Background: Cellular invasion is regulated by expression of COX-2 gene.
Results: VRK2 directly phosphorylates NFAT1 and promotes expression of COX-2, facilitating cellular invasion.
Conclusion: VRK2 hyperactivates NFAT1, activates COX-2 expression, and increases cellular invasion by tumor cells.
Implications: VRK2 forms part of a novel pathway regulating cellular invasion that might be targeted in cancer and immunosuppression.
Keywords: Cancer, Cell Invasion, Serine Threonine Protein Kinase, Transcription Factors, Tumor Metastases
Abstract
Human VRK2 (vaccinia-related kinase 2), a kinase that emerged late in evolution, affects different signaling pathways, and some carcinomas express high levels of VRK2. Invasion by cancer cells has been associated with NFAT1 (nuclear factor of activated T cells) activation and expression of the COX-2 (cyclooxygenase 2) gene. We hypothesized that VRK proteins might play a regulatory role in NFAT1 activation in tumor cells. We demonstrate that VRK2 directly interacts and phosphorylates NFAT1 in Ser-32 within its N-terminal transactivation domain. VRK2 increases NFAT1-dependent transcription by phosphorylation, and this effect is only detected following cell phorbol 12-myristate 13-acetate and ionomycin stimulation and calcineurin activation. This NFAT1 hyperactivation by VRK2 increases COX-2 gene expression through the proximal NFAT1 binding site in the COX-2 gene promoter. Furthermore, VRK2A down-regulation by RNA interference reduces COX-2 expression at transcriptional and protein levels. Therefore, VRK2 down-regulation reduces cell invasion by tumor cells, such as MDA-MB-231 and MDA-MB-435, upon stimulation with phorbol 12-myristate 13-acetate plus ionomycin. These findings identify the first reported target and function of human VRK2 as an active kinase playing a role in regulation of cancer cell invasion through the NFAT pathway and COX-2 expression.
Introduction
Cell invasion is one of the most clinically relevant biological characteristic of cancer cells. The nuclear factor of activated T cells (NFAT)3 pathway is functional in many cell types affecting different processes, such as metastasis, invasion (1, 2), proliferation (2–4), and angiogenesis. All of these processes are characteristic of the cancer phenotype (5).
Recently, a new subfamily of serine-threonine kinases was identified in the human kinome (6), known as vaccinia-related kinase (VRK) (7–9). These kinases have appeared late in evolution (10), and their target proteins are likely to be those that are already present in the cell and participate in different signaling pathways. Thus, the main role of these late kinases is probably to add an additional layer of control in complex organisms. VRK genes are expressed in many different cell types, and their protein levels are higher in proliferating cells (7, 11, 12). The best characterized member is VRK1 (13), which plays a role in the regulation of cell cycle initiation and behaves as an early response gene like c-myc and c-fos (13, 14). VRK1 is also involved in cellular stress and DNA damage responses mediated by p53 (8, 15–17), forming an autoregulatory loop (18, 19), and phosphorylates c-Jun (20), ATF-2 (21), and CREB1 (22). The serine-threonine kinase VRK2 is correlated with high levels of estrogen and progesterone receptors, whereas it is inversely correlated with ERBB2 in human breast cancers (23). VRK2 has two isoforms: VRK2A (VRK2), composed of 508 amino acids and localized in the cytoplasm anchored to the endoplasmic reticulum (24), and VRK2B, a shorter isoform generated by alternative splicing, which can partially replace VRK1 in the nucleus (24). VRK2A can modulate signaling pathways that are assembled on the JIP1 scaffold protein by a direct and stable interaction, independently of its kinase activity (25, 26). VRK2A interaction with JIP1 is able to reduce the stress response to hypoxia (25) and to interleukin-1β (26). Also, VRK2A directly interacts with the KSR1 scaffold protein and modulates the EGF-ERBB2-RAF-RAS signaling pathway (23, 27). Thus, VRK2 can alter the balance among the different pathways responding to a common stimulation.
NFAT1 is located in the cytosol in an inactive hyperphosphorylated state. Upon cell stimulation, there is an increase of intracellular calcium that activates the phosphatase calcineurin, which dephosphorylates NFAT1 (28–31). Then NFAT1 translocates to the nucleus (32), where it regulates gene transcription (33). Phosphorylation is a key process in the regulation of NFAT1 activity, so it is of interest to identify what kinases are implicated in the modulation of NFAT pathway. Calcineurin interacts with its inhibitor RCAN1 (MCIP1 or DSCR1) in the cytosol (34–36), and after stimulation RCAN1 can be phosphorylated by kinases, such as MEKK3 (34, 37). RCAN1 phosphorylation disrupts its interaction with calcineurin (34, 38) and the inhibitory effect on its phosphatase activity. In this way, kinases that phosphorylate RCAN1 indirectly regulate NFAT1 activation. The activated and dephosphorylated NFAT1 is translocated to the nucleus, but this activation can be further enhanced by additional phosphorylation in its N-terminal domain (39–41). Kinases that have been reported to mediate this phosphorylation are JNK (42), Cot/Tpl2 (39, 40), and PKC (43). Other kinases, such as CK1 (44), GSK3 (45), and DYRK (5), are involved in maintaining NFAT1 in the hyperphosphorylated state in the cytosol or rephosphorylating nuclear NFAT1 to inactivate it and export it to the cytoplasm.
The COX-2 gene promoter is regulated by NFAT1. In basal conditions, COX-2 expression is low in most cells, but it is induced by several stimuli. Distal and proximal NFAT1 response elements identified in the COX-2 promoter region are required for induction of COX-2 expression upon cell stimulation and suggest a role of NFAT1-induced COX-2 expression in tumor cells (46, 47). NFAT1 plays a significant role in promoting migration and invasion of breast and colon carcinoma cells (48, 49), and up-regulation of the COX-2, an important NFAT1 target gene, has been involved in cell invasion in a study of the gene expression profile of breast cancer cells expressing NFAT1 (50). It has been reported that invasion of breast cancer cells is associated with COX-2 expression at different stages of breast cancer development (51), and COX-2 is a marker of poorer prognosis (52). Also, COX-2 expression has been associated with breast cancer metastasis in bones and in the brain (5, 53, 54).
In this work, we have analyzed the role of VRK2 in the regulation of COX-2 expression in tumor cell lines, because VRK2 can identify two major groups in human breast cancer cells (23), and the contribution of this kinase to cell invasion potential. We have demonstrated that VRK2A induces COX-2 gene expression by a direct NFAT1 phosphorylation. Together, these results identified the first functional role of VRK2A as an active kinase by demonstrating its implication in regulation of cancer cell invasion through phosphorylation and activation of NFAT1-dependent COX-2 transcription. Therefore, these data detect a new regulatory component of the VRK2 pathway in higher eukaryotes and contribute to build the signaling network in which VRK2 participates.
EXPERIMENTAL PROCEDURES
Plasmids
HA-VRK2A, HA-VRK2B (24), and HA-VRK1 (16) were generated by cloning in pCEFL vector. GST-VRK2A wild type and the inactive kinase GST-VRK2AK169E, GST-VRK2B (24), and GST-VRK1 (8) were generated by cloning in pGEX4T1 vector for bacterial expression. The NFAT-Luc reporter plasmid containing three tandem copies of the distal NFAT1 site of the human IL-2 promoter fused to the minimal human IL-2 promoter and the full-length murine NFAT1-HA cloned in pEFBOS plasmid were kindly provided by Dr. J. M. Redondo (42). The GST-NFAT 4–57, GST-NFAT 4–68 and GST-NFAT 4–384 constructs were kindly provided by Dr. M López-Cabrera (43). The pCS2+MT-Myc-RCAN1 plasmid was used for eukaryotic expression, and the pCEFL-FLAG-MEK5-CA was kindly provided by Dr. A. Pandiella. The human COX-2 promoter constructs cloned in pXP2Luc reporter plasmid were kindly provided by Dr. M. Fresno (47).
Protein Expression and Purification
GST-VRK2, GST-VRK1, and GST-NFAT1 fusion proteins were expressed in Escherichia coli cells and purified using glutathione-Sepharose beads (GE Healthcare). Then fusion proteins were eluted with glutathione at 20 mm and stored at −20 °C (24, 55).
Cell Lines and Transfections
MDA-MB-435, MDA-MB-231, and HEK293T cells were growth in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum. All cell lines were obtained from the ATCC. Transfections were performed using JetPEI reagent (Polyplus, Illkirch, France) or Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The total amount of DNA was kept constant by completion with the corresponding empty vector (25, 26).
Luciferase Assay
Transcriptional activity was measured by luciferase reporter gene assays in the transiently transfected MDA-MB-435 cell line. Cells were cotransfected with 1 μg of the correspondent reporter plasmid and the indicated plasmids in each experiment. After 24 h, cells were stimulated with 100 ng/ml PMA (Sigma) and 0.5 μm ionomycin (Io) (Sigma) or left without stimulation for an additional 16 h. Then cells were harvested and lysed. Luciferase activity was determined by using a luciferase assay kit (Promega) with a luminometer Lumat LB 9507 (Berthold Technologies). Luciferase experiments were performed in triplicate, and the data presented are the mean of the determinations in relative activity -fold increase ± S.D. A representative immunoblot from each experiment is shown.
Immunoprecipitation and in Vitro Kinase Assay
HA-NFAT1 or Myc-RCAN1 plasmids were transfected in HEK293T cells, and proteins were immunoprecipitated using α-HA monoclonal antibody (Covance) and α-Myc polyclonal antibody (Upstate Biotechnology, Inc. (Lake Placid, NY)), respectively. Immunoprecipitates were washed and used for an in vitro kinase assay with 1 μg of GST-VRK2 in a final volume of 40 μl containing kinase buffer (20 mm Tris-HCl, pH 7.5, 5 mm MgCl2, 0,5 mm DTT, and 150 mm KCl), 5 μm ATP, and 5 μCi of [γ-32P]ATP. Purified GST-NFAT constructs and GST-VRK proteins were used for in vitro kinase assays in a final volume of 30 μl in the same conditions described previously (24, 55). The reactions were performed at 30 °C for 30 min and stopped by boiling in Laemmli buffer. The phosphorylated proteins were analyzed in 10% SDS-PAGE. Gels were stained with Coomassie Blue or transferred to Immobilon-P membrane (Millipore), and the incorporated radioactivity was detected.
Quantitative RT-PCR and Immunoblot
MDA-MB-231 and MDA-MB-435 cell lines were transfected with 200 nm specific siRNA to knock down VRK2 (siVRK2-06 (5′-GCAAGGUUCUGGAUGAUAU-3′; Dharmacon) or siVRK2-M (5′-GAUAUUGUCCCAAUGGGAA-3′; Mission series, Sigma)), and COX-2 mRNA and protein levels were determined. For quantitative RT-PCR analysis, cells were transfected with si-VRK2–06 or control siRNA, and after 3 days, cells were stimulated with 100 ng/ml PMA and 0.5 μm Io for 6 h or left without stimulation. Total RNA was extracted using the RNeasy extraction kit from Qiagen (Hilden, Germany). RNA was analyzed and quantified using the Bioanalyzer 2100 nanolab chip from Agilent Technologies (Germany), and 100 ng of total RNA were used in a one-step reverse transcription real-time PCR amplification reaction using the quantitative SYBR Green RT-PCR kit from Qiagen in an iCycler (Bio-Rad). The reaction was analyzed with iCycler software (Bio-Rad), and mRNA relative levels were represented as a mean of three independent experiments ± S.D. The following primers were used for human mRNA detection: human VRK2 (VRK2TA, 5′-AGTGAGAGAAGCGCTGAGTCCT-3′; VRK2TB, 5′-CAAAGGTTCTTGAGACTCTTG-3′), human COX-2 (COX-2F, 5′-CAAAAGCTGGGAAGCCTTCTCTAA-3′; COX-2R, 5′-GCCCAGCCCGTTGGTGAAAG-3′), human NFAT1 (hNFAT1-F, 5′-TGCATCTAACCCCATCGAGTG-3′; hNFAT1-R, 5′-TGAGGATCATTTGCTGGCC-3′). Human GAPDH amplification was used as internal control (GAPDH5′, 5′-GGTCTTACTCCTTGGAGGCCATGTG-3′; GAPDH3′, 5′-ACCTAACTACATGGTTTACATGTT-3′).
For Western blot analysis, cells were transfected with siVRK2 or control siRNA, and 3 days post-transfection, cells were stimulated with PMA plus ionomycin for 30 min and incubated for an additional 24 h. Cells were lysed with lysis buffer (50 mm HEPES, pH 7.5, 4 mm EDTA, 150 mm NaCl, 1,5 mm MgCl2, 10 mm NaF, 1% Triton-X-100, 0.1% SDS, and 10% glycerol and protease and phosphatase inhibitors), and 30 μg were loaded in a 7.5% or 10% PAGE. Proteins were transferred to an Immobilon-P membrane (Millipore), blocked in TBS-T buffer with 5% nonfat milk, and then incubated with the indicated specific antibody. Bands were detected using a chemiluminescence ECL kit (GE Healthcare).
Antibodies
VRK2 was detected with a rabbit polyclonal antibody (24), COX-2 with a monoclonal antibody from R&D Systems (Minneapolis, MN) and NFAT1 with a monoclonal antibody from BD Biosciences. Myc epitope was detected with monoclonal or polyclonal antibodies from Upstate Biotechnology, Inc. FLAG epitope was detected with polyclonal or monoclonal antibodies from Sigma. HA epitope was detected with a monoclonal antibody from Covance (Emeryville, CA), and β-actin was detected with a monoclonal antibody from Sigma.
Wound Healing and Matrigel Invasion Assays
Wound healing assays were performed with MDA-MB-231 cells transfected in p60 dishes with control siRNA or siVRK2–06. After 3 days, each dish was divided into 6-well plates, and cells were allowed to reach confluent monolayers. Cells were stimulated with PMA and Io for 30 min, and then a straight scratch was made in individual wells with a yellow pipette tip. Cells were washed with PBS, and then fresh medium was placed. Cells were allowed to grow, and wound size was photographed (×10 magnification) under the microscope at several time points using a digital camera (Canon PowerShot A640) connected to inverted microscope (Zeiss Axiovert 25). Measurements of wound width in each point of time were made in three different experiments, and the results were represented in graphs.
Invasion assays were performed in transwell chambers with 8-μm pore filters (BD Biosciences) coated with 1 mg/ml Matrigel (BD Biosciences). Cells were transfected with RNA interference for 3 days and were then stimulated or not for 30 min with PMA/ionomycin. Cells were harvested, and 50,000 cells were resuspended in serum-free medium to the upper chamber of the transwell. Medium with 10% FBS was added in the lower chamber, and cells were allowed to invade the Matrigel-coated filters at 37 °C. After 42 h, cells that had not invaded were removed with a cotton swab, and invasive cells were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. Invasive cells were photographed using a digital camera (Canon PowerShot A640) connected to an inverted microscope (Zeiss Axiovert 25) and counted using ImageJ version 1.45 (Wayne Rasband, National Institutes of Health). They were represented as the percentage of invasive cells mean ± S.D. of three independent experiments.
RESULTS
VRK2A Enhanced NFAT1 Transcriptional Activity after PMA/Io Stimulation in MDA-MB-435 Cells
Cell invasion is a malignant characteristic common to different types of carcinomas (56–58), lymphomas, or melanomas (50, 59). Tumor cell invasion is associated with COX-2 expression that is regulated by the NFAT1 transcription factor (50). Therefore, we studied if VRK2A might be regulating the NFAT1 transcriptional activity. For this aim, we determined the effect of VRK2A on the NFAT transcriptional response, performing a luciferase reporter assay in the MDA-MB-435 cell line. VRK2A increased NFAT-dependent transcription in a dose-dependent manner, but this effect was only detected in cells stimulated with PMA/ionomycin (Fig. 1A), indicating that the activation is secondary to the PMA/Io stimulation. Next, we determined if other members of the VRK family could also induce the same effect. NFAT1 activity increased with cytosolic VRK2A, whereas there was a lower effect with the free VRK2B isoform that is located in both nucleus and cytosol. The nuclear VRK1 has no effect on NFAT1 transcriptional activity. In the case of both VRK2 isoforms, the effect on NFAT1 activity required PMA/Io stimulation (Fig. 1B).
FIGURE 1.
VRK2A potentiated NFAT1-dependent transcription after PMA and ionomycin stimulation in MDA-MB-435 cells. A, melanoma MDA-MB-435 cells were transfected with NFAT-Luc reporter plasmid and increasing amounts of HA-VRK2A. After 24 h, cells were treated with 100 ng/μl PMA plus 0.5 μm Io or left without stimulation, and luciferase activity was measured after 16 h. B, MDA-MB-435 cells were transfected with NFAT-Luc reporter plasmid and each of the three VRK proteins, HA-VRK2A, HA-VRK2B, and HA-VRK1. After 24 h, cells were treated with 100 ng/μl PMA plus 0.5 μm Io for 16 h or left without stimulation, and luciferase activity was measured. Results are the mean of triplicate measurements of at least three independent experiments. The values were compared with the mock control. **, p < 0.005; ***, p < 0.0005. The expression of the transfected VRK proteins detected in Western blots is shown at the bottom. Error bars, S.D.
VRK2 also potentiated NFAT1 activity following PMA and Io stimulation in Jurkat cells (supplemental Fig. S1). This result suggests that the effect of VRK2A on NFAT1 activity is independent of cell type.
VRK2 in Vitro Phosphorylation of NFAT1 N-terminal Transactivation Domain on Serine 32
To determine if VRK2 was acting by either phosphorylation or interaction with NFAT1, we first performed in vitro kinase assays with GST-VRK2A and GST-VRK2B fusion proteins in the presence of radiolabeled ATP. The full-length NFAT1 protein tagged with HA was immunoprecipitated from HEK293T and used as substrate. Autoradiography revealed that both VRK2 isoforms phosphorylated NFAT1 (Fig. 2A). Next, we determined the region of NFAT1 phosphorylated by VRK2A, performing an in vitro kinase assay using different recombinant constructs containing the N-terminal region of NFAT1 fused to GST. Two of them (GST-NFAT1 4–57 and GST-NFAT1 4–68) involved only the N-terminal transactivation domain, and the last one (GST-NFAT1 4–384) also contained the regulatory domain. As shown in Fig. 2B, all of the NFAT1 fragments were phosphorylated by VRK2A. The kinase-dead VRK2(K169E) was used as a negative control, and it was not able to phosphorylate NFAT1. These data demonstrate that VRK2A phosphorylates NFAT1 in its N-terminal transactivation domain, between residues 4 and 57, although we cannot rule out that other residues could also be phosphorylated. Therefore, we performed in vitro kinase assays with several GST-NFAT1(4–68) mutants as substrate to determine the residue phosphorylated by VRK2A. As observed in Fig. 2C, only serine 32 mutation of NFAT1 showed a lower phosphorylation by VRK2A, whereas serine 53, 54, and 56 mutations did not show differences in phosphorylation. Serines 53, 54, and 56 had been reported as phosphorylation sites enhancing NFAT1 transactivation (43), but there are no previous data about serine 32. Luciferase assays showed that only the two VRK2 isoforms enhanced NFAT1 activity. We decided to determine if VRK2 was the only VRK member able to phosphorylate NFAT1. GST-VRK2A, GST-VRK2B, and GST-VRK1 fusion proteins were used for an in vitro kinase assay with the shortest NFAT1 construct (NFAT1 4–57) as substrate. As we expected, both VRK2 isoforms, but not VRK1, phosphorylated the NFAT1 construct (Fig. 2D). These results suggest that VRK2A could increase NFAT1 activity by a direct phosphorylation mechanism.
FIGURE 2.
NFAT1 phosphorylation by both VRK2 isoforms. A, VRK2A and VRK2B in vitro kinase assay with immunoprecipitated HA-NFAT1 from transfected 293T cells as substrate. The lysate used to prepare the substrate is shown at the top. The kinase assay with either GST-VRK2A or VRK2B is shown at the bottom. B, in vitro kinase assay with GST-VRK2A and its kinase-dead mutant VRK2(K169E), using different GST-NFAT1 constructs from its N-terminal domain as substrate. Top, kinase assay; bottom, stained gel. C, in vitro kinase assay with GST-VRK2A using different GST-NFAT1 4–68 mutants. Top, kinase assay; bottom, stained gel. D, in vitro kinase assay with VRK2A, VRK2B, and VRK1 fusion proteins and GST-NFAT1 4–57 as substrate. Top, kinase assay; bottom, stained gel. IP, immunoprecipitation; WB, Western blot.
Interaction between VRK Proteins and NFAT1
Next, it was determined if there was a direct interaction between VRK and NFAT1 proteins. For this aim, we performed a pull-down assay with GST-VRK and HA-NFAT1 proteins overexpressed in HEK293T cells. VRK2A and VRK2B, but not VRK1, were able to directly interact with NFAT1 (Fig. 3A). This interaction was also determined by immunoprecipitation of HA-NFAT1, and only VRK2A and VRK2B interacted with NFAT1 (Fig. 3B). Moreover, different VRK2A constructs fused to GST and HA-NFAT1 were used in a pull-down assay to determine the region of VRK2 interacting with NFAT1. The N-terminal domain of VRK2 between residues 1 and 320 interacted with NFAT1 (Fig. 3C), consistent with the interaction also seen in the case of VRK2B, because the two VRK2 isoforms have an identical sequence up to amino acid 364 (24). In addition, the region of NFAT1 interacting with endogenous VRK2A was mapped in an in vitro pull-down assay using NFAT1 constructs fused to GST. This NFAT1 interaction region was located between residues 68 and 384 (Fig. 3D).
FIGURE 3.
VRK2 and NFAT1 interaction. A, pull-down assay with GST-VRK2A, GST-VRK2B, and GST-VRK1 proteins showed interaction with full-length HA-NFAT1 protein. The result of the pull-down is shown at the top, and the result of the input lysate is shown at the bottom. B, immunoprecipitation of full-length HA-NFAT1 protein and its interaction with GST-VRK2A and GST-VRK2B but not with VRK1. Bottom, input lysate; top, immunoprecipitation. C, pull-down assay with different constructs of VRK2A and full-length HA-NFAT1. The N-terminal domain of VRK2A interacted with NFAT1. D, mapping the interaction region in NFAT1. An in vitro pull-down assay with different GST-NFAT1 constructs and MDA-MB-231 cell lysate was performed. The region between amino acids 68 and 384 of NFAT1 interacted with endogenous VRK2A. IP, immunoprecipitation.
VRK2A Did Not Increase NFAT1 Activity by an Indirect Mechanism Involving RCAN1 Phosphorylation
RCAN1 is a calcineurin-interacting protein that has an inhibitory role in calcineurin activity (36). It has been reported that RCAN1 phosphorylation by MAPK proteins disrupts its interaction with calcineurin, increasing calcineurin activity and NFAT1 activation (34). We tested if VRK2A might also indirectly contribute to regulate NFAT1 activity through a similar mechanism. First, we performed an in vitro kinase assay with recombinant GST-VRK2A fusion protein and immunoprecipitated Myc-RCAN1 from HEK293T cells as substrate. Immunoprecipitated HA-NFAT1 was used as positive control. As shown in Fig. 4A, VRK2A did not phosphorylate RCAN1, but it phosphorylated NFAT1.
FIGURE 4.
VRK2A did not phosphorylate RCAN1 and did not reverse its inhibitory effect on NFAT1 activity. A, in vitro kinase assay of VRK2A with Myc-RCAN1 immunoprecipitated from 293T cells as substrate. Immunoprecipitated HA-NFAT1 protein was used as a control of VRK2A phosphorylation substrate. B, analysis of NFAT-Luc activity in MDA-MB-435 cells transfected with Myc-RCAN1 and/or HA-VRK2A and stimulated or not with 100 ng/ml PMA plus 0.5 μm Io. FLAG-MEK5 CA was used as a positive control reversing RCAN1-dependent NFAT inhibition. Western blot of cell extracts with α-Myc antibody (Millipore) revealed that phospho-RCAN1 was not affected by VRK2A but was increased by MEK5 CA-positive control. Luciferase results are the mean of triplicate measurements of three independent experiments. *, p < 0.05; ***, p < 0.0005. IP, immunoprecipitation. Error bars, S.D.
Furthermore, to rule out an indirect effect of VRK2A on the NFAT1 pathway through RCAN1, we performed luciferase assays with the reporter plasmid NFAT-Luc in MDA-MB-435 cells. RCAN1 overexpression inhibited NFAT1 activation induced by PMA/Io, and VRK2A overexpression enhanced NFAT activity, as we expected. But when both plasmids were transfected together, VRK2A was not able to counteract the inhibitory effect of RCAN1 (Fig. 4B, top). However, MEK5 CA (constitutively active) counteracted the inhibitory effect of RCAN1, and NFAT activation was observed in this positive control. Therefore, phospho-RCAN1 presented different electrophoretic mobility and was detected by Western blot. Phospho-RCAN1 levels were not affected in the presence of VRK2A, but phospho-RCAN1 levels increased in the presence of MEK5 CA (Fig. 4B, bottom).
VRK2 Silencing Decreased COX-2 Gene Expression
We decided to study the effect of VRK2A down-regulation with a specific siRNA on COX-2 mRNA levels after PMA plus Io stimulation by performing quantitative real-time PCRs. COX-2 is an inducible gene, whose mRNA level is very low in non-stimulated cells, but after cell stimulation with PMA plus Io, COX-2 mRNA levels are increased. We transfected breast cancer MDA-MB-231 cells, which have high levels of endogenous NFAT1 and are an invasive cell line (50), with VRK2-specific siRNA, siVRK2-06, for 3 days, and we observed a reduction of COX-2 mRNA levels after PMA plus Io stimulation (Fig. 5A). The same results were observed in the MDA-MB-435 cell line (Fig. 5B). VRK2 knockdown had no effect on NFAT1 mRNA levels in either of these two cell lines (Fig. 5C). COX-2 mRNA was also reduced using a different siRNA for VRK2 (siVRK2-M) either in MDA-MB-231 cells (supplemental Fig. S2A) or in MDA-MB-435 cells (supplemental Fig. S2B).
FIGURE 5.
VRK2 silencing decreased COX-2 mRNA levels and COX-2 expression. A, total RNA from MDA-MB-231 breast cancer cells transfected with control siRNA (siC) or VRK2-specific siRNA (siVRK2-06) and stimulated or not with 100 ng/μl PMA and 0.5 μm Io was used for quantitative RT-PCR analysis to measure COX-2 and VRK2 mRNA levels. Data were normalized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels, and results are the mean of three independent experiments. **, p < 0.005; ***, p < 0.0005. B, total RNA from MDA-MB-435 melanoma cells treated as in A. ***, p < 0.0005. C, VRK2 knockdown by specific siRNA transfection did not affect NFAT1 mRNA levels in MDA-MB-435 and MDA-MB-231 cell lines. D, COX-2 protein expression decreased in MDA-MB-231 cells with reduced VRK2 protein levels. NFAT1 protein levels were not affected by VRK2 silencing. At the right is shown the quantification of protein levels. Error bars, S.D.
We also determined COX-2 protein levels in MDA-MB-231 cells stimulated or not with PMA/Io after VRK2 knockdown by siRNA transfection. In basal conditions, there was no detectable COX-2 protein expression, but after PMA/Io stimulation, there was an increase in COX-2 protein expression. When VRK2 was down-regulated, COX-2 protein levels decreased, but NFAT1 protein levels were not affected (Fig. 5D).
VRK2A Up-regulated COX-2 Transcription through NFAT Response Elements in the COX-2 Promoter
Because VRK2A increased NFAT1 transactivation activity, we tested if NFAT1 activation might be able to mediate NFAT1-dependent transcription in MDA-MB-435 cells. COX-2 is an NFAT1 target gene, so, we analyzed COX-2 promoter activity. For this aim, we performed luciferase assays with the reporter plasmid p2–274, which contains two NFAT1 binding sites: distal (dNFAT) and proximal (pNFAT) (47). VRK2A overexpression enhanced the transcriptional activity of the COX-2 gene promoter. This effect was not observed with either inactive kinase VRK2A(K169E) or VRK1 overexpression (Fig. 6A). Also, the increase in COX-2 promoter transcription induced by VRK2A was reduced in cells treated with the calcineurin inhibitor cyclosporin A (Fig. 6B, CsA). Cyclosporin A is a compound that inhibits calcineurin activity and consequently inhibits NFAT1 activation. We also carried out luciferase assays with the reporter plasmid p2–274, containing one or both NFAT binding sites mutated. As shown in Fig. 6C, VRK2A-increased transcription was significantly reduced when only the proximal NFAT1 binding site was mutated. Together, these results indicate that the effect of VRK2A on COX-2 transcription is NFAT1-dependent. Furthermore, we decided to perform the same experiments in a colon cancer cell line, because an increase in COX-2 promoter activity through activation of NFAT1 has been reported (46), and it is also known that COX-2 is a gene with relevant implications in colon cancer progression. In the SW-620 colon carcinoma cell line, we observed results similar to those in the MDA-MB-435 cell line (supplemental Fig. S3), indicating again that the effect of VRK2A on NFAT1-dependent COX-2 transcription was independent of cell type. Moreover, we performed in this SW-620 cell line luciferase-reporter experiments down-regulating VRK2 expression. Results showed that VRK2 overexpression enhanced COX-2 promoter activity, whereas VRK2 down-regulation reduced COX-2 promoter activity (supplemental Fig. S3).
FIGURE 6.
NFAT1 binding sites in the COX-2 promoter were required for increased COX-2 transcription induced by VRK2A. A, HA-VRK2A overexpression, but not HA-VRK1, increased COX-2 promoter transcription in MDA-MB-435 cells after 100 ng/μl PMA and 0.5 μm ionomycin stimulation for 16 h. VRK1(K179E) and VRK2A(K169E) are kinase-dead proteins. Western blot with the level of the transfected proteins is shown to the right. B, effect of 10 μm cyclosporin A (CsA) treatment for 1 h before PMA/Io stimulation on the NFAT1 activation induced by VRK2A. C, effect of VRK2A on the transcriptional activity of the reporter p2–274 mutated in the proximal (p) or both distal and proximal (d&p) NFAT binding sites. Levels of transfected HA-VRK2A are shown in the blot at the bottom. Graphs show the mean of the relative -fold increase in luciferase activity values of triplicate measurements of three independent experiments. *, p < 0.05; **, p < 0.005; ***, p < 0.0005. Error bars, S.D.
VRK2 Down-regulation Reduced Cell Invasion
To evaluate the role of VRK2A in COX-2-induced cell invasion through NFAT1 activation, we performed a Matrigel invasion assay in the MDA-MB-231 cell line. As shown in Fig. 7A, cells were more invasive after PMA/ionomycin stimulation, but in the case of VRK2 down-regulation with siVRK2-06, cell invasion decreased significantly. The same result was obtained in the MDA-MB-435 cell line. This effect was also detected using another siRNA (siVRK2-M) for VRK2 in this cell line and in MDA-MB-231 cells (supplemental Fig. S4). The difference in invasiveness between these two cell lines can be explained because MDA-MB-435 cells have a much lower level of NFAT1 (Fig. 7B), and thus the potential for activation of COX-2 expression is more limited. Therefore, we determined the effect of VRK2 down-regulation on cell migration, performing a wound healing assay. Control cells closed the wound area after 30 h, but cells with reduced VRK2 levels migrated more slowly than control cells and were not able to completely close the wound after 30 h (supplemental Fig. S5).
FIGURE 7.
Effect of VRK2 down-regulation on invasion of breast cancer MDA-MB-231 cells. A, MDA-MB-231 cells were transfected in p60 dishes with control siRNA (siControl) or VRK2-specific siRNA (siVRK2-06). After 3 days, cells were treated as control or stimulated with PMA/Io for 30 min; afterward, 50,000 cells were placed in serum-free medium in the upper chamber of a Transwell coated with Matrigel (1 mg/ml). Medium with 10% FBS was added in the lower chamber. The invasion assay was performed for 42 h. Invasive cells were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet dye. The microscopic image of the invasive cells is shown to the left. The mean values from duplicate samples of three independent experiments are shown to the right. ***, p < 0.0005. At the bottom is shown the level of COX-2 protein (left) and the level of VRK2 mRNA by quantitative RT-PCR (right). B, protein levels of VRK2 and NFAT1 in human cell lines of different origins. Jurkat (T-cells), SW-620 (colon carcinoma), MDA-MB-435 (melanoma), and MDA-MB-231 (breast cancer). The antibodies used are indicated under “Experimental Procedures.” Error bars, S.D.
DISCUSSION
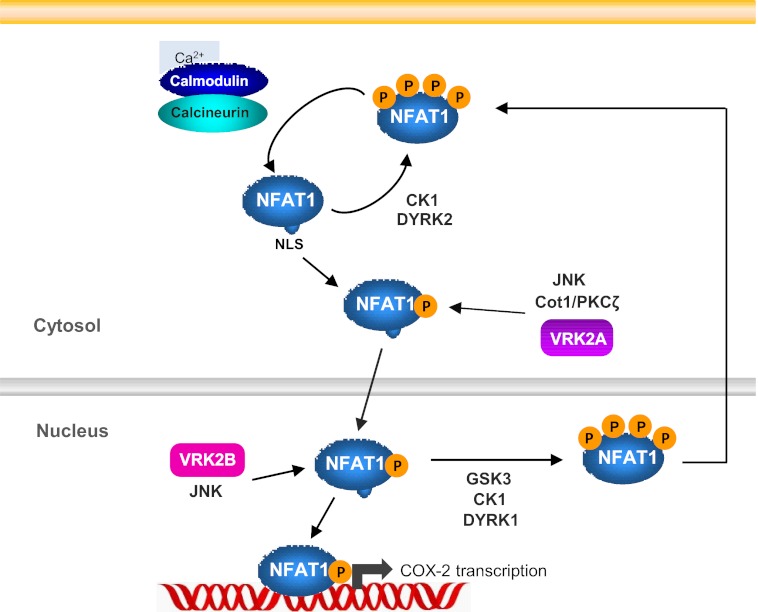
NFAT1 phosphorylation represents the first target identified for VRK2 as an active kinase because other VRK2 effects that have been reported are only mediated by protein-protein interactions (23, 25–27). Thus, this finding extends the significance of the biological role of VRK2 in cell signaling and cancer biology, where it has been mainly studied. Phosphorylation plays three different roles in NFAT1 regulation. One of them implicates an indirect mechanism by RCAN1 phosphorylation, which releases calcineurin from RCAN1 interaction and inhibition. Another effect is a direct phosphorylation in the NFAT1 N-terminal domain that enhances NFAT1 transcriptional activity. These additional activations might affect the interaction with other transcriptional factors or coactivators. Third, nuclear NFAT1 phosphorylation leads to inactivation and export of NFAT1 to the cytosol. Also, there are kinases that phosphorylate NFAT1 to maintain it in the cytosol in the inactive hyperphosphorylated state. Thus, NFAT1 phosphorylation in the cytosol has two different roles; one is to retain this transcription factor in the cytoplasm, and the other is to enhance the activity of activated NFAT1. This positive effect may also facilitate selection of other factors or interacting proteins. The N-terminal transactivation domain is the most important domain in the regulation of NFAT1 transactivation function. It has been reported that Cot kinase and PKC phosphorylate this domain in serine 53 and 56, increasing NFAT1 activity (41, 42, 60). In this report, we show that VRK2A is a new NFAT kinase and phosphorylates the NFAT1 transactivation domain in another residue, serine 32, that has not been previously reported, enhancing NFAT1 transcriptional activity. This effect occurs in the cytosol because it is mediated by the VRK2A isoform, which is anchored to the endoplasmic reticulum, but it might also occur in the nucleus in cells that also express the VRK2B isoform, as is the case with p53 (24). Thus, VRK2 can be incorporated in the complex regulation mediated by phosphorylation of NFAT1 (Fig. 8).
FIGURE 8.
Model of the regulation of NFAT1 activation and participation of different kinases.
The role of COX-2 in cancer has been mainly studied in the context of colorectal carcinoma (61), but it is likely that it plays a role in other cancers, such as breast carcinomas or melanomas. Also, COX-2 has been associated with bone and brain metastasis (52, 53, 62, 63). The requirement of NFAT1 phosphorylation for the regulation of COX-2 gene expression suggests that kinases might play a significant role. The increased NFAT1 activity by VRK2A is selective because it is dependent on NFAT1 response elements in the COX-2 gene promoter. The existence of two NFAT1 binding sites in this promoter might represent the possibility to respond to different input signals by the formation of alternative transcriptional complexes. COX-2 expression requires NFAT1 activation, and different levels of activated NFAT1 might have different functional effects.
VRK2 inhibits the mitogenic signal mediated by AP1 transcription factor (23) but promotes survival signals (23) and dissemination (this report). In tumors, VRK2 might alter the balance between these two properties. VRK2 might inhibit the proliferative signal and facilitate the invasive potential in breast cancer. Human breast cancer can be classified in different types, and VRK2 is positively associated with estrogen and progesterone receptor expression and inversely correlated with ERBB2 expression. This is explained by the fact that VRK2A has an inhibitory effect on the mitogenic signal mediated by the ERBB2-RAS-RAF-ERK pathway without affecting the survival pathway (23).
Targeting kinases is currently proving to be a useful therapeutic strategy in cancer (64, 65). The different phenotypes of breast carcinomas have different biological characteristics. Estrogen receptor-positive patients, in which VRK2 is expressed at high levels, can be controlled by antiestrogenic therapy, but if stimulatory signals are received, tumors might increase their invasive potential due to the action of VRK2 on NFAT1 activity. In breast cancer, NFAT1 expression and activation are relatively little characterized, but it is known that it plays a role in tumor dissemination. This might be associated with the inflammatory response triggered by interleukins present in the tumor environment as a consequence of COX-2 expression (54, 66). In this way, COX-2 expression might facilitate tumor invasion (67). Perhaps combined therapy of COX-2 and VRK2 inhibitors might be a feasible strategy in the future to reduce the invasiveness of tumor cells in breast cancer patients. Currently there are no inhibitors for VRK2, and this kinase family is very insensitive to current inhibitors due to their structural characteristics (55, 68).
In this work, we have identified the first reported role of VRK2 as an active kinase phosphorylating NFAT1 protein in a previously unidentified residue, serine 32, and enhancing the activity of this transcription factor. Also, we have demonstrated that VRK2 participates in the activation of NFAT1-dependent COX-2 transcription, and this effect on COX-2 expression by VRK2 can modulate the invasive properties of tumor cells. Thus, in the future, it will be necessary to study these proteins on human primary tumors in order to identify breast cancer subgroups, in which eventually an anti-VRK2 therapy might be used when available.
Supplementary Material
This work was supported by grants from Ministerio de Educación, Ciencia e Innovación Grant SAF2010-14935, Consolider-Ingenio 2010 Grant CSD07-0017, Junta de Castilla y León (Consejería de Educación, Grant CSI-006A11-2), Fundación Sandra Ibarra, and Kutxa-Fundación Inbiomed.

This article contains supplemental Figs. S1–S5.
The protein interactions from this publication have been submitted to the European Bioinformatics Institute-EMBL IMEx (www.imexconsortium.org/) consortium through IntAct (pmid 19850723) and assigned the identifier IM-17902.
- NFAT
- nuclear factor of activated T-cells
- VRK
- vaccinia-related kinase
- COX-2
- cyclooxygenase 2
- PMA
- phorbol 12-myristate 13-acetate
- Io
- ionomycin.
REFERENCES
- 1. Kim T. H., Kim H. I., Soung Y. H., Shaw L. A., Chung J. (2009) Integrin (α6β4) signals through Src to increase expression of S100A4, a metastasis-promoting factor. Implications for cancer cell invasion. Mol. Cancer Res. 7, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 2. Ryeom S., Baek K. H., Rioth M. J., Lynch R. C., Zaslavsky A., Birsner A., Yoon S. S., McKeon F. (2008) Targeted deletion of the calcineurin inhibitor DSCR1 suppresses tumor growth. Cancer Cell 13, 420–431 [DOI] [PubMed] [Google Scholar]
- 3. Buchholz M., Schatz A., Wagner M., Michl P., Linhart T., Adler G., Gress T. M., Ellenrieder V. (2006) Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway, EMBO J. 25, 3714–3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jiang X., Austin P. F., Niederhoff R. A., Manson S. R., Riehm J. J., Cook B. L., Pengue G., Chitaley K., Nakayama K., Nakayama K. I., Weintraub S. J. (2009) Mechanoregulation of proliferation. Mol. Cell. Biol. 29, 5104–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mancini M., Toker A. (2009) NFAT proteins. Emerging roles in cancer progression. Nat. Rev. Cancer 9, 810–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. (2002) The protein kinase complement of the human genome. Science 298, 1912–1934 [DOI] [PubMed] [Google Scholar]
- 7. Nezu J., Oku A., Jones M. H., Shimane M. (1997) Identification of two novel human putative serine/threonine kinases, VRK1 and VRK2, with structural similarity to vaccinia virus B1R kinase. Genomics 45, 327–331 [DOI] [PubMed] [Google Scholar]
- 8. Lopez-Borges S., Lazo P. A. (2000) The human vaccinia-related kinase 1 (VRK1) phosphorylates threonine-18 within the mdm-2 binding site of the p53 tumour suppressor protein. Oncogene 19, 3656–3664 [DOI] [PubMed] [Google Scholar]
- 9. Nichols R. J., Traktman P. (2004) Characterization of three paralogous members of the Mammalian vaccinia related kinase family. J. Biol. Chem. 279, 7934–7946 [DOI] [PubMed] [Google Scholar]
- 10. Klerkx E. P., Lazo P. A., Askjaer P. (2009) Emerging biological functions of the vaccinia-related kinase (VRK) family. Histol. Histopathol. 24, 749–759 [DOI] [PubMed] [Google Scholar]
- 11. Vega F. M., Gonzalo P., Gaspar M. L., Lazo P. A. (2003) Expression of the VRK (vaccinia-related kinase) gene family of p53 regulators in murine hematopoietic development. FEBS Lett. 544, 176–180 [DOI] [PubMed] [Google Scholar]
- 12. Santos C. R., Rodríguez-Pinilla M., Vega F. M., Rodríguez-Peralto J. L., Blanco S., Sevilla A., Valbuena A., Hernández T., van Wijnen A. J., Li F., de Alava E., Sánchez-Céspedes M., Lazo P. A. (2006) VRK1 signaling pathway in the context of the proliferation phenotype in head and neck squamous cell carcinoma. Mol. Cancer Res. 4, 177–185 [DOI] [PubMed] [Google Scholar]
- 13. Valbuena A., Sanz-García M., López-Sánchez I., Vega F. M., Lazo P. A. (2011) Roles of VRK1 as a new player in the control of biological processes required for cell division. Cell. Signal. 23, 1267–1272 [DOI] [PubMed] [Google Scholar]
- 14. Valbuena A., López-Sánchez I., Lazo P. A. (2008) Human VRK1 is an early response gene and its loss causes a block in cell cycle progression. PLoS ONE 3, e1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barcia R., López-Borges S., Vega F. M., Lazo P. A. (2002) Kinetic properties of p53 phosphorylation by the human vaccinia-related kinase 1. Arch. Biochem. Biophys. 399, 1–5 [DOI] [PubMed] [Google Scholar]
- 16. Vega F. M., Sevilla A., Lazo P. A. (2004) p53 Stabilization and accumulation induced by human vaccinia-related kinase 1. Mol. Cell. Biol. 24, 10366–10380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valbuena A., Vega F. M., Blanco S., Lazo P. A. (2006) p53 downregulates its activating vaccinia-related kinase 1, forming a new autoregulatory loop. Mol. Cell. Biol. 26, 4782–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valbuena A., Blanco S., Vega F. M., Lazo P. A. (2008) The C/H3 domain of p300 is required to protect VRK1 and VRK2 from their downregulation induced by p53. PLoS ONE 3, e2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valbuena A., Castro-Obregón S., Lazo P. A. (2011) Downregulation of VRK1 by p53 in response to DNA damage is mediated by the autophagic pathway. PLoS ONE 6, e17320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sevilla A., Santos C. R., Barcia R., Vega F. M., Lazo P. A. (2004) c-Jun phosphorylation by the human vaccinia-related kinase 1 (VRK1) and its cooperation with the N-terminal kinase of c-Jun (JNK). Oncogene 23, 8950–8958 [DOI] [PubMed] [Google Scholar]
- 21. Sevilla A., Santos C. R., Vega F. M., Lazo P. A. (2004) Human vaccinia-related kinase 1 (VRK1) activates the ATF2 transcriptional activity by novel phosphorylation on Thr-73 and Ser-62 and cooperates with JNK. J. Biol. Chem. 279, 27458–27465 [DOI] [PubMed] [Google Scholar]
- 22. Kang T. H., Park D. Y., Kim W., Kim K. T. (2008) VRK1 phosphorylates CREB and mediates CCND1 expression. J. Cell Sci. 121, 3035–3041 [DOI] [PubMed] [Google Scholar]
- 23. Fernández I. F., Blanco S., Lozano J., Lazo P. A. (2010) VRK2 inhibits mitogen-activated protein kinase signaling and inversely correlates with ErbB2 in human breast cancer. Mol. Cell. Biol. 30, 4687–4697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blanco S., Klimcakova L., Vega F. M., Lazo P. A. (2006) The subcellular localization of vaccinia-related kinase-2 (VRK2) isoforms determines their different effect on p53 stability in tumour cell lines. FEBS J. 273, 2487–2504 [DOI] [PubMed] [Google Scholar]
- 25. Blanco S., Santos C., Lazo P. A. (2007) Vaccinia-related kinase 2 modulates the stress response to hypoxia mediated by TAK1. Mol. Cell. Biol. 27, 7273–7283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blanco S., Sanz-García M., Santos C. R., Lazo P. A. (2008) Modulation of interleukin-1 transcriptional response by the interaction between VRK2 and the JIP1 scaffold protein. PLoS ONE 3, e1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fernández I. F., Pérez-Rivas L. G., Blanco S., Castillo-Dominguez A. A., Lozano J., Lazo P. A. (2012) VRK2 anchors KSR1-MEK1 to endoplasmic reticulum forming a macromolecular complex that compartmentalizes MAPK signaling. Cell Mol. Life Sci. 69, 3881–3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rao A., Luo C., Hogan P. G. (1997) Transcription factors of the NFAT family. Regulation and function. Annu. Rev. Immunol. 15, 707–747 [DOI] [PubMed] [Google Scholar]
- 29. Serfling E., Berberich-Siebelt F., Chuvpilo S., Jankevics E., Klein-Hessling S., Twardzik T., Avots A. (2000) The role of NF-AT transcription factors in T cell activation and differentiation, Biochim. Biophys. Acta 1498, 1–18 [DOI] [PubMed] [Google Scholar]
- 30. Hogan P. G., Chen L., Nardone J., Rao A. (2003) Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17, 2205–2232 [DOI] [PubMed] [Google Scholar]
- 31. Müller M. R., Rao A. (2010) NFAT, immunity and cancer. A transcription factor comes of age. Nat. Rev. Immunol. 10, 645–656 [DOI] [PubMed] [Google Scholar]
- 32. Luo C., Shaw K. T., Raghavan A., Aramburu J., Garcia-Cozar F., Perrino B. A., Hogan P. G., Rao A. (1996) Interaction of calcineurin with a domain of the transcription factor NFAT1 that controls nuclear import. Proc. Natl. Acad. Sci. U.S.A. 93, 8907–8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okamura H., Aramburu J., García-Rodríguez C., Viola J. P., Raghavan A., Tahiliani M., Zhang X., Qin J., Hogan P. G., Rao A. (2000) Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol. Cell 6, 539–550 [DOI] [PubMed] [Google Scholar]
- 34. Abbasi S., Lee J. D., Su B., Chen X., Alcon J. L., Yang J., Kellems R. E., Xia Y. (2006) Protein kinase-mediated regulation of calcineurin through the phosphorylation of modulatory calcineurin-interacting protein 1. J. Biol. Chem. 281, 7717–7726 [DOI] [PubMed] [Google Scholar]
- 35. Hilioti Z., Gallagher D. A., Low-Nam S. T., Ramaswamy P., Gajer P., Kingsbury T. J., Birchwood C. J., Levchenko A., Cunningham K. W. (2004) GSK-3 kinases enhance calcineurin signaling by phosphorylation of RCNs. Genes Dev. 18, 35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fuentes J. J., Genescà L., Kingsbury T. J., Cunningham K. W., Pérez-Riba M., Estivill X., de la Luna S. (2000) DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum. Mol. Genet. 9, 1681–1690 [DOI] [PubMed] [Google Scholar]
- 37. Abbasi S., Su B., Kellems R. E., Yang J., Xia Y. (2005) The essential role of MEKK3 signaling in angiotensin II-induced calcineurin/nuclear factor of activated T-cells activation. J. Biol. Chem. 280, 36737–36746 [DOI] [PubMed] [Google Scholar]
- 38. Sanna B., Brandt E. B., Kaiser R. A., Pfluger P., Witt S. A., Kimball T. R., van Rooij E., De Windt L. J., Rothenberg M. E., Tschop M. H., Benoit S. C., Molkentin J. D. (2006) Modulatory calcineurin-interacting proteins 1 and 2 function as calcineurin facilitators in vivo. Proc. Natl. Acad. Sci. U.S.A. 103, 7327–7332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsatsanis C., Patriotis C., Bear S. E., Tsichlis P. N. (1998) The Tpl-2 protooncoprotein activates the nuclear factor of activated T cells and induces interleukin 2 expression in T cell lines. Proc. Natl. Acad. Sci. U.S.A. 95, 3827–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Gregorio R., Iñiguez M. A., Fresno M., Alemany S. (2001) Cot kinase induces cyclooxygenase-2 expression in T cells through activation of the nuclear factor of activated T cells. J. Biol. Chem. 276, 27003–27009 [DOI] [PubMed] [Google Scholar]
- 41. Rainio E. M., Sandholm J., Koskinen P. J. (2002) Cutting edge. Transcriptional activity of NFATc1 is enhanced by the Pim-1 kinase. J. Immunol. 168, 1524–1527 [DOI] [PubMed] [Google Scholar]
- 42. Ortega-Pérez I., Cano E., Were F., Villar M., Vázquez J., Redondo J. M. (2005) c-Jun N-terminal kinase (JNK) positively regulates NFATc2 transactivation through phosphorylation within the N-terminal regulatory domain. J. Biol. Chem. 280, 20867–20878 [DOI] [PubMed] [Google Scholar]
- 43. Gómez-Casero E., San-Antonio B., Iñiguez M. A., Fresno M. (2007) Cot/Tpl2 and PKCζ cooperate in the regulation of the transcriptional activity of NFATc2 through the phosphorylation of its amino-terminal domain. Cell. Signal. 19, 1652–1661 [DOI] [PubMed] [Google Scholar]
- 44. Okamura H., Garcia-Rodriguez C., Martinson H., Qin J., Virshup D. M., Rao A. (2004) A conserved docking motif for CK1 binding controls the nuclear localization of NFAT1. Mol. Cell. Biol. 24, 4184–4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beals C. R., Sheridan C. M., Turck C. W., Gardner P., Crabtree G. R. (1997) Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275, 1930–1934 [DOI] [PubMed] [Google Scholar]
- 46. Duque J., Fresno M., Iñiguez M. A. (2005) Expression and function of the nuclear factor of activated T cells in colon carcinoma cells. Involvement in the regulation of cyclooxygenase-2. J. Biol. Chem. 280, 8686–8693 [DOI] [PubMed] [Google Scholar]
- 47. Iñiguez M. A., Martinez-Martinez S., Punzón C., Redondo J. M., Fresno M. (2000) An essential role of the nuclear factor of activated T cells in the regulation of the expression of the cyclooxygenase-2 gene in human T lymphocytes. J. Biol. Chem. 275, 23627–23635 [DOI] [PubMed] [Google Scholar]
- 48. Jauliac S., López-Rodriguez C., Shaw L. M., Brown L. F., Rao A., Toker A. (2002) The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat. Cell Biol. 4, 540–544 [DOI] [PubMed] [Google Scholar]
- 49. Corral R. S., Iñiguez M. A., Duque J., López-Pérez R., Fresno M. (2007) Bombesin induces cyclooxygenase-2 expression through the activation of the nuclear factor of activated T cells and enhances cell migration in Caco-2 colon carcinoma cells, Oncogene 26, 958–969 [DOI] [PubMed] [Google Scholar]
- 50. Yiu G. K., Toker A. (2006) NFAT induces breast cancer cell invasion by promoting the induction of cyclooxygenase-2. J. Biol. Chem. 281, 12210–12217 [DOI] [PubMed] [Google Scholar]
- 51. Sihto H., Lundin J., Lundin M., Lehtimäki T., Ristimäki A., Holli K., Sailas L., Kataja V., Turpeenniemi-Hujanen T., Isola J., Heikkilä P., Joensuu H. (2011) Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites. A nationwide cohort study. Breast Cancer Res. 13, R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim H. S., Moon H. G., Han W., Yom C. K., Kim W. H., Kim J. H., Noh D. Y. (2012) COX2 overexpression is a prognostic marker for Stage III breast cancer. Breast Cancer Res. Treat. 132, 51–59 [DOI] [PubMed] [Google Scholar]
- 53. Bos P. D., Zhang X. H., Nadal C., Shu W., Gomis R. R., Nguyen D. X., Minn A. J., van de Vijver M. J., Gerald W. L., Foekens J. A., Massagué J. (2009) Genes that mediate breast cancer metastasis to the brain, Nature 459, 1005–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Singh B., Berry J. A., Shoher A., Lucci A. (2006) COX-2 induces IL-11 production in human breast cancer cells. J. Surg. Res. 131, 267–275 [DOI] [PubMed] [Google Scholar]
- 55. Vázquez-Cedeira M., Barcia-Sanjurjo I., Sanz-García M., Barcia R., Lazo P. A. (2011) Differential inhibitor sensitivity between human kinases VRK1 and VRK2. PLoS ONE 6, e23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bergmann C., Strauss L., Zeidler R., Lang S., Whiteside T. L. (2007) Expansion of human T regulatory type 1 cells in the microenvironment of cyclooxygenase 2 overexpressing head and neck squamous cell carcinoma. Cancer Res. 67, 8865–8873 [DOI] [PubMed] [Google Scholar]
- 57. Tuynman J. B., Vermeulen L., Boon E. M., Kemper K., Zwinderman A. H., Peppelenbosch M. P., Richel D. J. (2008) Cyclooxygenase-2 inhibition inhibits c-Met kinase activity and Wnt activity in colon cancer. Cancer Res. 68, 1213–1220 [DOI] [PubMed] [Google Scholar]
- 58. Ogunwobi O. O., Wang T., Zhang L., Liu C. (2012) Cyclooxygenase-2 and Akt mediate multiple growth-factor-induced epithelial-mesenchymal transition in human hepatocellular carcinoma. J. Gastroenterol. Hepatol. 27, 566–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Flockhart R. J., Armstrong J. L., Reynolds N. J., Lovat P. E. (2009) NFAT signalling is a novel target of oncogenic BRAF in metastatic melanoma. Br. J. Cancer 101, 1448–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Diehn M., Alizadeh A. A., Rando O. J., Liu C. L., Stankunas K., Botstein D., Crabtree G. R., Brown P. O. (2002) Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc. Natl. Acad. Sci. U.S.A. 99, 11796–11801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vinogradova Y., Coupland C., Hippisley-Cox J. (2011) Exposure to cyclooxygenase-2 inhibitors and risk of cancer. Nested case-control studies. Br. J. Cancer 105, 452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li Z., Schem C., Shi Y. H., Medina D., Zhang M. (2008) Increased COX2 expression enhances tumor-induced osteoclastic lesions in breast cancer bone metastasis. Clin. Exp. Metastasis 25, 389–400 [DOI] [PubMed] [Google Scholar]
- 63. Singh B., Berry J. A., Shoher A., Ayers G. D., Wei C., Lucci A. (2007) COX-2 involvement in breast cancer metastasis to bone. Oncogene 26, 3789–3796 [DOI] [PubMed] [Google Scholar]
- 64. Fedorov O., Sundström M., Marsden B., Knapp S. (2007) Insights for the development of specific kinase inhibitors by targeted structural genomics. Drug Discov. Today 12, 365–372 [DOI] [PubMed] [Google Scholar]
- 65. Zhang J., Yang P. L., Gray N. S. (2009) Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 9, 28–39 [DOI] [PubMed] [Google Scholar]
- 66. Singh B., Berry J. A., Vincent L. E., Lucci A. (2006) Involvement of IL-8 in COX-2-mediated bone metastases from breast cancer. J. Surg. Res. 134, 44–51 [DOI] [PubMed] [Google Scholar]
- 67. Singh B., Berry J. A., Shoher A., Ramakrishnan V., Lucci A. (2005) COX-2 overexpression increases motility and invasion of breast cancer cells. Int. J. Oncol. 26, 1393–1399 [PubMed] [Google Scholar]
- 68. Scheeff E. D., Eswaran J., Bunkoczi G., Knapp S., Manning G. (2009) Structure of the pseudokinase VRK3 reveals a degraded catalytic site, a highly conserved kinase fold, and a putative regulatory binding site. Structure 17, 128–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.