, all data were re-analyzed as a percent of the previous baseline day (Figure 2B). With this transformation, there was a significant effect of dose [F(3,78) = 18.3, p < 0.001], but not a significant treatment × diet interaction [F(6,78) = 2.004, p < 0.08]. However, orthogonal analysis of trend revealed that, with the percent baseline transformed data, there was a significant interaction of the quadratic trends across diet groups [F(2,26) = 4.105, p < 0.05]. The source of this interaction is that the dose response curves in the three diet groups had different shapes, which appears to be due to the chow intake curve being different than the other two diet groups.
Figure 2.
Effect of AM6545 on intake of three different diets. A. Mean (± SEM) raw intake (expressed in grams) of three different diets during 30 min sessions. For the high carbohydrate and high fat diet groups every dose was significantly different from vehicle (p < 0.05) as measured by planned comparisons. There was also a significant dose × diet interaction. Chow intake was only reduced at the highest dose. B. Mean (± SEM) intake expressed as percent of baseline consumption (defined as the mean consumption of the previous two non-injection sessions) of the three different diets during 30 minute sessions. There was no significant dose × group interaction; however there was an overall effect of dose, and an interaction of the quadratic trends. (*p<0.05, different from vehicle)
3.3. Experiment 3 – Effects of AM6545 on Food Intake Parameters
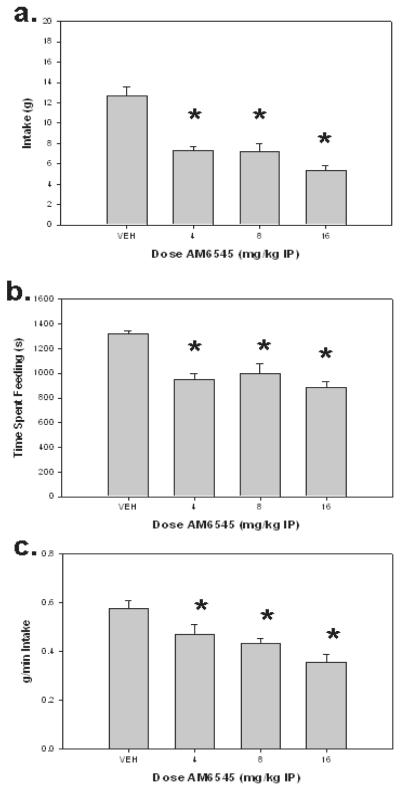
As in experiment 2, AM6545 decreased intake of high carbohydrate diet [F(3,21)=31.075, p<0.001]. In addition, planned comparisons showed a decrease at all doses relative to vehicle (p<0.01). As shown in Figure 3A, AM6545 decreased feeding rate or efficiency [F(3,21)=10.837, p<0.001]. Planned comparisons showed that efficiency under all doses of AM6545 was significantly different from vehicle (p<0.01). Time spent feeding was also decreased in the presence of AM6545 [F(3,21)=11.584, p<0.001; Figure 3B]. Planned comparisons showed that time spent feeding under all doses of AM6545 was different from vehicle (p<0.01). As shown in Table 1, there were no significant effects of AM6545 on forepaw usage during feeding, number of feeding bouts, or average bout duration.
Figure 3.
The effects of IP administration of AM6545 on various parameters of food intake (high carbohydrate diet) during the 30-min sessions for rats that received treatment with vehicle or 4.0, 8.0, or 16.0 mg/kg AM6545 IP. A. Mean (± SEM) amount of food consumed. B. Mean (± SEM) time spent feeding. C. Mean (± SEM) feeding rate or efficiency (grams/min spent feeding). (*p<0.05, different from vehicle)
Table 1.
Measures of intake and related behaviors from experiment 3.
| Measure | Vehicle | Dose AM 6545 (mg/kg) |
||
|---|---|---|---|---|
| 4.0 | 8.0 | 16.0 | ||
| Time Feeding with both forepaws (% of time feeding) |
99.8 (± 0.1) |
99.3 (± 0.5) |
99.3 (± 0.4) |
99.4 (± 0.3) |
|
| ||||
| Number of feeding bouts |
26 (± 3.1) |
25.88 (± 5.94) |
19.88 (± 4.14) |
20 (± 3.4) |
|
| ||||
| Average feeding bout length (s) |
61.63 (± 14.15) |
52.77 (± 13.1) |
61.6 (± 11.63) |
51.73 (± 7.69) |
4. Discussion
The novel CB1 receptor antagonist, AM6545, had a number of effects on food-motivated behavior. The first experiment showed that IP administration of AM6545 produced a dose-related reduction in food-reinforced lever pressing on an FR5 schedule of reinforcement. This result is consistent with previous studies showing that other drugs that interfere with CB1 transmission, including rimonabant, AM251, AM1387, AM6527 and AM4113, all produce similar effects (McLaughlin et al. 2003, 2006; Sink et al. 2008a, 2008b, 2009a). AM6545 reduced lever pressing in the dose range of 4.0-16.0 mg/kg. Over the same dose range, this drug also suppressed intake of three different types of foods in experiment 2. Intake of the high carbohydrate and high fat diets was reduced at 4.0-16.0 mg/kg doses. However, only the highest dose reduced intake of laboratory chow. Moreover, when the data were expressed as percent of baseline to correct for baseline differences in food intake across diets, orthogonal analysis of trend revealed that the shapes of the dose response curves for the effect of AM6545 on food intake differed across diet conditions. Overall, this pattern of effects across the three different food types is similar to that previously shown for rimonabant, AM251, AM1387 and AM4113 (McLaughlin et al. 2003, 2006; Sink et al. 2008a). Taken together, it appears that AM6545 suppresses food intake and food-reinforced behavior in a manner similar to other drugs that interfere with CB1 receptor transmission.
It is particularly noteworthy that AM6545 produced effects on food motivated behavior that closely resemble those produced by another neutral antagonist, AM4113 (Sink et al. 2008a). The fact that neutral antagonists can suppress food intake and food-reinforced behavior indicates that endogenous CB1 receptor tone is necessary for the maintenance of these behaviors (Sink et al. 2008a). Previous studies with AM4113 indicate that this drug fails to produce either nausea as measured by conditioned gaping, or conditioned taste avoidance (Chambers et al. 2007; Sink et al. 2008a). Parallel research has shown that AM6545, like AM4113, also does not induce conditioned gaping or conditioned taste avoidance in the same dose range that suppresses food intake (Cluny et al. in press). Thus, it appears that blockade of CB1 receptors can reduce food intake and food-reinforced behavior at doses that do not induce signs of nausea or malaise. This observation is in contrast to previously reported findings with CB1 receptor inverse agonists (McLaughlin et al. 2005; Sink et al. 2008a). These different effects of CB1 receptor inverse agonists vs. neutral antagonists may indicate that CB1 receptor neutral antagonists would be more useful than inverse agonists as appetite suppressant drugs in humans (Salamone et al. 2007; Sink et al. 2008a; La Foll et al. 2009).
The third experiment studied the effects of AM6545 on intake of the high carbohydrate diet using detailed behavioral observations in addition to gross measures of total intake. As in the second experiment, AM6545 reduced intake of the high carbohydrate diet over the dose range of 4.0-16.0 mg/kg. Some of this reduction in food intake was due to the fact that rats treated with AM6545 spent less time feeding. In addition, AM6545 also reduced the efficiency of feeding behavior (i.e., the rate of feeding in grams/minutes spent feeding). This pattern of effects is similar to that previously reported to occur after administration of some appetite suppressant drugs, such as the serotonergic agent fenfluramine (Blundell and Latham 1980; Blundell 1986). Moreover, despite these changes in time spent feeding and feeding rate, AM6545 did not produce a general disruption of feeding behavior. The number and average duration of feeding bouts was not affected by injections of AM6545. The lack of effect of AM6545 on these parameters of feeding behavior in experiment 3, when a high-carbohydrate diet was being consumed, was similar to what was reported previously in studies involving the effects of rimonabant on intake of a liquid sucrose diet (Higgs et al. 2003; Thornton-Jones et al. 2007). Furthermore, AM6545 did not affect forepaw usage or handling during feeding. In this sense, the effects of AM6545 on feeding behavior differed substantially from manipulations such as ventrolateral striatal dopamine depletions, which suppress food intake largely by producing motor impairments that are marked by disruptions in forepaw usage during feeding, and severe disruptions of feeding rate in the absence of effects on time spent feeding (Salamone et al. 1993).
In summary, the novel CB1 receptor antagonist AM6545 suppresses both food intake and food-reinforced behavior in the same dose range (4.0-16.0 mg/kg IP). In absolute terms, AM6545 produced a greater suppression of intake of high carbohydrate and high fat diets relative to intake of chow, although chow consumption also was significantly affected. Trend evidence indicated that the dose response curves for the effect of AM6545 may differ across diets. In addition, behavioral observations suggest that AM6545 is not disrupting food intake because of motor impairments specifically related to forepaw usage. However, the present data do not exclude other possible explanations for the effect of AM6545, such as sedative effects or the induction of competing behaviors (Tallett et al. 2007a, 2007b). Further studies should examine the effects of AM6545 on tests related to anxiety (Rodgers et al. 2005; Sink et al. 2010a), fear conditioning (Sink et al. 2010b) and the satiety sequence (Hodge et al. 2008), and should determine if this drug is active after oral administration (Sink et al. 2009b). Recent studies have indicated that AM6545 does not produce conditioned taste avoidance, and does not induce nausea or malaise as measured by the conditioned gaping test (Cluny et al. in press). Moreover, this drug has relatively poor penetrability into the brain relative to AM4113 (Cluny et al. in press). In view of data indicating that suppression of food intake induced by CB1 antagonists may be related to peripheral actions (Gomez et al. 2002; Sink et al. 2009b), future research should determine the extent to which peripheral or central mechanisms contribute to the suppression of food-motivated behaviors that results from administration of AM6545. Taken together, the present results suggest that AM6545 is a compound that warrants further study as a potential appetite suppressant drug.
Acknowledgements
This research was supported by grants to JDS and AM from the United States NIH/NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addy C, Rothenberg P, Li S, Majumdar A, Agrawal N, Li H, Zhong L, Yuan J, Maes A, Dunbar S, Cote J, Rosko K, Van Dyck K, De Lepeleire I, de Hoon J, Van Hecken A, Depré M, Knops A, Gottesdiener K, Stoch A, Wagner J. Multiple-dose pharmacokinetics, pharmacodynamics, and safety of taranabant, a novel selective cannabinoid-1 receptor inverse agonist, in healthy male volunteers. J. Clin. Pharmacol. 2008;48:734–744. doi: 10.1177/0091270008317591. [DOI] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiébot M-H, Poncelet M, Soubrié P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Blundell JE. Serotonin manipulations and the structure of feeding behaviour. Appetite. 1986;7(Suppl):39–56. doi: 10.1016/s0195-6663(86)80051-4. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Latham CJ. Characterisation of adjustments to the structure of feeding behaviour following pharmacological treatment: effects of amphetamine and fenfluramine and the antagonism produced by pimozide and methergoline. Pharmacol Biochem Behav. 1980;12:717–722. doi: 10.1016/0091-3057(80)90155-0. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Vemuri VK, Peng Y, Wood JT, Olszewska T, Pittman QJ, Makriyannis A, Sharkey KS. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2185–2193. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, Lutz B, Parker LA, Makriyannis A, Sharkey KA. A novel, peripherally restricted, cannabinoid 1 (CB1) receptor antagonist AM6545 reduces food intake and body weight, but does not cause malaise, in rodents. Brit J Pharmacol. doi: 10.1111/j.1476-5381.2010.00908.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:PL113–117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Cooper SJ. Endocannabinoids and food consumption: comparisons with benzodiazepine and opioid palatability-dependent appetite. Eur J Pharmacol. 2004;500:37–49. doi: 10.1016/j.ejphar.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Curioni C, Andre C. Rimonabant for overweight or obesity. Cochrane Database Syst. Rev. 2006;4 doi: 10.1002/14651858.CD006162.pub2. CD006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L. Rimonabant in Obesity-Lipids Study Group, 2005. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2006;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Poston JS, Porrino LJ. Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol Biochem and Behav. 2000;67:265–270. doi: 10.1016/s0091-3057(00)00359-2. [DOI] [PubMed] [Google Scholar]
- Gardner A, Mallet PE. Suppression of feeding, drinking, and locomotion by a putative cannabinoid receptor ‘silent antagonist’. Eur J Pharmacol. 2006;530:103–106. doi: 10.1016/j.ejphar.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, de Fonseca F Rodriguez. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs S, Williams CM, Kirkham TC. Cannabinoid influences on palatability: microstructural analysis of sucrose drinking after delta(9)-tetrahydrocannabinol, anandamide, 2-arachidonoyl glycerol and SR141716. Psychopharmacology. 2003;165(4):370–377. doi: 10.1007/s00213-002-1263-3. [DOI] [PubMed] [Google Scholar]
- Hodge J, Bow JP, Plyler KS, Vemuri VK, Wisniecki A, Salamone JD, Makriyannis A, McLaughlin PJ. The cannabinoid CB1 receptor inverse agonist AM 251 and antagonist AM 4113 produce similar effects on the behavioral satiety sequence in rats. Behav Brain Res. 2008;193:298–305. doi: 10.1016/j.bbr.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A researchers handbook. Prentice-Hall; Englewood Cliffs, NJ: 1982. [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharm (Berl) 2009;205:171–174. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse KL, Fournier DJ, Li X, Grzybowska J, Makriyannis A. A novel electrophilic high affinity irreversible probe for the cannabinoid receptor. Life Sci. 1995;56:1957–1962. doi: 10.1016/0024-3205(95)00176-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, Betz AJ, Ishiwari K, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonist AM251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology. 2005;180:286–293. doi: 10.1007/s00213-005-2171-0. (2005) [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Qian L, Wood JT, Wisniecki A, Winston KM, Swezey LA, Makriyannis A, Salamone JD. Suppression of food intake and food-reinforced behavior produced by the novel CB1 receptor antagonist/inverse agonist AM1387. Pharmacol Biochem Behav. 2006;83:396–402. doi: 10.1016/j.pbb.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J, RIO-North America Study Group Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Evans PM, Murphy A. Anxiogenic profile of AM-251, a selective cannabinoid CB1 receptor antagonist, in plus-maze-naïve and plus-maze-experienced mice. Behav Pharmacol. 2005;16:405–413. doi: 10.1097/00008877-200509000-00013. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Mahan K, Rogers S. Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol Biochem Behav. 1993;44:605–610. doi: 10.1016/0091-3057(93)90174-r. [DOI] [PubMed] [Google Scholar]
- Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB(1) receptor inverse agonists and neutral antagonists: Effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simiand J, Keane M, Keane PE, Soubrie P. SR141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, Pang Y, Olzewska T, Thakur GA, Makriyannis A, Parker LA, Salamone JD. The novel cannabinoid CB(1) receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008a;43:946–955. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology. 2008b;196:565–574. doi: 10.1007/s00213-007-0988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Wood J, Makriyannis A, Salamone JD. Oral bioavailability of the novel cannabinoid CB1 antagonist AM6527: effects on food-reinforced behavior and comparisons with AM4113. Pharmacol Biochem Behav. 2009a;91:303–306. doi: 10.1016/j.pbb.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Nunes EJ, Collins LE, Vemuri VK, Thakur G, Makriyannis A, Salamone JD. Intracerebroventricular administration of cannabinoid CB1 receptor antagonists AM251 and AM4113 fails to alter food-reinforced behavior in rats. Psychopharmacology. 2009b;206:223–232. doi: 10.1007/s00213-009-1602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Sink J, Randall PA, Collins LE, Correa M, Markus EJ, Vemuri VK, Makriyannis A, Salamone JD. Potential anxiogenic effects of cannabinoid CB1 receptor antagonists/inverse agonists in rats: Comparisons between AM4113, AM251, and the benzodiazepine inverse agonist FG-7142. Eur Neuropsychopharmacol. 2010a;20:112–122. doi: 10.1016/j.euroneuro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Collins LE, Markus EJ, Vemuri VK, Makriyannis A, Salamone JD. The CB1 inverse agonist AM251, but not the CB1 antagonist AM4113, enhances retention of contextual fear conditioning in rats. Pharmacol Biochem Behav. 2010b doi: 10.1016/j.pbb.2010.03.011. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallett AJ, Blundell JE, Rodgers JR. Acute anorectic response to cannabinoid CB1 receptor antagonist/inverse agonist AM 251 in rats: Indirect behavioural mediation. Behav Pharmacol. 2007a;18:591–600. doi: 10.1097/FBP.0b013e3282eff0a9. [DOI] [PubMed] [Google Scholar]
- Tallett AJ, Blundell JE, Rodgers RJ. Grooming, scratching and feeding: Role of response competition in acute anorectic response to rimonabant in male rats. Psychopharmacology. 2007b;195:27–39. doi: 10.1007/s00213-007-0880-2. [DOI] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Vickers SP, Clifton PG. The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology. 2005;179:452–460. doi: 10.1007/s00213-004-2047-8. [DOI] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Kennett GA, Vickers SP, Clifton PG. A comparison of the effects of the CB(1) receptor antagonist SR141716A, pre-feeding and changed palatability on the microstructure of ingestive behaviour. Psychopharmacology. 2007;193:1–9. doi: 10.1007/s00213-007-0745-8. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration Advisory Committee . FDA briefing document: zimulti (rimonabant) Tablets, 20 mg. FDA; Rockville: 2007. [WWW document]. URL ( http://www.fda.gov/OHRMS/DOCKETS/AC/07/briefing/2007-4306b1-00-index.htm) [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Roessner S, RIO-Europe Study Group Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Dykstra LA. The role of CB1 receptors in sweet versus fat reinforcement: Effect of CB1 receptor deletion, CB1 receptor antagonism (SR141716A) and CB1 receptor agonism (CP-55940) Behav Pharmacol. 2005;16:381–388. doi: 10.1097/00008877-200509000-00010. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Anandamide induces overeating: Mediation by central cannabinoid (CB1) receptors. Psychopharmacology. 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]