Abstract
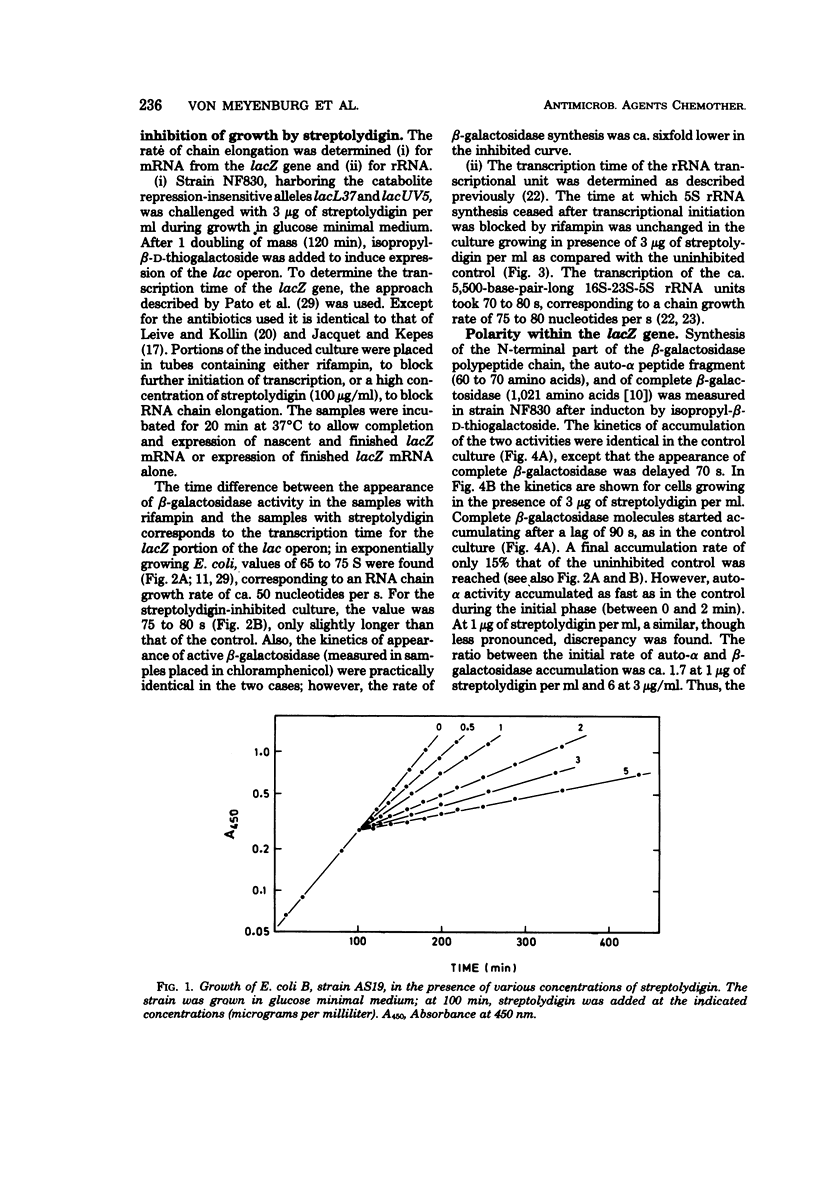
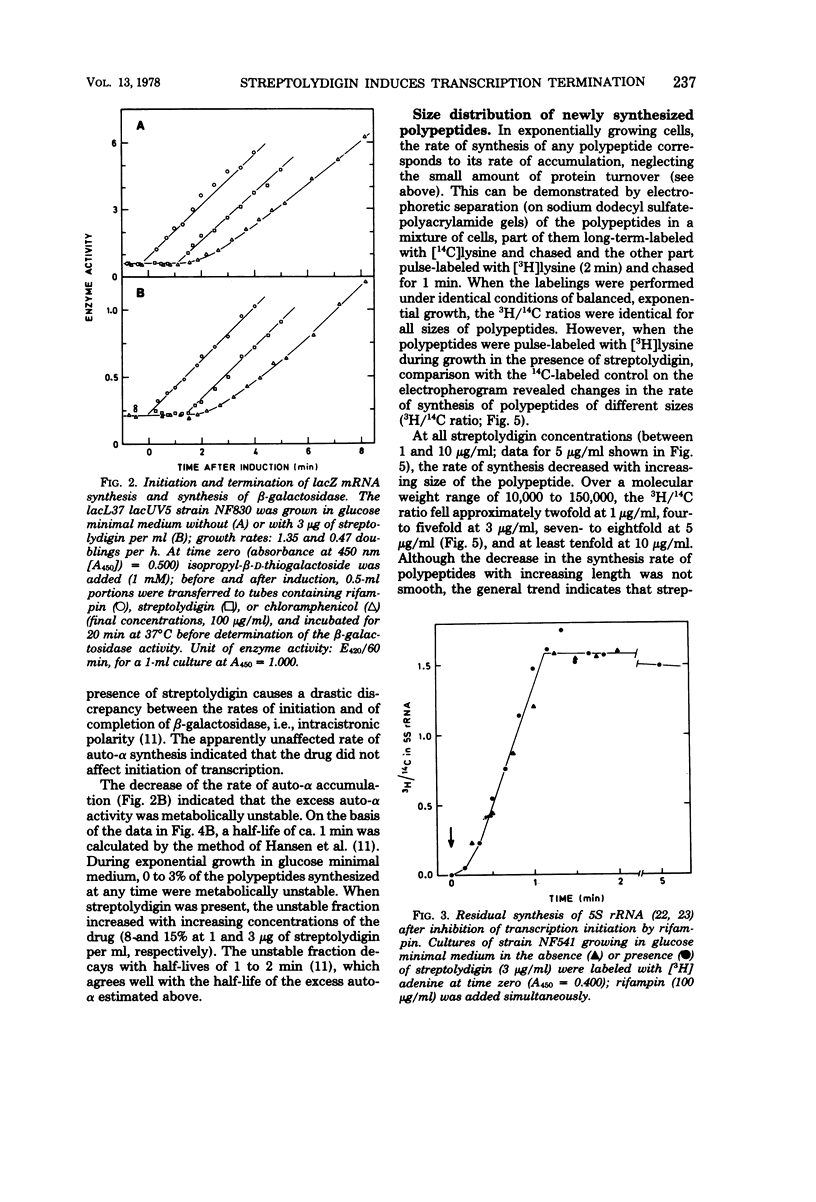
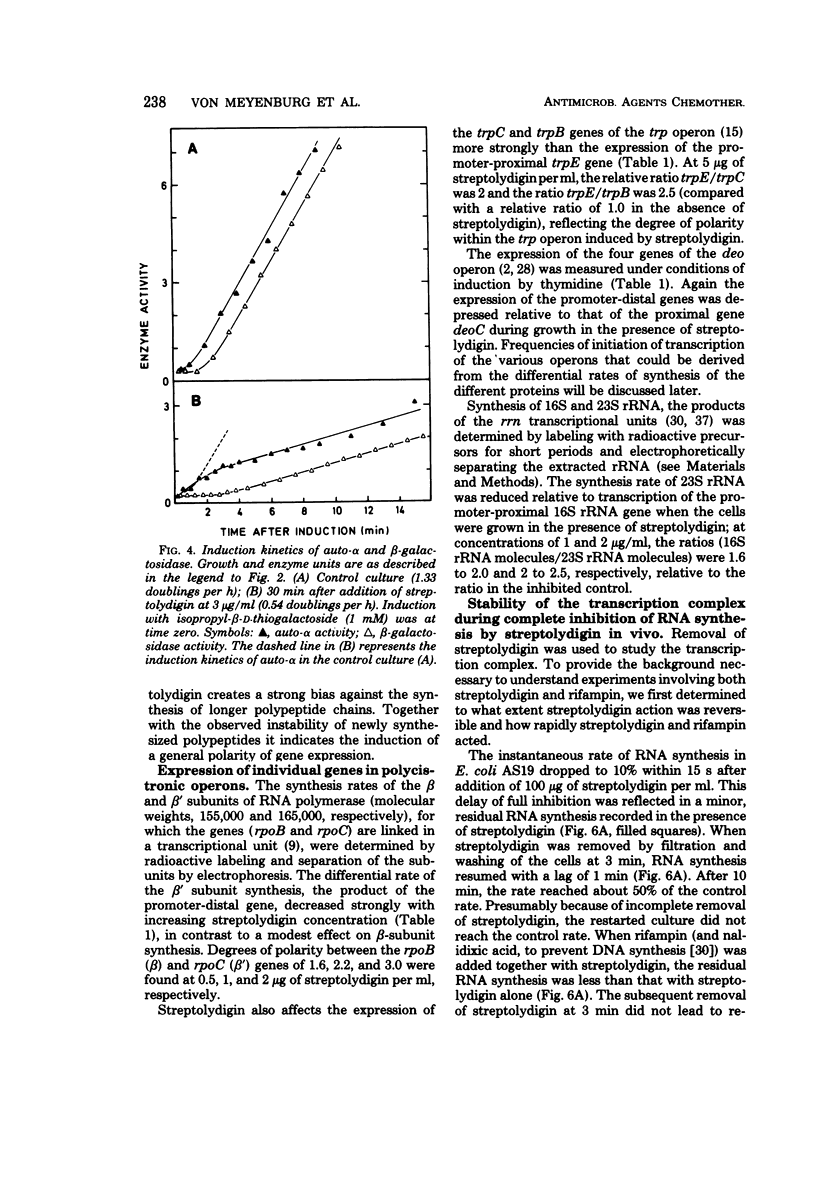
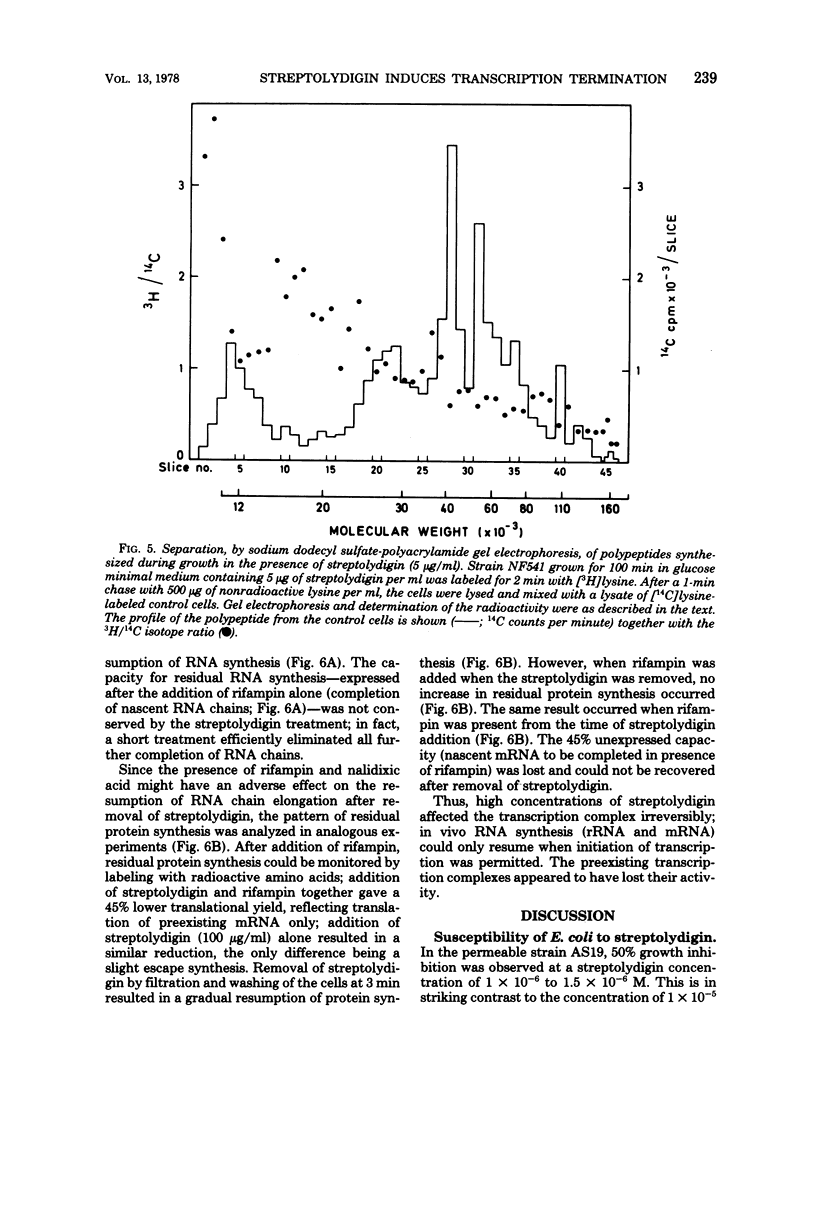
Growth of the permeable strain AS19 of Escherichia coli B is more sensitive to the antibiotic streptolydigin than is in vitro ribonucleic acid (RNA) synthesis. The in vivo chain elongation rates of lacZ messenger RNA and ribosomal RNA are not affected at 1.5 × 10−6 M, a concentration that reduces the growth rate threefold. The synthesis of large proteins is inhibited preferentially, and a considerable fraction of the polypeptides synthesized is unstable. The synthesis of complete β-galactosidase is inhibited relative to the synthesis of short, unstable polypeptides, which include the first 60 to 70 amino acids of β-galactosidase. The expression of the following polycistronic transcription units is strongly biased against promoter-distal genes: trp, deo, rpoBC, and rrn. The extent of polarity is proportional to the distance transcribed and to the streptolydigin concentration. Streptolydigin appears to destabilize active transcription complexes irreversibly irrespective of the type of transcript (messenger RNA, ribosomal RNA) and of transcription intensity. We suggest that streptolydigin leads to premature termination of transcription, resulting in release of incomplete transcripts and, thus, a decrease in overall messenger RNA concentration, which becomes limiting for protein synthesis, i.e., for growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M., De Crombrugghe B. Release of polarity in Escherichia coli by gene N of phage lambda: termination and antitermination of transcription. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2534–2538. doi: 10.1073/pnas.71.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrechtsen H., Hammer-Jespersen K., Munch-Petersen A., Fiil N. Multiple regulation of nucleoside catabolizing enzymes: effects of a polar dra mutation on the deo enzymes. Mol Gen Genet. 1976 Jul 23;146(2):139–145. doi: 10.1007/BF00268082. [DOI] [PubMed] [Google Scholar]
- Bleyman M., Kondo M., Hecht N., Woese C. Transcriptional mapping: functional organization of the ribosomal and transfer ribonucleic acid cistrons in the Bacillus subtilis genome. J Bacteriol. 1969 Aug;99(2):535–543. doi: 10.1128/jb.99.2.535-543.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassani G., Burgess R. R., Goodman H. M., Gold L. Inhibition of RNA polymerase by streptolydigin. Nat New Biol. 1971 Apr 14;230(15):197–200. doi: 10.1038/newbio230197a0. [DOI] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington L., Glass R. E., Hayward R. S., Scaife J. G. Structure and orientation of an RNA polymerase operon in Escherichia coli. Nature. 1974 Jun 7;249(457):519–522. doi: 10.1038/249519a0. [DOI] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. The amino acid sequence of beta-galactosidase of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1507–1510. doi: 10.1073/pnas.74.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. T., Bennett P. M., von Meyenburg K. Intracistronic polarity during dissociation of translation from transcription in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):589–604. doi: 10.1016/0022-2836(73)90225-8. [DOI] [PubMed] [Google Scholar]
- Hansen M. T., Pato M. L., Molin S., Fill N. P., von Meyenburg K. Simple downshift and resulting lack of correlation between ppGpp pool size and ribonucleic acid accumulation. J Bacteriol. 1975 May;122(2):585–591. doi: 10.1128/jb.122.2.585-591.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil A., Zillig W. Reconstitution of bacterial DNA-dependent RNA-polymerase from isolated subunits as a tool for the elucidation of the role of the subunits in transcription. FEBS Lett. 1970 Dec;11(3):165–168. doi: 10.1016/0014-5793(70)80519-1. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Morikawa N., Sato K. On the transcription of the tryptophan operon in Escherichia coli. 3. Multicistronic messenger RNA and polarity for transcription. J Mol Biol. 1965 Aug;13(1):169–182. doi: 10.1016/s0022-2836(65)80087-0. [DOI] [PubMed] [Google Scholar]
- Iwakura Y., Ishihama A., Yura T. RNA polymerase mutants of Escherichia coli. Streptolydigin resistance and its relation to rifampicin resistance. Mol Gen Genet. 1973 Mar 1;121(2):181–196. doi: 10.1007/BF00277531. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Kepes A. Initiation, elongation and inactivation of lac messenger RNA in Escherichia coli studied studied by measurement of its beta-galactosidase synthesizing capacity in vivo. J Mol Biol. 1971 Sep 28;60(3):453–472. doi: 10.1016/0022-2836(71)90181-1. [DOI] [PubMed] [Google Scholar]
- Johnsen K., Molin S., Karlström O., Maaloe O. Control of protein synthesis in Escherichia coli: analysis of an energy source shift-down. J Bacteriol. 1977 Jul;131(1):18–29. doi: 10.1128/jb.131.1.18-29.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoma T., Lark K. G. Characterization of the replication of Escherichia coli DNA in the absence of protein synthesis: stable DNA replication. J Mol Biol. 1975 May 15;94(2):243–256. doi: 10.1016/0022-2836(75)90081-9. [DOI] [PubMed] [Google Scholar]
- Leive L., Kollin V. Synthesis, utilization and degradation of lactose operon mRNA in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):247–259. doi: 10.1016/0022-2836(67)90330-0. [DOI] [PubMed] [Google Scholar]
- Matzura H., Molin S., Maaloe O. Sequential biosynthesis of the and ' subunits of the DNA-dependent RNA polymerase from Escherichia coli. J Mol Biol. 1971 Jul 14;59(1):17–25. doi: 10.1016/0022-2836(71)90410-4. [DOI] [PubMed] [Google Scholar]
- Molin S., Von Meyenburg K., Maaloe O., Hansen M. T., Pato M. L. Control of ribosome synthesis in Escherichia coli: analysis of an energy source shift-down. J Bacteriol. 1977 Jul;131(1):7–17. doi: 10.1128/jb.131.1.7-17.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. L., Zipser D. Polypeptide products of nonsense mutations. I. Termination fragments from nonsense mutations in the Z gene of the lac operon of Escherichia coli. J Mol Biol. 1970 Jun 14;50(2):359–371. doi: 10.1016/0022-2836(70)90198-1. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Guertin M. Regulation of mRNA utilization and degradation by amino-acid starvation. Nat New Biol. 1971 Aug 11;232(2):165–169. doi: 10.1038/newbio232165a0. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Mosteller R. D., Yanofsky C. Dynamics of synthesis, translation, and degradation of trp operon messenger RNA in E. coli. Cold Spring Harb Symp Quant Biol. 1969;34:725–740. doi: 10.1101/sqb.1969.034.01.082. [DOI] [PubMed] [Google Scholar]
- Munch-Petersen A., Nygaard P., Hammer-Jespersen K., Fiil N. Mutants constitutive for nucleoside-catabolizing enzymes in Escherichia coli K12. Isolation, charactrization and mapping. Eur J Biochem. 1972 May 23;27(2):208–215. doi: 10.1111/j.1432-1033.1972.tb01828.x. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Studies of the binding of actinomycin and related compounds to DNA. J Mol Biol. 1968 Jul 28;35(2):251–290. doi: 10.1016/s0022-2836(68)80024-5. [DOI] [PubMed] [Google Scholar]
- Pato M. L., Bennett P. M., von Meyenburg K. Messenger ribonucleic acid synthesis and degradation in Escherichia coli during inhibition of translation. J Bacteriol. 1973 Nov;116(2):710–718. doi: 10.1128/jb.116.2.710-718.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Yanofsky C. Metabolic regulation of the tryptophan operon of Escherichia coli: repressor-independent regulation of transcription initiation frequency. J Mol Biol. 1972 Aug 14;69(1):103–118. doi: 10.1016/0022-2836(72)90026-5. [DOI] [PubMed] [Google Scholar]
- Schleif R. Isolation and characterization of streptolydigin resistant RNA polymerase. Nature. 1969 Sep 6;223(5210):1068–1069. doi: 10.1038/2231068a0. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M., Iida S. Mutants of Escherichia coli permeable to actinomycin. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2315–2320. doi: 10.1073/pnas.58.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhikol C., Erbstoeszer J. W., Weisblum B. Mode of action of streptolydigin. J Bacteriol. 1969 Jul;99(1):151–155. doi: 10.1128/jb.99.1.151-155.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A. E., Arditti R. R., Magasanik B. Catabolite-insensitive revertants of lac promoter mutants. Proc Natl Acad Sci U S A. 1970 Jul;66(3):773–779. doi: 10.1073/pnas.66.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Svenningsen B. A. Regulated in vitro synthesis of the enzymes of the deo operon of Escerichia coli. properties of the DNA directed system. Mol Gen Genet. 1975;137(4):289–304. doi: 10.1007/BF00703255. [DOI] [PubMed] [Google Scholar]
- Tsai H., Suskind S. R. Enzymic properties of a mutant tryptophan synthase from Neurospora crassa. Biochim Biophys Acta. 1972 Sep 19;284(1):324–340. doi: 10.1016/0005-2744(72)90070-8. [DOI] [PubMed] [Google Scholar]
- Wegman J., DeMoss J. A. The enzymatic conversion of anthranilate to indolylglycerol phosphate in Neurospora crassa. J Biol Chem. 1965 Oct;240(10):3781–3788. [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]


