Abstract
Small cholangiocytes proliferate via activation of Ca2+-dependent signaling in response to pathological conditions that trigger the damage of large cAMP-dependent cholangiocytes. Although our previous studies suggest that small cholangiocyte proliferation is regulated by the activation of Ca2+-dependent signaling, the intracellular mechanisms regulating small cholangiocyte proliferation are undefined. Therefore, we sought to address the role and mechanisms of action by which phenylephrine, an α1-adrenergic agonist stimulating intracellular IP3/Ca2+ levels, regulates small cholangiocyte proliferation. Small and large bile ducts and cholangiocytes expressed all AR receptor subtypes. Small (but not large) cholangiocytes respond to phenylephrine with increased proliferation via the activation of IP3/Ca2+-dependent signaling. Phenylephrine stimulated the production of intracellular IP3. The Ca2+-dependent transcription factors, NFAT2 and NFAT4, were predominantly expressed by small bile ducts and small cholangiocytes. Phenylephrine stimulated the Ca2+-dependent DNA-binding activities of NFAT2, NFAT4, and Sp1 (but not Sp3) and the nuclear translocation of NFAT2 and NFAT4 in small cholangiocytes. To determine the relative roles of NFAT2, NFAT4, or Sp1, we knocked down the expression of these transcription factors with shRNA. We observed an inhibition of phenylephrine-induced proliferation in small cholangiocytes lacking the expression of NFAT2 or Sp1. Phenylephrine stimulated small cholangiocyte proliferation is regulated by Ca2+-dependent activation of NFAT2 and Sp1. Selective stimulation of Ca2+-dependent small cholangiocyte proliferation may be key to promote the repopulation of the biliary epithelium when large bile ducts are damaged during cholestasis or by toxins.
Keywords: biliary epithelial cells, calcineurin, intracellular calcium, phenylephrine, bile duct ligation
In human and experimental cholangiopathies, the proliferation/loss of bile ducts is restricted to specific-sized bile ducts (1-3). The secretory and proliferative capacity of large cholangiocytes depends on the activation of adenosine 3′, 5′-monophosphate (cAMP)-dependent mechanisms (2-4). Large (but not small) cholangiocytes are more susceptible to toxins (e.g., carbon tetrachloride, CCl4) that induce the loss of proliferative and secretory activity (3). Immortalized small cholangiocyte lines (5) proliferate via D-myo-Inositol 1,4,5-triphosphate (IP3)/Ca2+/calmodulin-dependent protein kinase (CaMK) I-dependent mechanisms regulated via activation of H1 histamine receptor subtypes (6). Ca2+-dependent small cholangiocyte proliferation may be a key compensatory mechanism for maintaining homeostasis and overall bile duct function in pathological ductopenic conditions associated with damage of large ducts (3, 7).
We have demonstrated that: (i) cholangiocytes express adrenergic receptors (AR) including α1A/1C, α1B, α2A, α2B, α2C, β1, and β2 subtypes; and (ii) administration of agonists for these receptors regulate large cholangiocyte function by modulation of cAMP-dependent signaling (8-10). For example, activation of α1A/1C, α1B AR (by phenylephrine) stimulates secretin-stimulated cholangiocyte choleresis of bile duct ligated (BDL) rats via Ca2+-dependent stimulation of cAMP signaling (10). The expression of α1-AR receptors, which are G-protein coupled receptors signaling via Ca2+ (11), in small and large cholangiocytes and the possible effects of their stimulation on proliferation has not been explored. In particular, activation of Ca2+-dependent signaling in small cholangiocytes by AR agonists, such as phenylephrine, that are known to trigger intracellular Ca2+ signaling (10), has not been studied.
Nuclear factor of activated T-cells (NFAT) is a ubiquitous transcription factor initially described in T-lymphocytes. Five NFAT family members have been described: NFAT1 (also known as NFATp or NFATc2), NFAT2 (NFATc or NFATc1), NFAT3, NFAT4 (NFATx or NFATc3) and NFAT5 (12). NFAT1, 2, 3 and 4 are regulated by calcium/calcineurin signaling (13), whereas activation of NFAT5 is calcineurin independent (14). In non-stimulated cells, NFAT proteins are located in the cytoplasm in a hyper-phosphorylated state. Following increases in [Ca2+]i, the Ca2+/calmodulin-dependent serine/threonine phosphatase, calcineurin, directly dephosphorylates NFAT, which induces rapid nuclear import providing a direct link between [Ca2+]i signaling and gene expression (13). In the nucleus, NFAT proteins bind to target promoter elements alone or in combination with other nuclear elements such as Sp1/Sp3 to regulate gene transcription. Nevertheless, the potential role of Ca2+/calcineurin dependent activation of NFAT in the regulation of small cholangiocyte proliferation has not been addressed.
Experimental Procedures
Materials
Reagents were obtained from Sigma Chemical Co. (St. Louis, MO) unless otherwise indicated. The mouse monoclonal antibodies for NFAT1 and NFAT2 were purchased from Novus Biologicals, Inc. (Littleton, CO). The rabbit polyclonal antibody for NFAT3 and the mouse monoclonal antibody for NFAT4 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The RIA kits for the determination of intracellular cAMP (cAMP [125I] Biotrak Assay System) and IP3 (IP3 [3H] Biotrak Assay System) levels were purchased from GE Healthcare (Piscataway, NJ).
Immortalized and freshly isolated small and large cholangiocytes
Small and large cholangiocytes from normal mice (BALB/c) were immortalized by the introduction of the SV40 large T antigen gene (5) and cultured as described (6). These cell lines display morphological, phenotypic and functional features similar to freshly isolated small and large mouse cholangiocytes (5, 6, 15). Freshly isolated small and large mouse cholangiocytes were purified as described (2, 6, 15-17).
Animal models
Male C57/Bl6N mice (20–25 g) were purchased from Charles River (Wilmington, MA). Animals received humane care according to the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health. Normal mice were treated with saline or phenylephrine (10 mg/kg BW, daily IP injections) (18) for 1 week in the absence/presence of: (i) 11R-VIVIT, a cell-permeable NFAT inhibitor peptide, 10 mg/kg, daily IP injections; or (ii) mithramycin A, MiA, an Sp1 inhibitor, 0.5 mg/kg, IP injections twice weekly) (19, 20) before evaluating intrahepatic bile duct mass (IBDM, by immunohistochemistry for cytokeratin-19, CK-19) (21) of small and large bile ducts. Stained sections were analyzed for each group using a BX-51 light microscopy (Olympus, Tokyo, Japan).
Expression of adrenergic receptors
The expression of α1A, α1B, α1D-AR was evaluated by: (i) immunohistochemistry in liver sections; and (ii) immunofluorescence and FACS analysis in immortalized small and large cholangiocytes. We evaluated the presence of the message for α1A, α1B, α1D, α2A, α2B, α2C, β1, β2 and β3 AR by real-time PCR in freshly isolated and immortalized small and large cholangiocytes. Immunohistochemistry was performed in paraffin-embedded liver sections (4-5 μm thick) (6). Light microscopy photographs of 10 non-overlapping fields liver sections were evaluated for receptor expression. Immunofluorescence in cell smears was performed as described (6). Negative controls were performed by usage of a pre-immune serum instead of the primary antibody. FACS analysis was performed as described (22) using a C6 flow cytometer (Accuri, Inc. Ann Arbor, MI) and analyzed by CFlow Software. At least 20,000 events in the light-scatter (SSC/FSC) were acquired. AR receptors were identified and gated on FL1-A/Count plots. The relative quantity of AR (mean AR fluorescence) was expressed as mean FL1-A (samples)/mean FL-1A (secondary antibodies only). For real-time PCR, a DDCT (delta delta of the threshold cycle) analysis was performed (23). The primers for α1A, α1B, α1D, α2A, α2B, α2C, β1, β2 and β3 AR subtypes were designed by SABiosciences (Frederick, MD) according to the sequences listed in NCBI. Data were expressed as fold-change of the ratio of relative mRNA levels ± SEM of AR to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Expression of NFAT isoforms
The expression of the different NFAT isoforms (NFAT1, NFAT2, NFAT3 and NFAT4) was evaluated by: (i) immunohistochemistry in normal liver sections (6); and (ii) immunofluorescence in immortalized small and large cholangiocytes (6). In liver sections, when 0-5% of bile ducts were positive for NFAT isoforms, we assigned a negative score; a +/− score was assigned when 6-10% of bile ducts were positive; a + score was assigned when 11-30% of bile ducts were positive; and a ++ score was assigned with 31-60% of bile ducts positive (15).
Effect of adrenergic receptor agonists on the proliferation of immortalized small and large cholangiocytes
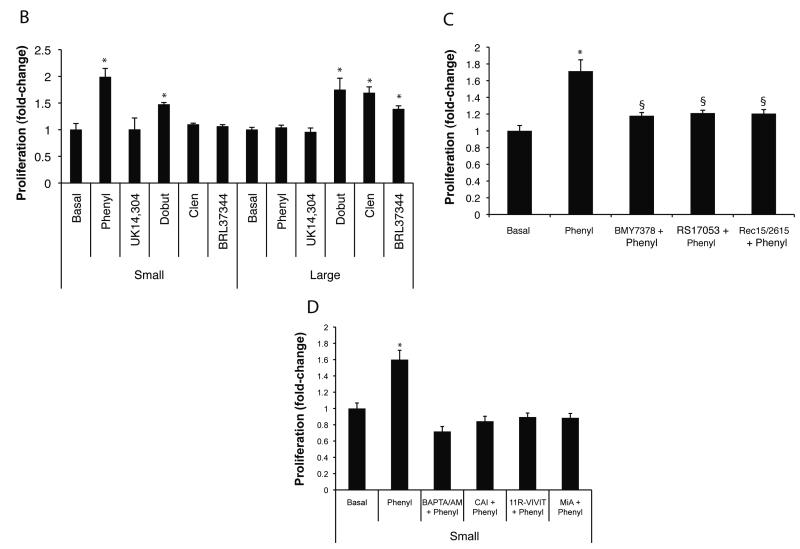
The effect of phenylephrine on small cholangiocyte proliferation was evaluated at varying dosages (10−11 to 10−3 M) and times (24-72 hrs) by MTS proliferation assay (6). The effects (24 hrs at 37°C) of α1 (phenylephrine, 10 μM) (10), α2 (UK14,304, 50 μM) (8), β1 (dobutamine, 10 μM) (9, 10), β2 (clenbuterol, 10 μM) (9) or β3 (BRL 37344, 10 μM) (24) AR agonists on the proliferation of small and large cholangiocytes were evaluated by MTS proliferation assays (6). The concentration of 10 μM for phenylephrine was chosen based on the fact that: (i) at this concentration (10 μM) phenylephrine stimulates cholangiocyte secretory activity (10); and (ii) the doses (10−11 to 10−5 M) used for phenylephrine induced a similar increase in small cholangiocyte proliferation (Figure 3A). Since phenylephrine was the only α1-AR agonist to increase small cholangiocyte proliferation (see results section), in separate sets of experiments we evaluated by MTS assay (6) the effect of phenylephrine on small cholangiocyte proliferation in the absence or presence of: (i) BAPTA/AM (intracellular Ca2+ chelator, 5 μM) (6); (ii) CAI (calcineurin autoinhibitory peptide, 50 μM) (4); (iii) 11R-VIVIT (1 nM) (19); or (iv) MiA (50 nM) (25). To demonstrate that the effects of phenylephrine on cholangiocyte proliferation are due to selective interaction with α-1 AR, we evaluated the effect of phenylephrine (10 μM for 24 hrs) on the proliferation of small cholangiocytes in the absence or presence of: (i) RS-17053 (selective α1A-AR antagonist, 1 nM) (26); (ii) Rec 15/2615 dihydrochloride (selective α1B-AR antagonist, 10 nM) (27); or (iii) BMY 7378 dihydrochloride (selective α1D-AR antagonist, 1 nM) (28).
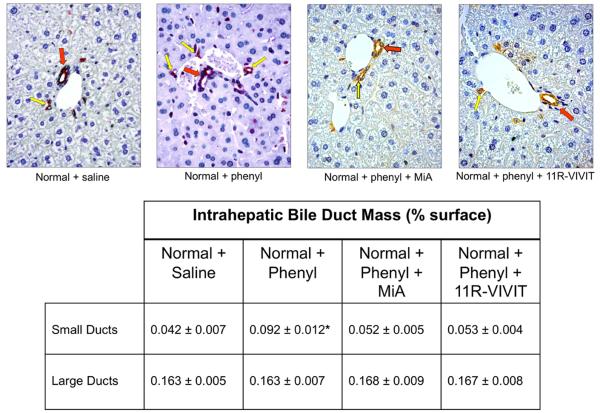
Fig. 3.
Expression of CK-19 by immunohistochemistry in liver sections of normal mouse treated with vehicle or phenylephrine for 1 week in the absence or presence of 11R-VIVIT or MiA. Large (red arrow) and small (yellow arrows) ducts are indicated. Original Magnification X 40. Measurement of IBDM of small and large cholangiocytes in liver sections from the selected groups of mice. Chronic administration of phenylephrine to normal mice induces a significant increase in IBDM of small but not large cholangiocytes, increase that was blocked by 11R-VIVIT and MiA. *p=0.0022 (by Mann-Whitney test) vs. IBDM of small cholangiocytes from normal mice treated with phenylephrine vs. normal mice treated with vehicle.
Effect of phenylephrine on intracellular cAMP and IP3 levels
Immortalized small and large cholangiocytes were stimulated at room temperature for 5 minutes with 0.2% BSA (basal) or phenylephrine (10 μM in 0.2% BSA) (10). Intracellular cAMP and IP3 levels were measured by commercially available kits according to the instructions provided by the vendor.
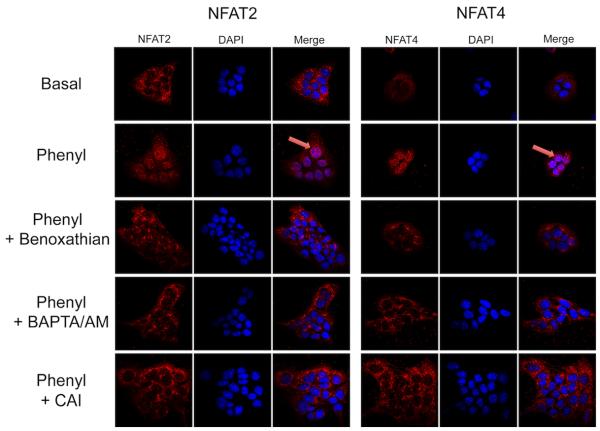
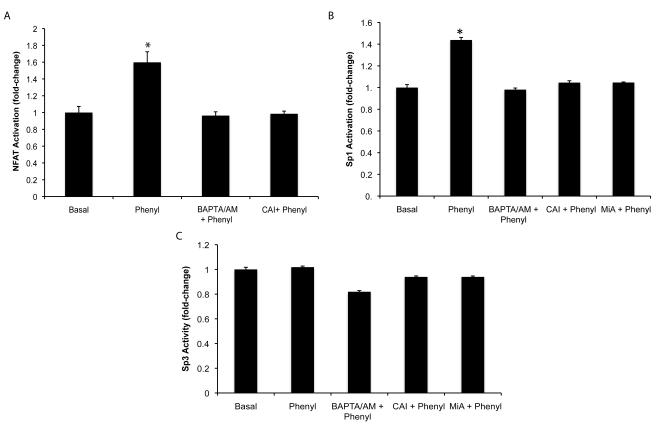
Effect of phenylephrine on the nuclear translocation and DNA-binding activity of NFAT2, NFAT4, Sp1 and Sp3 of immortalized small cholangiocytes
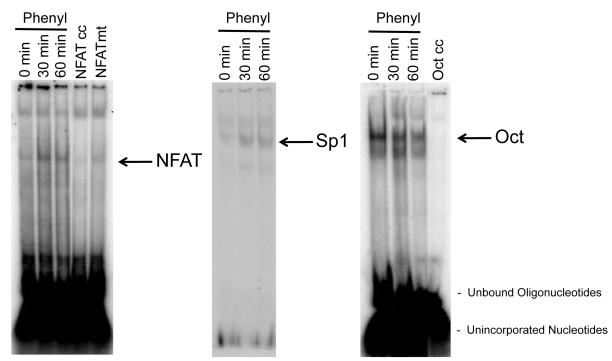
Experiments were performed to evaluate the effect of phenylephrine on: (i) the nuclear translocation of NFAT2 and NFAT4, the isoforms expressed by immortalized small cholangiocytes by immunofluorescence; and (ii) NFAT2, Sp1, and Sp3 DNA-binding activity by ELISA (29) and electrophoretic mobility shift assay (EMSA) (30) in immortalized small cholangiocytes. Nuclear translocation of NFAT2 and NFAT4 was evaluated by immunofluorescence (6) in small cholangiocytes treated with 0.2% BSA or phenylephrine (10 μM in 0.2% BSA) for 1 hr at 37°C in the presence/absence of pretreatment for 30 minutes with benoxathian (nonsubtype selective α1-AR antagonist, 50 μM) (31), BAPTA/AM or CAI. NFAT2 (a kit is not available for NFAT4), Sp1 and Sp3 DNA-binding activity was measured by a commercially available ELISA-based kit that detects transcription factor activation (TransAM™ transcription factor assay kit, Active Motif, Carlsbad, CA). Immortalized small cholangiocytes were stimulated with 0.2% BSA (basal) or phenylephrine (10 μM in 0.2% BSA) for 1 hr at 37°C in the presence/absence of BAPTA/AM, or CAI or MiA. Nuclear extracts were analyzed transcription for factor activation according to the manufacturer’s protocol (Active Motif, Carlsbad, CA). The relative DNA-binding of NFAT2/4 and Sp1was assessed by EMSA in immortalized small cholangiocytes treated with phenylephrine (10 μM) for 0, 30 and 60 min time-points at 37°C as described (30). Double-stranded oligonucleotides containing either the consensus binding motif for NFAT (Santa Cruz Biotechnology), Sp1 (Promega, Madison, WI) or Oct (Promega) were end labeled with 32P-dATP using T4 polynucleotide kinase for 10 minutes at room temperature. The NFAT consensus sequence binds both NFAT2 and NFAT4 isoforms (32). In parallel, to prove specificity of the relevant DNA-binding activities, cold competition assays were performed by adding 50 fold excess of unlabeled consensus sequences for NFAT, a mutant NFAT sequence that differs from the native sequence by 3 base pairs (Santa Cruz Biotechnology), Oct or Sp1 prior to the addition of the labeled sequence.
Knockdown of NFAT2, NFAT4 and Sp1 expression in immortalized small cholangiocytes
Immortalized small cholangiocyte cell lines lacking NFAT2, NFAT4 and Sp1 expression were established using SureSilencing shRNA (SuperArray, Frederick, MD) plasmids for mouse NFAT2, NFAT4 and Sp1, containing neomycin (for NFAT2) and puromycin (for NFAT4 and Sp1) resistance for the selection of stably transfected cells, according to the instructions provided by the vendor (33). Approximately 70% knockdown of NFAT2, NFAT4, and Sp1 mRNA expression was achieved in immortalized small cholangiocytes (Suppl. Fig 1A). Immunofluorescence and DNA-binding activity for NFAT2, NFAT4, and Sp1 by EMSA were used to validate the knockdown of protein expression in small cholangiocytes (Suppl. Fig 1B). There was no inadvertent knockdown of NFAT2, NFAT4, and Sp1 in each case. The small cholangiocyte cell line, mock-transfected clone (Neo-Control shRNA or Puro-Control shRNA), the NFAT2 knockdown clone, NFAT4 knockdown clone, and the Sp1 knockdown clone were stimulated with 0.2% BSA (basal) or phenylephrine (10 mM in 0.2% BSA) for 24 hrs before evaluation of proliferation by MTS assays (6).
Results
Cholangiocytes express α1-AR subtypes
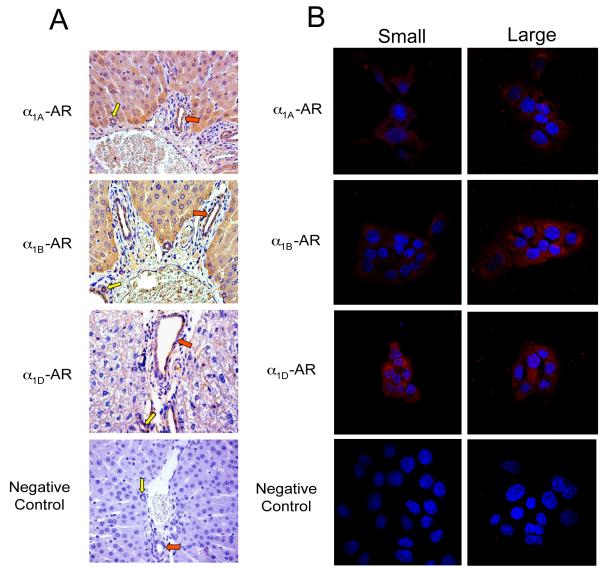
In normal liver sections, we demonstrated that α1A, α1B, α1D-AR are expressed by small (yellow arrow) and large (red arrow) bile ducts (Fig. 1A). Immortalized small and large cholangiocytes were positive for α1A, α1B, α1D-AR expression (Fig. 1B). By real-time PCR, freshly isolated and immortalized small and large cholangiocytes express the messages for α1A, α1B, α1D, α2A, α2B, α2C, β1, β2 and β3 AR (Suppl. Fig 2A). By FACS, we demonstrated that immortalized small and large cholangiocytes express the protein for α1A, α1B, α1D-AR (Suppl. Fig. 2B).
Fig. 1.
Expression of α1A, α1B, α1D AR by immunohistochemistry (A) in liver sections, and immunofluorescence (B) in immortalized small and large cholangiocytes. (A) Small (yellow arrows) and large (red arrows) bile ducts were positive for α1A-, α1B-, and α1D-AR expression. Original magnification, x20. (B) Immortalized small and large cholangiocytes were positive for α1A-, α1B-, and α1D-AR. AR expression is shown in red with nuclei counterstained with DAPI (blue). Original magnification, x60. The negative control performed without the primary antibody is presented at the bottom of the figure. Bile ducts (A) and immortalized small and large cholangiocytes (B) express α1A, α1B, and α1D-AR.
Small bile ducts and cholangiocytes express NFAT2 and NFAT4
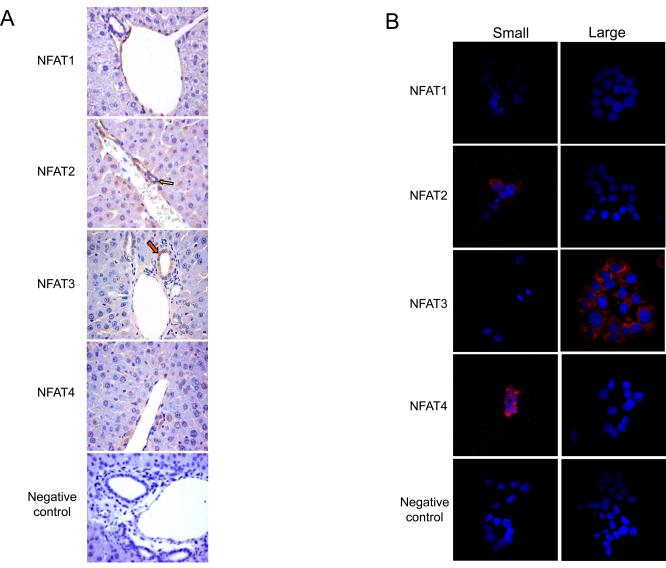
By immunohistochemistry, small bile ducts in liver sections express the NFAT2 and NFAT4 isoforms (Fig. 2A). Large bile ducts in liver sections expressed lower levels of NFAT2 and NFAT4 (Fig. 2A) as determined by semiquantitative immunohistochemical analysis (Suppl. Table 1). By immunofluorescence, we demonstrated that NFAT2 and NFAT4 were predominantly expressed by immortalized small cholangiocytes and that NFAT3 was expressed by large cholangiocytes (Fig. 2B). NFAT1 was not expressed by small or large bile ducts or immortalized small and large cholangiocytes (Fig. 2 A-B).
Fig. 2.
Expression of NFAT isoforms by immunohistochemistry (A) in liver sections, and immunofluorescence (B) in immortalized small and large cholangiocytes. (A) NFAT2 and 4 were expressed predominantly by small bile ducts (yellow arrows; for semiquantitative data see Suppl. Table 1). A small % of cholangiocytes in large bile ducts (red arrows) stained positively for NFAT2 and 4. NFAT3 was expressed only in large bile ducts, whereas NFAT1 was not expressed in either sized bile ducts. Original magnification, x20. (B) A similar expression profile was observed in immortalized small and large cholangiocytes. Small cholangiocytes were positive for NFAT2 and 4. Large cholangiocytes were positive for NFAT3, whereas neither cell type expressed NFAT1. Original magnification, x60. A representative negative control performed without the primary antibody is presented at the bottom of the figure.
Phenylephrine stimulates in vivo and in vitro the proliferation of small but not large cholangiocytes
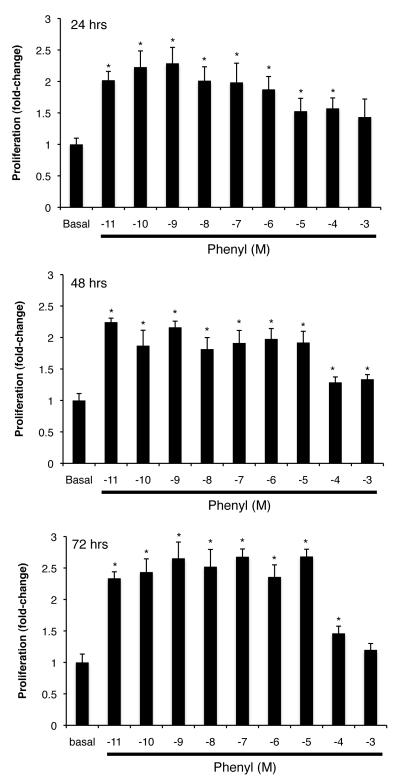
Chronic in vivo administration of phenylephrine to normal mice induces a significant increase in IBDM of small cholangiocytes, increase that was blocked by 11R-VIVIT and mithramycin A (Figure 3). The in vitro doses (10−11 to 10−5 M) used for phenylephrine induced a similar increase in the proliferation of immortalized small cholangiocytes (Fig. 4A). To determine the potential role of each of the AR subtypes on the proliferation of immortalized small and large cholangiocytes, we performed MTS proliferation assays in the presence/absence of α1 (phenylephrine), α2 (UK14,304), β1 (dobutamine), β2 (clenbuterol) or β3 (BRL 37344) AR agonists. In large cholangiocytes, we observed that dobutamine, clenbuterol, and BRL 37344 stimulated proliferation, whereas phenylephrine and UK14,304 had no effect on the growth of large cholangiocytes (Fig. 4B). In addition to phenylephrine, dobutamine (but not clenbuterol, and BRL 37344) increased small cholangiocyte proliferation (Fig. 4B). Since activation of β-adrenergic receptors regulates biliary functions by increased intracellular cAMP levels in cholangiocytes (9), we focused our studies on the role of phenylephrine (an α1-AR agonist stimulating IP3/Ca2+ levels) (10, 34) on Ca2+-dependent signaling in small cholangiocytes. We demonstrated that α1A (RS17053), α1B (Rec15/2615) and α1D (BMY7378) AR antagonists induced a partial yet significant reduction in phenylephrine-induced proliferation of immortalized small cholangiocytes (Fig. 4C). However, levels of proliferation stimulated by phenylephrine in the presence of the antagonists remain significant in comparison to basal control proliferation, which demonstrates that all three receptor subtypes are involved in phenylephrine-induced proliferation (Fig. 4C).
Fig. 4.
(A) Effect of different doses (10−11 to 10−3 M) of phenylephrine on the proliferation of immortalized small cholangiocytes. The doses (10−11 to 10−5 M) used for phenylephrine induced a similar increase in small cholangiocyte proliferation. *denotes significance (p<0.05) when compared with the respective basal treatment using a Kruskal-Wallis test (n=6-14). (B) In addition to phenylephrine, dobutamine increased small cholangiocyte proliferation. In immortalized large cholangiocytes, dobutamine, clenbuterol and BDL 37344 induced a significant increase in proliferation, whereas phenylephrine and UK14,304 had no effect. *denotes significance (p<0.05) when compared with the respective basal treatment using a Kruskal-Wallis test (n=14). (C) Effect of phenylephrine (10 μM for 24 hr) on the proliferation of immortalized small cholangiocytes in the absence or presence of selective AR antagonists. α1A-, α1B-, and α1D-AR antagonists induced a partial yet significant reduction in phenylephrine-induced small cholangiocyte proliferation. *denotes significance (p<0.05) when compared with the respective basal treatment using a t test (n=14). §denotes significance (p<0.05) when compared with phenylephrine-induced proliferation. (D) Phenylephrine stimulates small cholangiocytes proliferation in a Ca2+-dependent mechanism. Small mouse cholangiocytes were stimulated with phenylephrine in the presence/absence of BAPTA/AM; CAI; 11R-VIVIT; or MiA. Phenylephrine-stimulated small cholangiocyte proliferation was prevented by BAPTA/AM, CAI, 11R-VIVIT, or MiA. *denotes significance (p<0.05) when compared with the respective basal treatment using a Kruskal-Wallis test (n=14).
Phenylephrine stimulates the proliferation of immortalized small cholangiocytes via Ca2+-dependent signaling mechanism
Phenylephrine increased intracellular IP3 (but not cAMP, not shown) levels (basal: 0.39 ± 0.03 vs. phenylephrine: 0.62 ± 0.07 pmol/1 × 107 cells; P < 0.01) in immortalized small cholangiocytes. Phenylephrine-stimulated proliferation of immortalized small cholangiocytes was blocked by BAPTA/AM, CAI (4), 11R-VIVIT and MiA (Fig. 4D).
Phenylephrine stimulates the nuclear translocation of NFAT and DNA-binding activity of NFAT2/4 and Sp1 in Immortalized Small Cholangiocytes
To further define the role of NFAT in phenylephrine-stimulated proliferation, we performed experiments to evaluate nuclear translocation and DNA-binding activity of NFAT2 and NFAT4 in immortalized small cholangiocytes. By immunofluorescence, phenylephrine stimulates nuclear translocation of both NFAT2 and NFAT4 in small cholangiocytes (Fig. 5). This translocation that was blocked by inhibitors of upstream Ca2+-dependent signaling (i.e., benoxathian (non-subtype selective α1-AR antagonist) (31), BAPTA/AM, and CAI) (Fig. 5), which confirms the results of the proliferation studies (Fig. 4D). The activation of NFAT and Sp1/3 DNA-binding activity was determined by EMSA and DNA-binding activity ELISA. We found by EMSA that phenylephrine stimulates time-dependent activation of NFAT DNA-binding in small cholangiocytes (Fig. 6). The consensus sequence utilized in the EMSA will bind both NFAT2 and NFAT4 (elucidation of the involvement of isoforms was determined by knockdown experiments discussed later). NFAT2 DNA-binding activity was confirmed by DNA-binding activity ELISA. The ELISA kit utilized recognizes the specific DNA-binding activity of NFAT2 (and not other NFAT isoforms as there are no commercially available kits). Our results demonstrate that phenylephrine stimulates NFAT2 DNA-binding activity in small cholangiocytes, which was blocked by BAPTA/AM and CAI (Fig. 7A). We also found that phenylephrine stimulates the time-dependent increase in Sp1 DNA-binding activity in small cholangiocytes as determined by EMSA (Fig. 7B). The DNA-binding specificity of Sp1 when challenged during cold competition was determined and presented in Suppl. Fig. 3. Since both Sp1 and Sp3 are known to interact with NFAT2 and NFAT4, we determined by DNA-binding activity ELISA which isoforms (i.e., Sp1 and Sp3) are activated by phenylephrine. In small immortalized cholangiocytes, phenylephrine stimulated Sp1 (but not Sp3), which was blocked by BAPTA/AM, CAI and MiA (Fig. 7B-C).
Fig. 5.
Phenylephrine stimulates the nuclear translocation of NFAT2 and NFAT4. Immortalized small cholangiocytes were stimulated with phenylephrine in the presence/absence of benoxathian, BAPTA/AM, and CAI for 60 minutes. By immunofluorescence, phenylephrine stimulates the nuclear translocation of NFAT2 and NFAT4 (arrows), which was blocked by benoxathian, BAPTA/AM, and CAI. Original magnification, x60.
Fig. 6.
Evaluation of phenylephrine-induced NFAT and Sp1 DNA-binding activity by EMSA. Immortalized small cholangiocytes were stimulated with phenylephrine for 0, 30 and 60 min at 37°C and DNA-binding activity was assessed by EMSA. Phenylephrine stimulated a time-dependent increase in DNA-binding for both NFAT (NFAT2 and 4 can both bind the consensus sequence) and Sp1. DNA-binding activity to the Oct consensus sequence was used as a loading control. Specificity of binding was demonstrated by adding 50 fold excess of either unlabeled NFAT consensus sequence, (NFAT cc), mutated NFAT consensus sequence (NFAT mt) or Oct sequence to the nuclear extract taken at time 0. NFAT = nuclear factor of activated T-cells; NFAT cc = NFAT cold consensus sequence; NFAT mt = NFAT mutated competitor; Oct = octamer binding factor; and Oct cc = Oct cold consensus sequence.
Fig. 7.
Phenylephrine induces NFAT2 and Sp1 (but not Sp3) DNA-binding activity. (A) Immortalized small cholangiocytes were stimulated with phenylephrine in the presence/absence of BAPTA/AM and CAI for 60 min. NFAT2 DNA-binding activity was determined by ELISA. Phenylephrine stimulates the DNA-binding activity of NFAT2, which is blocked by BAPTA/AM and CAI. *denotes significance (p<0.05) when compared with the respective basal treatment using a t test (n=4). (B-C) Small cholangiocytes were stimulated with phenylephrine in the presence/absence of BAPTA/AM, CAI and MiA for 60 min. Sp1 and Sp3 DNA-binding ac8ivity was determined by ELISA. Phenylephrine stimulates the DNA-binding activity of Sp1 (B) (but not Sp3 (C)), which is blocked by BAPTA/AM, CAI and MiA. *denotes significance (p<0.05) when compared with the respective basal treatment using a t test (n=4).
Knockdown of NFAT2 and Sp1 expression in immortalized small cholangiocytes prevents phenylephrine-induced proliferation
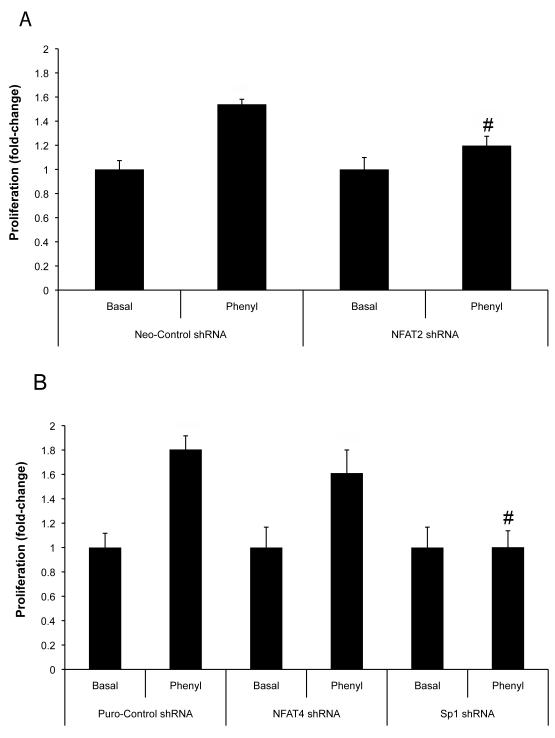
We established small cholangiocyte lines that have NFAT2, NFAT4 and Sp1 expression stably knockdown. Knockdown of NFAT2 expression prevented phenylephrine stimulated proliferation of small cholangiocytes (Fig. 8A). Knockdown of NFAT4 only slightly depressed phenylephrine-stimulated proliferation of small cholangiocytes (Fig. 8B). In NFAT4 knockdown cells phenylephrine stimulated a significant increase in small cholangiocyte proliferation versus basal (Fig. 8B). Phenylephrine had no effect on small cholangiocyte proliferation in cells with knockdown of Sp1 expression (Fig. 8B).
Fig. 8.
Knockdown of NFAT2 and Sp1 expression prevents phenylephrine-induced proliferation of immortalized small cholangiocytes. The expression of NFAT2, NFAT4, or Sp1 was knockdown in small cholangiocytes by stable transfection of the respective shRNA. The knockdown of NFAT2 (A) and Sp1 (B) resulted in the prevention of phenylephrine-stimulated small cholangiocyte proliferation. (B) Knockdown of the expression NFAT4 did not significantly inhibit phenylephrine stimulation of small cholangiocyte proliferation. Data was expressed as fold-change relative to the respective basal values. #denotes significance (p<0.05) when compared with the respective phenyl-stimulated treatment group using a t test (n=7).
Discussion
We demonstrated that: (i) small and large bile ducts and freshly isolated and immortalized cholangiocytes express all of the AR subtypes; (ii) NFAT2 and NFAT4 are predominantly expressed by small bile ducts and immortalized small cholangiocytes; (iii) phenylephrine stimulates both in vivo and in vitro the proliferation of small cholangiocytes via activation of Ca2+-dependent signaling, which is blocked by in vivo and in vitro inhibition of NFAT and Sp1; (iv) phenylephrine stimulates Ca2+-dependent DNA-binding activities of NFAT2 and Sp1 (but not Sp3) and nuclear translocation of NFAT2 and NFAT4 in immortalized small cholangiocytes; and (v) knockdown of NFAT2 or Sp1 gene expression prevents phenylephrine-induced small cholangiocyte proliferation, whereas NFAT4 knockdown had a minimal effect on phenylephrine-induced proliferation of immortalized small cholangiocytes. The regulation of small cholangiocyte proliferation (via activation of α1A, α1B, α1D AR by phenylephrine) is dependent upon activation of Ca2+/NFAT2/Sp1 signaling mechanisms.
The possible influence on the results by using small and large immortalized cholangiocytes are minimal, since these cells are derived from small and large bile ducts (5, 6); and have similar morphological, phenotypical and functional characteristics of freshly isolated small and large murine cholangiocytes (5, 6, 35). These cell preparations express similar levels of the biliary markers, cytokeratin-7 and -19 (5, 6), and display similar morphological differences in size (5, 6). At the functional level immortalized large (but not small) cholangiocytes express secretin receptor, CFTR and Cl−/HCO3− exchanger and selectively respond to secretin with changes in cAMP levels similar to that of freshly isolated cholangiocytes (5, 6). Immortalized small and large cholangiocytes display proliferative capacities similar to freshly isolated small and large mouse cholangiocytes since large cholangiocytes proliferate by a cAMP-dependent pathway, whereas IP3/Ca2+-dependent signaling regulate the growth of small cholangiocytes (5, 6, 15). These findings support the validity of immortalized small and large cholangiocytes for evaluating functions of small and large bile ducts.
Small and large cholangiocytes express α1-AR (α1A, α1B, α1D). However, only immortalized small cholangiocytes respond in vitro to phenylephrine with increased proliferation that was blocked by all three α1-AR antagonists (Fig. 4C). Although dobutamine induced in vitro a significant increase in the proliferation of immortalized small cholangiocytes, we did not address the mechanisms of such increase since dobutamine is a racemic mixture, in which one enantiomer is an agonist at β1 and β2 AR, and the other enantiomer is an agonist at α1 AR (36). Thus, dobutamine-induced increase in small cholangiocyte proliferation may be due to the activation of α1 AR. A specific β1-AR agonist is not available. We have demonstrated that phenylephrine increases secretin-induced choleresis of large cholangiocytes when administered to BDL rats (10). In in vitro studies, phenylephrine did not alter basal but increased secretin-stimulated large bile duct secretory activity and cAMP levels, which were blocked by BAPTA/AM and Gö6976 (a PKC antagonist) (10). Phenylephrine increased IP3 and Ca2+ levels and activated PKCα and PKCβII (10). Since large cholangiocytes are normally hormonally responsive to secretin (16, 37) and regulated by cAMP-dependent signaling (3, 16, 23), we propose that this acute effect of phenylephrine on secretin-stimulated large bile duct secretion is likely mediated by activation of the Ca2+-dependent adenylyl cyclase, AC8, which is key in the secretory activity of large cholangiocytes (38). We postulated that phenylephrine has differential effects on small and large cholangiocytes. In immortalized small cholangiocytes, phenylephrine stimulated intracellular IP3 levels and plays a role in stimulating proliferation. Activation of small cholangiocyte proliferation by endogenous catecholamines (such as, norepinephrine and epinephrine) and other Ca2+ agonists (including phenylephrine) may be key during pathological conditions when large cholangiocytes are damaged, and the de novo proliferation of small cholangiocytes is necessary for the replenishment of the biliary system and compensation for loss of hormonal responsiveness (3, 7). Other studies have shown that α1-AR agonists like phenylephrine can induce proliferation in various cell types including hepatocytes (39). We found a similar profile in small cholangiocytes, since phenylephrine-induced proliferation was blocked by inhibition of Ca2+, calcineurin activity, and NFAT activity. In addition, phenylephrine-induced proliferation was blocked by MiA implicating the involvement of Sp1/3. NFAT and Sp1/3 isoforms play a critical role in the regulation of cell proliferation. NFAT2 stimulates proliferation of several cell types including lymphocytes (40). NFAT4 deficiency results in incomplete liver regeneration following partial hepatectomy (41). NFAT2 and 4 have also been shown to crosstalk with Sp1/Sp3 to co-operatively regulate membrane type 1 matrix metalloproteinase gene transcription and cellular differentiation in keratinocytes (42). Utilizing several molecular approaches, we found that phenylephrine stimulates the Ca2+-dependent DNA-binding activities of NFAT2/4, and Sp1 (but not Sp3) and the nuclear translocation of NFAT2 and NFAT4 suggesting the involvement of these transcription factors in phenylephrine-induced proliferation of small cholangiocytes. We confirmed their involvement using shRNA to knockdown the expression of these transcription factors.
In summary, we demonstrated that small cholangiocyte proliferation is regulated by the activation of α1-ARs and occurs through Ca2+/calcineurin-dependent activation of NFAT2 and Sp1. Modulation of the Ca2+-dependent transcription factors, NFAT2 and SP1, may be an important therapeutic approach for inducing ductular proliferation for maintaining the homeostasis of the biliary during the damage of large cAMP-responsive bile ducts (1, 3, 7).
Supplementary Material
Suppl. Fig. 1. Evaluation of the levels of knockdown for NFAT2, NFAT4, and Sp1 in small cholangiocytes stably transfected with shRNA. (A, left) Evaluation of the message expression by real-time PCR for NFAT2, NFAT4, and Sp1 in RNA isolated from small cholangiocytes stably transfected with shRNA for NFAT2, NFAT4, Sp1, Neo-control or Puro-control. The message expression was ≈70% knocked-down for each shRNA for NFAT2, NFAT4, and Sp1. There was no unwanted knockdown of any of the specific messages for the other proteins (i.e., the shRNA for NFAT2 did not alter the message expression levels of NFAT4 and Sp1, etc.). Data (n =6-8) were expressed as fold-change relative mRNA levels ± SEM of the gene of interest to GAPDH ratio with samples compared to the respective freshly isolated normal wild-type mouse small cholangiocytes. *denotes significance (p<0.05) when compared with the respective control shRNA treatment using a Kruskal-Wallis test. (A, right) Evaluation of DNA-binding activity in nuclear extracts from small cholangiocytes stably transfected with shRNA for NFAT2, NFAT4, and Sp1 as determined by EMSA. The shRNAs for NFAT2, NFAT4, and Sp1 significantly knockdown the protein expression (as evidenced by decreased DNA-binding activity shown by EMSA) for the target gene in each case. (B) We also confirm the down regulation of protein expression by the shRNAs by immunofluorescence for NFAT2, NFAT4, and Sp1. Immunofluorescence was performed in untransfected small mouse cells, neo-control shRNA, puro-control shRNA, NFAT2 shRNA, NFAT4 shRNA, and Sp1 shRNA stably transfected cells. NFAT2, NFAT4, and Sp1 expression is shown in red with nuclei counterstained with DAPI (blue). Original magnification, x60. A representative negative control performed without the primary antibody is presented at the bottom of each set of images.
Suppl. Fig. 2. (A) Evaluation of the message expression (by real-time PCR) for α1A, α1B, α1D, α2A, α2B, α2C, β1, β2 and β3 AR subtypes in freshly isolated and immortalized small and large cholangiocytes. Freshly isolated cholangiocytes and immortalized small and large cholangiocytes express all of the AR subtypes. Data (n =6) were expressed as fold-change relative mRNA levels ± SEM of AR to GAPDH ratio. (B) Evaluation of the expression of α1A, α1B, and α1D-AR subtypes by FACS. Both small and large immortalized cholangiocytes express α1A, α1B, and α1D-AR subtypes. Expression of all three subtypes was elevated in large compared to small cholangiocytes. *denotes significance (p<0.05) when large cholangiocytes are compared to small cholangiocytes (Kruskal-Wallis test).
Acknowledgments
We thank Anna Webb of the Texas A&M Health Science Center Microscopy Imaging Center for assistance with confocal microscopy and Bryan Moss (Medical Illustration, Scott & White) for the help on the preparation of the figures and Dr. Marco Marzioni (Università Politecnica delle Marche, Italy) for the comments related to the revision of the manuscript.
Financial Support: This work was supported partly by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, the VA Research Scholar Award, a VA Merit Award and the NIH grants DK58411, and DK76898 to Dr. Alpini, an NIH RO1 grant award to Dr. Glaser (DK081442) an NIH K01 grant award (DK078532) to Dr. DeMorrow, and by University funds to Dr. Onori and PRIN 2007 (# 2007HPT7BA_001) and Federate Athenaeum funds from University of Rome “La Sapienza” to Prof. Gaudio and by a grant-in-aid for Scientific Research (C) from Japan Promotion of Science (21590822), and by Health and Labour Sciences Research Grants for the Research on Measures for Intractable Diseases (from the Ministry of Health, Labour and Welfare of Japan) to Dr. Ueno.
Abbreviations
- AR
adrenergic receptor
- BDL
bile duct ligated
- [Ca2+]i
intracellular calcium
- BAPTA/AM
1,2-bis-(o-Aminophenoxy)-ethane-N,N,N’,N’-tetraacetic acid, tetraacetoxymethyl ester
- BMY 7378 dihydrochloride
(8-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-8-azaspiro[4.5]decane-7,9-dione dihydrochloride)
- B R L 3 7 3 4 4
(±)-(R*,R*)-[4-[2-[[2-(3-Chlorophenyl)-2-hydroxyethyl]amino]propyl]phenoxy]acetic acid
- BSA
bovine serum albumin
- CAI
calcineurin autoinhibitory peptide
- cAMP
3′-5′-cyclic adenosine monophosphate
- CaMK
calmodulin-dependent protein kinase
- CK-19
cytokeratin-19
- clen
clenbuterol
- CCl4
carbon tetrachloride
- DAPI
4,6-diamidino-2-phenylindole
- ΔΔCT
delta delta of the threshold cycle
- dobut
dobutamine
- EMSA
electrophoretic mobility shift assay
- IBDM
intrahepatic bile duct mass
- IP3
D-myo-inositol-1,4,5-triphosphate
- MiA
mithramycin A
- MTS
3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt
- NFAT
nuclear factor of activated T-cells
- PBST
phosphate buffered saline Tween-20
- PCNA
proliferating cell nuclear antigen
- phenyl
phenylephrine
- Rec 15/2615 dihydrochloride
(1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-[[2-methoxy- 6-(1-methylethyl)phenoxy]acetyl]piperazine dihydrochloride
- RS-17053
(N-[2-(2-cyclopropylmethoxyphenoxy)ethyl]-5-chloro-alpha, alpha-dimethyl-1H-indole-3-ethanamine hydrochloride)
- Sp1/3
specificity protein 1/3
REFERENCES
- 1.Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: Arias IM, Boyer JL, Chisari FV, Fausto N, Jakoby W, Schachter D, Shafritz DA, editors. The Liver; Biology & Pathobiology. 4th Ed Lippincott Williams & Wilkins; Philadelphia, PA: 2001. pp. 421–435. [Google Scholar]
- 2.Alpini G, Glaser S, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, et al. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1998;274:G767–775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 3.LeSage G, Glaser S, Marucci L, Benedetti A, Phinizy JL, Rodgers R, Caligiuri A, et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1289–1301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- 4.Francis H, Glaser S, Ueno Y, LeSage G, Marucci L, Benedetti A, Taffetani S, et al. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–537. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Ueno Y, Alpini G, Yahagi K, Kanno N, Moritoki Y, Fukushima K, Glaser S, et al. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int. 2003;23:449–459. doi: 10.1111/j.1478-3231.2003.00876.x. [DOI] [PubMed] [Google Scholar]
- 6.Francis H, Glaser S, DeMorrow S, Gaudio E, Ueno Y, Venter J, Dostal DE, et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CAMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mancinelli R, Franchitto A, Gaudio E, Onori P, Glaser S, Francis H, Venter J, et al. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol Gastrointest Liver Physiol. 2010;176:1790–1800. doi: 10.2353/ajpath.2010.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis H, LeSage G, DeMorrow S, Alvaro D, Ueno Y, Venter J, Glaser S, et al. The alpha2-adrenergic receptor agonist UK 14,304 inhibits secretin-stimulated ductal secretion by downregulation of the cAMP system in bile duct-ligated rats. Am J Physiol Cell Physiol. 2007;293:C1252–1262. doi: 10.1152/ajpcell.00031.2007. [DOI] [PubMed] [Google Scholar]
- 9.Glaser S, Alvaro D, Francis H, Ueno Y, Marucci L, Benedetti A, De Morrow S, et al. Adrenergic receptor agonists prevent bile duct injury induced by adrenergic denervation by increased cAMP levels and activation of Akt. Am J Physiol Gastrointest Liver Physiol. 2006;290:G813–826. doi: 10.1152/ajpgi.00306.2005. [DOI] [PubMed] [Google Scholar]
- 10.LeSage G, Alvaro D, Glaser S, Francis H, Marucci L, Roskams T, Phinizy JL, et al. Alpha-1 adrenergic receptor agonists modulate ductal secretion of BDL rats via Ca(2+)- and PKC-dependent stimulation of cAMP. Hepatology. 2004;40:1116–1127. doi: 10.1002/hep.20424. [DOI] [PubMed] [Google Scholar]
- 11.Hein P, Michel MC. Signal transduction and regulation: are all alpha1-adrenergic receptor subtypes created equal? Biochem Pharmacol. 2007;73:1097–1106. doi: 10.1016/j.bcp.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen T, Di Giovanni S. NFAT signaling in neural development and axon growth. Int J Dev Neurosci. 2008;26:141–145. doi: 10.1016/j.ijdevneu.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipskaia L, Lompre AM. Alteration in temporal kinetics of Ca2+ signaling and control of growth and proliferation. Biol Cell. 2004;96:55–68. doi: 10.1016/j.biolcel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Ho SN. The role of NFAT5/TonEBP in establishing an optimal intracellular environment. Arch Biochem Biophys. 2003;413:151–157. doi: 10.1016/s0003-9861(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 15.Glaser S, Gaudio E, Rao A, Pierce LM, Onori P, Franchitto A, Francis HL, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89:456–469. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, LeSage G, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 17.Alpini G, Ulrich C, Roberts S, Phillips JO, Ueno Y, Podila PV, Colegio O, et al. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1997;272:G289–297. doi: 10.1152/ajpgi.1997.272.2.G289. [DOI] [PubMed] [Google Scholar]
- 18.Marinese D, Patel R, Walden PD. Mechanistic investigation of the adrenergic induction of ventral prostate hyperplasia in mice. Prostate. 2003;54:230–237. doi: 10.1002/pros.10170. [DOI] [PubMed] [Google Scholar]
- 19.Noguchi H, Matsushita M, Okitsu T, Moriwaki A, Tomizawa K, Kang S, Li ST, et al. A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat Med. 2004;10:305–309. doi: 10.1038/nm994. [DOI] [PubMed] [Google Scholar]
- 20.Jia Z, Gao Y, Wang L, Li Q, Zhang J, Le X, Wei D, et al. Combined treatment of pancreatic cancer with mithramycin A and tolfenamic acid promotes Sp1 degradation and synergistic antitumor activity. Cancer Res. 2010;70:1111–1119. doi: 10.1158/0008-5472.CAN-09-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeSage G, Glaser S, Gubba S, Robertson WE, Phinizy JL, Lasater J, Rodgers RE, et al. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology. 1996;111:1633–1644. doi: 10.1016/s0016-5085(96)70027-6. [DOI] [PubMed] [Google Scholar]
- 22.Onori P, Wise C, Gaudio E, Franchitto A, Francis H, Carpino G, Lee V, et al. Secretin inhibits cholangiocarcinoma growth via dysregulation of the cAMP-dependent signaling mechanisms of secretin receptor. Int J Cancer. 2009 doi: 10.1002/ijc.25028. [DOI] [PubMed] [Google Scholar]
- 23.Francis H, Franchitto A, Ueno Y, Glaser S, DeMorrow S, Venter J, Gaudio E, et al. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest. 2007;87:473–487. doi: 10.1038/labinvest.3700533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oriowo MA, Chapman H, Kirkham DM, Sennitt MV, Ruffolo RR, Jr., Cawthorne MA. The selectivity in vitro of the stereoisomers of the beta-3 adrenoceptor agonist BRL 37344. J Pharmacol Exp Ther. 1996;277:22–27. [PubMed] [Google Scholar]
- 25.Jia Z, Zhang J, Wei D, Wang L, Yuan P, Le X, Li Q, et al. Molecular basis of the synergistic antiangiogenic activity of bevacizumab and mithramycin A. Cancer Res. 2007;67:4878–4885. doi: 10.1158/0008-5472.CAN-06-3494. [DOI] [PubMed] [Google Scholar]
- 26.Ford AP, Arredondo NF, Blue DR, Jr., Bonhaus DW, Jasper J, Kava MS, Lesnick J, et al. RS-17053 (N-[2-(2-cyclopropylmethoxyphenoxy)ethyl]-5-chloro-alpha, alpha-dimethyl-1H-indole-3-ethanamine hydrochloride), a selective alpha 1A-adrenoceptor antagonist, displays low affinity for functional alpha 1-adrenoceptors in human prostate: implications for adrenoceptor classification. Mol Pharmacol. 1996;49:209–215. [PubMed] [Google Scholar]
- 27.Morton JS, Daly CJ, Jackson VM, McGrath JC. α1A-Adrenoceptors mediate contractions to phenylephrine in rabbit penile arteries. Br J Pharmacol. 2007;150:112–120. doi: 10.1038/sj.bjp.0706956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goetz AS, King HK, Ward SD, True TA, Rimele TJ, Saussy DLJ. BMY 7378 is a selective antagonist of the D subtype of alpha 1-adrenoceptors. Eur J Pharmacol. 1995;272:R5–6. doi: 10.1016/0014-2999(94)00751-r. [DOI] [PubMed] [Google Scholar]
- 29.Ma P, Wang Z, Pflugfelder SC, Li DQ. Toll-like receptors mediate induction of peptidoglycan recognition proteins in human corneal epithelial cells. Exp Eye Res. 2009 doi: 10.1016/j.exer.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodenough S, Davidson M, Chen W, Beckmann A, Pujic Z, Otsuki M, Matsumoto I, et al. Immediate early gene expression and delayed cell death in limbic areas of the rat brain after kainic acid treatment and recovery in the cold. Exp Neurol. 1997;145:451–461. doi: 10.1006/exnr.1997.6471. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs R, Stelzer I, Haas HS, Leitinger G, Schauenstein K, Sadjak A. The alpha1-adrenergic receptor antagonists, benoxathian and prazosin, induce apoptosis and a switch towards megakaryocytic differentiation in human erythroleukemia cells. Ann Hematol. 2009;88:989–997. doi: 10.1007/s00277-009-0704-z. [DOI] [PubMed] [Google Scholar]
- 32.Badran BM, Wolinsky SM, Burny A, Willard-Gallo KE. Identification of three NFAT binding motifs in the 5′-upstream region of the human CD3gamma gene that differentially bind NFATc1, NFATc2, and NF-kappa B p50. J Biol Chem. 2002;277:47136–47148. doi: 10.1074/jbc.M206330200. [DOI] [PubMed] [Google Scholar]
- 33.DeMorrow S, Francis H, Gaudio E, Ueno Y, Venter J, Onori P, Franchitto A, et al. Anandamide inhibits cholangiocyte hyperplastic proliferation via activation of thioredoxin 1/redox factor 1 and AP-1 activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G506–519. doi: 10.1152/ajpgi.00304.2007. [DOI] [PubMed] [Google Scholar]
- 34.J OU, Komukai K, Kusakari Y, Obata T, Hongo K, Sasaki H, Kurihara S. alpha1-adrenoceptor stimulation potentiates L-type Ca2+ current through Ca2+/calmodulin-dependent PK II (CaMKII) activation in rat ventricular myocytes. Proc Natl Acad Sci U S A. 2005;102:9400–9405. doi: 10.1073/pnas.0503569102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glaser S, Lam IP, Franchitto A, Gaudio E, Onori P, Chow BK, Wise C, et al. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology. 2010;52:204–214. doi: 10.1002/hep.23657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parker K, Brunton L, Goodman LS, Blumenthal D, Buxton I. Goodman & Gilman’s manual of pharmacology and therapeutics. McGraw-Hill Medical; 2008. p. 159. [Google Scholar]
- 37.Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, LeSage G. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1064–1074. doi: 10.1152/ajpgi.1997.272.5.G1064. [DOI] [PubMed] [Google Scholar]
- 38.Strazzabosco M, Fiorotto R, Melero S, Glaser S, Francis H, Spirli C, Alpini G. Differentially expressed adenylyl cyclase isoforms mediate secretory functions in cholangiocyte subpopulation. Hepatology. 2009;50:244–252. doi: 10.1002/hep.22926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han C, Bowen WC, Michalopoulos GK, Wu T. Alpha-1 adrenergic receptor transactivates signal transducer and activator of transcription-3 (Stat3) through activation of Src and epidermal growth factor receptor (EGFR) in hepatocytes. J Cell Physiol. 2008;216:486–497. doi: 10.1002/jcp.21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida H, Nishina H, Takimoto H, Marengere LE, Wakeham AC, Bouchard D, Kong YY, et al. The transcription factor NF-ATc1 regulates lymphocyte proliferation and Th2 cytokine production. Immunity. 1998;8:115–124. doi: 10.1016/s1074-7613(00)80464-1. [DOI] [PubMed] [Google Scholar]
- 41.Pierre KB, Jones CM, Pierce JM, Nicoud IB, Earl TM, Chari RS. NFAT4 deficiency results in incomplete liver regeneration following partial hepatectomy. J Surg Res. 2009;154:226–233. doi: 10.1016/j.jss.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santini MP, Talora C, Seki T, Bolgan L, Dotto GP. Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expression in keratinocyte differentiation. Proc Natl Acad Sci U S A. 2001;98:9575–9580. doi: 10.1073/pnas.161299698. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Fig. 1. Evaluation of the levels of knockdown for NFAT2, NFAT4, and Sp1 in small cholangiocytes stably transfected with shRNA. (A, left) Evaluation of the message expression by real-time PCR for NFAT2, NFAT4, and Sp1 in RNA isolated from small cholangiocytes stably transfected with shRNA for NFAT2, NFAT4, Sp1, Neo-control or Puro-control. The message expression was ≈70% knocked-down for each shRNA for NFAT2, NFAT4, and Sp1. There was no unwanted knockdown of any of the specific messages for the other proteins (i.e., the shRNA for NFAT2 did not alter the message expression levels of NFAT4 and Sp1, etc.). Data (n =6-8) were expressed as fold-change relative mRNA levels ± SEM of the gene of interest to GAPDH ratio with samples compared to the respective freshly isolated normal wild-type mouse small cholangiocytes. *denotes significance (p<0.05) when compared with the respective control shRNA treatment using a Kruskal-Wallis test. (A, right) Evaluation of DNA-binding activity in nuclear extracts from small cholangiocytes stably transfected with shRNA for NFAT2, NFAT4, and Sp1 as determined by EMSA. The shRNAs for NFAT2, NFAT4, and Sp1 significantly knockdown the protein expression (as evidenced by decreased DNA-binding activity shown by EMSA) for the target gene in each case. (B) We also confirm the down regulation of protein expression by the shRNAs by immunofluorescence for NFAT2, NFAT4, and Sp1. Immunofluorescence was performed in untransfected small mouse cells, neo-control shRNA, puro-control shRNA, NFAT2 shRNA, NFAT4 shRNA, and Sp1 shRNA stably transfected cells. NFAT2, NFAT4, and Sp1 expression is shown in red with nuclei counterstained with DAPI (blue). Original magnification, x60. A representative negative control performed without the primary antibody is presented at the bottom of each set of images.
Suppl. Fig. 2. (A) Evaluation of the message expression (by real-time PCR) for α1A, α1B, α1D, α2A, α2B, α2C, β1, β2 and β3 AR subtypes in freshly isolated and immortalized small and large cholangiocytes. Freshly isolated cholangiocytes and immortalized small and large cholangiocytes express all of the AR subtypes. Data (n =6) were expressed as fold-change relative mRNA levels ± SEM of AR to GAPDH ratio. (B) Evaluation of the expression of α1A, α1B, and α1D-AR subtypes by FACS. Both small and large immortalized cholangiocytes express α1A, α1B, and α1D-AR subtypes. Expression of all three subtypes was elevated in large compared to small cholangiocytes. *denotes significance (p<0.05) when large cholangiocytes are compared to small cholangiocytes (Kruskal-Wallis test).