Background: Cerebrospinal fluid Tau levels are altered in tauopathies; however, the mechanism of Tau secretion is poorly understood.
Results: Tau isoforms and mutations alter extracellular Tau levels in cultured cells.
Conclusion: Tau is actively released, and Tau release is modified by variability in Tau that is associated with tauopathies.
Significance: Defining factors that influence Tau release is crucial to understanding Tau metabolism in tauopathies.
Keywords: Alzheimers Disease, Calcium, Cell Culture, Secretion, Tau, Frontotemporal Dementia, Tauopathy
Abstract
Tauopathies are a class of neurodegenerative diseases marked by intracellular aggregates of hyperphosphorylated Tau. These diseases may occur by sporadic mechanisms in which genetic variants represent risk factors for disease, as is the case in Alzheimer disease (AD). In AD, cerebrospinal fluid (CSF) levels of soluble Tau/pTau-181 are higher in cases compared with controls. A subset of frontotemporal dementia (FTD) cases occur by a familial mechanism in which MAPT, the gene that encodes Tau, mutations are dominantly inherited. In symptomatic FTD patients expressing a MAPT mutation, CSF Tau levels are slightly elevated but are significantly lower than in AD patients. We sought to model CSF Tau changes by measuring extracellular Tau in cultured cells. Full-length, monomeric extracellular total Tau and pTau-181 were detectable in human neuroblastoma cells expressing endogenous Tau, in human non-neuronal cells overexpressing wild-type Tau, and in mouse cortical neurons. Tau isoforms influence the rate of Tau release, whereby the N terminus (exons 2/3) and microtubule binding repeat length contribute to Tau release from the cell. Compared with cells overexpressing wild-type Tau, cells overexpressing FTD-associated MAPT mutations produce significantly less extracellular total Tau without altering intracellular total Tau levels. This study demonstrates that cells actively release Tau in the absence of disease or toxicity, and Tau release is modified by changes in the Tau protein that are associated with tauopathies.
Introduction
Tauopathies, including Alzheimer disease (AD)2 and frontotemporal dementia (FTD), belong to a class of neurodegenerative diseases sharing the characteristic pathology of intracellular neurofibrillary tangles and extensive neuronal loss in the brain. Intracellular neurofibrillary tangles are composed of hyperphosphorylated Tau aggregates. Tau is an intracellular microtubule-associated protein, which functions to stabilize and facilitate the assembly of microtubules (1).
For more than a decade, extracellular Tau has been measured in human studies (reviewed in Ref. 2). Cerebrospinal fluid (CSF) levels of soluble total Tau and pTau-181 are associated with clinical AD (2) and are used as biomarkers for AD (3–9). CSF total Tau and pTau-181 levels are significantly elevated in clinically demented individuals compared with age-matched, cognitively normal controls (3–9). In symptomatic FTD patients expressing MAPT mutations, CSF Tau levels are slightly elevated but are significantly lower than in AD patients (5, 10). In stroke (11) and traumatic brain injury (12), CSF total Tau levels are briefly elevated after the initial insult, reflective of Tau release due to neuronal death; however, CSF phospho-Tau levels remain unchanged. CSF total Tau and pTau-181 levels can be used as a quantitative endophenotype to identify genes that influence Tau levels and contribute to AD pathogenesis (13, 14).
Tau protein in the CSF represents an intracellular protein in the extracellular space of the central nervous system. Recent studies suggest that extracellular Tau is detectable in cell and mouse models. Tau is detectable in the media of immortalized cells transiently overexpressing human Tau (15–17), and human/mouse Tau are detectable in the brain interstitial fluid and CSF of non-transgenic and Tau-P301S transgenic mice (18). In cultured cells, a robustly aggregating fragment of Tau, containing the microtubule-binding region, is taken up by neighboring cells and induces intracellular wild-type (WT) Tau protein to aggregate (19). In mouse models, Tau aggregates can be propagated to distal regions of the brain (20, 21). Taken together, these data demonstrate that the Tau protein has prion-like properties and that once outside the cell, Tau proteins may induce toxicity and/or aggregation.
In this study, we sought to measure extracellular Tau in cultured neuronal cells and to characterize the mechanism by which WT Tau is released. In immortalized and primary neuronal cultures, we demonstrate that full-length, monomeric extracellular Tau is present in phosphorylated and dephosphorylated states. Furthermore, we demonstrate that the release of Tau is influenced by calcium and is likely mediated by the unconventional secretory pathway. We also provide evidence that Tau isoforms are released at different rates and that pathogenic mutations within Tau further alter Tau release. Together, we demonstrate that cells actively release Tau in the absence of disease or toxicity, and Tau release is modified by changes in the Tau protein that are associated with tauopathies.
EXPERIMENTAL PROCEDURES
Site-directed Mutagenesis
Human WT Tau cDNA (3R0N, 3R2N, 4R0N, or 4R2N) was expressed in the pcDNA3.1 vector (generously provided by Virginia Lee and Michael Goedert). Tau isoform nomenclature defines the length of the microtubule binding repeat (3R or 4R) and the presence or absence of exons 2 and 3 at the N terminus (0N or 2N). FTD-associated Tau mutations were introduced into the 3R2N or 4R2N Tau constructs by site-directed mutagenesis using the QuikChange II Site-directed Mutagenesis kit (Agilent). The sequence of each construct was verified by Sanger sequencing.
Cell Lines and Transient Transfection
Undifferentiated human neuroblastoma (SH-SY5Y) cells were cultured in Iscove's modified Dulbecco's medium supplemented with 10% fetal bovine serum (FBS), 1% l-glutamine, and penicillin/streptomycin. Human embryonic kidney (HEK293T) cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% l-glutamine, and penicillin/streptomycin. For transient transfection, HEK293T cells were cultured in 6-well lysine-coated plates. Upon reaching 90% confluence, cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol and harvested after 24 h.
Primary Mixed Neuronal Cultures
Glial cultures, to serve as a feeder layer, were isolated from 2-day-old postnatal Swiss Webster mouse pups following standard protocols (22). Neuronal cultures were isolated from mice at embryonic day 18 as previously described (22). Neocortices were extracted and plated on glial cultures and grown at 37 °C for 14 days.
Tau ELISA
Media was collected and centrifuged at 3000 × g for 10 min at 4 °C to remove cell debris. Cell lysates were extracted in lysis buffer (50 mm Tris, pH 7.6, 1 mm EDTA, 150 mm NaCl, 1% Triton X-100, phosphatase and protease inhibitors) and incubated on ice for 5 min. Lysates were then centrifuged at 14,000 × g for 10 min at 4 °C, and the resulting supernatant was saved for analysis. Media and cell lysates were analyzed for total Tau and phospho-Tau using commercially available ELISA kits (Invitrogen) specific to human total Tau, mouse total Tau, and pTau-181. To account for protein concentration, total protein in cell lysates were measured by BCA assay according to manufacturer's protocol. ELISA values were obtained (pg/ml) and corrected for total intracellular protein (μg/ml), producing a final unit of pg/μg.
Immunoprecipitation
Media and cell lysates were pre-cleared with Protein G beads (Thermo Scientific). Pre-cleared supernatants were incubated overnight at 4 °C with the antibodies indicated. Supernatant-antibody complexes were then incubated with Protein G beads at room temperature for 2 h. After washing, proteins were dissociated from the Protein G beads by incubating the beads in Laemmli sample buffer (23) supplemented with 5% β-mercaptoethanol at 95 °C for 10 min.
Pulse-Chase
To measure the half-life of intracellular Tau protein and the release rate of Tau protein in SH-SY5Y cells, cells were plated in 60-mm lysine-coated dishes (Biocoat) and grown at 37 °C overnight. Cells were conditioned in methionine-free media (Invitrogen) for 1 h and pulsed with 300 μCi of [35S]methionine (PerkinElmer Life Sciences) in methionine-free media for 1 h. Cells were then washed and chased with complete media for 1, 3, 6, 12, 18, and 24 h. Culture media and cell lysates were extracted and immunoprecipitated as described above. Immunoblots were exposed to autoradiography film and developed to visualize Tau protein bands.
Inhibition of Protein Secretion
Cells were treated with the following chemicals to inhibit protein trafficking: 2 μg/ml of brefeldin A (BFA, Sigma), 2 μm BAPTA-AM (Sigma), 0.5 mm EDTA (Fisher), 100 nm thapsigargin (Sigma), 100 nm ionomycin (Sigma), 2 μm chloroquine (CQ, Sigma), 2 mm methylamine (Fisher), 1 μm paclitaxel (Sigma), 25 μm pseudoloric acid B (PAB, Enzo Life Science). All reagents were diluted in DMSO. After growing SH-SY5Y cells overnight at 37 °C, culture media was removed and replaced with media containing the compounds listed above, diluted 1:1000, for 6 h. Culture media and cells were subsequently collected and prepared for ELISA analysis (as described above). Results were expressed relative to DMSO-treated controls.
Lactate Dehydrogenase (LDH) Assay
Cytotoxicity was measured in SH-SY5Y cells using an LDH assay kit (Clontech). Cells were incubated with untreated LDH assay media (low control) or with LDH assay media supplemented with 1% Triton X-100 (high control), DMSO (1:1000), or protein trafficking compounds for 6 h. Cytotoxicity was also measured in HEK293T cells transiently transfected with vectors expressing GFP, WT, or mutant Tau for 24 h. Media from these cells was then incubated with a reaction mixture containing tetrazolium salt and measured at 490 nm. Cytotoxicity was expressed relative to the percentage of maximal cell lysis.
Exosome Preparation
SH-SY5Y cells (10 10-cm plates) were incubated for 48 h in exosome-free media. Exosomes were then isolated as previously described (24).
Biotinylation
Tau at the cell surface was measured by biotinylation of cultured cells with NHS-SS biotin (Thermo Scientific). Untransfected SH-SY5Y cells were treated with biotin (1 mg/ml) for 30 min, washed, and collected.
To measure Tau internalization, cells were biotinylated (1 mg/ml) for 30 min on ice, which inhibits endocytosis. The internalized protein fraction was measured by incubating biotinylated cells at 37 °C for 10 min. Cells were subsequently treated with biotin stripping buffer (50 mm 2-mercaptoethanesulfonic acid, 100 mm NaCl, and 2.5 mm CaCl2 in 50 mm Tris-HCl, pH 7.6) to digest biotinylated proteins at the cell surface.
Cell lysates were extracted as described above and incubated with strepavidin beads (Thermo Scientific). Protein-antibody complexes were dissociated by incubation in Laemmli sample buffer (23).
Immunoblotting
Standard SDS-PAGE was performed in 4–20% Criterion Tris-HCl gels (Bio-Rad). Samples were boiled in Laemmli sample buffer (23) prior to electrophoresis. Immunoblots were probed with the antibodies listed below.
Immunocytochemistry
Cells were grown on lysine-coated coverslips in 12-well plates overnight at 37 °C. When appropriate, cells were treated with compounds to inhibit protein trafficking or transiently transfected (as described above). Culture media was aspirated, and cells were then washed and fixed with 4% paraformaldehyde (Sigma). After several washing steps, cells were permeabilized with cold 100% methanol. Cells were then blocked in normal goat serum (Invitrogen) and treated with primary and secondary antibodies diluted in 10% normal goat serum. Cell-coated coverslips were then mounted with Aquamount on histology slides and imaged (Nikon Eclipse 80i fluorescent microscope).
Antibodies
The antibodies used in this study include: Tau12, Tau1, Tau5, Tau7 (generously provided by Lester Binder), HJ9.3 (generously provided by David Holtzman), DAPI (Sigma), Giantin (Abcam), Tub2.1 conjugated to Alexa Fluor 594 (Invitrogen), tubulin (Abcam), Alexa Fluor 594 (Invitrogen), and anti-mouse Trueblot Ultra (Ebiosciences).
RNA Extraction and Reverse Transcription
RNA was extracted from HEK293T cell lysates with an RNeasy kit (Qiagen) according to the manufacturer's protocol. Extracted RNA (10 ug) was converted to cDNA by PCR using the High Capacity cDNA Reverse Transcriptase kit (ABI). Exon 10 was measured by PCR.
Cycloheximide Treatment of Cultured Cells
HEK293T cells were transiently transfected with vectors expressing human 4R2N WT and P301S Tau (as described above). Media was aspirated after 24 h and replaced with media supplemented with 100 μm cycloheximide. Media and cells were collected at the designated time points after cycloheximide treatment.
Tau Uptake by HEK293T Cells
HEK293T cells were transiently transfected with wild-type (4R2N) Tau in the pcDNA3.1 vector or empty vector (as described above). Media was replaced 24 h after transfection. After an additional 24 h in culture, media was removed and placed on untransfected HEK293T cells, which do not express Tau. Cells were collected 24 and 48 h after treatment. Media and cell lysates were collected and analyzed by ELISA, immunoblotting, and immunocytochemistry.
RESULTS
Increasing evidence in human subjects and cell/mouse models suggests that Tau is detectable outside the cell (2, 15–17, 25). We sought to measure extracellular Tau in cells that endogenously express Tau, to determine the mechanism by which Tau is released, and to determine how genetic variants associated with tauopathies influence extracellular Tau levels.
Extracellular Tau Is Full-length and Monomeric
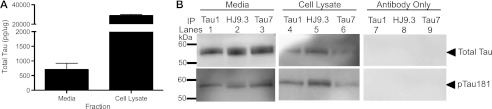
We have previously demonstrated that extracellular total and phospho-Tau (pTau-181 and pTau-396) are detectable in the media in human neuroblastoma SH-SY5Y cells endogenously expressing human Tau (17). To determine whether mouse cortical cells also release Tau into the media, cortical neurons were isolated from the brains of mouse embryos (E18) and grown on an astrocyte monolayer. At DIV14, media and cell lysates were collected and analyzed by ELISA. Total Tau was detectable in both the media and cell lysates of mouse cortical cultures (Fig. 1A). In accordance with observations that we made in human neuroblastoma cells (17), extracellular Tau represents a small fraction of the total Tau detected (Fig. 1A). We were unable to detect extracellular pTau-181 in the media by ELISA (data not shown). However, by immunoprecipitation, we demonstrate that total Tau and pTau-181 are detectable in the media of mouse cortical cultures (Fig. 1B).
FIGURE 1.
Modeling extracellular Tau in neuronal cells. A, intracellular and extracellular total Tau were measured by ELISA in mouse primary mixed neuronal cultures. Values are expressed relative to total intracellular protein, as measured by a BCA assay. Graph represents average ± S.E. Data shown are representative of 4 replicate experiments. B, media (1.5 ml) and cell lysates (100 μl) from mouse primary mixed neuronal cultures were immunoprecipitated with antibodies specific to the central domain (Tau1) and C terminus (HJ9.3 and Tau7) of Tau. Controls were included in which the immunoprecipitated antibody was incubated with the Protein G beads. Immunoblots were probed with an antibody specific to the central domain, which detects all forms of Tau (top, Tau5) and to Tau phosphorylated at Thr-181 (bottom, AT270). Immunoblots are representative of 2 replicate experiments.
A subset of intracellular Tau is truncated in cultured cells (26) and in tangles from autopsy-confirmed AD brain tissue (27, 28). We have previously demonstrated that extracellular Tau is predominantly full-length in immortalized cell lines expressing human Tau (17). To determine whether extracellular Tau is predominantly full-length or truncated in mouse cortical cells, Tau was immunoprecipitated with antibodies with epitopes directed to the central domain (Tau1 and HJ9.3) and the C terminus (Tau7). Immunoblotting with antibodies to total Tau in the central domain (top, Tau5) and pTau-181 (bottom, AT270) resulted in bands corresponding to full-length Tau in the media (Fig. 1B). Together, we demonstrate that mouse cortical cells release full-length Tau that is dephosphorylated and, to a lesser extent, phosphorylated at pTau-181. Thus, intracellular and extracellular Tau levels are comparable in mouse cortical cultures and human neuroblastoma cells (17). We chose to perform the subsequent studies in human neuroblastoma cells, which allowed us to specifically study the human Tau protein.
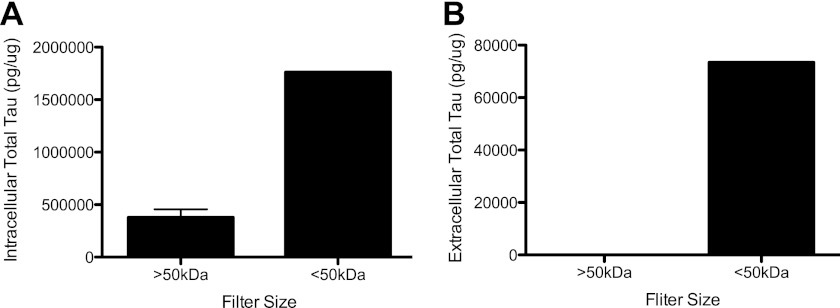
Frost and colleagues (19) have demonstrated that Tau fibrils can propagate from cell to cell. The presence of extracellular Tau monomers, oligomers, or fibrils could explain the propagation of Tau aggregates. To determine which forms of Tau are present outside the cell, cell lysates and media from SH-SY5Y cells were passed through a 50-kDa size exclusion column, which captures proteins that are 70 kDa and smaller in size. In this cell system, intracellular Tau was predominantly 70 kDa and smaller (Fig. 2A). To a lesser extent, we were able to detect intracellular Tau in the fraction representing proteins greater than 70 kDa (Fig. 2A). Thus, our assay is capable of detecting species of Tau that are larger than a monomer. In the media, we could only detect extracellular Tau proteins at or below 70 kDa (Fig. 2B). The absence of extracellular Tau in the >70 kDa fraction does not eliminate the possibility that multimeric Tau exists outside the cell. This ELISA may not be sufficiently sensitive to detect multimeric forms of Tau if they represent a small fraction of the total extracellular Tau. Together, these findings demonstrate that extracellular Tau detected in this assay is predominantly monomeric.
FIGURE 2.
Extracellular tau is predominantly monomeric. Cell lysates (A) and media (B) from SH-SY5Y cells were passed through a 50-kDa size exclusion spin column, and the fractions were analyzed using a total Tau ELISA. Values are expressed relative to total intracellular protein, as measured by a BCA assay. Graphs represent average ± S.E. Figs. are representative of 3 replicate experiments.
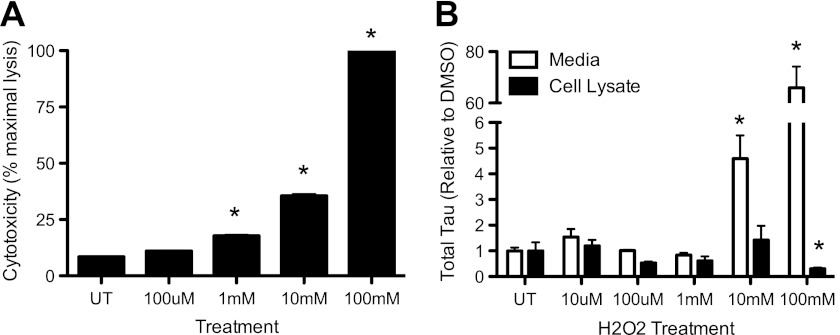
It has been proposed that Tau present in the CSF of AD patients represents Tau released from dying neuronal cells in the brain. To determine the extent to which cell death contributes to changes in extracellular Tau levels in our cell model, we treated SH-SY5Y cells with increasing concentrations of H2O2. Cytotoxicity and intracellular/extracellular Tau levels were then measured. Treatment of cells with 1 mm H2O2 produced significant cytotoxicity compared with untreated controls (Fig. 3A). Cytotoxicity was increased with increasing H2O2 concentrations, achieving 100% toxicity, relative to maximal cell lysis by 1% Triton X-100, with treatment of 100 mm H2O2 (Fig. 3A). Extracellular Tau levels, however, were only significantly elevated in cells achieving at least 40% cell death: 10 and 100 mm H2O2 (Fig. 3B). Extracellular Tau changes that were induced by cytotoxicity were 5–80-fold above controls (Fig. 3B). Thus, low levels of cytotoxicity are not sufficient to increase extracellular Tau levels in our cell model.
FIGURE 3.
Cytotoxicity induces extracellular Tau release. SH-SY5Y cells were treated with increasing concentrations of H2O2 for 6 h. A, cytotoxicity was measured by LDH assay. B, intracellular and extracellular total Tau was measured by ELISA in treated cells. Values are expressed relative to total intracellular protein, as measured by a BCA assay. Graphs represent average ± S.E. White bar, media. Black bar, cell lysate. Data are representative of 4 replicate experiments. *, p < 0.05.
Isoform-specific Release of Extracellular Tau
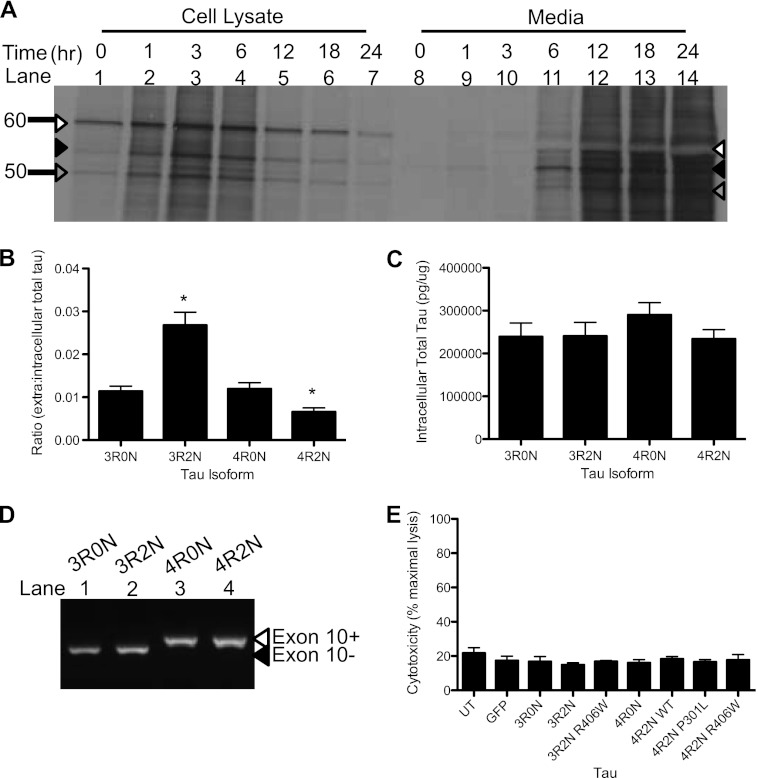
To determine the rate at which endogenous human Tau is released from the cell, we radiolabeled SH-SY5Y cells. In cell lysates, three species of Tau are prominent (Fig. 4A). The most abundant Tau species is very stable (Fig. 4A, white arrow). The other two Tau species are much less abundant and are turned over more rapidly (Fig. 4A, black and gray arrows). In the media, Tau is detectable 6 h after chase (Fig. 4A). Interestingly, the most abundant Tau isoform outside the cell corresponds to a band of lesser abundance in the cell lysates (Fig. 4A, black arrow). Thus, neuroblastoma cells release Tau species at different rates, independent of their abundance in the cell. These findings are in agreement with a recent report in which Tau is measured in mouse brain tissue and interstitial fluid (18). We are unable to define the species of Tau that are most abundant inside and outside these cells. These species may represent phosphorylated and dephosphorylated forms of Tau or Tau isoforms produced by alternative splicing. The specific release of a subset of Tau species in these cells points to an active mechanism of Tau transport out of the cell.
FIGURE 4.
Tau isoforms influence extracellular Tau levels. A, Tau stability in neuroblastoma cells. SH-SY5Y cells, expressing endogenous human Tau, were pulsed with [35S]methionine and chased with complete media. Cell lysates and media were immunoprecipitated with the Tau12 antibody. Arrows mark the Tau species. White arrow, Tau species abundant in cells. Black arrow, Tau species abundant in media. Gray arrow, Tau species equally detectable in cells and media. Image is representative of 3 replicate experiments. B–E, isoform-specific effects on extracellular Tau levels. Vectors containing WT Tau 3R0N, 3R2N, 4R0N, and 4R2N were expressed in HEK293T cells for 24 h. Intracellular and extracellular Tau was measured by total Tau ELISA. Values are expressed relative to total intracellular protein, as measured by a BCA assay. B, extracellular/intracellular Tau ratio. C, intracellular total Tau. D, RNA was extracted from cell lysates and converted to cDNA. Exon 10 of Tau, which is alternatively spliced to produce 4R Tau, was amplified by PCR. White arrow, inclusion of exon 10. Black arrow, exclusion of exon 10. E, Tau isoforms and FTD-associated Tau mutations do not induce cytotoxicity. LDH assay measured as percent maximal lysis. Graphs represent average ± S.E. Data are representative of 4 replicate experiments. *, significantly different from 3R0N and 4R0N (p < 0.01).
SH-SY5Y cells express Tau isoforms that differ in the N-terminal insert and microtubule binding repeat length. SH-SY5Y cells endogenously express the 2N and 0N isoforms of Tau at relative equal levels, and both are more highly expressed than the 1N isoforms of Tau (29). To determine whether the differences in Tau species that we observed inside and outside the cell (Fig. 4A) reflect isoform-specific differences in Tau release, we cloned wild-type (WT) Tau cDNA differing at the N terminus and in the microtubule repeat length (3R0N, 3R2N, 4R0N, 4R2N) into the pcDNA3.1 vector and transiently expressed each construct in HEK293T cells. After 24 h in culture, cells expressing Tau 3R2N exhibited the highest ratio of extracellular/intracellular Tau (Fig. 4B), whereas Tau 4R2N exhibited the lowest ratio of extracellular/intracellular Tau (Fig. 4B). Cells expressing Tau isoforms that do not contain N-terminal exons 2 and 3 (3R0N and 4R0N) have a similar ratio of extracellular/intracellular Tau (Fig. 4B), which is significantly lower than 3R2N and significantly higher than 4R2N. Intracellular Tau protein levels (Fig. 4C) and mRNA levels (Fig. 4D) do not differ between these Tau isoforms, illustrating that differences in extracellular/intracellular Tau ratios are driven by changes in extracellular Tau. Furthermore, Tau isoforms do not induce cytotoxicity (Fig. 4E). Our findings that Tau isoforms influence extracellular Tau levels provides further evidence that Tau release is an active and specific process.
Extracellular Tau Is Released via the Unconventional Secretory Pathway
To determine the mechanism by which neuroblastoma cells release Tau, SH-SY5Y cells, expressing endogenous human Tau, were treated with compounds that inhibit components of the classical and unconventional secretory pathways. Total Tau was then measured in cell lysates and media by ELISA. Cytotoxicity, as assessed by LDH assay, was not observed in any treatment conditions (Fig. 5B). Heat shock of SH-SY5Y cells at 40 °C produced a significant increase in extracellular Tau levels compared with cells incubated at 37 °C (Fig. 5A). Passive transport of proteins is unaffected by temperature change (30). Thus, Tau is not transported out of the cell by passive diffusion. Instead, fitting with our previous observations (Fig. 4), it is likely that Tau is actively transported out of the cell.
FIGURE 5.
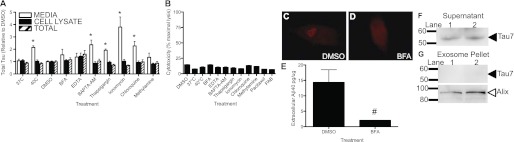
Tau is actively released in human neuroblastoma cells. A, SH-SY5Y cells, expressing endogenous Tau, were treated with compounds that inhibit protein secretory pathways and extracellular (white bar), intracellular (black bar), and total (striped bar) Tau were measured by total Tau ELISA. Values are expressed relative to total intracellular protein, as measured by a BCA assay. Values adjusted for total intracellular protein were then normalized to DMSO controls. Data shown are representative of 6 replicate experiments. B, compounds that do not induce cytotoxicity. LDH assay measured as percent maximal lysis. SH-SY5Y cells, expressing endogenous Tau, were treated with compounds that inhibit protein secretory pathways for 6 h at 37 °C. Data are representative of at least 3 replicates. C-E, cellular response to compounds affecting protein transport. SH-SY5Y cells were fixed in 4% paraformaldehyde. Cells were visualized at ×40 magnification. Cells were stained with an antibody specific to the Golgi apparatus (giantin) and secondary antibody anti-Alexa Fluor 594. C, SH-SY5Y cells were treated with a control compound (DMSO) for 6 h. D, cells were treated with a compound that causes disassembly of the Golgi apparatus (BFA) for 6 h. E, extracellular Aβ40 levels are reduced in the presence of BFA. Media from SH-SY5Y cells treated with BFA for 6 h were analyzed by ELISA (Invitrogen) for Aβ40 levels. Values are expressed relative to total intracellular protein, as measured by a BCA assay. Data shown are representative of 3 replicate experiments. F and G, Tau is not packaged and released in exosomes. SH-SY5Y cells were cultured in exosome-free media for 48 h. Exosomes were purified from the media by a series of high-speed centrifugation steps. Immunoblots were probed with antibodies specific to the C terminus of Tau or Alix, an exosomal marker. F, supernatant. G, exosome pellet. Immunoblots are representative of 2 replicate experiments. Graphs represent average ± S.E. PAB, pseudoloric acid B. *, p < 0.01; #, p < 0.05.
To determine whether Tau is released via the classical secretory pathway, in which proteins are transported from the endoplasmic reticulum to the Golgi apparatus to secretory vesicles, SH-SY5Y cells were treated with 2 μg/ml of BFA for 6 h. BFA causes disassembly of the Golgi complex (Fig. 5, C and D) (31), resulting in inhibition of retrograde and anterograde transport. Total Tau was unchanged in the intracellular and extracellular fractions in the BFA-treated cells compared with DMSO-treated controls (Fig. 5A). Aβ40 levels decrease, as expected, with BFA treatment (Fig. 5E) (32). Thus, it is unlikely that Tau is released primarily via the classical secretory pathway.
Calcium signaling is important in early endosome fusion, exocytosis, and exosome-mediated release (33–35). Thus, we sought to determine the effects of altering calcium levels on extracellular Tau. Treatment of cells with a calcium chelator, 0.5 mm EDTA, did not produce changes in intracellular or extracellular total Tau compared with DMSO-treated controls (Fig. 5A). EDTA, however, is not membrane permeable. Treatment of cells with BAPTA-AM, a membrane permeable calcium chelator, resulted in a significant increase in extracellular total Tau without altering intracellular total Tau levels (Fig. 5A). Conversely, increasing intracellular calcium levels by treatment with 50 nm thapsigargin, which causes cytosolic Ca2+ release from Ca2+ stores in the ER (36), or 10 nm ionomycin, a calcium ionophore, produced significantly elevated extracellular Tau levels without changing intracellular Tau levels (Fig. 5A). Thus, whereas calcium is not required for Tau release, calcium homeostasis is a critical mediator of Tau release.
Calcium signaling is important in the endocytic pathway (33). We examined the effect of inhibition of the endocytic pathway on extracellular Tau. Treatment of cells with 2 mm methylamine, which prevents endosome and lysosome acidification (37, 38), resulted in a slight increase in extracellular Tau (Fig. 5A); however, this increase was not significantly different from DMSO controls. Treatment of cells with 25 μm CQ, which perturbs endosome acidification (34, 39), produced a significant increase in extracellular Tau without changing intracellular Tau (Fig. 5A). CQ also perturbs calcium signaling in the cell (40), providing further evidence that calcium homeostasis influences Tau release.
Aggregation-prone proteins such as prion protein, α-synuclein, and β-amyloid are packaged in multivesicular bodies and released by the cell in exosomes (41–43). To determine whether Tau is also packaged and released in the exosome. SH-SY5Y cells, which express endogenous human Tau, were grown in exosome-free media for 48 h and the exosomal fraction was isolated by a series of centrifugation steps (24). Immunoblotting with antibodies directed to Tau demonstrates that we were able to detect Tau in the supernatant (Fig. 5F); however, we were unable to detect Tau in the exosomal fraction (Fig. 5G). To confirm that exosomes were isolated, the immunoblots were probed with an antibody specific to Alix, a protein highly expressed in exosomes (Fig. 5G). Thus, it is unlikely that the primary mechanism of Tau release is mediated by exosomes.
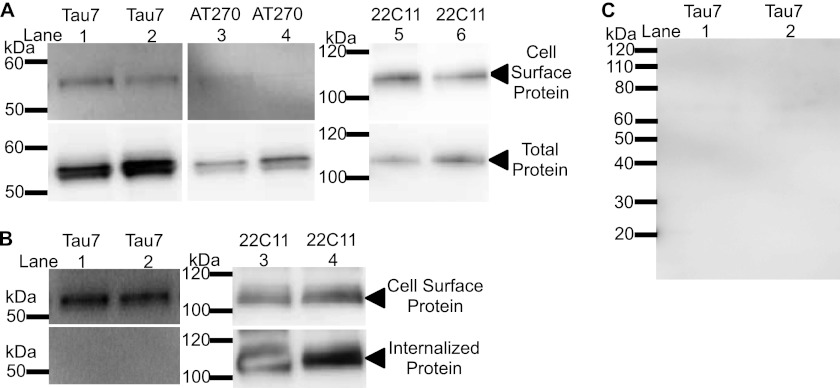
Several mechanisms of unconventional protein secretion involve the release of proteins in the absence of vesicular packaging (reviewed in Ref. 44). Tau is reportedly present at the cell surface (45, 46). First, we confirmed that Tau is detectable at the cell surface in our cell model (Fig. 6A). The Tau detected at the cell surface represents a small fraction of the total Tau detected in the cell (compare Fig. 6A, top and bottom). Some proteins, like APP, are transported to the cell surface only to be recycled back into the cell via the endocytic pathway (Fig. 6B) (47). To determine what happens to Tau proteins after reaching the cell surface, we biotinylated cells on ice to inhibit protein transport and then incubated biotinylated cells at 37 °C to allow biotinylated proteins to be internalized. Biotinylated proteins at the cell surface were then stripped so that biotinylated proteins are exclusively located inside the cell. We failed to detect biotinylated intracellular Tau in this assay (Fig. 6B). However, we also failed to capture biotinylated Tau protein in the culture media 6 h after cells were biotinylated (Fig. 6C). This does not eliminate the possibility that cell surface Tau is released into the media. Because we are attempting to capture a subset of extracellular Tau, which was biotinylated at the cell surface, our methods of measurement may not be sufficiently sensitive. Alternatively, biotinylated extracellular Tau may be processed quickly or proteolyzed in such a way that limits detection. The presence of Tau at the cell surface suggests that Tau may be released via protein conducting channels in the plasma membrane.
FIGURE 6.
Tau is present at the cell surface. Biotinylated proteins and total cell lysates were immunoblotted with an antibody specific to the C terminus of Tau (Tau7), phospho-Tau181 (AT270), and APP (22C11). A, proteins at the cell surface were biotinylated. B, once at the cell surface, Tau is not endocytosed. Endocytosis was inhibited by incubating cells on ice and cell surface proteins were biotinylated. Endocytosis was restarted and internalized, biotinylated proteins were captured. Immunoblots are representative of 6 replicate experiments. C, biotinylated Tau is not detectable in culture media. SH-SY5Y cells were biotinylated for 30 min and the culture media was replaced for 6 h. Biotinylated Tau in the media was immunoblotted with an antibody specific to the C terminus of Tau (Tau7). Immunoblots are representative of 3 replicate experiments.
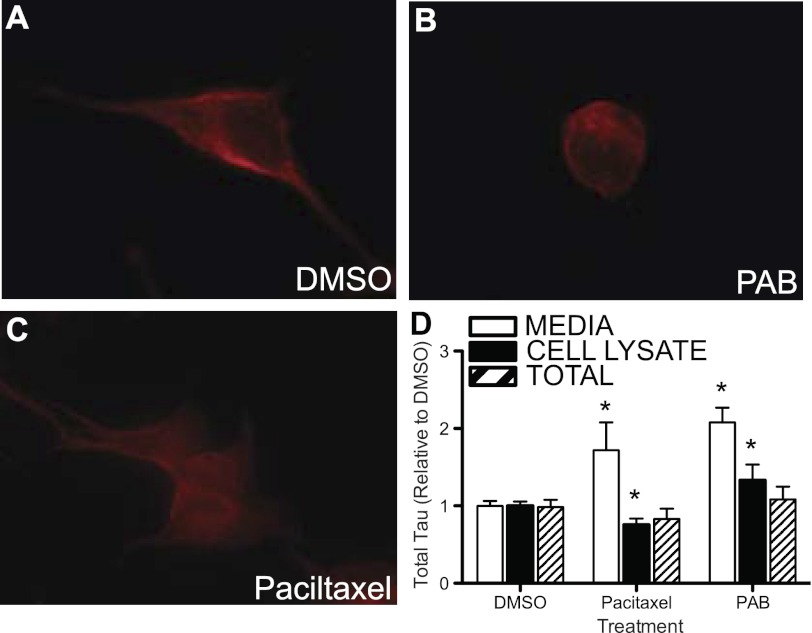
Tau functions to stabilize microtubules. The stabilization or destabilization of microtubules with paclitaxel or PAB, respectively (Fig. 7, A–C), produced significant changes in intracellular and extracellular total Tau levels (Fig. 7D). This indicates that perturbation of Tau function in the cell can lead to altered homeostasis of Tau inside and outside the cell; however, the ability of Tau to bind to microtubules is not required for Tau retention or release.
FIGURE 7.
Tau release is modified by microtubule stability. A–C, microtubule binding stability. SH-SY5Y cells were stained with an antibody specific to tubulin (tub2.1). A, SH-SY5Y cells were treated with a control compound (DMSO) for 6 h. B, cells were treated with a compound that causes destabilization of microtubules (pseudoloric acid B, PAB) for 6 h. C, cells were treated with a compound that stabilizes microtubules (Paciltaxel) for 6 h. D, SH-SY5Y cells were treated with compounds that stabilize or destabilize microtubules. Extracellular (white bar), intracellular (black bar), and total (striped bar) Tau were measured by total Tau ELISA. Values are expressed relative to total intracellular protein, as measured by a BCA assay. Graphs represent average ± S.E. Data are representative of 3 replicate experiments. *, p = 0.01.
FTD-associated Tau Mutations Alter Extracellular Tau Levels
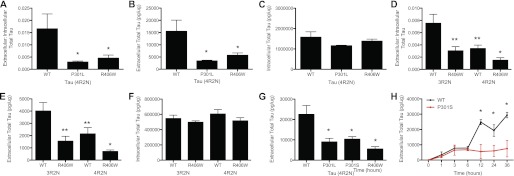
FTD-associated Tau mutations are associated with Tau tangles in the brain. To determine whether FTD-associated Tau mutations alter intracellular or extracellular Tau levels, we overexpressed vectors containing 4R2N Tau WT, P301L, and R406W in HEK293T cells and measured intracellular and extracellular Tau. Extracellular Tau was significantly lower in cells expressing FTD-associated Tau mutations (P301L and R406W) compared with Tau WT-expressing cells (Fig. 8, A and B). Intracellular Tau levels did not differ between mutant and WT expressing cells (Fig. 8C). Similarly, an FTD-associated Tau mutation (R406W) in the 3R2N Tau isoform produced significantly less extracellular Tau compared with 3R2N WT (Fig. 8, D and E) without altering intracellular Tau levels (Fig. 8F). LDH assays demonstrate that the observed differences are not due to cytotoxic effects of the Tau constructs (Fig. 4E). Thus, FTD-associated Tau mutations in 4R and 3R Tau isoforms influence extracellular Tau levels in cultured cells.
FIGURE 8.
FTD-associated Tau mutations produce less extracellular total Tau than WT Tau expressing cells. A–E, vectors containing WT and FTD-associated Tau mutations were expressed in HEK293T cells for 24 h. Total Tau was measured by ELISA. Values are expressed relative to total intracellular protein, as measured by a BCA assay. A–C, FTD-associated Tau mutations were introduced into 4R2N Tau. A, extracellular/intracellular Tau ratio. B, extracellular Tau. C, intracellular Tau. D–F, the FTD-associated Tau mutation R406W was introduced into 3R2N Tau and compared with 4R2N Tau. P301L was not introduced into the 3R2N cDNA because P301L is located in exon 10, which is spliced out of 3R Tau. D, extracellular/intracellular Tau ratio. E, extracellular Tau. F, intracellular Tau. Data are representative of 6 replicate experiments. G and H, Tau P301S is released at a slower rate than WT Tau. G, vectors containing 4R2N Tau WT, P301L, P301S, and R406W were expressed in HEK293T cells for 24 h. Total Tau was measured by ELISA. H, WT and P301S Tau were transiently expressed in HEK293T cells for 24 h and subsequently treated with cycloheximide. Media was then collected at 1, 6, 12, 24, and 36 h and analyzed by total Tau ELISA. Values are expressed relative to total intracellular protein, as measured by a BCA assay. Data are representative of 5 replicate experiments. Graphs represent average ± S.E. * significantly different from Tau WT 4R2N (p < 0.001). **, significantly different from Tau WT 3R2N (p < 0.001).
Because we observed differences in the rate at which certain Tau species are released from the cell (Fig. 4A), we tested whether the observed differences in extracellular Tau levels in WT and mutant-expressing cells are due to the rate of Tau release. Cells expressing 4R2N Tau P301S produced a significant decrease in extracellular Tau levels similar to cells expressing 4R2N Tau P301L and R406W (Fig. 8G). To determine how an FTD-associated Tau mutation influences Tau release rates, cells expressing WT and P301S Tau cells were treated with cycloheximide, which inhibits protein synthesis, and total Tau was measured in the media by ELISA. WT and P301S Tau were released at a similar level for the first 6 h post-cycloheximide treatment; however, after 6 h, WT extracellular Tau levels continued to increase, whereas P301S extracellular Tau levels were stabilized (Fig. 8H). Thus, Tau mutations influence the rate of Tau release in cultured cells.
Cells Propagate Full-length Wild-type Tau
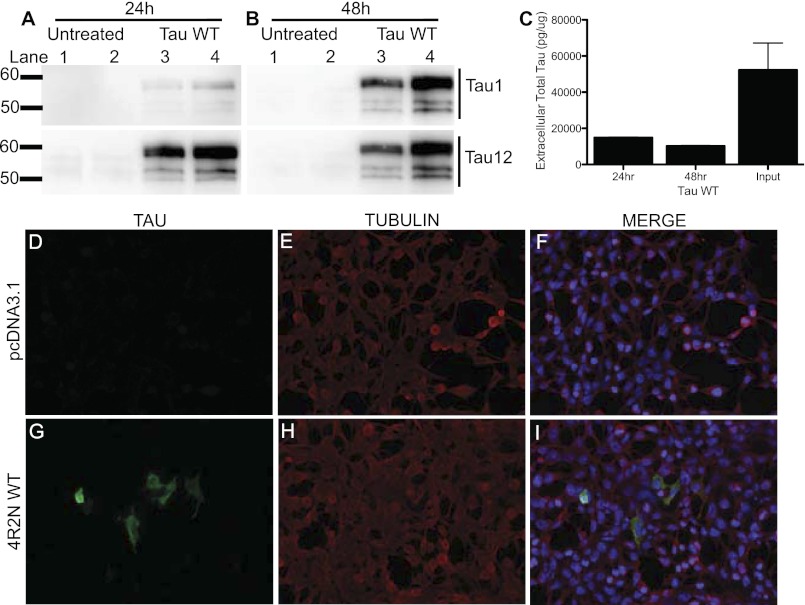
Cells can internalize extracellular aggregates of the microtubule-binding region of Tau (19, 48). To determine whether cells can take up extracellular full-length Tau in this cell system, we treated naive HEK293T cells, which do not express endogenous Tau, with conditioned media from cells overexpressing Tau. Within 24 h of treatment with conditioned Tau media, Tau was detectable in the intracellular HEK293T cell lysate (Fig. 9A). The proportion of total intracellular Tau was significantly increased 48 h after treatment (Fig. 9B). The amount of Tau in the media was significantly reduced after the 24- and 48-h treatments (Fig. 9C). This observed decrease likely represents Tau that is taken up by the cell. This reduction may also represent some turnover of the Tau protein; however, we demonstrate that Tau is very stable in this system. In cells treated with conditioned Tau media, Tau and microtubules co-localize, indicating that once taken up by the cell, Tau retains its normal function (Fig. 9, G–I). Thus, Tau metabolism represents a dynamic balance between intracellular and extracellular Tau protein.
FIGURE 9.
Extracellular full-length Tau is taken up by the cell. HEK293T cells were cultured with media from WT Tau-expressing cells for 24 or 48 h. A and B, cell lysates were extracted in non-ionic detergent, and immunoblots were probed with antibodies to the N terminus of Tau (Tau12) and the central domain of Tau (Tau1). A, 24 h. B, 48 h. C, media from cells overexpressing WT Tau (input) and from cells after treatment for 24 or 48 h were assayed with a total Tau ELISA. Values are expressed relative to total intracellular protein, as measured by a BCA assay. Graphs represent average ± S.E. Data are representative of 4 replicate experiments. Media from cells transiently expressing pcDNA3.1-empty vector (D–F) or from cells transiently expressing Tau WT-4R2N (G–I) were incubated on naive HEK293T cells for 48 h. Cells were fixed in 4% paraformaldehyde and stained with the Tub2.1 antibody. Cells were visualized at ×20 magnification. Images are representative of 2 replicate experiments.
DISCUSSION
The presence of Tau in human CSF from healthy and diseased individuals suggests that Tau is released by the cell under normal and pathologic conditions (2). In this study, we sought to characterize the mechanism by which endogenous and mutant Tau is released in cultured cells. Full-length, monomeric extracellular Tau is present in immortalized cell lines and primary neuronal cultures. Endogenous Tau release is influenced by calcium via the unconventional secretory pathway. Furthermore, Tau isoforms and FTD-associated Tau mutations influence the rate of Tau release. Thus, cells actively release endogenous Tau in the absence of toxicity, and Tau release is modified by genetic changes in Tau that are associated with tauopathies.
Previous studies in immortalized cell lines overexpressing Tau have demonstrated that a fraction of total Tau is present outside the cell (15–17). In this study, we demonstrate that extracellular Tau (total and phospho-Tau) is present in the media of neuroblastoma cells (SH-SY5Y) and in primary mouse cortical cultures in the absence of overexpression. We have further demonstrated that endogenous Tau is actively released by the cell. We provide two independent lines of evidence for this conclusion: 1) in pulse-chase experiments, specific Tau species are more rapidly released than other Tau species and 2) heat shocked cells produce more extracellular Tau than cells cultured at 37 °C. Thus, our cell culture studies, together with observations in human CSF studies in healthy individuals, provides evidence for the hypothesis that during normal Tau metabolism, a fraction of Tau is released by the cell.
The mechanism by which Tau is actively released from the cell is complex. We demonstrate that endogenous human Tau is actively released via the unconventional secretory pathway, as Tau levels are not influenced by Golgi apparatus disassembly. The unconventional secretory pathway involves vesicular and non-vesicular pathways that are independent of the ER/Golgi-mediated secretory pathway (49); however, these pathways are poorly characterized. Calcium signaling plays many important roles in the cell. Our findings that alteration of intracellular calcium levels in either direction increases extracellular Tau levels suggests that calcium homeostasis is critical for controlling intracellular and extracellular Tau levels.
Several reports suggest that extracellular recombinant Tau induces an increase in intracellular calcium levels by binding to and activating muscarinic receptors (50, 51). Exogenous α-synuclein, β-amyloid, and prion protein also induce calcium dysregulation in cultured neurons (52, 53). Dysregulation of intracellular calcium signaling may play a role in AD pathogenesis (54–56). Based on our findings, we hypothesize that dysregulation of intracellular calcium levels in the neurons of AD brains could contribute to higher levels of extracellular Tau, which are reflected in increased CSF Tau levels in AD patients.
Aggregation prone proteins that are associated with neurodegenerative diseases have been reported to be released from the cell in exosomes (41–43, 57, 58). Despite recent reports (59, 60), we failed to detect extracellular Tau in exosomes from neuroblastoma cells expressing endogenous human Tau. Similarly, Tau is not detectable in exosomes purified from primary mouse neuronal cultures (61, 62). Extracellular Tau is reported to co-localize with exosomes; however, Tau only appears to co-localize with exosomes when Tau expression levels are high (59, 60). The cell may package Tau in exosomes to compensate for protein overexpression; however, this is not relevant to normal Tau metabolism or disease. Alternatively, Tau may stick to the outside of exosomes. If extracellular Tau were primarily packaged into exosomes, we would not detect the protein in the nondenaturing conditions used in this study. Additionally, Yamada and colleagues (18) demonstrate that human and mouse Tau are detectable in the interstitial fluid and CSF using a 100-kDa microdialysis probe, which is not large enough to capture exosomes. Thus, the majority of extracellular Tau is not packaged in vesicular structures when transported out of the cell.
All 4 isoforms of Tau expressed in HEK293T cells (3R0N, 3R2N, 4R0N, and 4R2N) were detected outside of the cell. We propose that all Tau isoforms are released by a similar mechanism. We hypothesize that the differences that we observe between the 4 isoforms are due to the way that each protein folds, which may facilitate or hinder transport across the plasma membrane.
We hypothesize that the cell actively releases Tau and that genetic factors enriched in patients with tauopathies alter the release of Tau from cells in the brain. CSF total/pTau-181 levels are significantly elevated in AD patients compared with age-matched, cognitively normal controls (4, 8). In symptomatic FTD patients carrying the P301L MAPT mutation, CSF total Tau levels are elevated relative to controls but lower than symptomatic AD patients (10); however, little is known of how CSF Tau changes over the course of this tauopathy. We demonstrate that extracellular Tau levels are significantly lower in cells expressing FTD-associated Tau mutations compared with WT-expressing cells. This difference may reflect a slower rate of Tau release from the cell. If this is the case, retention of mutant Tau by the cell has implications for aggregation and downstream toxic affects. Alternatively, the Tau released by mutant-expressing cells may adopt protein conformations that cannot be captured by this assay. Yamada and colleagues (18) have demonstrated that interstitial fluid Tau levels drop in symptomatic Tau-P301S transgenic mice (12 months) compared with young Tau-P301S mice, which correlates with the accumulation of formic acid soluble Tau in the hippocampus. Together, we provide evidence for a shared phenotype in cells expressing FTD-associated Tau mutations that are located in 2 different exons (P301L and R406W) and which have differing effects on microtubule assembly (63).
Tau splicing has also been implicated as a risk factor for tauopathies. Several FTD-associated Tau mutations produce altered Tau splicing, resulting in higher expression levels of Tau with exon 10 (4R) in the brain. Additionally, two extended haplotypes, spanning 1.8 Mb, occur in the MAPT region. The H1 Tau haplotype is associated with increased risk for several sporadic tauopathies: progressive supranuclear palsy (64), corticobasal degeneration (65), Alzheimer disease (66), and Parkinson disease (67). In AD brains, the H1 Tau haplotype is associated with an increase in the ratio of 4R to 3R Tau mRNA (68). SH-SY5Y cells, which express endogenous human Tau, are homozygous for the Tau H1 haplotype (data not shown). Our studies indicate that Tau isoforms are released at differing rates, whereby both the N terminus and microtubule binding repeat length contribute to release rate: 4R Tau isoforms (4R2N and 4R0N) are much less abundant outside the cell compared with 3R Tau isoforms (3R2N and 3R0N). Our observations that 4R Tau isoforms are less abundant outside the cell than 3R Tau isoforms suggests that the differential splicing of the Tau protein observed in FTD may also influence the retention of certain Tau isoforms within the cell. Together, we demonstrate that Tau is an intracellular and extracellular protein. The relative ratio of intracellular and extracellular Tau may be important for normal cell function, as FTD and Tau isoforms associated with tauopathies alter these ratios.
Acknowledgments
We thank Joy Snider and the Hope Center Viral Vector Core at Washington University for assistance in preparation of the primary mouse cortical cells. We thank Virginia Lee for providing 3R0N and 4R2N Tau constructs and Michel Goedert for providing 3R2N and 4R0N Tau constructs.
This work was supported, in whole or in part, by National Institutes of Health Grant 5P01AG003991–29 (to John C. Morris) and AstraZeneca.
- AD
- Alzheimer disease
- FTD
- frontotemporal dementia
- DMSO
- dimethyl sulfoxide
- PAB
- pseudoloric acid B
- LDH
- lactate dehydrogenase
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- BFA
- brefeldin A
- CSF
- cerebrospinal fluid.
REFERENCES
- 1. Cleveland D. W., Hwo S. Y., Kirschner M. W. (1977) Purification of Tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J. Mol. Biol. 116, 207–225 [DOI] [PubMed] [Google Scholar]
- 2. Craig-Schapiro R., Fagan A. M., Holtzman D. M. (2009) Biomarkers of Alzheimer disease. Neurobiol. Dis. 35, 128–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Csernansky J. G., Miller J. P., McKeel D., Morris J. C. (2002) Relationships among cerebrospinal fluid biomarkers in dementia of the Alzheimer type. Alzheimer Dis. Assoc. Disord. 16, 144–149 [DOI] [PubMed] [Google Scholar]
- 4. Galasko D., Chang L., Motter R., Clark C. M., Kaye J., Knopman D., Thomas R., Kholodenko D., Schenk D., Lieberburg I., Miller B., Green R., Basherad R., Kertiles L., Boss M. A., Seubert P. (1998) High cerebrospinal fluid tau and low amyloid β42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch. Neurol. 55, 937–945 [DOI] [PubMed] [Google Scholar]
- 5. Green A. J., Harvey R. J., Thompson E. J., Rossor M. N. (1999) Increased Tau in the cerebrospinal fluid of patients with frontotemporal dementia and Alzheimer disease. Neurosci. Lett. 259, 133–135 [DOI] [PubMed] [Google Scholar]
- 6. Jensen M., Basun H., Lannfelt L. (1995) Increased cerebrospinal fluid Tau in patients with Alzheimer disease. Neurosci. Lett. 186, 189–191 [DOI] [PubMed] [Google Scholar]
- 7. Tato R. E., Frank A., Hernanz A. (1995) Tau protein concentrations in cerebrospinal fluid of patients with dementia of the Alzheimer type. J. Neurol. Neurosurg. Psychiatr. 59, 280–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vigo-Pelfrey C., Seubert P., Barbour R., Blomquist C., Lee M., Lee D., Coria F., Chang L., Miller B., Lieberburg I. (1995) Elevation of microtubule-associated protein Tau in the cerebrospinal fluid of patients with Alzheimer disease. Neurology 45, 788–793 [DOI] [PubMed] [Google Scholar]
- 9. Fagan A. M., Roe C. M., Xiong C., Mintun M. A., Morris J. C., Holtzman D. M. (2007) Cerebrospinal fluid Tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol. 64, 343–349 [DOI] [PubMed] [Google Scholar]
- 10. Hampel H., Teipel S. J. (2004) Total and phosphorylated Tau proteins. Evaluation as core biomarker candidates in frontotemporal dementia. Dement. Geriatr. Cogn. Disord. 17, 350–354 [DOI] [PubMed] [Google Scholar]
- 11. Hesse C., Rosengren L., Andreasen N., Davidsson P., Vanderstichele H., Vanmechelen E., Blennow K. (2001) Transient increase in total Tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci. Lett. 297, 187–190 [DOI] [PubMed] [Google Scholar]
- 12. Ost M., Nylén K., Csajbok L., Ohrfelt A. O., Tullberg M., Wikkelsö C., Nellgård P., Rosengren L., Blennow K., Nellgård B. (2006) Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology 67, 1600–1604 [DOI] [PubMed] [Google Scholar]
- 13. Kauwe J. S., Cruchaga C., Mayo K., Fenoglio C., Bertelsen S., Nowotny P., Galimberti D., Scarpini E., Morris J. C., Fagan A. M., Holtzman D. M., Goate A. M. (2008) Variation in MAPT is associated with cerebrospinal fluid Tau levels in the presence of amyloid-β deposition. Proc. Natl. Acad. Sci. U.S.A. 105, 8050–8054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cruchaga C., Kauwe J. S. K., Mayo K., Spiegel N., Bertelsen S., Nowotny P., Shah A. R., Abraham R., Hollingworth P., Harold D., Owen M. M., Williams J., Lovestone S., Peskind E. R., Li G., Leverenz J. B., Galasko D., Initiative A. s. D. N., Morris J. C., Fagan A. M., Holtzman D. M., Goate A. M. (2010) SNPs associated with cerebrospinal fluid phospho-Tau levels influence rate of decline in Alzheimer disease. PLoS Genet. 6, e31039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim W., Lee S., Hall G. F. (2010) Secretion of human Tau fragments resembling CSF-Tau in Alzheimer disease is modulated by the presence of the exon 2 insert. FEBS Lett. 584, 3085–3088 [DOI] [PubMed] [Google Scholar]
- 16. Kim W., Lee S., Jung C., Ahmed A., Lee G., Hall G. F. (2010) Interneuronal transfer of human Tau between Lamprey central neurons in situ. J. Alzheimers Dis. 19, 647–664 [DOI] [PubMed] [Google Scholar]
- 17. Karch C. M., Jeng A. T., Goate A. M. (2012) Calcium phosphatase calcineurin influences Tau metabolism. Neurobiol. Aging, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamada K., Cirrito J. R., Stewart F. R., Jiang H., Finn M. B., Holmes B. B., Binder L. I., Mandelkow E. M., Diamond M. I., Lee V. M., Holtzman D. M. (2011) In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid Tau levels in P301S human Tau transgenic mice. J. Neurosci. 31, 13110–13117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frost B., Jacks R. L., Diamond M. I. (2009) Propagation of Tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 284, 12845–12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clavaguera F., Bolmont T., Crowther R. A., Abramowski D., Frank S., Probst A., Fraser G., Stalder A. K., Beibel M., Staufenbiel M., Jucker M., Goedert M., Tolnay M. (2009) Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 11, 909–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu L., Drouet V., Wu J. W., Witter M. P., Small S. A., Clelland C., Duff K. (2012) Trans-synaptic spread of Tau pathology in vivo. PLoS One 7, e31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi D. W. (1987) Ionic dependence of glutamate neurotoxicity. J. Neurosci. 7, 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 24. Théry C., Amigorena S., Raposo G., Clayton A. (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. Suppl. 30, 3.22.1–3.22.29 [DOI] [PubMed] [Google Scholar]
- 25. Ono T., Yamamoto H., Tashima K., Nakashima H., Okumura E., Yamada K., Hisanaga S., Kishimoto T., Miyakawa T., Miyamoto E. (1995) Dephosphorylation of abnormal sites of Tau factor by protein phosphatases and its implication for Alzheimer disease. Neurochem. Int. 26, 205–215 [DOI] [PubMed] [Google Scholar]
- 26. Wang Y. P., Biernat J., Pickhardt M., Mandelkow E., Mandelkow E. M. (2007) Stepwise proteolysis liberates Tau fragments that nucleate the Alzheimer-like aggregation of full-length Tau in a neuronal cell model. Proc. Natl. Acad. Sci. U.S.A. 104, 10252–10257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gamblin T. C., Chen F., Zambrano A., Abraha A., Lagalwar S., Guillozet A. L., Lu M., Fu Y., Garcia-Sierra F., LaPointe N., Miller R., Berry R. W., Binder L. I., Cryns V. L. (2003) Caspase cleavage of Tau. Linking amyloid and neurofibrillary tangles in Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 100, 10032–10037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horowitz P. M., Patterson K. R., Guillozet-Bongaarts A. L., Reynolds M. R., Carroll C. A., Weintraub S. T., Bennett D. A., Cryns V. L., Berry R. W., Binder L. I. (2004) Early N-terminal changes and caspase-6 cleavage of Tau in Alzheimer disease. J. Neurosci. 24, 7895–7902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agholme L., Lindström T., Kågedal K., Marcusson J., Hallbeck M. (2010) An in vitro model for neuroscience. Differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheimers Dis. 20, 1069–1082 [DOI] [PubMed] [Google Scholar]
- 30. Melchior F., Gerace L. (1995) Mechanisms of nuclear protein import. Curr. Opin. Cell Biol. 7, 310–318 [DOI] [PubMed] [Google Scholar]
- 31. Lee H. J., Patel S., Lee S. J. (2005) Intravesicular localization and exocytosis of α-synuclein and its aggregates. J. Neurosci. 25, 6016–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. (1992) Production of the Alzheimer amyloid β protein by normal proteolytic processing. Science 258, 126–129 [DOI] [PubMed] [Google Scholar]
- 33. Mills I. G., Urbé S., Clague M. J. (2001) Relationships between EEA1 binding partners and their role in endosome fusion. J. Cell Sci. 114, 1959–1965 [DOI] [PubMed] [Google Scholar]
- 34. Savina A., Furlán M., Vidal M., Colombo M. I. (2003) Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 278, 20083–20090 [DOI] [PubMed] [Google Scholar]
- 35. Barclay J. W., Morgan A., Burgoyne R. D. (2005) Calcium-dependent regulation of exocytosis. Cell Calcium 38, 343–353 [DOI] [PubMed] [Google Scholar]
- 36. Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. (1990) Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl. Acad. Sci. U.S.A. 87, 2466–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lindstedt R., Apodaca G., Barondes S. H., Mostov K. E., Leffler H. (1993) Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J. Biol. Chem. 268, 11750–11757 [PubMed] [Google Scholar]
- 38. Geetha T., Wooten M. W. (2008) TrkA receptor endolysosomal degradation is both ubiquitin and proteasome dependent. Traffic 9, 1146–1156 [DOI] [PubMed] [Google Scholar]
- 39. Andrei C., Dazzi C., Lotti L., Torrisi M. R., Chimini G., Rubartelli A. (1999) The secretory route of the leaderless protein interleukin 1β involves exocytosis of endolysosome-related vesicles. Mol. Biol. Cell 10, 1463–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Misra U. K., Gawdi G., Pizzo S. V. (1997) Chloroquine, quinine and quinidine inhibit calcium release from macrophage intracellular stores by blocking inositol 1,4,5-trisphosphate binding to its receptor. J. Cell. Biochem. 64, 225–232 [DOI] [PubMed] [Google Scholar]
- 41. Rajendran L., Honsho M., Zahn T. R., Keller P., Geiger K. D., Verkade P., Simons K. (2006) Alzheimer disease β-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. U.S.A. 103, 11172–11177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fevrier B., Vilette D., Archer F., Loew D., Faigle W., Vidal M., Laude H., Raposo G. (2004) Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. U.S.A. 101, 9683–9688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Emmanouilidou E., Melachroinou K., Roumeliotis T., Garbis S. D., Ntzouni M., Margaritis L. H., Stefanis L., Vekrellis K. (2010) Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 30, 6838–6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nickel W. (2005) Unconventional secretory routes. Direct protein export across the plasma membrane of mammalian cells. Traffic 6, 607–614 [DOI] [PubMed] [Google Scholar]
- 45. Lee G., Newman S. T., Gard D. L., Band H., Panchamoorthy G. (1998) Tau interacts with src-family non-receptor tyrosine kinases. J. Cell Sci. 111, 3167–3177 [DOI] [PubMed] [Google Scholar]
- 46. Bhaskar K., Yen S. H., Lee G. (2005) Disease-related modifications in Tau affect the interaction between Fyn and Tau. J. Biol. Chem. 280, 35119–35125 [DOI] [PubMed] [Google Scholar]
- 47. Thinakaran G., Koo E. H. (2008) Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 283, 29615–29619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo J. L., Lee V. M. (2011) Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J. Biol. Chem. 286, 15317–15331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nickel W., Rabouille C. (2009) Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 10, 148–155 [DOI] [PubMed] [Google Scholar]
- 50. Gómez-Ramos A., Díaz-Hernández M., Rubio A., Miras-Portugal M. T., Avila J. (2008) Extracellular Tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Mol. Cell Neurosci. 37, 673–681 [DOI] [PubMed] [Google Scholar]
- 51. Gómez-Ramos A., Díaz-Hernández M., Rubio A., Díaz-Hernández J. I., Miras-Portugal M. T., Avila J. (2009) Characteristics and consequences of muscarinic receptor activation by Tau protein. Eur. Neuropsychopharmacol. 19, 708–717 [DOI] [PubMed] [Google Scholar]
- 52. Danzer K. M., Haasen D., Karow A. R., Moussaud S., Habeck M., Giese A., Kretzschmar H., Hengerer B., Kostka M. (2007) Different species of α-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 27, 9220–9232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Demuro A., Mina E., Kayed R., Milton S. C., Parker I., Glabe C. G. (2005) Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J. Biol. Chem. 280, 17294–17300 [DOI] [PubMed] [Google Scholar]
- 54. LaFerla F. M. (2002) Calcium dyshomeostasis and intracellular signalling in Alzheimer disease. Nat. Rev. Neurosci. 3, 862–872 [DOI] [PubMed] [Google Scholar]
- 55. Mattson M. P., Chan S. L. (2003) Neuronal and glial calcium signaling in Alzheimer disease. Cell Calcium 34, 385–397 [DOI] [PubMed] [Google Scholar]
- 56. Stutzmann G. E. (2005) Calcium dysregulation, IP3 signaling, and Alzheimer disease. Neuroscientist 11, 110–115 [DOI] [PubMed] [Google Scholar]
- 57. Saman S., Kim W., Raya M., Visnick Y., Miro S., Saman S., Jackson B., McKee A. C., Alvarez V. E., Lee N. C., Hall G. F. (2012) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 287, 3842–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Simón D., García-García E., Gómez-Ramos A., Falcón-Pérez J. M., Díaz-Hernández M., Hernández F., Avila J. (2012) Tau overexpression results in its secretion via membrane vesicles. Neurodegener. Dis. 10, 73–75 [DOI] [PubMed] [Google Scholar]
- 59. Chang H. C., Samaniego F., Nair B. C., Buonaguro L., Ensoli B. (1997) HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 11, 1421–1431 [DOI] [PubMed] [Google Scholar]
- 60. Simón D., García-García E., Royo F., Falcón-Pérez J. M., Avila J. (2012) Proteostasis of Tau. Tau overexpression results in its secretion via membrane vesicles. FEBS Lett. 586, 47–54 [DOI] [PubMed] [Google Scholar]
- 61. Fauré J., Lachenal G., Court M., Hirrlinger J., Chatellard-Causse C., Blot B., Grange J., Schoehn G., Goldberg Y., Boyer V., Kirchhoff F., Raposo G., Garin J., Sadoul R. (2006) Exosomes are released by cultured cortical neurones. Mol. Cell Neurosci. 31, 642–648 [DOI] [PubMed] [Google Scholar]
- 62. Santa-Maria I., Varghese M., Ksiezak-Reding H., Dzhun A., Wang J., Pasinetti G. M. (2012) Paired helical filaments from Alzheimer disease brain induce intracellular accumulation of Tau in aggresomes. J. Biol. Chem. 287, 20522–20533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dayanandan R., Van Slegtenhorst M., Mack T. G., Ko L., Yen S. H., Leroy K., Brion J. P., Anderton B. H., Hutton M., Lovestone S. (1999) Mutations in Tau reduce its microtubule binding properties in intact cells and affect its phosphorylation. FEBS Lett. 446, 228–232 [DOI] [PubMed] [Google Scholar]
- 64. Baker M., Litvan I., Houlden H., Adamson J., Dickson D., Perez-Tur J., Hardy J., Lynch T., Bigio E., Hutton M. (1999) Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum. Mol. Genet. 8, 711–715 [DOI] [PubMed] [Google Scholar]
- 65. Houlden H., Baker M., Morris H. R., MacDonald N., Pickering-Brown S., Adamson J., Lees A. J., Rossor M. N., Quinn N. P., Kertesz A., Khan M. N., Hardy J., Lantos P. L., St George-Hyslop P., Munoz D. G., Mann D., Lang A. E., Bergeron C., Bigio E. H., Litvan I., Bhatia K. P., Dickson D., Wood N. W., Hutton M. (2001) Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology 56, 1702–1706 [DOI] [PubMed] [Google Scholar]
- 66. Myers A. J., Kaleem M., Marlowe L., Pittman A. M., Lees A. J., Fung H. C., Duckworth J., Leung D., Gibson A., Morris C. M., de Silva R., Hardy J. (2005) The H1c haplotype at the MAPT locus is associated with Alzheimer disease. Hum. Mol. Genet. 14, 2399–2404 [DOI] [PubMed] [Google Scholar]
- 67. Simón-Sánchez J., Schulte C., Bras J. M., Sharma M., Gibbs J. R., Berg D., Paisan-Ruiz C., Lichtner P., Scholz S. W., Hernandez D. G., Krüger R., Federoff M., Klein C., Goate A., Perlmutter J., Bonin M., Nalls M. A., Illig T., Gieger C., Houlden H., Steffens M., Okun M. S., Racette B. A., Cookson M. R., Foote K. D., Fernandez H. H., Traynor B. J., Schreiber S., Arepalli S., Zonozi R., Gwinn K., van der Brug M., Lopez G., Chanock S. J., Schatzkin A., Park Y., Hollenbeck A., Gao J., Huang X., Wood N. W., Lorenz D., Deuschl G., Chen H., Riess O., Hardy J. A., Singleton A. B., Gasser T. (2009) Genome-wide association study reveals genetic risk underlying Parkinson disease. Nat. Genet. 41, 1308–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Myers A. J., Pittman A. M., Zhao A. S., Rohrer K., Kaleem M., Marlowe L., Lees A., Leung D., McKeith I. G., Perry R. H., Morris C. M., Trojanowski J. Q., Clark C., Karlawish J., Arnold S., Forman M. S., Van Deerlin V., de Silva R., Hardy J. (2007) The MAPT H1c risk haplotype is associated with increased expression of Tau and especially of 4 repeat containing transcripts. Neurobiol. Dis. 25, 561–570 [DOI] [PubMed] [Google Scholar]