Summary
The purpose of this study was to evaluate the feasibility of photocrosslinkable chitosan as an embolization material for aneurysms. Three experimental aneurysms were created in three Japanese white rabbits. All of the aneurysms were packed with chitosan hydrogel.
Histopathologic data were analyzed on two, seven, and 30 days after embolization. Unorganized clots and minimal inflammation around the applied chitosan hydrogel were observed two days after implantation. After seven days, the chitosan was reduced and inflammatory response appeared. At 30 days, most of the aneurysm lumen was replaced with inflammatory cells, and the remaining chitosan was not observed. Severe complications such as anaphylaxis did not occur after the embolization with the chitosan. These results suggest that photocros-slinkable chitosan might be a candidate for an embolization material for endovascular treatment of cerebral aneurysms.
Key words: chitosan, embolization
Introduction
Current therapeutic management of cerebral aneurysms includes endovascular embolization as an alternative to standard surgical clipping. The Gudlielmi detachable coil has become the gold standard for the endovascular occlusion of ruptured or unruptured intracranial aneurysms worldwide1,2. A critical limitation of this system is a possibility of aneurysm recanalization, particularly in wide-necked or large/giant aneurysms1,3,4. Even in densely packed aneurysms, the cavities are filled with only 20% to 30% of platinum, with the remaining 70% to 80% filled with thrombus5.
Chitin is a linear homopolymer of ß(1,4)-linked N-acetyl-D-glucosamine, and chitosan is a partially deacetylated chitin. Chitin and chitosan have been proposed as biomaterials with a range of biomedical and industrial applications attributable to their biocompatibility 6. Chitosan was observed to accelerate wound healing 7-10 by inducing infiltration of inflammatory cells into the wound area9,10, activation of macrophages 11, and production of cytokines 12,13, and by its anti-infection activity. Application of ultraviolet (UV) irradiation to a photocrosslinkable chitosan (Az-CH-LA) aqueous solution resulted within 10 s in an insoluble, flexible hydrogel. The chitosan is a viscous solution, able to crosslink upon ultraviolet light (UV) irradiation. The chitosan hydrogel is a stronger tissue adhesive when compared with fibrin glue. It effectively seals air leakages from lung or trachea and completely stops bleeding from a pinhole of rabbit carotid artery within 10 s of UV irradiation 14. Furthermore, the gel has been found to induce wound contraction and healing15.
We previously reported the laser-dispensed ultraviolet light (UV) irradiation successfully prevented the development of experimental vasospasm of in the rabbit common carotid artery(CCA) model16,17. For possible clinical application, we also previously developed an optical fiber technique in which the artery is irradiated with UV light in a cylindrically symmetric pattern via an endovascular approach 17. Using the optical fiber, endovascular UV irradiation is able to convert the Az-CH-LA aqueous solution to an insoluble, flexible hydrogel. The purpose of this study was to evaluate the photocrosslinkable chitosan as an embolization material for aneurysms.
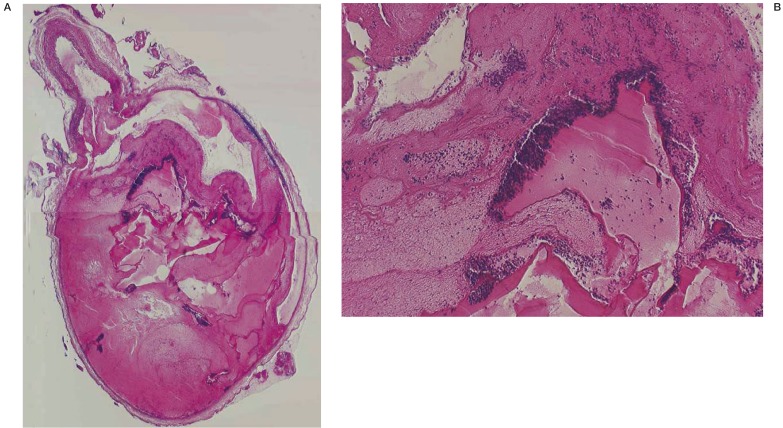
Figure 1.
A) Low-magnification light microscopy view of a chitosan-treated aneurysm two days after embolization (H&E, original magnification X4). B) High-magnification light microscopy view of a chitosan-treated aneurysm two days after embolization (H&E, original magnification X40). Unorganized clot and minimal inflammation was present around the chitosan hydrogel in the sac of the aneurysm treated with chitosan.
Methods
Photocrosslinkable chitosan molecules (Az-CH-LA) have been prepared as previously reported18. The chitosan used in this study had a molecular weight of 800 to approximately 1000 kDa, with a deacetylation ratio of 0.8 (Yaizu Suisankagaku Industry Co., Ltd., Shizuoka, Japan). For the first condensation reaction, chitosan (125 g) was added to 3L (pH = 4.75) of 50 mM of TEMED (N, N, N', N'-tetramethyleth-ylenediamine; Wako Pure Chemical Industry, Ltd., Japan) containing 56.25 mL of concentrated HCl. Then EDC [32.5 g, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide; Wako Pure Chemical Industry] and 4-O-ß-D-galactopyranosyl-(1,4)-D-gluconic acid (0.25 g of lactovionic acid; Wako Pure Chemical Industry) were added. This mixture was stirred at room temperature for 24h, followed by an ultrafiltration membrane passing unreacted substances below 10 kDa (Diaflo Ultrafilters YM10, Amicon, Ontario, Canada). Finally, a powder of lactose-linked chitosan (CH-LA) was obtained by freezedrying. It has been estimated by a conventional phenolsulfuric acid colormetric assay for carbohydrate that about 2% of amino groups in the chitosan molecule were replaced by lactobionic acid. Subsequently the product (CH-LA, 1 g), EDC (0.35 g), and 4-azidobenzoic acid (0.2 g; Tokyo Chemical Industry Co., Ltd., Japan) were added to 100mL of 50mM of the TEMED solution. The mixture was stirred at room temperature for 72h. Condensation and purification steps to obtain the photocrosslinkable chitosan (Az-CH-LA) were carried out as described above. It has been estimated by specific absorbance (275 nm) and NMR of FIIR spectrum of azidobenzoic acid (data not shown) that about 2.5% of amino groups in the chitosan molecule were replaced by azidbenzoic acid.
Azide (p-azidebenzoic acid) and lactose (lactobionic acid) moieties have been introduced through the condensation reaction with amino groups. The introduction of lactose resulted in a water-soluble chitosan at neutral pH values. It has been estimated that about 2.5 and 2.0% of the amino groups in the chitosan were substituted with p-azide benzoic acid and lactobionic acid, respectively. A viscous Az-CH-LA aqueous solution (30-5 mg/mL) has been converted into an insoluble hydrogel within 10 s upon UV irradiation at a lump distance of 2 cm through crosslinking of azide and amino groups of the Az-CH-LA molecules.
Three Japanese white rabbits of either sex, between 2.5 and 3 kg each, were used in this study. The experimental protocol was evaluated and approved by the University of Nagoya Animal and Ethics Review Committee. Anesthesia was induced with an intramuscular injection of 25 mg/kg sodium pentobarbital. Sodium pentobarbital solution was administered as a supplemental anesthesia as needed. The animals were allowed to breathe room air spontaneously. A longitudinal skin incision, measuring 4 cm in length, was made at the midline in the cervical area in each animal. Both common carotid artery (CCA) and ipsilateral internal jugular vein (IJV) in the animals were exposed and dissected free from any loose connective tissue. Small vascular clamps were then placed at each end of the isolated CCAs and IJVs to achieve temporary vessel occlusion. Using microsurgical technique, the CCAs and the IJVs were anastomosed side to side, and the insoluble hydrogel were inserted into the IJVs, and the both ends of the IJVs were ligated. Thus, experimental lateral aneurysms were embolized by the chitosan hydrogels. The animals were sacrificed on 2, 7, and 30 days by using a lethal injection of pentobarbital. The aneurysms were removed and placed in 10% neutral buffered formalin. To prepare samples for light microscopy, the aneurysms were embedded in paraffin. Next, the sections were stained with hematoxylin and eosin.
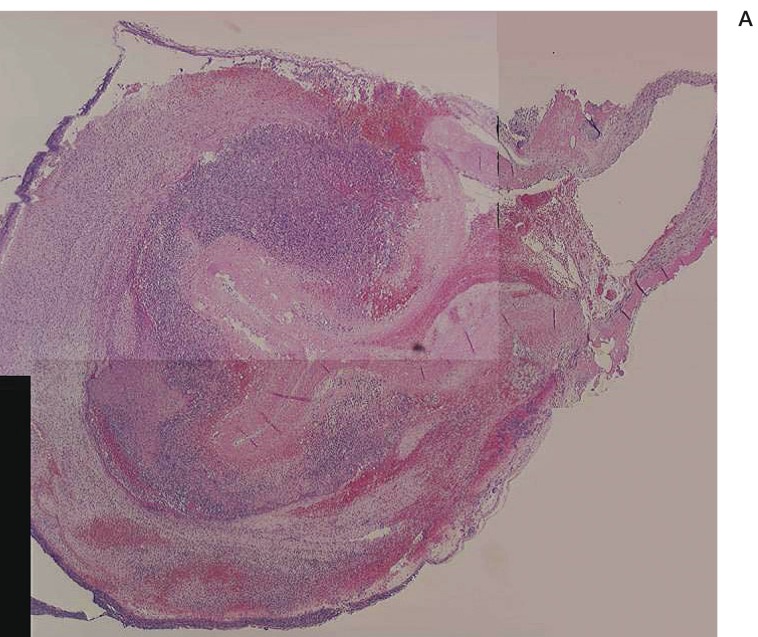
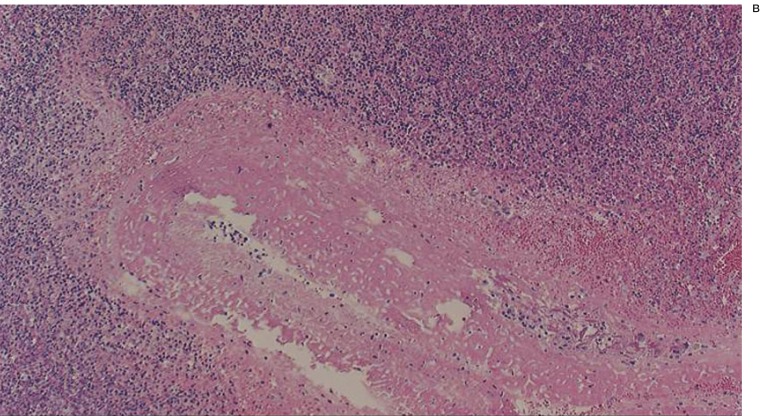
Figure 2.
A) Low-magnification light microscopy view of a chitosan-treated aneurysm seven days after embolization (H&E, original magnification X4). B) High-magnification light microscopy view of a chitosan-treated aneurysm seven days after embolization (H&E, original magnification X40). The volume of chitosan was reduced and moderate inflammatory reaction was present.
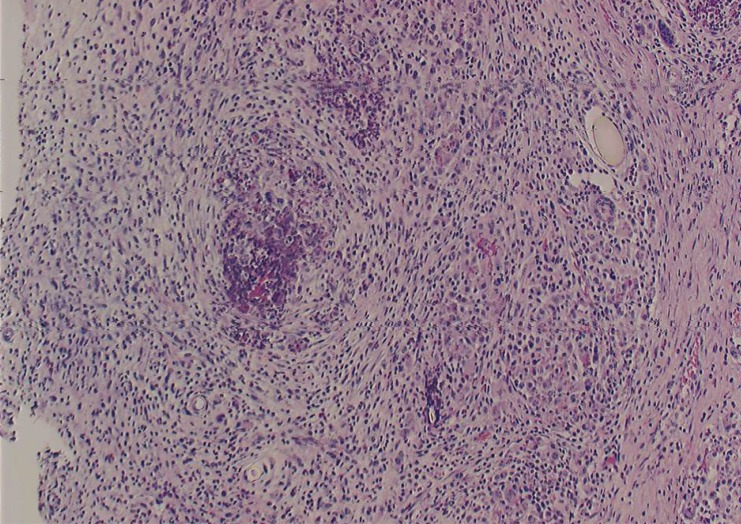
Figure 3.
A) High-magnification light microscopy view of a chitosan-treated aneurysm 30 days after embolization (H&E, original magnification X40). Most of the aneurysm lumen was replaced with inflammatory cells, and chitosan was almost absent.
Results
The severe complications such as anaphylaxis did not occur after packing of chitosan into the aneurysm lumen. The animal that died 2 days after implantation exhibited unorganized clot and minimal inflammation around the chitosan in the sac of the aneurysm treated with the chitosan. On seven days, chitosan was reduced and inflammatory response was increased compared with the findings on 2 days. On 30 days, most of the aneurysm lumen was replaced with inflammatory cells, and chitosan was almost absent.
Discussion
In this study, we showed feasibility of aneurysm embolization with photocrosslinkable and bioadsorbable chitosan hydrogel. A noticeable limitation of endovascular occlusion of an intracranial aneurysm with GDCs is the potential risk of aneurysm recanalization. Platinum GDCs produce a mild biological response when delivered into an aneurysm. Experimental and clinical reports of aneurysms embolized with GDCs have demonstrated a limited cellular reaction induced by platinum coils5,19,20. The presence of an unorganized clot within the aneurysm does not provide strong anatomic support for GDCs in the early phases of treatment. Controlled biological cellular response to the presence of intra-aneurysmal embolic material is another promising method that may improve acute and long-term morphological outcomes in aneurysms embolized with this type of embolic materials. Murayama et Al applied the concept of tissue engineering to aneurysm therapy using bioabsorbable polymeric material (BPM), and developed a newly designed hybrid platinum/BPM coil (Matrix). Though the use of Matrix accelerated aneurysm healing and might decrease and probably prevent aneurysmal recanalization after endovascular treatment of cerebral aneurysms, platinum coils are not bioabsorbable and remains permanently in the lumens of the aneurysms. Onyx (Micro Therapeutics, Inc., Irvine, CA, USA), which is a liquid embolic material designed for endovascular use, might produce occlusion rates that are significantly better than the reported endovascular occlusion rates achieved with existing coil devices. However, there is a significant incidence of delayed occlusion of the parent artery, when the material spilled into the parent artery, or when there was extensive reconstruction of the parent vessel in very wide-neck aneurysms 21. As chitosan has comparatively high viscosity, it might be less dangerous than a liquid material in spilling into the parent artery, while a system to deliver the chitosan to aneurysm lumen safely and easily should be required. Chitosan originates from crustacean such as shrimps and crabs, and is absorbed by phagocytic cells and aneurismal cavity is replaced with the inflamed tissue.
Chitosan has been proven to be safe in standard animal toxicologic studies. Chitosan has been used in food and skin wound management22. Azide residues may be toxic and highly reactive to tissue components, such as proteins. However, with a complete crossreaction of azide residues to amino groups, this toxic effect may disappear. Our previous results in mice also indicated that the Az-CH-LA aqueous solution and the chitosan hydrogel were found to be safe. Mice administered about 1200 mg/kg of the Az-CH-LA solution (n = 6) intraperitoneally all survived the entire one month observation 18. Cytotoxicity has not been observed with in vitro assays. In addition, preliminary toxicity tests of mutagenecity and cytotoxicity have shown the safety of both Az-CH-LA and its hydrogel (data not shown). Furthermore, subcutaneously implanted chitosan hydrogels were not detected at the site of implantation even after one month. These results suggest the safety of Az-CH-LA and chitosan gels in medical use. In order to fully evaluate its safety in practical use, however, the in vivo properties of chitosan gels, such as biodegradation, induction of inflammation, antigenicity, and adhesiveness to tissues, should be investigated in detail using appropriate animal models. However, sufficient data are not yet available on the complete toxicity profile of Az-CH-LA aqueous solutions and chitosan hydrogels in humans, and standard toxicologic studies should be performed before this chitosan hydrogel is used as an embolization material.
Ultraviolet light (UV) irradiation has immnosuppressive and/or anti-inflammatory properties. Inflammation has long been suspected to play an important role in the development of cerebral aneurysm after subarachnoid haemorrhage. On the basis of these observations, we hypothesized that irradiation with UV light could be one of the treatment modalities for cerebral vasospasm after subarachnoid haemorrhage, and we have previously reported the preventive effect of UV irradiation16,23. In order to anticipate possible clinical application, we developed an optical fiber technique in which the artery is irradiated with UV light in a cylindrically symmetric pattern via endovascular approach 17. The advantage of UV irradiation via endovascular approach is not only to convert a viscous aqueous solution into an insoluble hydrogel, but also to prevent the development of delayed vasospasm.
Conclusions
This preliminary animal study shows that the experimental aneurysm lumen embolized with photocrosslinkable chitosan hydrogel was replaced with products induced by inflammatory response. Chitosan might be feasible as an embolization material for endovascular treatment of cerebral aneurysms.
References
- 1.Murayama Y, Vinuela F, Tateshima S, et al. Cellular responses of bioabsorbable polymeric material and Guglielmi detachable coil in experimental aneurysms. Stroke. 2002;33:1120–1128. doi: 10.1161/01.str.0000014423.20476.ee. [DOI] [PubMed] [Google Scholar]
- 2.Byrne JV, Sohn MJ, %olyneux AJ, et al. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg. 1999;90:656–663. doi: 10.3171/jns.1999.90.4.0656. [DOI] [PubMed] [Google Scholar]
- 3.Hayakawa M, Murayama Y, Duckwiler GR, et al. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg. 2000;93:561–568. doi: 10.3171/jns.2000.93.4.0561. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez Zubillaga A, Guglielmi G, Vinuela F, et al. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatment results. Am J Neuroradiol. 1994;15:815–820. [PMC free article] [PubMed] [Google Scholar]
- 5.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg. 1999;91:284–293. doi: 10.3171/jns.1999.91.2.0284. [DOI] [PubMed] [Google Scholar]
- 6.Chandy T, Sharma CP. Chitosan--as a biomaterial. Biomater Artif Cells Artif Organs. 1990;18:1–24. doi: 10.3109/10731199009117286. [DOI] [PubMed] [Google Scholar]
- 7.Cho YW, Cho YN, Chung SH, et al. Water-soluble chitin as a wound healing accelerator. Biomaterials. 1999;20:2139–2145. doi: 10.1016/s0142-9612(99)00116-7. [DOI] [PubMed] [Google Scholar]
- 8.Prudden JF, Migel P, Hanson P, et al. The discovery of a potent pure chemical wound-healing accelerator. Am J Surg. 1970;119:560–564. doi: 10.1016/0002-9610(70)90175-3. [DOI] [PubMed] [Google Scholar]
- 9.Ueno H, Yamada H, Tanaka I, et al. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials. 1999;20:1407–1414. doi: 10.1016/s0142-9612(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto Y, Shibazaki K, Minami S, et al. Evaluation of chitin and chitosan on open would healing in dogs. J Vet Med Sci. 1995;57:851–854. doi: 10.1292/jvms.57.851. [DOI] [PubMed] [Google Scholar]
- 11.Peluso G, Petillo O, Ranieri M, et al. Chitosan-mediated stimulation of macrophage function. Biomaterials. 1994;15:1215–1220. doi: 10.1016/0142-9612(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 12.Mori T, Okumura M, Matsuura M, et al. Effects of chitin and its derivatives on the proliferation and cytokine production of fibroblasts in vitro. Biomaterials. 1997;18:947–951. doi: 10.1016/s0142-9612(97)00017-3. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura K, Ishihara C, Ukei S, et al. Stimulation of cytokine production in mice using deacetylated chitin. Vaccine. 1986;4:151–156. doi: 10.1016/0264-410x(86)90002-2. [DOI] [PubMed] [Google Scholar]
- 14.Ono K, Ishihara M, Ozeki Y, et al. Experimental evaluation of photocrosslinkable chitosan as a biologic adhesive with surgical applications. Surgery. 2001;130:844–850. doi: 10.1067/msy.2001.117197. [DOI] [PubMed] [Google Scholar]
- 15.Ishihara M, Nakanishi K, Ono K, et al. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials. 2002;23:833–840. doi: 10.1016/s0142-9612(01)00189-2. [DOI] [PubMed] [Google Scholar]
- 16.Nakai K, Morimoto Y, Wada K, et al. Pretreatment with continuous-wave ultraviolet irradiation to prevent the development of delayed vasospasm in the rabbit common carotid artery model. J Neurosurg. 2000;92:671–675. doi: 10.3171/jns.2000.92.4.0671. [DOI] [PubMed] [Google Scholar]
- 17.Nakai K, Numaguchi Y, Foster TH, et al. Endovascular treatment using low-power ultraviolet laser for delayed vasospasm in the rabbit carotid artery model. Am J Neuroradiol. 2002;23:1725–1731. [PMC free article] [PubMed] [Google Scholar]
- 18.Ono K, Saito Y, Yura H, et al. Photocrosslinkable chitosan as a biological adhesive. J Biomed Mater Res. 2000;49:289–295. doi: 10.1002/(sici)1097-4636(200002)49:2<289::aid-jbm18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Molyneux AJ, Ellison DW, Morris J, et al. Histological findings in giant aneurysms treated with Guglielmi detachable coils. Report of two cases with autopsy correlation. J Neurosurg. 1995;83:129–132. doi: 10.3171/jns.1995.83.1.0129. [DOI] [PubMed] [Google Scholar]
- 20.Mawad ME, Mawad JK, Cartwright J, Jr., et al. Longterm histopathologic changes in canine aneurysms embolized with Guglielmi detachable coils. Am J Neuroradiol. 1995;16:7–13. [PMC free article] [PubMed] [Google Scholar]
- 21.Molyneux AJ, Cekirge S, Saatci I, et al. Cerebral Aneurysm Multicenter European Onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. Am J Neuroradiol. 2004;25:39–51. [PMC free article] [PubMed] [Google Scholar]
- 22.Rao SB, Sharma CP. Use of chitosan as a biomaterial: studies on its safety and haemostatic potential. J Biomed Mater Res. 1997;34:21–28. doi: 10.1002/(sici)1097-4636(199701)34:1<21::aid-jbm4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 23.Nakai K, Morimoto Y, Kikuchi M, et al. Inhibition of experimental vasospasm by pretreatment with ultraviolet light irradiation in a rat femoral artery model. Neurosurgery. 2001;48:1318–1327. doi: 10.1097/00006123-200106000-00028. [DOI] [PubMed] [Google Scholar]