Abstract
Background
Melanomas are aggressive neoplasms with limited therapeutic options. Therefore, developing new therapies with low toxicity is of utmost importance. Honokiol is a natural compound that has recently shown promise as an effective anti-cancer agent.
Methods
Honokiol effect on melanoma cancer cells was assessed in vitro. Proliferation and physiological changes were determined using hexoseaminidase assay and transmission electron microscopy. Protein expression was assessed by immunoblotting.
Results
Honokiol treatment inhibited cell proliferation and induced death. Electron microscopy demonstrated autophagosome formation. Reduced levels of cyclin D1 accompanied cell cycle arrest. Honokiol also decreased phosphorylation of AKT (known as Protein Kinase B) and mTOR (mammalian target of rapamycin), and inhibited γ-secretase activity by downregulating the expression of γ-secretase complex proteins especially APH-1 (anterior pharynx-defective 1).
Conclusions
Honokiol is highly effective in inhibiting melanoma cancer cells by attenuating AKT/mTOR and Notch signaling. These studies warrant further clinical evaluation for honokiol alone or with present chemotherapeutic regimens for treatment of melanomas.
Keywords: Melanoma, AKT, cyclinD1, cell cycle arrest, AKT, mTOR, γ-secretase
INTRODUCTION
Malignant melanoma is an aggressive form of skin cancer with extremely poor survival and few therapeutic options. The incidence of melanoma in the United States has increased more rapidly in the past few decades than any other malignancy1,2. Approximately 40 to 60% of cutaneous melanomas may also carry B-Raf mutations that lead to the constitutive activation of downstream signaling cascade through the mitogen-activated protein kinase (MAPK) pathway3,4. Approximately 90% of these mutations result in the substitution of glutamic acid for valine at codon 600 (BRAF V600E) and renders B-RAF in constitutively active form that confers mitogenic responses for the growth of cancerous cells5. Finally, alterations of the chromosome 10q24–26 region are present in 30–50% of melanoma cell lines and 5–20% of uncultured melanomas6. This region includes the lipid phosphatase PTEN (Phosphatase and tensin homologue deleted from chromosome 10), which functions by negatively regulating the phosphatidylinositol 3′-kinase (PI3K)/AKT pathway. Loss of PTEN function in melanoma needs further investigation6. The PI3K/AKT and Raf/MEK/ERK pathways promote tumorigenesis essentially by positively regulating cell survival, cell cycle progression7,8,9, tumor angiogenesis10,11,12, and tumor invasion13,14,15. All these events together activate a multitude of downstream signaling cascades including the Raf/MAPK kinase (MEK)/extracellular signal-regulated kinase (ERK) and PI3K/AKT pathways. B-RAF mutations activate the MAPK cascade and loss of PTEN activates PI3K; these two pathways are important mediators of melanoma downstream of Ras.
Aberrant activation of the Notch signaling has been also associated with the development of skin cancer. In melanomas, levels of Notch expression are associated with progression, and metastasis16. Notch signaling is initiated when a ligand such as Jagged interacts with the Notch transmembrane receptor, leading to translocation of NICD and binding of two cofactors to transactivate target genes, such as those in the hairy and enhancer of split (Hes), related with YRPW motif (Hey) and cyclin D1 proteins16,17. The importance of Ras-activation and the Notch pathways in melanoma suggests that they may be effective targets for prevention and treatment of melanomas. The real challenge in inhibiting these signaling cascades is that they regulate important growth and survival pathways shared by both neoplastic and normal cells. Therefore, it is of prime importance to develop a therapeutic strategy that able to exert the maximum effect on cancer cells with the minimum toxicity on normal cells.
Honokiol (Fig. 1A) is a biphenolic compound from Magnolia officianalis that is used in -traditional Chinese and Japanese medicine for the treatment of various ailments including ulcer, allergy, and bacterial infections. It is also used as a muscle relaxant and possesses antithrombotic activity18. Recent studies have shown that it has antitumor activity with low toxicity18. In this article, we investigated the effect of honokiol on melanoma cancer cells. This article focuses on defining nontoxic approaches toward eradicating melanoma cancer cells.
Figure 1.
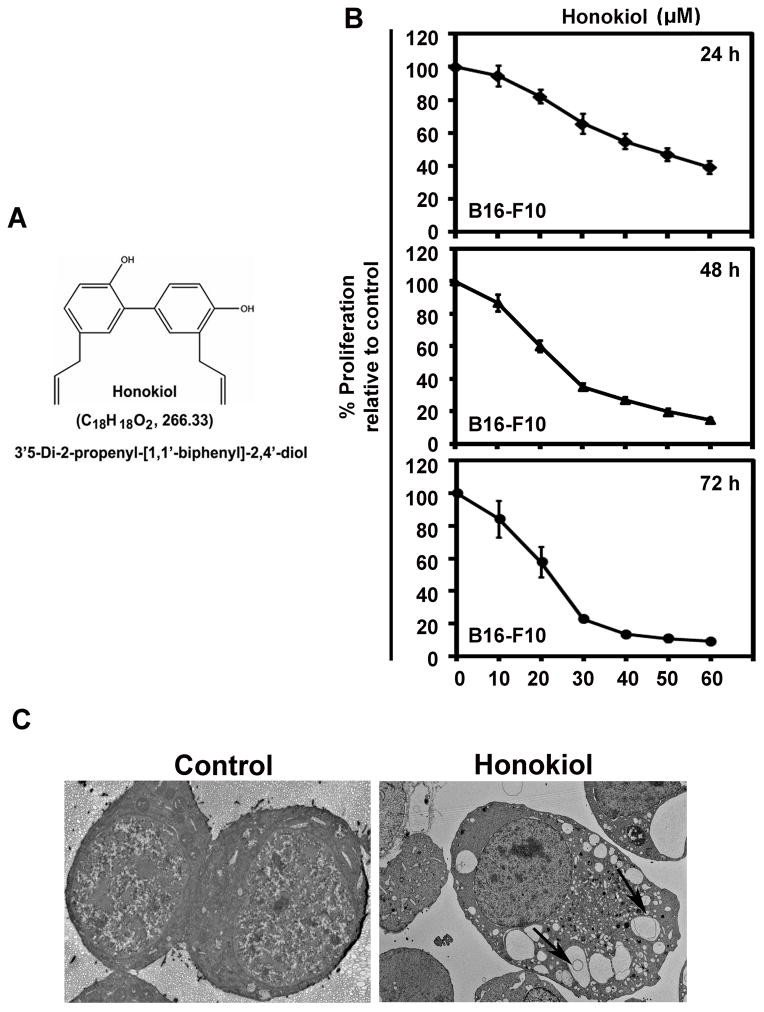
Effect of Honokiol on melanoma cancer cells. A) Chemical structure of honokiol. B) B16-F10 cells were incubated with increasing doses of honokiol (0–50 μM) at different time intervals (24, 48 and 72h) to determine the cell proliferation of B16-F10 cells. The honokiol treatment resulted in a significant dose and time-dependent decrease in cell proliferation compared to their respective controls (P < 0.05). C) Transmission electron micrograph showing honokiol induced vacuoles formation in B16-F10 cells at 24 h of honokiol (30 μM) treatment. All images were taken at 2000 magnification.
MATERIALS AND METHODS
Cell line and reagents
B16-F10 cells (American Type Culture Collection) were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS (Sigma-Aldrich) and antibiotic solution (Mediatech Inc.) at 37° C in a humidified atmosphere containing 5% CO2. Cells used in this study were within 20 passages after receipt or resuscitation (approximately 3 months of noncontinuous culturing). Growth medium was changed after every 3 day and cells were split (1:3) when they reached 80% confluence. For honokiol treatment, stock solution of honokiol was prepared in DMSO, stored at − 20°C in aliquots, and diluted with fresh medium immediately before use. Honokiol was purchased from Sigma Aldrich.
Cell Viability Assay
Assessment of cell proliferation after honokiol treatment was performed by Hexoseaminidase assay19. Cells were plated in 96 well plate, grown over night and treated next day with increasing concentrations of honokiol (0–50 μM) for 24, 48 and 72h. Cell growth was calculated as percent viability = [(A/B)x100], where A and B are the absorbance of treated and control cells, respectively.
Immunoblot Analysis
Cell lysates after honokiol treatment were extracted and quantitated by using Pierce BCA protein assay kit (Thermo Scientific, IL, USA). Cell lysates were subjected to SDS-PAGE and blotted onto nitro cellulose membrane (GE Healthcare) and proteins of interest were detected by using enhanced chemiluminescence system (GE Healthcare). Antibodies for immunoblotting were purchased from Cell Signaling Technology Inc. USA, GenScript Inc. USA, and Santa Cruz Biotechnology Inc. USA. For equal loading of protein in each well, each blot were normalized with their respective internal controls (β-actin or β-tubulin, total AKT and mTOR).
Statistical analysis
All experiments were performed in triplicates. Significant differences were analyzed using student’s t test. Data were considered to be statistically significant if p<0.05. Data was expressed as mean ± SD between triplicate experiments performed thrice.
RESULTS
Honokiol affect viability of B16-F10 melanoma cells
To study the effect of honokiol on melanoma cell growth, B16-F10 melanoma cells were treated with increasing concentrations of honokiol (0–50 μM ) for 24, 48 and 72h and performed cell proliferation by using Hexoseaminidase assay. Honokiol treatment significantly decreased cell proliferation of melanoma cells in dose and time dependent manner with respect to their respective controls (p<0.05). Decrease in cell proliferation was observed within 24 h of Honokiol treatment which was further suppressed over the next 72 h in a dose dependent manner respectively (Fig. 1B). We further, studied the morphological changes in B16-F10 by transmission electron microscopy. Honokiol treatment increased the autophagosomes like vacuoles formation in B16-F10 cells (Fig. 1C). All together, these findings indicate that honokiol showed cytotoxic effects on melanoma cancer cells.
Honokiol down regulates the expression of Cyclin D1 in melanoma cells
Honokiol effectively attenuated the levels of cyclin D1 protein in B16-F10 cells in time and dose dependent manner (Fig. 2A) and thus resulted in cell cycle arrest of melanoma cancer cells. Honokiol attenuated cyclin D1 levels in B16-F10 cells further strengthen the fact that significant decrease in cell growth of B16-F10 cells by honokiol was partly due to inhibition of cell proliferation and induction cell death and partly due to cell cycle arrest. Altogether, this data demonstrated that honokiol has both cytostatic and cytotoxic properties against melanoma cancer cells.
Figure 2.
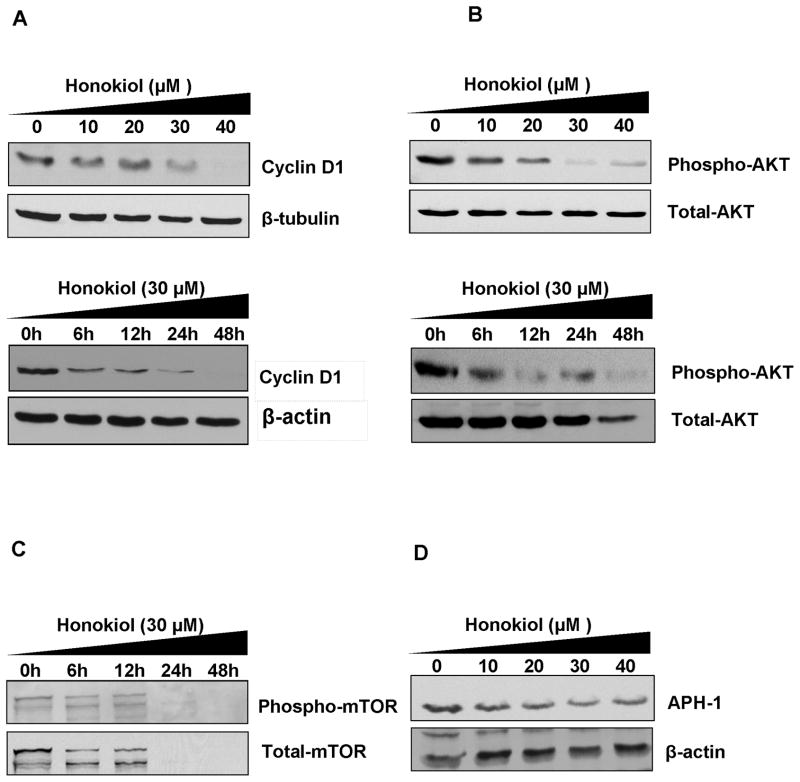
Honokiol attenuates AKT/mTOR and Notch signaling in B16-F10 melanoma cells. A) Immunoblotting was done for cyclin D1 protein levels in B16-F10 cells. Cells were treated with increasing concentrations of honokiol (0–40 μM) for 24 h of time period to study the dose course effect of honkiol. For time course effects, cells were treated with 30 μM of honokiol for 12, 24 and 48 h. After honokiol treatment, protein lysates were prepared and analyzed by Immunoblotting for Cyclin D1 protein. B) and C) Honokiol affects AKT/mTOR signaling. Immunoblot analyses of lysates after honokiol treatment showed significant reduction in phosphorylation of AKT and mTOR phosphorylation and hence their activities in dose and time dependent manner. D) Honokiol affects Notch signaling. Honokiol inhibits Notch signaling by inhibiting the γ-secretase complex proteins. Honokiol showed significant reduction in the expression of APH1.
Signaling affected by honokiol in melanoma cells
Most of the chemotherapeutic agents against melanoma are partly or fully designed for targeting of malfunctioned Ras and Raf signaling molecules in melanoma cancer cells. Therefore we further tried to explore the pathways involved in honokiol induced cell death and growth inhibition of melanoma cells. We observed that honokiol significantly affected the Ras and Raf signaling in melanoma cells by dephosphorylating the constitutively phosphorylated AKT and mTOR protein in B16-F10 cells in dose and time dependent manner (Fig. 2B and 2C). This substantiate the fact that honokiol is inhibiting the Ras and Raf signaling in melanoma cells and thus induced the cell death, cell cycle arrest and inhibited the growth of aggressive melanoma cells. In addition to conventional melanoma signaling, honokiol was also found to inhibit cancer stem cells related signaling i.e. Notch signaling. Honokiol significantly attenuated the levels of APH1 in B16-F10 cells in time dependent manner (Fig. 2D). This protein is the part of the γ-secretase enzyme complex. Gamma-secretase is the key enzyme involved in cleavage of Notch intracellular domain (NICD) and this cleaved notch get translocated to nucleus for transcriptional up-regulation of downstream client genes specially cyclin D1.
DISCUSSION
Honokiol, because of its diverse anti-oxidative, anti-inflammatory, anti-tumor, and antimicrobial pharmacological properties, is a putative candidate for both therapeutic interventions and chemoprevention. Previous studies have shown efficacy of honokiol against several types of cancer like pancreatic, colon and breast without any toxicity20. Unlike other polyphenolic agents, which have been hindered by poor absorption and rapid excretion, honokiol exhibits a significant systemic levels in blood in preclinical models, and it can also cross the blood brain barrier20. We have demonstrated honokiol’s effect on inducing B16-F10 melanoma cancer cells cell death studied by using hexoseaminidase assay and by electron microscopy. Honokiol began to induce these changes within 24 h of treatment and the effect remained at 48 and 72 h of treatment. Honokiol mediated cell death was partly due to the formation of autophagosomes like vacuoles in B16-F10 cells, as indicated by arrow in electron micrograph. Additional studies are required to confirm the types of vacuoles in B16-F10 cells after honokiol treatment. Subsequently we demonstrated that honokiol also exert cytostatic effects by significantly down regulating the cyclin D1 levels in B16-F10 cell in dose and time dependent manner. Cyclin D1, often found to be overexpressed in human melanomas, is an important positive regulator of the G1-S cell cycle transition through its binding and activation of CDK4/6 kinases. Activation of CDKs further inactivate retinoblastoma protein and blocks its growth-inhibitory activity, promotes release of bound E2F which finally leads to cell cycle progression21,22. Recent studies also reported the honokiol induced G0/G1 cell cycle arrest in colon and lung cancer cells18,23. Further studies are required to study the transition of the cells through the various phases of the cell cycle are along with the checkpoint-related markers to confirm this phenomenon.
We further explored the honokiol attenuated molecular pathways involved in cell death and cystatic effects in B16-F10 cells. Melanomas often consist of mutant BRAF or N-RAS, and consequently increased ERK-MAPK and AKT activities24. Preclinical studies have demonstrated a close and complex interconnection of the RAS-RAF-MEK-ERK and the PI3K-AKT-mTOR signaling pathways which are involved in growth, progression and drug resistance of melanomas. AKT has a central role in regulating apoptosis and tumor regression25. In BRAF V600E mutant cells, AKT activation was required for initiation of melanoma, demonstrating the interdependence of these two pathways in melanoma. Our data reveal that honokiol significantly inhibited the AKT and mTOR phosphorylation in dose and time dependent in B16-F10 melanoma cells. This strengthen the facts that honokiol act on PI3K-AKT-mTOR signaling pathway. In this connection, others have shown that topical treatment of melanomas with specific PI3K inhibitor (Ly294002) results in significant reduction of TPras transgenic melanoma growth in severe combined immunodeficient (SCID) mice26. Additionally, inhibition of either the Raf/MEK/ERK pathway by the MEK1/2-specific inhibitor U0126 or PI3K leads to a reduction in tumor invasion and angiogenesis26,27,28.
Role of Notch expression has been reported in progression and metastasis of melanomas16. Notch signaling is 10- to 30-fold higher in the stem cells when compared with other cell types29. In this study, we have determined that honokiol inhibited the Notch signaling by inhibiting the essential members of the γ-secretase complex, the critical enzyme that cleaves and releases the NICD from the membrane. Honokiol decreased the APH1 protein levels in dose dependent manner. Therefore, honokiol–mediated inhibition of melanoma cancer cell growth is also partly mediated via inactivation of Notch-1 signaling cascade. Recent study suggested the role of Notch1 in prevention of cancer cells from cytotoxic insults. This was substantiated by ectopic expression of the Notch intracellular domain partially rescuing the colon and breast cancer cells from cytotoxic compound’s effect18,30. One more recent study also showed that honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine by affecting NF-κB31,32. Recent studies proposed that honokiol alone or in combination of radio- or chemotherapy resulted in a growth inhibition of colon, pancreatic and glioma in vitro and in vivo18,32,33. In addition, the honokiol significantly suppressed Notch-1 activation. Taken together, these data suggest that the honokiol to target cancer stem cells is an attractive novel potential agent for the treatment and prevention of various cancers. Further studies are warranted to show the efficacy of the honokiol in the clinical setting. It would also be interesting to determine whether there are other clients for the γ-secretase complex and the role of these client proteins in cancer stem cell biogenesis.
Acknowledgments
Grant Support:
The work was supported by grants from the NIH (S. Anant) and from the Department of Surgery, KUMC (J. Mammen).
Abbreviations
- AKT
known as Protein Kinase B
- APH-1
Anterior pharynx-defective 1
- BCA
Bicinchoninic acid
- CDK4
Cyclin-dependent kinase 4
- CDK6
Cyclin-dependent kinase 6
- ERK
Extracellular signal-regulated kinases
- mTOR
Mammalian target of rapamycin
- PTEN
Phosphatase and tensin homologue deleted from chromosome 10
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal MJ, Thun LA, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linos E, Swetter SM, Cockburn MG, et al. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–1674. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 4.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 5.Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–9. [PubMed] [Google Scholar]
- 6.Wu H, Goel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113–3122. doi: 10.1038/sj.onc.1206451. [DOI] [PubMed] [Google Scholar]
- 7.Liang J, Zubovitz J, Petrocelli T, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–6. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 8.Welsh CF, Roovers K, Villanueva J, et al. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat Cell Biol. 2001;3:950–7. doi: 10.1038/ncb1101-950. [DOI] [PubMed] [Google Scholar]
- 9.Fulton D, Gratton JP, McCabe TJ, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zundel W, Schindler C, Haas-Kogan D, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Schulze A, Lehmann K, Jefferies HB, et al. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 2001;15:981–94. doi: 10.1101/gad.191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park BK, Zeng X, Glazer RI. AKT1 induces extracellular matrix invasion and matrix metalloproteinase-2 activity in mouse mammary epithelial cells. Cancer Res. 2001;61:7647–53. [PubMed] [Google Scholar]
- 13.Liu E, Thant AA, Kikkawa F, et al. The Ras-mitogen-activated protein kinase pathway is critical for the activation of matrix metalloproteinase secretion and the invasiveness in v-crk-transformed 3Y1. Cancer Res. 2000;60:2361–4. [PubMed] [Google Scholar]
- 14.Benbow U, Tower GB, Wyatt CA, et al. High levels of MMP-1 expression in the absence of the 2G single nucleotide polymorphism is mediated by p38 and ERK1/2 mitogen-activated protein kinases in VMM5 melanoma cells. J Cell Biochem. 2002;86:307–19. doi: 10.1002/jcb.10225. [DOI] [PubMed] [Google Scholar]
- 15.Vlahos CJ, Matter WF, Hui KY, et al. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–8. [PubMed] [Google Scholar]
- 16.Massi D, Tarantini F, Franchi A, et al. Evidence for differential expression of Notch receptors and their ligands in melanocytic nevi and cutaneous malignant melanoma. Mod Pathol. 2006;19:246–54. doi: 10.1038/modpathol.3800526. [DOI] [PubMed] [Google Scholar]
- 17.Ronchini C, Capobianco AJ. Induction of cyclin D1 transcription and CDK2 activity by Notch(ic): implication for cell cycle disruption in transformation by Notch(ic) Mol Cell Biol. 2001;21:5925–34. doi: 10.1128/MCB.21.17.5925-5934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponnurangam S, Mammen JM, Ramalingam S, et al. Honokiol in combination with radiation targets notch signaling to inhibit colon cancer stem cells. Mol Cancer Ther. 2012;11:963–72. doi: 10.1158/1535-7163.MCT-11-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landegren U. Measurement of cell numbers by means of the endogenous enzyme Hexoseaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984;67:379–88. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- 20.Fried LE, Arbiser JL. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid Redox Signal. 2009;11:1139–48. doi: 10.1089/ars.2009.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnellan R, Chetty R. Cyclin D1 and human neoplasia. J Clin Pathol:Mol Pathol. 1998;51:1–7. doi: 10.1136/mp.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sauter ER, Yeo UC, von Stemm A, et al. Cyclin D1 is a candidate oncogene in cutaneous melanoma. Cancer Res. 2002;62:3200–6. [PubMed] [Google Scholar]
- 23.Hu J, Chen LJ, Liu L, et al. Liposomal honokiol, a potent anti-angiogenesis agent, in combination with radiotherapy produces a synergistic antitumor efficacy without increasing toxicity. Exp Mol Med. 2008;40:617–28. doi: 10.3858/emm.2008.40.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platz A, Egyhazi S, Ringborg U, et al. Human cutaneous melanoma; a review of NRAS and BRAF mutation frequencies in relation to histogenetic subclass and body site. Mol Oncol. 2008;1:395–405. doi: 10.1016/j.molonc.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung M, Sharma A, Madhunapantula SV, et al. AKT3 and mutant V600E B-Raf cooperate to promote early melanoma development. Cancer Res. 2008;68:3429–39. doi: 10.1158/0008-5472.CAN-07-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bedogni B, O’Neill MS, Welford SM, et al. Topical treatment with inhibitors of the phosphatidylinositol 3′-kinase/AKT and Raf/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways reduces melanoma development in severe combined immunodeficient mice. Cancer Res. 2004;1:2552–60. doi: 10.1158/0008-5472.can-03-3327. [DOI] [PubMed] [Google Scholar]
- 27.Favata MF, Horiuchi KY, Manos EJ, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–32. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 28.Davies SP, Reddy H, Caivano M, et al. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sikandar SS, Pate KT, Anderson S, et al. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res. 2010;70:1469–78. doi: 10.1158/0008-5472.CAN-09-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondratyev M, Kreso A, Hallett RM, et al. Gamma-secretase inhibitors target tumor-initiating cells in a mouse model of ERBB2 breast cancer. Oncogene. 2012;31:93–103. doi: 10.1038/onc.2011.212. [DOI] [PubMed] [Google Scholar]
- 31.Lee SY, Yuk DY, Song HS, et al. Growth inhibitory effects of obovatol through induction of apoptotic cell death in prostate and colon cancer by blocking of NF-kappaB. Eur J Pharmacol. 2008;582:17–25. doi: 10.1016/j.ejphar.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Arora S, Bhardwaj A, Srivastava SK, et al. Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PLoS One. 2011;6:e21573. doi: 10.1371/journal.pone.0021573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bello L, Carrabba G, Giussani C, et al. Low-dose chemotherapy combined with an antiangiogenic drug reduces human glioma growth in vivo. Cancer Res. 2001;61:7501–6. [PubMed] [Google Scholar]