Summary
One hundred and seventy patients with ruptured cerebral aneurysms were treated by coil embolization from September 1997 to December 2002. After January 2000, coil embolization was selected as the first-choice treatment for ruptured aneurysms. During this period, the authors investigated the number of aborted cases, the number of complications, and how many patients could be treated by coil embolization according to the locations of ruptured cerebral aneurysms. One hundred and ninety-five sessions were performed on 170 patients, and 13 sessions (6.7%) were aborted mainly because of the difficulty of the approach and the wide necks of the aneurysms. In four patients, although procedural perforation and haemorrhage occurred, the outcome was good or excellent. Eight poorgrade patients experienced haemorrhage after coil embolization and seven patients died. The volume embolization ratios of small and large aneurysms were 27% and 21%, and the recanalization of small and large aneurysms occurred in 9% and 38% of patients, respectively. From January 2000 to December 2002, 119 (66%) of 180 ruptured cerebral aneurysms were treated by coil embolization. According to the location of aneurysms, 89% vertebrobasilar, 87% anterior cerebral, 65% internal carotid and 24% middle cerebral artery aneurysms could be treated by coil embolization. Because the tight packing of large aneurysms was difficult, the recanalization rate of large aneurysms was high. However, the results of small aneurysms were satisfactory. Almost 90% of vertebrobasilar and anterior cerebral artery aneurysms could be treated by coil embolization.
Key words: subarachnoid haemorrhage, cerebral aneurysm, endovascular surgery
Introduction
Since 1997, the authors have performed endovascular surgery of ruptured cerebral aneurysms using Guglielmi detachable coils (GDCs). After 2000, the authors have selected endovascular surgery as the first-choice treatment for ruptured cerebral aneurysms. The purpose of this paper is to present our protocols for the management of patients with ruptured cerebral aneurysms to prevent perioperative haemorrhage and ischemic complications, and to estimate the number ratio of patients treated by coil embolization to those treated by clipping surgery under the protocol of endovascular surgery as the first-choice treatment.
Patients and Methods
Patient population
One hundred ninety-five sessions of coil embolization performed on 170 patients with ruptured cerebral aneurysms, between September 1,1997 and December 31, 2002, were included in this study. The number of aborted sessions was thirteen (6.7%). Haemorrhagic and ischemic complications, and the outcome of patients treated by coil embolization were estimated. From January 1, 2000 to December 31, 2002, coil embolization was selected as the first-choice treatment for 180 ruptured cerebral aneurysms, and the number ratio of patients treated by coil embolization to those treated by clipping surgery was estimated.
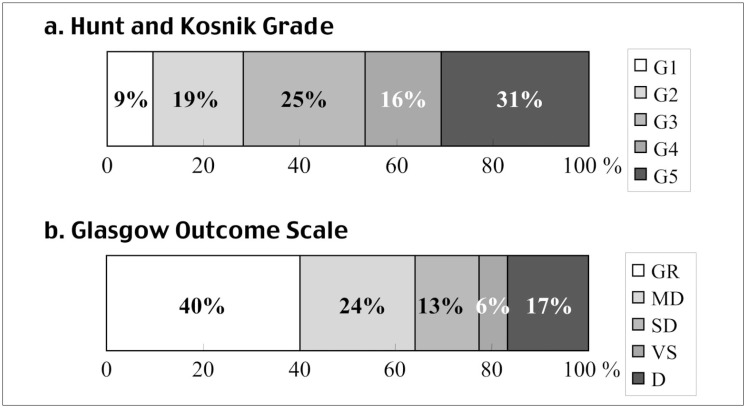
The patients were admitted with the following Hunt and Kosnik grades1: 9.4% grade I, 18.8% grade II, 25.3% grade III, 15.9% grade IV, and 30.6% grade V (figure 2A). There were 65 men (38%) and 105 women (62%). The mean age of the 170 patients was 61.9 years (range, 34-88 years). Ruptured aneurysms were located in the following arteries: 62 (36.5%) anterior cerebral, 54 (31.8%) internal carotid, nine (5.3%) middle cerebral, 24 (14.1%) basilar, three (1.8%) posterior cerebral, and 18 (10.6%) vertebral (table 1). There were 130 small saccular (< 10 mm), 29 large saccular (10 mm ≤, < 25 mm), and 11 dissecting aneurysms.
Table 1.
Locations of Ruptured Aneurysms Treated by Coil Embolization
| Location | No. of patients | Location | No. | ||
|---|---|---|---|---|---|
| AcomA | 54 | ||||
| ACA distal | 6 | 62 (36.5%) | |||
| ACA (A1 dissecting) | 2 | ||||
| IC bifurcation | 7 | ||||
| IC-Ach | 8 | Anterior circulation | 125 (73.5 %) | ||
| IC-PC | 33 | 54 (31.8%) | |||
| IC-paraclinoid | 2 | ||||
| IC-dorsal | 4 | ||||
| MCA | 9 | 9 (5.3%) | |||
| BA-tip | 16 | ||||
| BA-SCA | 6 | 24 (14.1%) | |||
| BA-AICA | 2 | ||||
| PCA distal | 3 | 3 (1.8%) | Posterior circulation | 45 (26.5%) | |
| VA union | 1 | ||||
| VA-PICA | 8 | 18 (10.6%) | |||
| VA dissecting | 9 | ||||
| Total | 170 | 170 | |||
|
AcomA, anterior communicating artery; ACA distal, distal anterior cerebral artery; IC, internal cerebral artery; Ach, anterior choroidal artery; PC, posterior communicating artery; MCA, middle cerebral artery; BA, basilar artery; SCA, superior cerebellar artery; AICA, anterior inferior cerebellar artery; PCA, posterior cerebral artery; VA, vertebral artery; PICA, posterior inferior cerebellar artery. | |||||
General management
All the patients were managed in an intensive care unit under the collaboration of neurosurgeons and a group of critical care specialists. Following the diagnosis of subarachnoid haemorrhage (SAH), the systolic blood pressure of all the patients was controlled at less than 120 mm Hg under a continuous administration of nicardipine and/or nitroglycerine. Tracheal intubation was performed under deep sedation using propofol and/or midazolam in all the patients except those who were completely conscious and with good blood pressure control. After these procedures cerebral angiography was performed.
When acute hydrocephalus was detected, ventricular or spinal lumbar drainage was performed before the cerebral angiography. Following the identification of the ruptured cerebral aneurysm, three-dimensional digital subtraction angiography (3D DSA) was performed and the size and three-dimensional structure of the aneurysms were estimated.
Indications and methods of endovascular surgery
The indications of coil embolization for ruptured cerebral aneurysms were as follows: 1) no large intracerebral haematoma, 2) dome/neck ratio2 not smaller than 1.0, 3) no branch from aneurysmal dome, and 4) no severe renal failure. In grade V patients, ventricular drainage and/or lumbar drainage was performed before endovascular surgery and when brain stem reflexes were preserved under deep sedation, endovascular surgery was selected as the firstchoice treatment.
Coil embolization was performed mainly on day 0 or one day after SAH (day one), under general anesthesia. When anesthesiologist was not available, embolization was performed under tracheal intubation and deep sedation using propofol anesthesia. In the first half of this study, systemic heparinization was started following the insertion of the first or second coil into the aneurysm. However, in the second half, systemic heparinization was started right after the sheath insertion and activated clotting time (ACT) was controlled between 200 to 250 sec. After the insertion of the first or second coil into the aneurysm, ACT was extended up to 250 to 300 sec. Guiding catheters were irrigated using heparinized saline (4000U/1000ml).
Volume embolization ratio (VER) 3 was used as one of the parameters of the degree of embolization, and 25-30% of VER was considered to be the goal of embolization. For wide-neck aneurysms located on internal carotid or basilar arteries, the neck remodeling technique 4 was utilized for tight packing of the aneurysms.
After endovascular surgery, systemic heparinization was continued for 12-18 hr with an ACT of approximately 200 sec. However, in the second half of this study, the natural reversal of heparin after embolization was applied for small-neck or incompletely embolized aneurysms, to prevent postoperative rebleeding. Furthermore deep sedation and blood pressure control were continued at least overnight after embolization. After the heparin reversal, spinal lumbar drainage was performed continuously for 7-10 days to drain the subarachnoid clot.
Postoperative follow-up was performed by digital subtraction angiography (DSA), MR angiography (MRA), and skull X-rays, and the rate of recanalization (coil compaction) was estimated. Follow-up DSA was performed once during hospitalization, and again 6-12 months later. MRA and skull X-rays were performed at 3-6 month intervals. The outcome was assessed at more than six months after the endovascular surgery.
The mean follow-up period was 23.4 months, excluding the data of 28 patients deceased within six months. The follow-up rate was 98%.
Results
Incidence of complications
Perioperative complications are shown in table 2. There were four (2.4%) cases of procedural aneurysmal perforation and haemorrhage among the 170 patients. These four cases of haemorrhage were treated by the heparin reversal and blood pressure control under general anesthesia, avoiding the immediate removal of the coil and microcatheter. Only one patient underwent clipping surgery, because an additional coil insertion failed (figure 1). The outcome of these patients was moderate disability (one patient) and good recovery (three patients). Postoperative haemorrhage occurred in eight (4.7%) patients, seven of which died. The aneurysms of these patients were internal carotid dorsal aneurysms (two patients), wideneck and/or large aneurysms (two patients), partially thrombosed aneurysm (one patient), and others (three patients). After encountering postoperative haemorrhage, the authors abandoned the postoperative systemic heparinization for incompletely embolized aneurysms and small neck aneurysms. Following this protocol modification, no postoperative haemorrhage occurred.
Table 2.
Complications and Symptomatic Vasospasm
| Complication | No. | Comment | |
|---|---|---|---|
| Haemorrhagic complications | Procedural | 4 (2.4%) | BA-tip, BA-AICA, IC dorsal, MCA GR, 3, MD, 1 |
| Postembolization | 8 (4.7%) | IC dorsal, 2; wide neck/large, 2; others, 2 Dead, 7 |
|
| Ischemic complications | Minor infarction | 9 (5.3%) | Permanent, 4 (2.4%) Reversible, 5 (2.9%) |
| Coil compaction | Small aneurysm | 12 (9.2%) | 2nd treatment, 8 |
| Large aneurysm | 11 (38%) | 2nd treatment, 10 | |
| Symptomatic vasospasm | 11 (6.5%) | ||
|
BA, basilar artery; AICA, anterior inferior cerebellar artery; IC, internal carotid artery; MCA, middle cerebral artery; GR, good recovery; MD, moderate disability | |||
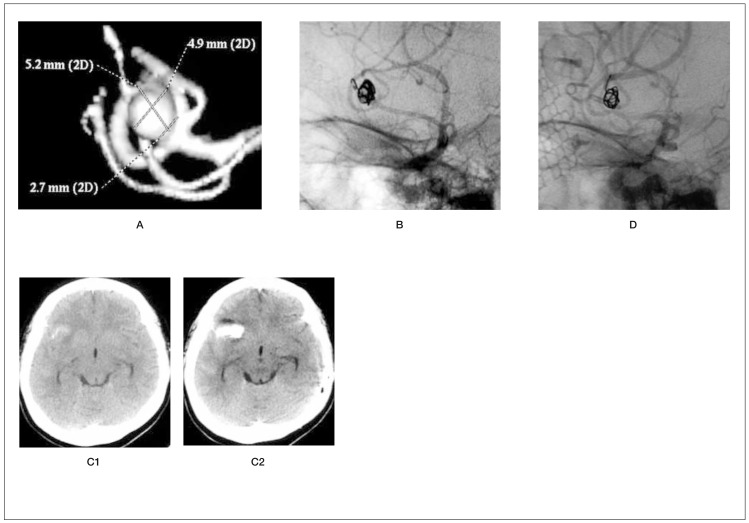
Figure 1.
A) preoperative 3D-DSA of a right middle cerebral artery aneurysm. B) Protrusion of end of first coil and microcatheter from the aneurysmal dome. C1) Preoperative CT scan. C2) CT scan immediately after perforation demonstrates intracerebral haematoma. D) DSA following clipping surgery. Thirty-seven-year-old woman, Hunt and Kosnik grade 2. The perforation of the dome of the right middle cerebral artery aneurysm occurred at the end of the first-coil insertion. Avoiding immediate removal of the coil and microcatheter, heparin reversal was performed. Following the detachment of the first coil, the insertion of the second coil from the outside into the inside of the perforated dome was attempted, but failed. After this procedure, extravasation was identified. Brain CT scan revealed an intracerebral haematoma. Subsequently, clipping surgery was performed successfully. She was discharged 31 days after the clipping surgery without any neurological deficits.
There were nine cases (5.3%) of minor ischemic complications, four (2.4%) of which resulted in permanent infarction. All these ischemic complications occurred in the first half of this study. Following the change in the timing of systemic heparinization, that is, starting systemic heparinization right after the sheath insertion, no perioperative ischemic complications occurred.
Volume embolization ratio and recanalization
The mean volume embolization ratios (VERs) of small and large aneurysms were 27.1 ±5.4 (mean±SD) and 21.0±5.4, respectively.
The mean VER of small aneurysms was significantly higher than that of large aneurysms (P<0.01). Recanalization (coil compaction) occurred in 9% of patients with small aneurysms and in 38% of patients with large aneurysms.
Glasgow outcome scale
Six months postoperatively, 40% of the patients had good recovery, 24% had moderate disability, 13% had severe disability, 6% were in a persistent vegetative state, and 17% died. figure 2A,B show the outcome of endovascular treatment compared with the preoperative grade.
Figure 2.
A) Preoperative Hunt and Kosnik (H&K) grade. 47% of the patients treated by coil embolization were classified as poor grade (grades 4-5). B) Glasgow outcome scale (GOS) score six months after coil embolization. 64% of the patients treated by coil embolization resulted in good recovery (GR) or moderate disability (MD). SD, severe disability;VS, vegetative state; D, dead.
Ratio of the numbers of patients treated by coil embolization and clipping surgery
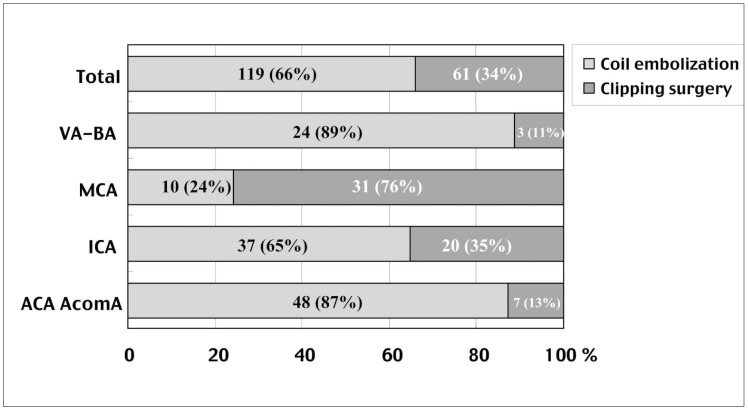
From January 1, 2000 to December 31, 2002, coil embolization was selected as the first choice treatment for ruptured aneurysms. Of 180 patients with ruptured aneurysms, 119 (66%) were treated by coil embolization and 61 (34%) by clipping surgery (figure 3). According to the location of the aneurysms, 89% vertebrobasilar, 24% middle cerebral, 65% internal carotid, and 87% anterior cerebral artery aneurysms could be treated by coil embolization.
Figure 3.
From 2000 to 2002, coil embolization was selected as the first-choice treatment for ruptured aneurysms. Of 180 patients, 119 (66%) were treated by coil embolization and 61 (34%) by clipping surgery. In particular, in the vertebrobasilar and anterior cerebral arteries, almost 90% of the aneurysms could be treated by coil embolization. Abbreviations, refer to table 1.
Discussion
ISAT 2002 (5) has reported one of the most significant lines of evidence that endovascular coil embolization can be a superior treatment for ruptured cerebral aneurysms compared with clipping surgery. The authors have applied coil embolization as the first-choice treatment for ruptured aneurysms for more than four years from January 1, 2000, following a preparation period from 1997 to 1999. Because endovascular treatment includes both haemorrhagic and ischemic complication risks, one of our concerns was the timing of systemic heparinization. In the first half of this study, systemic heparinization was started after the first or second-coil insertion into the aneurysm following the protocol of Raymond J et Al6. Because of the occurrence of minor infarctions under this protocol, in the second half of this study, systemic heparinization was started right after the sheath insertion following the protocol of Cronqvist and Moret 7, under controlled systemic blood pressure less than 120 mmHg. An early start of systemic heparinization effectively prevented perioperative ischemic complications.
Procedural aneurysmal perforation and haemorrhage occurred in four patients. All these perforations occurred in small aneurysms. Avoiding the immediate removal of the coil and/or microcatheter, systemic heparin reversal using protamine sulfate was performed. This procedure apparently prevented massive haemorrhage from the aneurysms in four patients, the outcome of whom was good or excellent. However, postoperative haemorrhage, which occurred in eight patients, resulted in a poor outcome.
Therefore, postoperative systemic heparinization for 12-18 hr on small-neck or incompletely embolized aneurysms was abandoned in the second half of this study, and deep sedation and systemic blood pressure control were continued at least overnight after embolization. After this protocol modification, no postoperative haemorrhage occurred.
The recanalization rate of large aneurysms was 38% (11/29), and all these aneurysms required additional embolization. However the recanalization rate of small aneurysms was 9% (12/130) and seven patients with these aneurysms underwent additional coil embolization or clipping surgery. These results are satisfactory in comparison with other major medical centers 8,9,10. Therefore, our protocol of 25-30% of VER as a goal of embolization may be reasonable at least for small ruptured aneurysms. The overall outcome after a six-month follow-up compared with the preoperative grade of the patients (figure 2A,B) indicates that our protocol, that is, endovascular treatment as the first choice treatment, is reasonable and useful for the treatment of ruptured aneurysms.
Under the protocol of endovascular surgery as the first-choice treatment, 66% of ruptured aneurysms were treated by coil embolization. Mase et Al11 reported that 62.8% of 35 ruptured aneurysms could be treated by coil embolization under the GDC first protocol. Although, unlike this report, we utilized the neck remodeling technique for wide-neck aneurysms of the internal carotid and basilar arteries, we found that 34% of the aneurysms still required clipping surgery.
On the other hand, almost 90% of the vertebro-basilar and anterior cerebral arteries aneurysms could be treated by coil embolization (figure 3). Because the prevention of psychomotor function is one of the most important issues in treating ruptured aneurysms, coil embolization may play an important part in treating ruptured anterior cerebral artery aneurysms.
Conclusions
66% of ruptured cerebral aneurysms could be treated by coil embolization under the protocol of endovascular surgery as the firstchoice treatment. In particular, in the vertebrobasilar and anterior cerebral arteries, almost 90% of the aneurysms could be treated by coil embolization. Because the tight packing of large aneurysms was difficult, the recanalization rate of large aneurysms was high. However, the results of small aneurysms were satisfactory. The timing of systemic heparinization is one of the most important factors in preventing perioperative haemorrhagic and ischemic complications.
References
- 1.Hunt E, Kosnik J. Timing and perioperative care in intracranial aneurysm surgery. Clin Neurosurg. 1974;21:79–89. doi: 10.1093/neurosurgery/21.cn_suppl_1.79. [DOI] [PubMed] [Google Scholar]
- 2.Debrun GM, Aletich VA, Kehrli P, et al. Selection of cerebral aneurysms for treatment using Guglielmi detachable coils: the preliminary University of Illinois at Chicago experience. Neurosurgery. 1998;43:1281–1295. doi: 10.1097/00006123-199812000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Satoh K, Matsubara S, et al. Intracranial aneurysm embolization using interlocking detachable coils. Interventional Neuroradiology. 1977;3(Suppl 2):125–128. doi: 10.1177/15910199970030S226. [DOI] [PubMed] [Google Scholar]
- 4.Moret J, Cognard C, et al. Reconstruction technic in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases. J Neuroradiol. 1997;24:30–44. [PubMed] [Google Scholar]
- 5.International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 6.Raymond J, Roy D, et al. Endovascular treatment of acutely ruptured and unruptured aneurysms of the basilar bifurcation. J Neurosurg. 1997;86:211–219. doi: 10.3171/jns.1997.86.2.0211. [DOI] [PubMed] [Google Scholar]
- 7.Cronqvist ME, Moret J. Detachable coil embolization of intracranial aneurysms. In: Connors JJ III, Wojak JC, editors. Interventional Neuroradiology. Saunders; 1999. pp. 294–316. [Google Scholar]
- 8.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475–482. doi: 10.3171/jns.1997.86.3.0475. [DOI] [PubMed] [Google Scholar]
- 9.Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysm. Neurosurgery. 1997;41:1235–1246. doi: 10.1097/00006123-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Murayama Y, Nien YL, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. 2003;98:959–966. doi: 10.3171/jns.2003.98.5.0959. [DOI] [PubMed] [Google Scholar]
- 11.Mase M, Yamada K, et al. Limitation of endovascular treatment for ruptured cerebral aneurysms: Results from the protocol "GDC as the first choice". Interventional Neuroradiology. 2000;6(Suppl 1):43–47. doi: 10.1177/15910199000060S104. [DOI] [PMC free article] [PubMed] [Google Scholar]