Abstract
GM (grey matter) changes of thalamus and basal ganglia have been demonstrated to be involved in AD (Alzheimer's disease). Moreover, the increase of a specific EEG (electroencephalogram) marker, α3/α2, have been associated with AD-converters subjects with MCI (mild cognitive impairment). To study the association of prognostic EEG markers with specific GM changes of thalamus and basal ganglia in subjects with MCI to detect biomarkers (morpho-physiological) early predictive of AD and non-AD dementia. Seventy-four adult subjects with MCI underwent EEG recording and high-resolution 3D MRI (three-dimensional magnetic resonance imaging). The α3/α2 ratio was computed for each subject. Three groups were obtained according to increasing tertile values of α3/α2 ratio. GM density differences between groups were investigated using a VBM (voxel-based morphometry) technique. Subjects with higher α3/α2 ratios when compared with subjects with lower and middle α3/α2 ratios showed minor atrophy in the ventral stream of basal ganglia (head of caudate nuclei and accumbens nuclei bilaterally) and of the pulvinar nuclei in the thalamus; The integrated analysis of EEG and morpho-structural markers could be useful in the comprehension of anatomo-physiological underpinning of the MCI entity.
Keywords: Alzheimer's disease, basal ganglia, electroencephalogram (EEG), mild cognitive impairment, thalamus, voxel-based morphometry (VBM)
Abbreviations: AD, Alzheimer's disease; DARTEL, Diffeomorphic Anatomical Registration using Exponentiated Lie; EEG, electroencephalogram; fMRI, functional magnetic resonance imaging; GM, grey matter; IAF, individual α frequency; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; PET, positron-emission tomography; TF, transition frequency; TIV, total intracranial volume; VBM, voxel-based morphometry
INTRODUCTION
EEG (electroencephalogram) has been demonstrated as a reliable diagnostic tool in dementia research (Stam et al., 2003; Jeong, 2004; Babiloni et al., 2006; Rossini et al., 2008; Dauwels et al., 2010, 2011) The increase of high α relative to low α power has been recently demonstrated as a reliable EEG marker of hippocampal atrophy as well as conversion of patients with MCI (mild cognitive impairment) in AD (Alzheimer's disease; Moretti et al., 2011). Moreover, the increase of the θ/γ ratio has been found to be associated with the atrophy of amygdala complex as well as with the conversion of MCI patients in non-AD dementias (Moretti et al., 2009a, 2009b).
fMRI (functional magnetic resonance imaging) and PET (positron-emission tomography)-based studies, approaching the large-scale neural connectivity issue, showed that impaired visual working memory correlated with brain activity within the posterior parietal association cortex, prefrontal cortex, as well as thalamus nuclei in AD (Collette et al., 1997; Desgranges et al., 1998; Bokde et al., 2009). A study using an associate memory task with healthy controls, MCI subjects and AD patients (Celone et al., 2006) found a pattern of normal activation in the hippocampus-related network in healthy controls, a pattern of hyperactivation in more mildly impaired MCI subjects, a pattern of hypoactivation in more severe MCI subjects and a pattern of no activation in AD patients. The non-linear changes in the memory network activation across the various groups provided further evidence of previous studies suggesting this non-linear dynamic in the hippocampus (Dickerson et al., 2005; Moretti et al., 2007b). Other studies of interest found that the activation during a task was not altered between the MCI subjects and the healthy controls (Bokde et al., 2008), suggesting that connectivity within a network is first altered due to the putative AD neuropathology, and then changes in activation occur in the brain. A possible explanation is that the functional connectivity would be the first step leading to increased activation in a region that would activate as a compensatory mechanism.
The large neural network altered in AD encompasses also deep GM (grey matter) structures (Frisoni et al., 2006, 2007, 2008, 2009; van Strien et al., 2009). In particular, atrophy of thalamus and basal ganglia has been demonstrated to be involved in AD (Canu et al., 2010; Cherubini et al., 2010). In particular, AD is associated with neuronal loss not only in the hippocampus and amygdala but also in the thalamus and basal ganglia. Anterodorsal, centromedial and pulvinar nuclei are the main sites of degeneration in AD (Zarei et al., 2010). Moreover, volumes of putamen and thalamus were found significantly reduced in patients diagnosed with probable AD, and the decrease in volume correlated linearly with impaired global cognitive performance (de Jong et al., 2008). These findings strongly suggest that, besides neo-cortical atrophy, deep GM structures in AD suffer structural changes and that degenerative processes in the basal ganglia and thalamus may contribute to cognitive decline in AD.
The relationship between the sources of different EEG rhythms and thalamus–basal ganglia structure have been widely studied and accepted. For instance, findings in human and animal studies suggest that coordinated simultaneous θ activity is observed in two networks linked, respectively, to striatal nucleus (Llinás et al., 1999) and to the frontal–anterior thalamic system (Kirk and Mackay, 2003; Schmiedt et al., 2005). The α rhythm generation is quite more complex. The α dominant rhythm arises from the continuous interplay among posterior thalamo–caudate loop, posterior cingulated and parieto–occipital cortical areas (Steriade, 2006; Cantero et al., 2009). As a consequence, the generation of α rhythm is linked to the cortico–thalamo–caudate loops Of note, changes in α and γ oscillatory activity, impinging on thalamo–cortical posterior networks, have been shown to play a relevant functional role during perceptual, executive and mnemonic processes (Klimesch, 1997). Moreover, episodic memory process studies have demonstrated the modulation of the EEG rhythms by deep brain structure, such as basal ganglia and thalamus (Hart et al., 2012). Depending on the context, caudate modulation of interactions between cortical and the thalamus or cortical and more posterior cortical regions could suppress incorrect objects and enhance representations of the correct object, followed by termination of the retrieval process revealed by increase in upper α or β band (Crosson et al., 2003). Thalamic function is essential to effective semantic-memory retrieval and is disrupted to varying degrees in cognitive impaired patients. In particular, it has been demonstrated that in the most impaired patients, who made more errors and had longer reaction times, there was an increase in thalamic BOLD signal as reaction times increased. These findings demonstrate that thalamic dysfunction correlates with object memory retrieval errors.
An important component of the circuit is the pulvinar synchronization of primary and non-primary visual cortices via α/γ EEG rhythms propagated through corticothalamic pathways (Bekisz and Wrobel, 1999; Sherman and Guillery, 2002; Shipp, 2003; Wrobel et al., 2007). An important question is what semantic operations engage this mechanism or specific aspects of it. A recent work (Hart et al., 2012) has proposed that the cortical–thalamus–caudate circuit is engaged for complex, controlled semantic search and retrieval. This notion extends from the proposition that the thalamus and basal ganglia (caudate) are engaged by higher-order language processing that cannot rely on automatic processing, but which recruits controlled processes and might reflect a strategic semantic search mechanism (Ketteler et al., 2008). These processes are reflected by modulation of upper α frequency.
Recently, it has been demonstrated that the increase of high α relative to low α power is a reliable EEG marker of hippocampal atrophy (Moretti et al., 2007b) and amigdalo-hippocampal complex atrophy (Moretti et al., 2009b). Furthermore, the increase in α3/α2 power ratio has been demonstrated predictive of conversion of patients with MCI in AD, but not in non-AD dementia (Moretti et al., 2011). The same increase of α3/α2 power ratio was found to be correlated with hippocampal atrophy in subjects with AD (Moretti et al., 2012). For these previous findings, the α3/α2 power ratio analysis has been focused on in the present study.
In the present study, the association of EEG index with GM changes in thalamus and basal ganglia has been studied in subjects with MCI. The working hypothesis was that modifications of the EEG marker could be underpinned by specific deep brain structures, unveiling the possibility to identify different MCI populations. Results show that subjects with higher α3/α2 ratios when compared with subjects with lower and middle α3/α2 ratios showed minor atrophy in the ventral stream of basal ganglia (head of caudate nuclei and accumbens nuclei bilaterally) and of the pulvinar nuclei in the thalamus.
MATERIALS AND METHODS
Subjects
For the present study, 74 subjects with MCI were recruited from the memory IRCCS (Clinic of the Scientific Institute for Research and Care) of Alzheimer's and psychiatric diseases ‘Fatebenefratelli’ in Brescia. The data of same subjects were used in previously published works of our group (Moretti et al., 2009a,b,c). All experimental protocols had been approved by the local Ethics Committee. Informed consent was obtained from all participants or their caregivers, according to the Code of Ethics of the World Medical Association (Declaration of Helsinki). The research was undertaken with the understanding and written consent of each participant.
Diagnostic criteria
Patients were taken from a prospective project on the natural history of MCI. The project was aimed to study the natural history of non-demented persons with apparently primary cognitive deficits, i.e. deficits not due to psychic (anxiety, depression etc.) or physical (hypothyroidism, vitamin B12 and folate deficiency, uncontrolled heart disease, uncontrolled diabetes etc.) conditions.
Patients were rated with a series of standardized diagnostic and severity instruments, including the MMSE (Mini-Mental State Examination; Folstein et al., 1975), the CDRS (Clinical Dementia Rating Scale; Hughes et al., 1982), the HIS (Hachinski Ischemic Scale ; Rosen et al., 1980) and the IADL and BADL (Instrumental Activities of Daily Living and Basic Activities of Daily Living; Lawton and Brodie, 1969). In addition, patients underwent diagnostic neuroimaging procedures (MRI), and laboratory testing to rule out other causes of cognitive impairment. These inclusion and exclusion criteria for MCI were based on previous seminal studies (Petersen et al., 2001; Portet et al., 2006; Geroldi et al., 2006; Dubois et al., 2007). Inclusion criteria of the study were all of the following: (i) complaint by the patient or report by a relative or the general practitioner, of memory or other cognitive disturbances; (ii) MMSE score of 24–27/30 or MMSE of 28 and higher plus low performance (score of 2–6 or higher) on the clock drawing test (Lezak et al., 2004); (iii) sparing of IADL and BADL or functional impairment steadily due to causes other than cognitive impairment, such as physical impairments, sensory loss, gait or balance disturbances etc. Exclusion criteria were any one of the following: (i) patients aged 90 years and older; (ii) history of depression or juvenile-onset psychosis; (iii) history or neurological signs of major stroke; (iv) other psychiatric diseases, epilepsy, drug addiction, alcohol dependence; (v) use of psychoactive drugs, including acetylcholinesterase inhibitors or other drugs enhancing brain cognitive functions; and (vi) current or previous uncontrolled or complicated systemic diseases (including diabetes mellitus) or traumatic brain injuries.
All patients underwent: (i) semi-structured interview with the patient and – whenever possible – with another informant (usually, the patient's spouse or a child of the patient) by a geriatrician or neurologist; (ii) physical and neurological examinations; (iii) performance-based tests of physical function, gait and balance; (iv) neuropsychological battery assessing memory (Babcock Story Recall-Rey-Osterrieth Complex Figure, Recall-Auditory-Verbal Learning Test, immediate and delayed recall; Lezak et al., 2004) verbal and non-verbal memory, attention and executive functions (Trail Making Test B, A and B-A; Inverted Motor Learning-Clock Drawing Test; Lezak et al., 2004), abstract reasoning thinking (Raven Coloured Progressive Matrices; Lezak et al., 2004), frontal functions (Inverted Motor Learning), language (Phonological and Semantic fluency-Token test, Lezak et al., 2004) and apraxia and visuo-constructional abilities (Rey-Osterrieth Complex Figure, Rey figure copy, Clock Drawing Test; Lezak et al., 2004); (v) assessment of depressive symptoms by means of the CES-D (Center for Epidemiologic Studies Depression scale; Radloff, 1977). As the aim of our study was to evaluate the relationship between GM loss and α2/α3 or θ/γ ratios, we did not consider the clinical subtype of MCI, i.e., amnesic or non-amnesic, single or multiple domains.
EEG recordings
The EEG activity was recorded continuously from 19 sites by using electrodes set in an elastic cap (Electro-Cap International, Inc.) and positioned according to the 10–20 international systems (Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1 and O2). The ground electrode was placed in front of Fz. The left and right mastoids served as reference for all electrodes. The recordings were used off-line to re-reference the scalp recordings to the common average. Data were recorded with a band-pass filter of 0.3–70 Hz, and digitized at a sampling rate of 250 Hz (BrainAmp, BrainProducts). Electrodes–skin impedance was set below 5 kΩ. Horizontal and vertical eye movements were detected by recording the EOG (electrooculogram). The recording lasted 5 min, with subjects with closed eyes. Longer recordings would have reduced the variability of the data, but they would also have increased the possibility of slowing of EEG oscillations due to reduced vigilance and arousal. EEG data were then analysed and fragmented offline in consecutive epochs of 2 s, with a frequency resolution of 0.5 Hz. The average number of epochs analysed was 140 ranging from 130 to 150. The EEG epochs with ocular, muscular and other types of artefact were preliminarily identified by a computerized automatic procedure (Moretti et al., 2003). Two expert electroencephalographists manually double-checked and confirmed the automatic selections. The epochs with ocular, muscular and other types of artefacts were discarded. We are confident about the stationarity of EEG signal in our traces. Our recordings were performed at rest state, without any external stimulation that could bias the signal, maintaining the stochastic nature of spontaneous ongoing EEG. Moreover, it is widely accepted that the duration of a so-called quasi-stationary interval of continuous EEG recordings is expected not to exceed 2–4 s (Kaplan, 1999), but some authors found much longer fragments of even 12 s (Cohen and Sances, 1977), 25 s (Kawabata, 1976) or 40–60 s (McEwen and Anderson 1975) to be approximately stationary. Of note, our spectral analysis has been evaluated on 2 s epoch in each subject. Finally, the spectral power was averaged across all electrodes to obtain sort of global field power, which would have reduced the channel-to-channel variability, with the advantage to extract a high stationary measure. Recently, it has been demonstrated that the analysis of the EEG recording in frequency domain (i.e. power spectra) results in high stationary signal (Kipiński et al., 2011). Anyway as a control analysis, the stability of the EEG signal was tested as follows: the power spectra of ten epochs lasting 2 s each were averaged both at the beginning at the end of the free-artefact EEG trace of any subjects, since testing for stationarity of the variability of trial-to-trial power spectra required equal time intervals between consecutive observations (Kipiński et al., 2011). ANOVA showed no statistical difference (P = 0.2) between the beginning and the ending epochs in each subject and among all subjects.
Analysis of individual frequency bands
All recordings were obtained in the morning with subjects resting comfortably. Vigilance was continuously monitored in order to avoid drowsiness. A digital FFT-based power spectrum analysis (Welch technique, Hanning windowing function, no phase shift) computed-ranging from 2 to 45 Hz–the power density of EEG rhythms with a 0.5 Hz frequency resolution. Two anchor frequencies were selected according to the literature guidelines (Klimesch, 1997, 1999), that is, the θ/α TF (transition frequency) and the IAF (individual α frequency) peak. The TF marks the TF between the θ and α bands and represents an estimate of the frequency at which the θ and α spectra intersect. TF was computed as the minimum power in the α frequency range, since our EEG recordings were performed at rest. The IAF represents the frequency with the maximum power peak within the extended α range (5–14 Hz). Based on TF and IAF, we estimated the frequency band range for each subject, as follows: δ from TF-4 to TF-2, θ from TF-2 to TF, low α band (α1 and α2) from TF to IAF and high α band (or α3) from IAF to IAF+2. The α1 and α2 bands were computed for each subject as follows: α1 from TF to the middle point of the TF–IAF range, and α2 from such middle point to the IAF peak (Moretti et al., 2004, 2007a,b; 2008a,b; 2009a,b,c). Moreover, individual β and γ frequencies were computed. Three frequency peaks were detected in the frequency range from the individual α3 frequency band and 45 Hz. These peaks were named β1 (IBF 1), β2 (IBF 2) and γ (IGF). Based on peaks, the frequency ranges were determined. β1 ranges from α3 to the lower spectral power value between β1 and β2 peak; β2 frequency ranges from β1 to the lower spectral power value between β2 and γ peak; γ frequency ranges from β2 to 45 Hz, which is the end of the range considered. Moreover, within θ frequency, the frequency peak ITF (individual theta frequency) was also individuated. The mean frequency ranges computed in MCI subjects considered as a whole are: δ 2.9–4.9 Hz; θ 4.9–6.9 Hz; α1 6.9–8.9 Hz; α2 8.9–10.9 Hz; α3 10.9–12.9 Hz; β1 12.9–19.2 Hz; β2 19.2–32.4; γ 32.4–45. Finally, in the frequency bands determined on an individual basis, we computed the relative power spectra for each subject. The relative power density for each frequency band was computed as the ratio between the absolute power and the mean power spectra from 2 to 45 Hz. The relative band power at each band was defined as the mean of the relative band power for each frequency bin within that band. The α3/α2 and θ/γ ratio was computed in all subjects. Three groups were obtained according to increasing tertile values of α3/α2: low (α3/α2<1); middle (1<α3/α2<1.16); and high (α3/α2>1.17). A tertile subdivision was chosen in which each tertile is statistical significantly different from others. It should be possible to obtain three groups with equal size or only two groups, but the analysis of the results would be less sensible as compared with three groups, whose average values were significantly different from each other.
MRI scans
For each subject, a high-resolution sagittal T1 weighted volumetric MR scan was acquired by using a 1.0 T Philips Gyroscan scanner, with a gradient echo 3D technique: TR = 20 ms, TE = 5 ms, flip angle = 30, field of view = 220 mm, acquisition matrix 256×256, slice thickness 1.3 mm.
The pattern of GM atrophy was studied using the VBM (voxel-based morphometry) technique (Ashburner, 2007).
Voxel-based morphometry
The 3D images were processed through the SPM5 software package (Statistical Parametric Mapping, Version 5; Welcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm), running on Matlab 7.0.1 (Math-Works). DICOM files were converted into ANALYZE format image, the extra-cranial voxels were removed and the AC (anterior commissure) was manually set for all images as the origin of the spatial coordinates for an anatomical normalization algorithm implemented in SPM. Converted files were then segmented into GM and white matter and normalized to the GM population templates, generated from the complete image set, using the DARTEL (Diffeomorphic Anatomical Registration using Exponentiated Lie) algebra registration method (Ashburner, 2007). This non-linear warping technique minimizes between-subject structural variations.
Spatially normalized images were modulated by the Jacobian determinants derived from the spatial normalization to ensure that the overall amount of each tissue class was not altered by the spatial normalization procedure. The final voxel resolution after DARTEL was 1.5×1.5×1.5 mm. Finally, each modulated, warped GM image was transformed to MNI space and smoothed with an 8 mm FWHM (full-width at half-maximum) Gaussian kernel filter. The experimenter performing the MRN computations was blinded to the results of previous EEG works so that there were not biases in the analysis.
Statistical analysis
VBM results were assessed at an uncorrected threshold of P<0.001. This threshold has an important limit in that it allows the type I statistical error. Anyway, a more permissive threshold could be more adequate to this explorative study, in order to avoid the β (or type II) statistic error, with the risk to neglect interesting results. The power of the study was allowed by size of the sample and by the robust results of the subsequent analyses. The sample was disaggregated into three groups according to three increasing values of α3/α2 ratio: low-α3/α2 (α3/α2<1), middle-α3/α2 (1<α3/α2<1.16), high-α3/α2 (α3/α2>1.17).
Voxel-based analyses were carried out first comparing the three patient groups with increasing values of the α3/α2 ratio (high-α3/α2; middle-α3/α2; low-α3/α2).
For each EEG group, between-group regional differences in GM volumes were assessed by using an ANCOVA model, modelling the effects of groups (high, middle, low) and parametric nuisance covariates (age, gender, education, MMSE scores as covariates). For continuous variables, post-hoc pairwise comparisons among groups were performed with the Games-Howell or Bonferroni tests depending on homogeneity of variance tested with Levene's test.
Moreover, the TIV (total intracranial volume) was introduced in the statistical analysis as a covariate to avoid the confounding item of the global cortical atrophy. The TIV was computed by manually tracing the entire intracranial cavity on 7 mm thick coronal slices, by the use of the software DISPLAY 1.3 tools.
All the analyses were restricted to the thalamus and basal ganglia as regions of interest in order to focus on the relationship between the brain areas and EEG markers. It should be possible to perform a computation encompassing other brain areas, but this was beyond the scope of the present work. Moreover, the relationship of EEG markers with hippocampus and amygdala was addressed previously (Moretti et al., 2009b). For this purpose, a mask including caudate nucleus, putamen, globus pallidus, accumbens nucleus and thalamus was entered into the models as explicit mask. It was manually traced, through the software MRIcroN, on the previous template generated from the complete image set.
The detection of the anatomical regions was based on the localization of the thalamic nuclei and basal ganglia in histological sections from a human atlas (Mai et al., 1997).
RESULTS
EEG α3/α2 ratio
Low-α3/α2 group
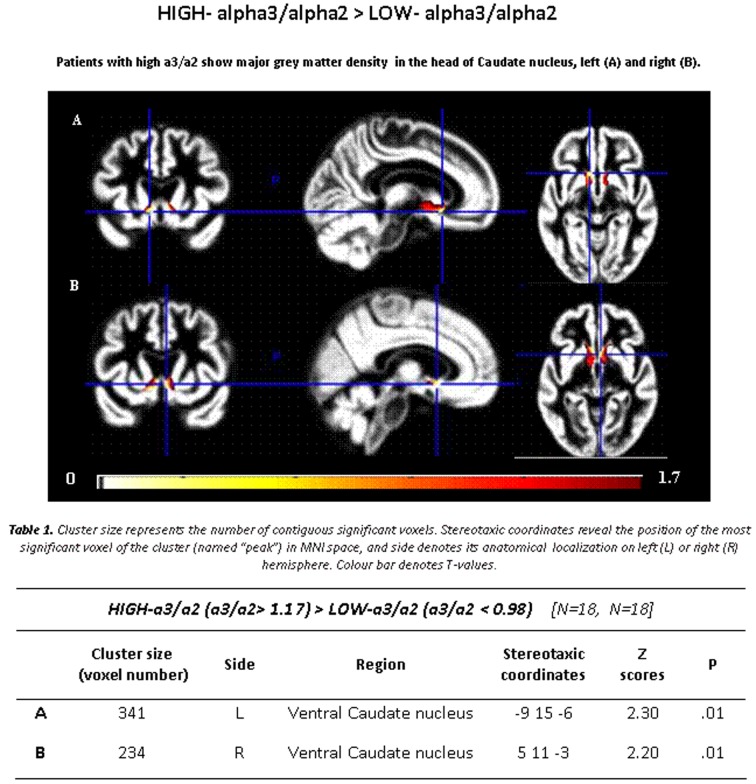
Subjects with low α3/α2 ratio exhibited a region of GM more atrophic than subjects with high α3/α2 ratios located in the head of caudate, specifically in the ventral part and accumbens nuclei bilaterally, slightly wider on the right side (see Figure 1).
Figure 1. GM density in high versus low α3/α2 power ratio.
T-map showing greater regional GM density in patients with high α3/α2 ratio contrasted with patients with low α3/α2 ratio (P≤0.05 uncorrected, the symbol ‘>‘ denotes ‘major GM density then’; see also Table 1).
Table 1. Demographic and cognitive characteristics in the whole sample disaggregated for increased levels of α3/α2 ratio.
Numbers denote means±S.D., number and (range).
| α3/α2 | |||||
|---|---|---|---|---|---|
| All | High | Middle | Low | P | |
| Number of subjects | 74 | 18 | 38 | 18 | |
| Age (years) | 69.4±0.6 (52–85) | 70.4±6.7 (60–85) | 68.4±8.2 (52–83) | 70.4±7.4 (57–80) | 0.55 |
| Sex (percentage female) | 51 | 13 | 24 | 14 | 0.51 |
| Education (years) | 7.6±3.9 (3–18) | 6.6±3.6 (4–18) | 7.6±3.7 (3–17) | 8.3±4.7 (3–18) | 0.42 |
| Mini mental state exam | 27.2±1.7 (23–30) | 26.9±1.3 (23–29) | 27±1.7 (24–30) | 27.4±1.2 (23–30) | 0.46 |
| α3/α2 | 1.09±0.15 (0.77–1.52) | 1.29±0.1 (1.17–1.52) | 1.08±0.0 (1–1.16) | 0.9±0.1 (0.77–0.98) | 0.000 |
P denotes significance on ANOVA (continuous variables) and chi-square test (dichotomous variables).
No regions of GM tissue loss were found when patients with low α3/α2 ratio were compared with those with middle α3/α2 ratio.
Middle-α3/α2 group
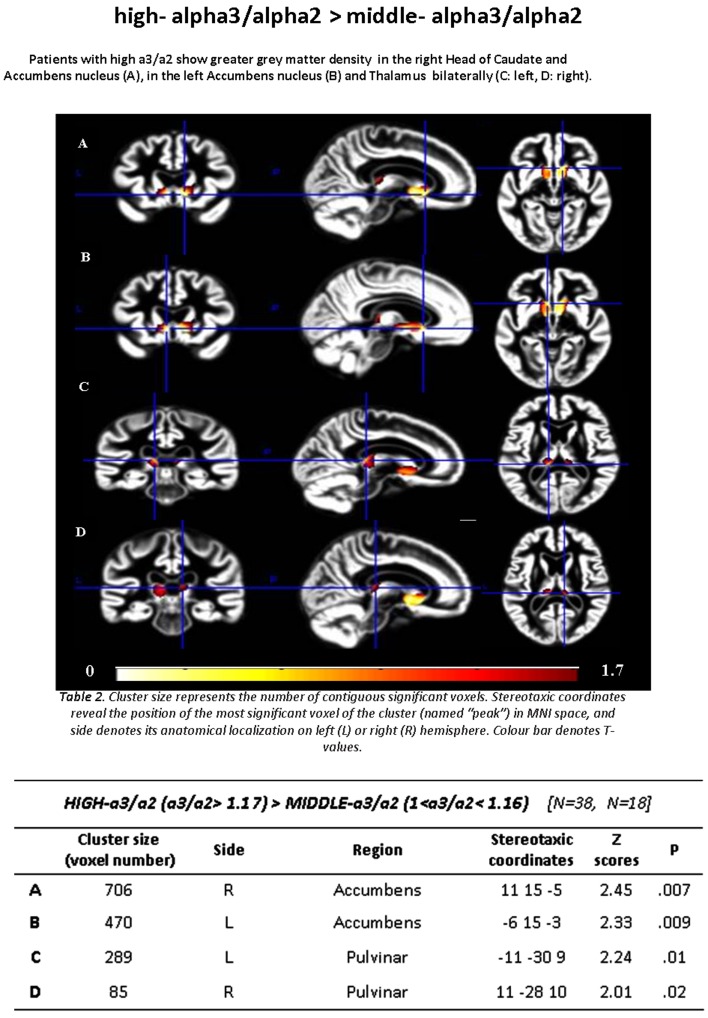
Subjects with middle α3/α2 ratio, contrasted with individuals with high α3/α2 ratios, showed the same cerebral atrophic areas observed in the previous contrast (low α3/α2 compared with high α3/α2 ratio) including the bilateral head of caudate. Specifically, the GM loss was located in the medial, ventral caudate and accumbens nuclei on the right brain hemisphere, whereas the atrophy was restricted to the ventral part of caudate and in the accumbens nucleus on the left one (see Figure 2).
Figure 2. GM density in high versus middle α3/α2 power ratio.
T-map showing greater regional GM density in patients with high α3/α2 ratio contrasted with patients with middle α3/α2 ratio (P≤0.05 uncorrected, the symbol ‘>’ denotes ‘major GM density then’; see also Table 1).
No regions of significant GM tissue loss were found in other comparisons in this group.
DISCUSSION
Preliminary remarks
In this study, we have considered the GM changes of deep brain structures, basal ganglia and thalamus, based on brain electrical activity markers. As a consequence, the analysis of anatomical structural changes in MCI patients was EEG marker driven. This is a crucial point for considering the results of the present study. Indeed, it is not a simple detection of atrophy pattern between two clinically different populations of subjects, but it would investigate the association of EEG markers with specific GM changes of thalamus and basal ganglia in subpopulations of subjects with MCI in order to detect biomarkers (morphophysiological) early predictive of AD and non-AD dementia. The α3/α2 frequency ratio has been chosen for two principal reasons: (1) previous studies demonstrated their association with important anatomical substrates for atrophy, namely the hippocampus for α3/α2 (Moretti et al., 2009b); and (2) a previous study has demonstrated that those EEG indexes have different diagnostic and prognostic value: the increase in α3/α2 ratio is associated with the conversion of MCI subjects in AD (Moretti et al., 2009c). Moreover, the increase of this EEG index has been previously correlated with impairment in psychometric tests in MCI subjects (Moretti et al., 2009a,b). Of note, the present results derive from comparisons within masked regions along with relatively liberal statistical thresholds. This implies that these findings need to be confirmed by further studies with larger size patient populations. On the other hand, the presence of a peculiar pattern of association between EEG and MRI markers in a subgroup of MCI subjects could be useful in the comprehension of anatomo-physiological underpinning of the MCI entity. Indeed, in the previous studies published by our group, the increase of α3/α2 ratio was associated with a decrease (or atrophy) of the hippocampal volume. On the contrary, in the present study, the α3/α3 ratio is associated with a bigger volume (or minor atrophy) in the basal ganglia and thalamus.
Association between EEG markers and GM changes
The increase of α3/α2 ratio is associated with minor atrophy of caudate nuclei, accumbens nuclei in the basal ganglia, and of the pulvinar nuclei of the thalamus. These results confirm previous studies on AD patients, showing both basal ganglia and thalamic involvement in AD (de Jong et al., 2008; Canu et al., 2010; Cherubini et al., 2010; Zarei et al., 2010). The principal difference with the most of the other studies is that they reported a greater atrophy of thalamus and basal ganglia. There are some possible explanations: (1) the EEG-driven evaluation of atrophy, focusing on specific morpho-structural features of patients, have magnified only some GM patterns; (2) previous observed atrophy patterns could be linked to specific regressive processes due to aging (Cabeza et al., 2004; Logan et al., 2002; Park et al., 2003, Grady et al., 2006; (3) previous studies were mostly performed on AD patients whereas subjects with MCI could have a different, perhaps compensatory, mechanism as compared with AD patients (Dickerson et al., 2004, 2005; Golob et al. 2007).
Anatomo-physiological relationship between EEG markers and GM changes
Our results show the presence of different patterns of GM changes associated with EEG marker. This could suggest the existence of specific neural networks underlying the degenerative disease. We suggest an interesting, although speculative, hypothesis that could explain the novelty emerging from our results. A ventral stream, encompassing striatum (ventral caudate nuclei), and accumbens nuclei together with pulvinar nuclei of the thalamus, seems to be less affected in MCI patients who will develop AD. The relatively preserved anatomical structure could suggest a state of compensatory hyperfunction of this circuitry, determining both cognitive and psychiatric symptoms of prodromal AD (Spalletta et al., 2010). Indeed, the ventral caudate and accumbens nuclei are related to the regulation of emotional control function, in particular with the impulsivity and fear behaviour control (Basar et al., 2010). In turn, the pulvinar nuclei are involved in cognitive visuo-spatial function (Berman and Wurtz, 2010). The brain electrical activity marker of these structural changes is represented by the increase of α3/α2 ratio. This could be due to the prevalence of an anterior circuit impinging on anterior cingulate cortex, ventral (limbic) striatum and orbito-frontal cortex, relatively spared (in the initial time of disease) in prodromal AD patients. The disrupture of the posterior circuit, encompassing hippocampal cortex, posterior cingulate, precuneus, posterior parietal cortices, will give rise to the decrease of low α (α2 in our analysis) rhythm, whereas the dominance of an anterior circuit gives rise to the increase of high α. This explanation is supported by two well-known neurophysiological phenomena in AD: (1) the decrease of power of the dominant α rhythm on posterior cerebral areas; and (2) the so-called ‘anteriorization’ of α frequency (Klimesch et al., 2007). The anterior α is higher in frequency for two possible reasons: (1) the lack of large areas to synchronize, as the posterior sibling usually does; and (2) the association with attentive phenomena, more than with visuo-spatial analysis, usually related to higher α rhythm (Klimesch, 1999).
The lack of results in the comparison between middle and low ratios of α3/α2 is counterintuitive and puzzling to be interpreted. The most plausible reason is that the neurophysiological phenomena become evident when the atrophy of deep GM nuclei reaches a threshold level, highlighting the strength of the EEG markers-guided analysis in a prognostic view. Indeed, the other groups could present MCI subjects that will remain stable or with depression or with prevalent cerebrovascular disease.
Anatomo-physiological implications at network level
Ventral stiatum (ventral caudate and accumbens nuclei) is a key entry point structure for afferent information from the periphery as well as for afferents and efferents of wider CSPTC (cortico–striatal–pallido–thalamo–cortical functionally segregated loops; Kopell and Greenberg, 2008). Of note, this network has peculiar functions within the loop associated with characteristic modulation effects.
The ventral striatum/accumben complex is related to orbito-frontal cortex and is involved in the weighting of stimuli in the reward against punishment evaluation. Moreover, these structures provide a vital link between the internal state of emotion and the external state of motor behaviour (Kopell and Greenberg, 2008; Haber and Knutson, 2010). The involvement of pulvinar nuclei is well associated with visuo-spatial associative function as well as with a modulation of the limbic system through the connection between medial pulvinar and the amygdala (Berman and Wurtz, 2010).
There are some limitations due to the explorative nature of the present study: (1) the lack of the estimation of other EEG frequencies; and (2) the lack of the estimation of synchrony or coherence measures (see also Dauwels et al., 2009). Anyway, along the conceptual frame of the study, both of them need to be associated with morpho-structural parameters. These aspects need to be addressed in future studies.
Previous studies, facing the neural network connectivity issue, using fMRI and PET as well as EEG, demonstrate that connectivity of brain activity is altered in AD and correlates with cognitive deficits (Bokde et al., 2009). Grady et al. suggested a functional disconnection between the hippocampus and the frontal cortices in the AD patients, and that the disconnection was underlying the memory deficit in the AD patients (Grady et al., 2001, 2003). On the other hand, a hyperactivation of memory networks was found in subjects with MCI with clinical memory impairment (Dickerson et al., 2005; Bokde et al., 2008, 2009). Our present results could offer a possible comprehensive explanation. As a speculative hypothesis, it could be possible that in MCI subjects at risk to develop AD, discrete networks prevail on long-range networks (disconnection theory). At the same time, the survival of hyperactive, although short-range, networks permit the maintenance of a normal performance (compensation theory), at least in the early phases of disease.
Conclusion
The integrated analysis of EEG and morpho-structural markers could be useful in the comprehension of anatomo-physiological underpinning of the MCI entity.
Footnotes
This work was supported by the Fatebenefratelli Association for Research (AFaR).
REFERENCES
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Binetti G, Cassetta E, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Frisoni G, Hirata K, Lanuzza B, Miniussi C, Moretti DV, Nobili F, Rodriguez G, Romani GL, Salinari S, Rossini PM. Sources of cortical rhythms change as a function of cognitive impairment in pathological aging: a multi-centric study. Clin Neurophysiol. 2006;117:252–268. doi: 10.1016/j.clinph.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Basar K, Sesia T, Groenewegen H, Steinbusch HW, Visser-Vandewalle V, Temel Y. Nucleus accumbens and impulsivity. Prog Neurobiol. 2010;92:533–557. doi: 10.1016/j.pneurobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Bekisz M, Wrobel A. Coupling of beta and gamma activity in cortico-thalamic system of cats attending to visual stimuli. Neuroreport. 1999;10:3589–3594. doi: 10.1097/00001756-199911260-00023. [DOI] [PubMed] [Google Scholar]
- Berman RA, Wurtz RH. Functional identification of a pulvinar path from superior colliculus to cortical area MT. J Neurosci. 2010;30:6342–6354. doi: 10.1523/JNEUROSCI.6176-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokde AL, Lopez-Bayo P, Born C, Dong W, Meindl T, Leinsinger G, Teipel SJ, Faltraco F, Reiser M, Möller HJ, Hampel H. Functional abnormalities of the visual processing system in subjects with mild cognitive impairment: an fMRI study. Psychiatr Res Neuroimaging. 2008;163:248–259. doi: 10.1016/j.pscychresns.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Ewers M, Hampel H. Assessing neuronal networks: understanding Alzheimer's disease. Prog Neurobiol. 2009;89:125–133. doi: 10.1016/j.pneurobio.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Cruz-Vadell A, Suarez-Gonzalez A, Gil-Neciga E. Increased synchronization and decreased neural complexity underlie thalamocortical oscillatory dynamics in mild cognitive impairment. Neuroimage. 2009;46:938–948. doi: 10.1016/j.neuroimage.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Canu E, McLaren DG, Fitzgerald ME, Bendlin BB, Zoccatelli G, Alessandrini F, Pizzini FB, Ricciardi GK, Beltramello A, Johnson SC, Frisoni GB. Microstructural diffusion changes are independent of macrostructural volume loss in moderate to severe Alzheimer's disease. J Alzheimers Dis. 2010;19:963–976. doi: 10.3233/JAD-2010-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini A, Péran P, Spoletini I, Di Paola M, Di Iulio F, Hagberg GE, Sancesario G, Gianni W, Bossù P, Caltagirone C, Sabatini U, Spalletta G. Combined volumetry and DTI in subcortical structures of mild cognitive impairment and Alzheimer's disease patients. J Alzheimers Dis. 2010;19:1273–1282. doi: 10.3233/JAD-2010-091186. [DOI] [PubMed] [Google Scholar]
- Cohen BA, Sances A. Stationarity of the human electroencephalogram. Med Biol Eng Comput. 1977;15:513–518. doi: 10.1007/BF02442278. [DOI] [PubMed] [Google Scholar]
- Collette F, Salmon E, Van der Linden M, Degueldre C, Franck G. Functional anatomy of verbal and visuo-spatial span tasks in Alzheimer's disease. Hum Brain Mapp. 1997;5:110–118. doi: 10.1002/(sici)1097-0193(1997)5:2<110::aid-hbm4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Crosson B, Benefield H, Cato MA, Sadek JR, Moore AB, Wierenga CE, Gopinath K, Soltysik D, Bauer RM, Auerbach EJ, Gökçay D, Leonard CM, Briggs RW. J Int Neuropsychol Soc. 2003;9:1061–1077. doi: 10.1017/S135561770397010X. [DOI] [PubMed] [Google Scholar]
- Dauwels J, Srinivasan K, Ramasubba Reddy M, Musha T, Vialatte FB, Latchoumane C, Jeong J, Cichocki A. Slowing and loss of complexity in Alzheimer's EEG: two sides of the same coin? Int J Alzheimers Dis. 2011;2011:539–621. doi: 10.4061/2011/539621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwels J, Vialatte F, Cichocki A. Diagnosis of Alzheimer's disease from EEG signals: where are we standing? Curr Alzheimer Res. 2010;7:487–505. doi: 10.2174/156720510792231720. [DOI] [PubMed] [Google Scholar]
- Dauwels J, Vialatte F, Latchoumane C, Jeong J, Cichocki A. EEG synchrony analysis for early diagnosis of Alzheimer's disease: a study with several synchrony measures and EEG data sets. In Conf Proc IEEE Engineering in Medicine and Biology Society. 2009:pp. 2224–2227. doi: 10.1109/IEMBS.2009.5334862. [DOI] [PubMed] [Google Scholar]
- de Jong LW, van der Hiele K, Veer IM, Houwing JJ, Westendorp RG, Bollen EL, de Bruin PW, Middelkoop HA, van Buchem MA, van der Grond J. Strongly reduced volumes of putamen and thalamus in Alzheimer's disease: an MRI study. Brain 131. 2008:3277–3285. doi: 10.1093/brain/awn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgranges B, Baron JC, de la Sayette V, Petit-Taboué MC, Benali K, Landeau B, Lechevalier B, Eustache F. The neural substrates of memory systems impairment in Alzheimer's disease: a PET study of resting brain glucose utilization. Brain. 1998;121:611–631. doi: 10.1093/brain/121.4.611. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippomcapal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O'Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini mental state’: a practical method for grading the cognitive state of patients for clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Ganzola R, Canu E, Rüb U, Pizzini FB, Alessandrini F, Zoccatelli G, Beltramello A, Caltagirone C, Thompson PM. Mapping local hippocampal changes in Alzheimer's disease and normal ageing with MRI at 3 Tesla. Brain. 2008;131:3266–3276. doi: 10.1093/brain/awn280. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Pievani M, Testa C, Sabattoli F, Bresciani L, Bonetti M, Beltramello A, Hayashi KM, Toga AW, Thompson PM. The topography of grey matter involvement in early and late onset Alzheimer's disease. Brain. 2007;130:720–730. doi: 10.1093/brain/awl377. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Prestia A, Rasser PE, Bonetti M, Thompson PM. In vivo mapping of incremental cortical atrophy from incipient to overt Alzheimer's disease. J Neurol. 2009;256:916–924. doi: 10.1007/s00415-009-5040-7. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Sabattoli F, Lee AD, Dutton RA, Toga AW, Thompson PM. In vivo neuropathology of the hippocampal formation in AD: a radial mapping MR-based study. Neuroimage. 2006;32:104–110. doi: 10.1016/j.neuroimage.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Geroldi C, Rossi R, Calvagna C, Testa C, Bresciani L, Binetti G, Zanetti O, Frisoni GB. Medial temporal atrophy but not memory deficit predicts progression to dementia in patients with mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2006;77:1219–1222. doi: 10.1136/jnnp.2005.082651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golob EJ, Irimajiri R, Starr A. Auditory cortical activity in amnestic mild cognitive impairment: relationship to subtype and conversion to dementia. Brain. 2007;130:740–752. doi: 10.1093/brain/awl375. [DOI] [PubMed] [Google Scholar]
- Grady CL, Furey ML, Pietrini P, Horwitz B, Rapoport SI. Altered brain functional connectivity and impaired short-term memory in Alzheimer’s disease. Brain. 2001;124:739–756. doi: 10.1093/brain/124.4.739. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black SE. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer’s disease. J Neurosci. 2003;23:986–993. doi: 10.1523/JNEUROSCI.23-03-00986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J Jr, Maguire MJ, Motes M, Mudar RA, Chiang HS, Womack KB, Kraut MA. Semantic memory retrieval circuit: Role of pre-SMA, caudate, and thalamus. Brain Lang. 2012 doi: 10.1016/j.bandl.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Cohen LA, Martin RL. A new clinical rating scale for the staging of dementia. Br J Psychiatry. 1982;140:1225–1230. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Jeong J. EEG dynamics in patients with Alzheimer's disease. Clin Neurophysiol. 2004;115:1490–1505. doi: 10.1016/j.clinph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Kaplan AY. The problem of segmental description of human electroencephalogram. Human Physio. l999;25:107–114. [Google Scholar]
- Kawabata N. Test of statistical stability of the electroencephalogram. Biol Cybern. 1976;22:235–238. doi: 10.1007/BF00365089. [DOI] [PubMed] [Google Scholar]
- Kipiński L, König R, Sielużycki C, Kordecki W. Application of modern tests for stationarity to single-trial MEG data: transferring powerful statistical tools from econometrics to neuroscience. Biol Cybern. 2011;105:183–195. doi: 10.1007/s00422-011-0456-4. [DOI] [PubMed] [Google Scholar]
- Kirk IJ, Mackay JC. The role of theta-range oscillations in synchronising and integrating activity in distributed mnemonic networks. Cortex. 2003;39:993–1008. doi: 10.1016/s0010-9452(08)70874-8. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG-alpha rhythms and memory processes. Int J Psychophysiol. 1997;26:319–340. doi: 10.1016/s0167-8760(97)00773-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kopell BH, Greenberg BD. Anatomy and physiology of the basal ganglia: implications for DBS in psychiatry. Neurosci Biobehav Rev. 2008;32:408–422. doi: 10.1016/j.neubiorev.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brodie EM. Assessment of older people: self-maintaining and instrumental activity of daily living. J Gerontol. 1969;9:179–186. [PubMed] [Google Scholar]
- Lezak M, Howieson D, Loring DW. Neuropsychological Assessment, 4th edn, Oxford: University Press, Oxford; 2004. [Google Scholar]
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and non-selective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Ketteler D, Kastrau F, Vohn R, Huber W. The subcortical role of language processing. High level linguistic features such as ambiguity-resolution and the human brain; an fMRI study. Neurolmage. 2008;39:2002–2009. doi: 10.1016/j.neuroimage.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G. San Diego: Academic Press; 1997. Atlas of the Human Brain. [Google Scholar]
- McEwen JA, Anderson GB. Modeling the stationarity and gaussianity of spontaneous electroencephalographic activity. IEEE Trans Biomed Eng. 1975;22:361–369. doi: 10.1109/tbme.1975.324504. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Babiloni C, Binetti G, Cassetta E, Dal Forno G, Ferreri F, Ferri R, Lanuzza B, Miniussi C, Nobili F, Rodriguez G, Salinari S, Rossini PM. Individual analysis of EEG frequency and band power in mild Alzheimer's disease. Clin Neurophysiol. 2004;115:299–308. doi: 10.1016/s1388-2457(03)00345-6. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Babiloni F, Carducci F, Cincotti F, Remondini E, Rossini PM, Salinari S, Babiloni C. Computerized processing of EEG-EOG-EMG artifacts for multi-centric studies in EEG oscillations and event-related potentials. Int J Psychophysiol. 2003;47:199–216. doi: 10.1016/s0167-8760(02)00153-8. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Fracassi C, Pievani M, Geroldi C, Binetti G, Zanetti O, Sosta K, Rossini PM, Frisoni GB. Increase of theta/gamma ratio is associated with memory impairment. Clin Neurophysiol. 2009a;120:295–303.. doi: 10.1016/j.clinph.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Frisoni GB, Fracassi C, Pievani M, Geroldi C, Binetti G, Rossini PM, Zanetti O. MCI patients' EEGs show group differences between those who progress and those who do not progress to AD. Neurobiol Aging. 2011;32:563–571. doi: 10.1016/j.neurobiolaging.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Frisoni GB, Pievani M, Rosini S, Geroldi C, Binetti G, Rossini PM. Cerebrovascular disease and hippocampal atrophy are differently linked to functional coupling of brain areas: an EEG coherence study in MCI subjects. J Alzheimers Dis. 2008a;14:285–299. doi: 10.3233/jad-2008-14303. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Miniussi C, Frisoni G, Zanetti O, Binetti G, Geroldi C, Galluzzi S, Rossini PM. Vascular damage and EEG markers in subjects with mild cognitive impairment. Clin Neurophysiol. 2007a;118:1866–1876. doi: 10.1016/j.clinph.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Miniussi C, Frisoni GB, Geroldi C, Zanetti O, Binetti G, Rossini PM. Hippocampal atrophy and EEG markers in subjects with mild cognitive impairment. Clin Neurophysiol. 2007b;118:2716–2729. doi: 10.1016/j.clinph.2007.09.059. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Pievani M, Fracassi C, Binetti G, Rosini S, Geroldi C, Zanetti O, Rossini PM, Frisoni GB. Increase of theta/gamma and alpha3/alpha2 ratio is associated with amygdalo-hippocampal complex atrophy. J Alzheimers Dis. 2009b;120:295–303. doi: 10.3233/JAD-2009-1059. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Pievani M, Fracassi C, Geroldi C, Calabria M, DeCarli C, Rossini PM. Brain vascular damage of cholinergic pathways and E.E.G. markers in mild cognitive impairment. J Alzheimers Dis. 2008b;15:357–372. doi: 10.3233/jad-2008-15302. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Pievani M, Geroldi C, Binetti G, Zanetti O, Cotelli M, Rossini PM, Frisoni GB. Increasing hippocampal atrophy and cerebrovascular damage is differently associated with functional cortical coupling in MCI patients. Alzheimer Dis Assoc Disord. 2009c;23:323–332. doi: 10.1097/WAD.0b013e31819d4a9d. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Prestia A, Fracassi C, Binetti G, Zanetti O, Frisoni GB. Specific EEG changes associated with atrophy of hippocampus in subjects with mild cognitive impairment and Alzheimer's disease. Int J Alzheimers Dis. 2012;2012:253153. doi: 10.1155/2012/253153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Welsh RC, Marshuetz C, Gutchess AH, Mikels J, Polk TA, Noll DC, Taylor SF. Working memory for complexes scenes: age differences in frontal and hippocampal activations. J Cogn Neurosci. 2003;15:1122–1134. doi: 10.1162/089892903322598094. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Portet F, Ousset PJ, Visser PJ, Frisoni GB, Nobili F, Scheltens Ph, Vellas B, Touchon J, the MCI Working Group of the European Consortium on Alzheimer's Disease (EADC) Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer's Disease. J Neurol Neurosurg Psychiatry. 2006;77:714–718. doi: 10.1136/jnnp.2005.085332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Buscema M, Capriotti M, Grossi E, Rodriguez G, Del Percio C, Babiloni C. Is it possible to automatically distinguish resting EEG data of normal elderly vs. mild cognitive impairment subjects with high degree of accuracy? Clin Neurophysiol. 2008;119:1534–1545. doi: 10.1016/j.clinph.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Schmiedt C, Brand A, Hildebrandt H, Basar-Eroglu C. Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res Cogn Brain Res. 2005;25:936–947. doi: 10.1016/j.cogbrainres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Phil Trans R Soc London B: Biol Sci. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S. The functional logic of cortico-pulvinar connections. Phil Trans R Soc London B: Biol Sci. 2003;358:1605–1624. doi: 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalletta G, Musicco M, Padovani A, Rozzini L, Perri R, Fadda L, Canonico V, Trequattrini A, Pettenati C, Caltagirone C, Palmer K. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer’s disease. Am J Geriatr Psychiatry. 2010;18:1026–1035. doi: 10.1097/JGP.0b013e3181d6b68d. [DOI] [PubMed] [Google Scholar]
- Stam CJ, van der Made Y, Pijnenburg YA, Scheltens P. EEG synchronization in mild cognitive impairment and Alzheimer's disease. Acta Neurol Scand. 2003;108:90–96. doi: 10.1034/j.1600-0404.2003.02067.x. [DOI] [PubMed] [Google Scholar]
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Wrobel A, Ghazaryan A, Bekisz M, Bogdan W, Kaminski J. Two streams of attention-dependent beta activity in the striate recipient zone of cat’s lateral posterior-pulvinar complex. J Neurosci. 2007;27:2230–2240. doi: 10.1523/JNEUROSCI.4004-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Patenaude B, Damoiseaux J, Morgese C, Smith S, Matthews PM, Barkhof F, Rombouts SA, Sanz-Arigita E, Jenkinson M. Combining shape and connectivity analysis: an MRI study of thalamic degeneration in Alzheimer's disease. NeuroImage. 2010; 49:1–8. doi: 10.1016/j.neuroimage.2009.09.001. [DOI] [PubMed] [Google Scholar]