Background: Sonic hedgehog (Shh) is a secreted protein that needs to be palmitoylated to signal.
Results: The first six amino acids of mature Shh are sufficient for palmitoylation.
Conclusion: Hedgehog acyltransferase, the enzyme that palmitoylates Shh, recognizes N-terminal substrate residues.
Significance: This serves as a model for understanding how secreted morphogens are modified by distinct palmitoyl acyltransferases.
Keywords: Hedgehog, Post-translational Modification, Protein Acylation, Protein Palmitoylation, Secretion, Hhat, Rasp, Hedgehog Acyltransferase
Abstract
Sonic Hedgehog (Shh) is a secreted morphogen that regulates embryonic development. After removal of the signal peptide, Shh is processed to the mature, active form through autocleavage and a series of lipid modifications, including the attachment of palmitate. Covalent attachment of palmitate to the N-terminal cysteine of Shh is catalyzed by Hedgehog acyltransferase (Hhat) and is critical for proper signaling. The sequences within Shh that are responsible for palmitoylation by Hhat are not known. Here we show that the first six amino acids of mature Shh (CGPGRG) are sufficient for Hhat-mediated palmitoylation. Alanine scanning mutagenesis was used to determine the role of each amino acid and the positional sequence requirement in a cell-based Shh palmitoylation assay. Mutation of residues in the GPGR sequence to Ala had no effect on palmitoylation, provided that a positively charged residue was present within the first seven residues. The N-terminal position exhibited a strong but not exclusive requirement for Cys. Constructs with an N-terminal Ala were not palmitoylated. However, an N-terminal Ser served as a substrate for Hhat, but not the Drosophila melanogaster ortholog Rasp, highlighting a critical difference between the mammalian and fly enzymes. These findings define residues and regions within Shh that are necessary for its recognition as a substrate for Hhat-mediated palmitoylation. Finally, we report the results of a bioinformatics screen to identify other potential Hhat substrates encoded in the human genome.
Introduction
Sonic Hedgehog (Shh) is a secreted signaling protein that acts in a concentration-dependent manner to regulate embryonic patterning, differentiation, and growth (1, 2). Mutations in the Shh signaling pathway result in defective neural tube and limb development and are responsible for a number of human congenital malformations such as holoprosencephaly and congenital ataxia (3–5). In adults, aberrant Shh signaling promotes the initiation and progression of multiple cancers, including gastrointestinal tumors, medulloblastoma, and prostate and pancreatic cancer (6, 7).
Shh undergoes a series of processing events, beginning with the removal of the N-terminal signal sequence as the protein enters the secretory pathway (8). The C-terminal region of the Shh precursor facilitates an autocleavage reaction that produces the 19-kDa N-terminal product, ShhN, and a 26-kDa C-terminal product, ShhC. ShhN undergoes modification by two distinct lipids (8). During autoprocessing, cholesterol is attached through an ester linkage to the C-terminal glycine (8, 9). In a separate reaction, Hedgehog acyltransferase (Hhat)2 catalyzes the attachment of palmitate to the N terminus of ShhN via an amide linkage (8, 10, 11). The dually lipidated protein, ShhNp, represents the mature, activated signaling molecule, which is then secreted from the cell (8). A similar set of processing reactions occurs on the Drosophila melanogaster protein Hedgehog, Hh, which is palmitoylated by the Hhat ortholog, Rasp (10, 12).
Attachment of both cholesterol and palmitate is required for efficient long and short range Shh signaling (13). Modification of Shh by cholesterol increases Shh membrane binding, restricts Shh diffusion and generates a defined Shh spatial gradient (9, 14, 15). A critical role for palmitoylation of Shh has been established both in vitro and in vivo. Palmitate attachment to Shh increases its signaling potency when assayed in cell culture (13, 16, 17). Palmitoylation has been shown to be required for the formation of active soluble multimeric Shh complexes, which mediate Shh signaling in reporter-based assays (13, 18). When nonpalmitoylated Shh is expressed in transgenic mice, its distribution in the developing embryos is disrupted, resulting in defects in neural tube and limb patterning (13). Furthermore, Hhat knock-out mice exhibit neural tube and limb patterning defects similar to those observed in Shh knock-out mice and nonpalmitoylated Shh transgenic animals (13). The phenotype in flies is more severe, because nonpalmitoylated Hh has essentially no patterning activity (19, 20).
A recent study from our laboratory identified regions and amino acid residues in Hhat that are required to catalyze attachment of palmitate to Shh (21). However, little is known about the regions within Shh that are involved in palmitoylation. Attachment of palmitate to Shh requires an N-terminal cysteine with a free N terminus (11), but the role of the surrounding amino acids has not been explored. To determine the minimal sequence for palmitoylation and to identify the regions of Shh that are involved in facilitating palmitate attachment, we created a series of Shh truncation mutants and tested their ability to incorporate palmitate in a cell-based assay. Here we report that a “mini-Shh” construct, Shh(1–29) EGFP, contains sequence information sufficient for Hhat-mediated palmitoylation in cells. Shh(1–29) EGFP consists of the signal peptide (amino acids 1–23) and the first six amino acids of mature Shh (CGPGRG) fused to EGFP. Alanine scanning mutagenesis was performed to determine the role of each individual amino acid in this region and the positional sequence requirements for palmitoylation. Using this information, we created an algorithm that defines an initial set of parameters for Hhat-mediated palmitoylation. We then used a bioinformatics screen to derive a list of potential Hhat substrates.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Anti-HA, anti-GFP (rabbit), and anti-FLAG (rabbit and mouse) antibodies were purchased from Sigma. Anti-GFP (mouse) antibody was purchased from Roche Applied Science. Anti-Shh antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Protein A+G-agarose beads were obtained from Alpha Diagnostic International Inc. (Burlington, Canada). [125I]NaI was purchased from PerkinElmer Life Sciences. The QuikChange site-Directed mutagenesis kit was purchased from Stratagene. Lipofectamine 2000 transfection reagent was purchased from Invitrogen.
cDNA Constructs
Plasmids encoding HA-tagged Hhat, HA-Rasp, Spitz-EGFP, and full-length Shh were generated as previously described (11, 22). A plasmid for expression of D. melanogaster Hh was constructed by replacing the signal sequence of Hh with that of Spitz, as follows. The cDNA sequence encoding residues 85–471 of Hh was amplified by PCR. The PCR product was then ligated to the 3′ end of the Spitz signal sequence in pcDNA3.1 using two StuI restriction sites. Shh EGFP fusions were constructed as follows: Shh(1–29), Shh(1–34), Shh(1–78), Shh(1–119), Shh(1–169), and Shh(1–197) fragments were generated by PCR. PCR products were ligated into pEGFPN-1 (Clontech) using EcoRI and BamHI restriction sites. Shh(1–24) EGFP was engineered using the QuikChange site-directed mutagenesis kit (Stratagene). Full-length Shh and Shh EGFP point mutations were introduced using the QuikChange site-directed mutagenesis kit (Stratagene). All of the constructs were confirmed using DNA sequencing. Signal P was used to verify that each mutant maintained the correct signal peptidase cleavage site.
Cell-based Palmitoylation Assay
Synthesis of [I125]iodopalmitate was carried out as previously described (11, 23, 24). COS-1 cells were maintained in DMEM supplemented with 10% FBS at 37 °C. All transfections were performed using Lipofectamine (Invitrogen). The cells were labeled 48 h post-transfection. Briefly, transfected COS-1 cells were incubated for 1 h at 37 °C with DMEM containing 2% dialyzed FBS and then labeled for 4 h with 10–20 μCi of [I125]iodopalmitate. The cells were lysed and subjected to immunoprecipitation with either anti-GFP or anti-Shh antibody, and the immunoprecipitates were diluted in 50 μl of 2× SDS-PAGE sample buffer containing 1 mm or 10 mm DTT. The samples were analyzed on 12.5% or 10% SDS-PAGE gels, followed by phosphorimaging on a Fuji FLA-7000 phosphorimager. [I125]Iodopalmitate incorporation into each construct was quantified using Image Gauge (Fujifilm Science Lab 2003, version 4.1) and normalized to protein expression. An aliquot of each immunoprecipitate was analyzed for ShhN or Shh-EGFP protein expression by Western blotting. The expression level of each point mutant was quantified from the Western blots using Quantity One (Bio-Rad) on either a GS-800 Calibrated Densitometer or a ChemiDoc XRS+ System (Bio-Rad). Protein expression from total cell lysates was monitored by SDS-PAGE and Western blotting.
RESULTS
The First Six Amino Acids of Mature Shh Are Sufficient for Palmitoylation
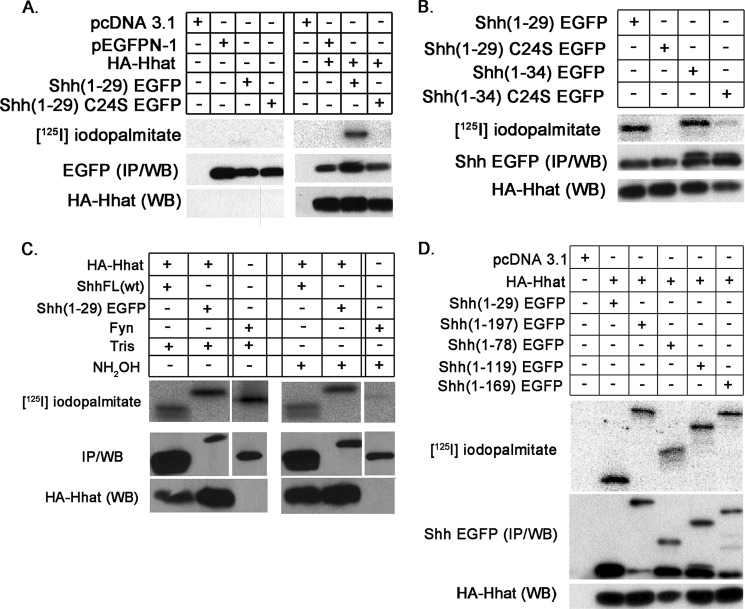
The minimal sequence for palmitoylation of Shh by Hhat is unknown. We previously reported that an EGFP fusion construct containing the first 44 amino acids of Shh, Shh(1–44) EGFP, was palmitoylated when co-expressed with Hhat in COS-1 cells (11). Shh(1–44) EGFP contains the Shh signal sequence (amino acids 1–23) and the first 21 amino acids of the mature protein (24–44). To determine whether fewer residues would suffice for palmitoylation, we generated constructs consisting of amino acids 1–29 and 1–34 of Shh fused to EGFP in the pEGFP-N1 vector (Table 1). These constructs were tested in a cell-based palmitoylation assay. COS-1 cells were co-transfected with plasmids encoding either Shh(1–29) EGFP, Shh(1–34) EGFP, or EGFP and empty vector or HA-tagged Hhat (HA-Hhat). The cells were labeled for 4 h with [125I]iodopalmitate, a radioiodinated palmitate analog that accurately monitors protein palmitoylation (24, 25). Cell lysates were subjected to immunoprecipitation with anti-GFP antibody followed by SDS-PAGE, and [125I]iodopalmitate incorporation was determined by phosphorimager analysis. Both Shh(1–29) EGFP and Shh(1–34) EGFP were palmitoylated, whereas mutant constructs in which the cysteine modification site was changed to a serine (C24S) had no or vastly decreased palmitate incorporation (Fig. 1, A and B). No radiolabel incorporation into the fusion constructs was observed in the absence of co-transfected Hhat or when EGFP was co-expressed with Hhat (Fig. 1A). Radiolabel incorporation into Shh(1–29) EGFP was resistant to treatment with 1 m NH2OH, consistent with attachment of palmitate via amide linkage (Fig. 1C). A similar result was obtained for ShhNp, which is palmitoylated via amide linkage. We used Fyn as a control for a protein that is palmitoylated via thioester linkage. As expected, radiolabel incorporation was sensitive to 1 m NH2OH treatment (Fig. 1C). Longer constructs that contained Shh residues 1–78, 1–119, 1–169, or 1–197 (which represents the entire ShhN signaling domain) fused to EGFP incorporated [125I]iodopalmitate to levels approximately equivalent to those obtained for Shh(1–29) EGFP (Fig. 1D). A band corresponding in size to EGFP was evident in the anti-GFP Western blots, and this band remained even when the ATG start site codon for EGFP within the pEGFP-N1 vector was removed. However, no radiolabel incorporation into the EGFP band occurred, consistent with our finding that EGFP itself is not palmitoylated. Taken together, these findings imply that the first six amino acids of mature Shh are sufficient for Hhat-mediated palmitoylation.
TABLE 1.
Sequences and palmitoylation status of Shh-EGFP constructs
Each construct contains the Shh signal sequence (amino acids 1–23) and then the indicated sequence from mature ShhN, fused in frame to EGFP. After signal sequence cleavage, Cys-24 becomes the N terminus.
| Construct | Mature Shh sequence | Linker sequence | Palmitoylation |
|---|---|---|---|
| 1–29 WT | CGPGRG | KDPPVAT | + |
| 1–29 G25A | CAPGRG | KDPPVAT | + |
| 1–29 G27A | CGPARG | KDPPVAT | + |
| 1–29 G25A,G27A | CAPARG | KDPPVAT | + |
| 1–29 P26A | CGAGRG | KDPPVAT | + |
| 1–29 R28A | CGPGAG | KDPPVAT | + |
| 1–29 P26A,R28A | CGAGAG | KDPPVAT | + |
| 1–29 K30A | CGPGRG | ADPPVAT | + |
| 1–29 R28A,K30A | CGPGAG | ADPPVAT | − |
| 1–29 G25S | CSPGRG | KDPPVAT | + |
| 1–29 G25K | CKPGRG | KDPPVAT | − |
| 1–24 | C | KDPPVAT | − |
| 1–24 K25S | C | SDPPVAT | − |
FIGURE 1.
Palmitoylation of Shh-EGFP fusion constructs. COS-1 cells were transfected with the indicated constructs and labeled for 4 h with [125I]iodopalmitate. Cell lysates were immunoprecipitated with anti-GFP antibody and analyzed by SDS-PAGE and phosphorimaging ([125I]iodopalmitate, top panels) and Western blotting with anti-GFP antibody (EGFP (IP/WB), middle panels). HA-tagged Hhat expression was determined by Western blotting of total cell lysates with anti-HA antibody (HA-Hhat (WB), bottom panels). A, analysis of EGFP and Shh(1–29) EGFP in the absence and presence of co-transfected HA-Hhat. B, analysis of Shh(1–29) EGFP and Shh(1–34) EGFP constructs with co-expressed Hhat. C, COS-1 cells were co-transfected with plasmids encoding Hhat and either full-length Shh or Shh(1–29)EGFP or with a plasmid encoding Fyn. Cells were labeled for 4 h with [125I]iodopalmitate. Cell lysates were immunoprecipitated with either anti-Shh or anti-GFP or anti-Fyn antibody, as appropriate. Immunoprecipitates were treated with 1 m Tris, pH 7.0, or 1 m NH2OH, pH 7.0, for 30 min at 20 °C, as indicated and then analyzed as described above. The 19-kDa ShhNp product is shown in the first lane. D, analysis of Shh(1–29) EGFP and longer fusion constructs.
A Single Cysteine Following the Signal Sequence Is Not Sufficient for Hhat-mediated Palmitoylation
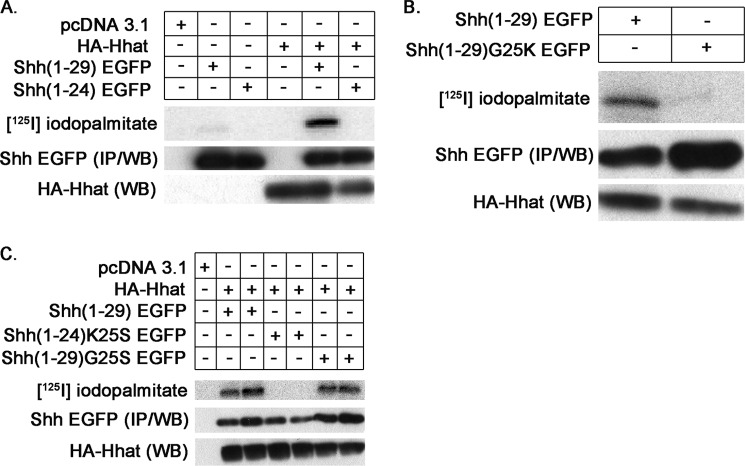
Mammalian cells express three Hedgehog family members: Sonic, Indian (Ihh), and Desert (Dhh) (26). The N-terminal five amino acids (CGPGR) are conserved in mature Shh, Dhh, Ihh, and Hh. To determine whether the conserved sequence is required for palmitoylation, we generated Shh(1–24) EGFP, a construct that contains the 23-amino acid signal sequence of Shh followed by a single Cys fused to EGFP (Table 1). Analysis with the SignalP signal sequence cleavage predictor program confirmed that the correct cleavage site is maintained in this construct, leaving Cys-24 as the N-terminal residue of the processed protein. When Shh(1–24) EGFP was co-expressed with HA-Hhat and tested in a cell-based palmitoylation assay, it failed to incorporate palmitate (Fig. 2A). One explanation for this finding is that, in the absence of Shh residues 25–29, a single cysteine following the signal sequence is not sufficient for palmitoylation. However, Shh-EGFP fusion proteins generated in the pEGFP-N1 vector contain a seven-amino acid linker sequence from the vector (KDPPVAT) that is present between the Shh and EGFP sequences. This places a large, positively charged residue (Lys) adjacent to the N-terminal cysteine in Shh(1–24) EGFP, which might interfere with recognition of the substrate by Hhat. To determine whether the residue adjacent to Cys-24 affects palmitoylation of Shh, we generated Shh(1–29)G25K EGFP and Shh(1–29)G25S EGFP, constructs with the Gly at position 25 replaced by Lys or Ser (Table 1). When tested in a cell-based palmitoylation assay, no detectable label was incorporated into Shh (1–29)G25K EGFP (Fig. 2B), whereas Shh(1–29)G25S EGFP incorporated [125I]iodopalmitate to the same extent as Shh(1–29) EGFP (Fig. 2C and Table 1). These data indicate that Ser but not Lys can be placed next to Cys-24. However, the shorter construct Shh(1–24)K25S EGFP, which places Ser adjacent to Cys-24 in the context of Shh(1–24), was not palmitoylated (Fig. 2C). These findings reveal two features of Shh palmitoylation: palmitoylation of Shh by Hhat is influenced by the residue adjacent to the N-terminal cysteine, and a single cysteine following the signal sequence is likely not sufficient for palmitoylation.
FIGURE 2.
A single Cys following the signal sequence is not sufficient for Shh palmitoylation. COS-1 cells were transfected with the indicated constructs, labeled with [125I]iodopalmitate and analyzed as described for Fig. 1. A, Shh(1–24) EGFP is not palmitoylated. B, substitution of Lys for Gly at position 25 prevents palmitoylation of Shh(1–29) EGFP. C, substitution of Ser at position 25 allows palmitoylation of Shh(1–29) EGFP but not Shh(1–24) EGFP. IP, immunoprecipitation; WB, Western blot.
Role of the N-terminal Conserved Sequence of Hedgehog Proteins
To further explore the importance of this core conserved sequence, we mutated these residues to Ala and monitored the effect on Shh(1–29) EGFP palmitoylation. Surprisingly, except for the N-terminal Cys, mutation of the core sequence residues, alone or in pairs, had no effect on palmitoylation of Shh(1–29) EGFP constructs (Fig. 3). Next, we generated the same substitutions in the context of full-length Shh which, when expressed in cells, will generate fully processed (palmitoylated and cholesteroylated) ShhNp. Mutation of Gly-25, Pro-26, and/or Gly-27 had no effect on palmitoylation of ShhNp, but the R28A mutant exhibited a 5-fold decrease in [125I]iodopalmitate incorporation compared with wild-type Shh (Fig. 4A).
FIGURE 3.
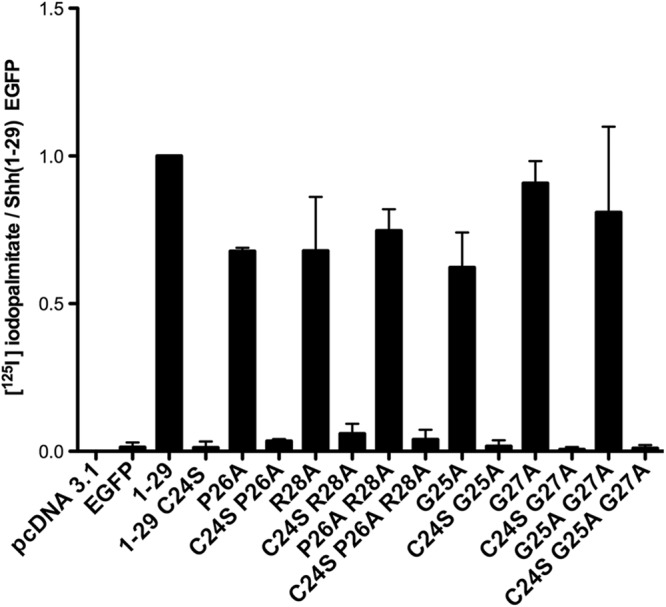
Palmitoylation is preserved when N-terminal conserved residues are mutated within Shh(1–29) EGFP. COS-1 cells were transfected with the indicated constructs, labeled with [125I]iodopalmitate, and analyzed as described in Fig. 1. Levels of [125I]iodopalmitate incorporation were divided by the expression level of each construct and normalized to that of Shh(1–29) EGFP (set at 1.0). Each bar represents the average of experiments that were performed in duplicate and repeated two or three times.
FIGURE 4.
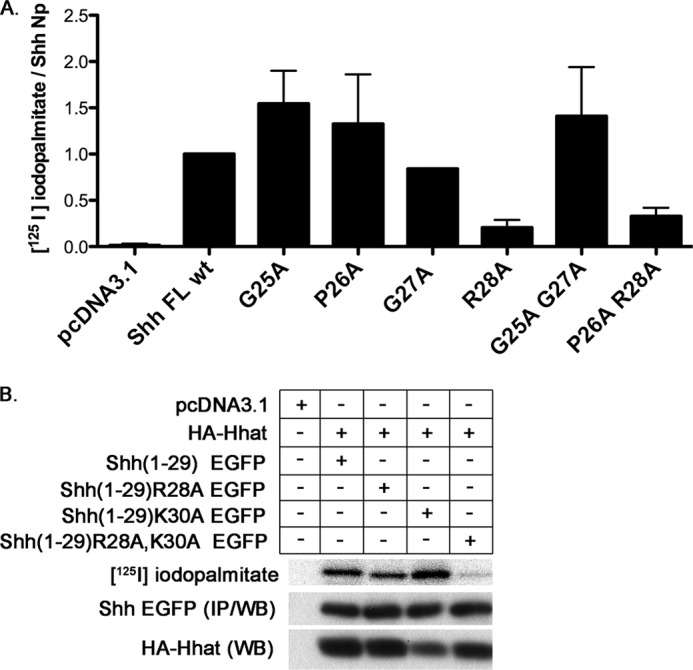
Influence of positively charged residues within the Shh N-terminal region on palmitoylation. A, COS-1 cells were co-transfected with constructs encoding full-length Shh and Hhat and labeled for 4 h with [125I]iodopalmitate. Cell lysates were immunoprecipitated with anti-Shh antibody and analyzed as described in Fig. 1. The levels of [125I]iodopalmitate incorporation were divided by the expression level of each construct and normalized to that of WT Shh (set at 1.0). Each bar represents the average of experiments that were performed in duplicate and repeated two times. B, palmitoylation of Shh(1–29) EGFP constructs containing mutations of Arg and/or Lys in the N-terminal region was analyzed as described in Fig. 1. IP, immunoprecipitation; WB, Western blot.
One possible explanation for the difference observed between the R28A mutants of Shh(1–29) EGFP and ShhNp might be the differences in the amino acids in the N-terminal region downstream of the core conserved sequences. Hedgehog proteins (Shh, Ihh, Dhh, and Hh), and the D. melanogaster EGF-like ligands Spitz, Keren, and Gurken are all validated or likely substrates for Hhat/Rasp (22). Each of these proteins contain an Arg either three or four residues downstream from the N-terminal, palmitoylated Cys. In addition to Arg at position 5 (Arg-28), Shh(1–29) EGFP contains another positively charged residue, Lys at position 7 (Lys-30) (Table 1). We hypothesized that loss of Arg-28 might be compensated by the presence of the Lys residue two residues downstream. To test this hypothesis, we generated point mutants in the context of Shh(1–29) EGFP, in which the Lys in the linker was mutated to Ala either alone, Shh(1–29)K30A EGFP, or in conjunction with Arg-28, Shh(1–29)R28A,K30A EGFP. When tested in a cell-based palmitoylation assay, Shh(1–29)K30A EGFP incorporated palmitate, but Shh(1–29)R28A,K30A EGFP did not (Fig. 4B). These results imply that a positively charged amino acid within the N-terminal region of Shh is required for palmitoylation and that Lys-30, derived from the pEGFP-N1 linker, can compensate for the loss of Arg-28.
An N-terminal Serine in Full-length Shh Can Be Palmitoylated by Hhat but Not by Rasp
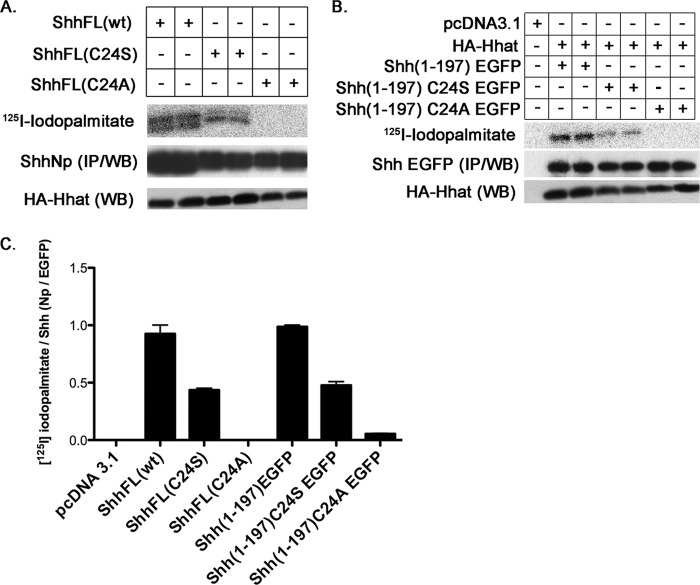
To generate nonpalmitoylated mutants of Shh for functional analysis, previous investigators have mutated the N-terminal Cys-24 to either Ser or Ala (13, 18, 19). It has been assumed, but never demonstrated, that these two mutations are equivalent and that neither can serve as an acceptor for palmitate. However, recent studies have shown that Ser can serve as an acceptor for fatty acylation. For example, Wnt proteins contain a monounsaturated fatty acid, palmitoleic acid, attached to a serine residue via oxyester linkage (27). We therefore directly compared the ability of Shh C24A and C24S mutants to incorporate palmitate in the context of both EGFP fusions and full-length Shh proteins. As depicted in Fig. 5, an N-terminal Ala cannot serve as a palmitate acceptor: none of the C24A mutants incorporated detectable levels of [125I]iodopalmitate. However, ShhNp and Shh(1–197) EGFP proteins containing the C24S mutation incorporated [125I]iodopalmitate (Fig. 5, A and B) to levels ∼50% of the corresponding WT proteins (Fig. 5C). A similar result was observed for Shh(1–34)C24S EGFP (Fig. 1B). These findings indicate that an N-terminal Ser can act as a palmitate acceptor for Hhat-mediated palmitoylation of Shh.
FIGURE 5.
An N-terminal Ser in Shh can be palmitoylated by Hhat. A and B, palmitoylation of wild-type and C24S and C24A mutants of ShhNp (A) and Shh(1–197) EGFP (B) was analyzed in COS-1 cells co-transfected with Hhat. A doublet for palmitoylated ShhNp was observed occasionally and may represent degradation or additional processing. C, quantification of the data from A and B, from one to three experiments, each performed in duplicate or triplicate. IP, immunoprecipitation; WB, Western blot.
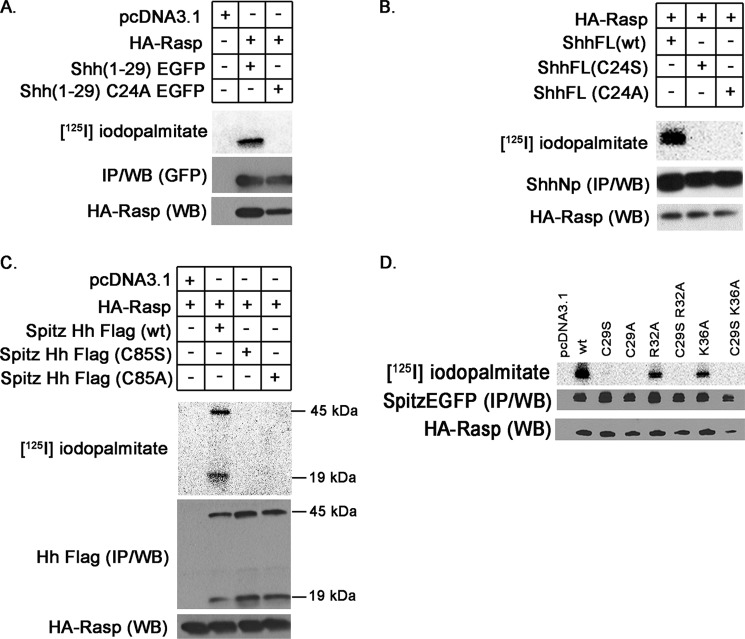
We then explored whether Rasp, the D. melanogaster ortholog of Hhat, exhibited substrate specificity similar to that of Hhat. Shh(1–29) EGFP was palmitoylated by Rasp, indicating that the first six amino acids (which are identical in Shh and Hh) are also sufficient for recognition by Rasp (Fig. 6A). When full-length Shh was co-transfected with Rasp, [125I]iodopalmitate was incorporated into wild-type ShhNp (Fig. 6B). No radiolabel incorporation was detected into the C24A mutant of Shh. However, in sharp contrast to Hhat, the C24S Shh mutant was not palmitoylated by Rasp (Fig. 6B).
FIGURE 6.
Substrate specificity of Rasp. A, COS-1 cells were co-transfected with Rasp and the indicated Shh(1–29) EGFP constructs, labeled with [125I]iodopalmitate, and analyzed as described for Fig. 1. B, palmitoylation of wild-type and mutant ShhNp was analyzed in COS-1 cells co-transfected with Rasp. C, palmitoylation of wild-type and mutant HhNp (expressed from a plasmid containing the signal sequence from Spitz) was analyzed in COS-1 cells co-transfected with Rasp. The migration positions of the 45-kDa Hh precursor and 19-kDa HhNp product are marked. D, palmitoylation of wild-type and mutant Spitz-EGFP constructs was analyzed in COS-1 cells co-transfected with Rasp. IP, immunoprecipitation; WB, Western blot.
We next examined the ability of Rasp to palmitoylate its natural targets, Hh and Spitz. When cells were transfected with a cDNA clone encoding full-length Hh, expression levels of Hh were very low and barely detectable. We reasoned that the extremely long signal sequence (84 residues) of Hh might interfere with Hh expression in mammalian cells. Accordingly, when the signal sequence of Hh was replaced with that of Spitz (28 amino acids), robust Hh expression was achieved, and processing to the 19-kDa HhNp product was clearly observed (Fig. 6C). When full-length Hh was co-transfected with Rasp, [125I]iodopalmitate was incorporated into wild-type Hh, but not into the C29A or C29S mutants of Hh (Fig. 6C). Both the 45-kDa Hh precursor and the 19-kDa product were palmitoylated, as we have previously observed for Shh (11).
A similar result was obtained when Spitz-EGFP was used as a substrate for Rasp. Wild-type Spitz was palmitoylated by Rasp, but no detectable radiolabel incorporation was observed for the Spitz C29A or C29S mutants (Fig. 6D). Thus, unlike Hhat, Rasp cannot promote palmitate incorporation into an N-terminal Ser. Rasp does, however, share with Hhat the requirement for basic residues within the N-terminal region for efficient palmitoylation (compare the R32A and K36A mutants of Spitz with WT Spitz; Fig. 6D). Taken together, these findings highlight critical similarities and differences in the way that Hhat and Rasp recognize and/or catalyze the attachment of palmitate to protein substrates.
A Bioinformatics Screen to Search for Additional Hhat Substrates
Although Spitz has been shown to be a substrate for N-palmitoylation by Rasp in D. melanogaster (22), no Spitz ortholog has been identified in mammals. These findings raise the possibility that Hhat could palmitoylate other proteins in mammalian cells. To identify potential Hhat substrates, we screened the Secreted Protein Database for proteins predicted to contain an N-terminal Cys after the signal sequence cleavage site (28). Eighty-seven protein sequences were identified, including all three hedgehog family members (supplemental Table S1). We grouped the proteins into categories based on function. In addition to the 3 Hh proteins, the list includes 11 MHC Class I heavy chain members, 10 interferons, 11 proteases or protease inhibitors, 11 membrane-bound receptors, 3 adhesion proteins, 23 proteins of miscellaneous function, and 15 hypothetical proteins or proteins of unknown function. Sequences were further analyzed via literature searches to determine whether the modification status of the N-terminal Cys was known. Of note, there are 32 proteins whose N-terminal Cys is reported to be linked to another Cys in the protein via an intramolecular disulfide bond. These include the interferons, serine proteases (elastase and chymotrypsinogen), tissue inhibitor of metalloproteinases, Nogo receptors, hormone preproteins, and leucine-rich repeat proteins that contain a cysteine knot motif. These proteins are probably not Hhat substrates because it is unlikely, although not impossible, that the N-terminal Cys is both disulfide-bonded through the thiol side chain and N-palmitoylated via the N-terminal amine. We eliminated five other proteins because the N-terminal Cys is reported to be unmodified, the N-terminal region is cleaved off, an N-terminal Cys is not predicted by Signal P, or the protein is excluded from lipid rafts. In total, 37 of the protein entries on the list are unlikely to be palmitoylated.
Analysis of the remaining protein sequences revealed that 12 proteins contained Pro and 8 had Gln as the amino acid following the predicted N-terminal cysteine. We tested whether Pro or Gln adjacent to the N-terminal Cys affected Hhat-mediated palmitoylation by generating G25P and G25Q Shh constructs. As depicted in Fig. 7, ShhNp proteins containing G25P and G25Q substitutions were palmitoylated to levels approximately equal to that of WT ShhNp. This finding demonstrates that Pro and Gln can be accommodated at position 2, at least within the Shh sequence, and suggests that proteins containing CP and CQ at the N terminus might be substrates for Hhat. Taken together, the data in Figs. 4, 5, and 7 can be combined into a working model defining a motif for Hhat-mediated palmitoylation (Fig. 8).
FIGURE 7.
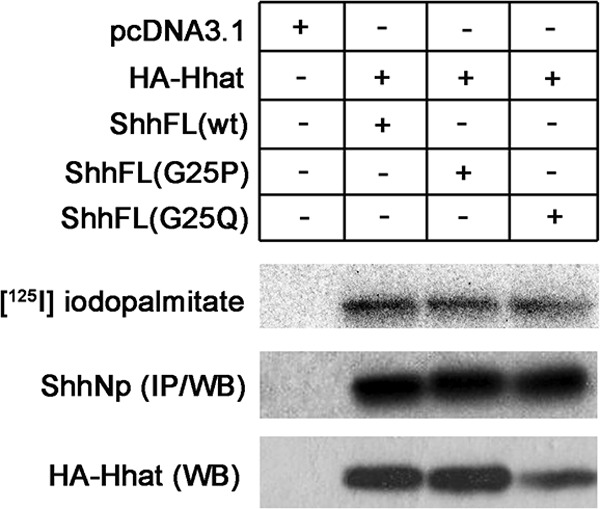
ShhNp containing Pro or Gln at position 2 are palmitoylated. COS-1 cells were transfected with constructs encoding full-length Shh, labeled with [125I]iodopalmitate, and analyzed as described for Fig. 4. IP, immunoprecipitation; WB, Western blot.
FIGURE 8.
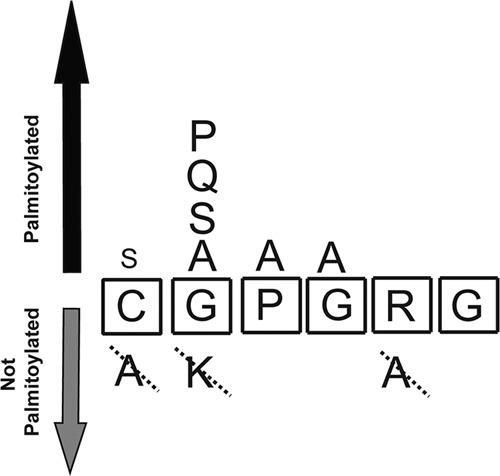
Schematic model for the Hhat palmitoylation motif within Shh. See “Discussion” for details.
DISCUSSION
Identification of a Minimal Shh Palmitoylation Sequence
In this study, we define a mini-Shh construct that contains sequences sufficient for N-palmitoylation. A cell-based system was used to mimic the in situ intracellular environment for palmitoylation, a reaction that likely occurs in the lumen of the ER and/or Golgi. Each EGFP fusion construct contains the Shh signal sequence, because passage through the secretory pathway is required for palmitoylation in the cell (11). Moreover, each sequence was analyzed with SignalP to ensure that the correct signal peptidase cleavage site was retained. After signal sequence cleavage, the N terminus of Shh(1–29) EGFP contains the first six amino acids, residues that are conserved in Shh, Dhh, and Hh.
Analyses of the cell-based labeling results obtained for Shh(1–29) EGFP and ShhNp establish an initial set of parameters that define Hhat-mediated palmitoylation of Shh: 1) The N-terminal residue (position 1) exhibits a strong, but not exclusive, requirement for Cys. Ala is not allowed, but Ser can substitute and provide partial activity. 2) Residues at positions 2, 3, and 4 (GPG) can be mutated to Ala without affecting palmitoylation. Although these residues are conserved in all hedgehog protein family members, the other known (Spitz) or potential (Keren and Gurken) Rasp substrates contain Ser residues at the 2 and 3 positions. 3) Palmitoylation is sensitive to the identity of the amino acid at position 2: Gly, Ser, Ala, Pro, and Gln are tolerated, but Lys is not. Of note, N-myristoyl transferase, which catalyzes attachment of myristate to an N-terminal glycine of N-myristoylated proteins, cannot myristoylate substrate target proteins with Lys at the 2 position (29). 4) At least one basic amino acid needs to be present within the first seven residues of the N-terminal region. Hedgehog proteins contain Arg at position 5, and Spitz, Keren, and Gurken contain Arg at position 4. Mutation of these Arg residues within Spitz or Shh inhibits palmitoylation. However, the R28A Shh mutant phenotype can be rescued by the presence of a Lys at position 7 in Shh(1–29)R28A EGFP. This Lys is provided by the EGFP linker, a sequence unrelated to Shh. It is possible that additional downstream Shh sequences contribute to palmitoylation. We also cannot exclude the possibility that other amino acids in the linker sequence have an influence on the palmitoylation status of Shh(1–29) EGFP.
In addition to promoting Shh palmitoylation, the N-terminal region of ShhNp regulates Shh multimerization and signaling activity (13, 18, 30). The crystal structure of human Shh reveals that the N terminus of ShhN extends away from the rest of the protein and forms contacts with adjacent Shh molecules (18). It is possible that this could place the free thiol group on the N-terminal Cys in a position to form disulfide bonds with other Shh monomers. Medium from cells overexpressing ShhNp is enriched in a freely diffusable, multimeric form of ShhNp that forms a gradient and is biologically active (18). N-terminal Cys mutants of Shh migrate as monomers, indicating that palmitoylation or the Cys thiol group is required for ShhNp multimerization (13). The data in Fig. 3 suggest that the amino acid sequence requirements for Shh palmitoylation and multimerization differ and that multimerization is not required for Shh palmitoylation. Mutation of the Pro in the conserved N-terminal sequence (Pro-27 in rat Shh) produces a protein that is unable to multimerize, yet mutation of this residue in human Shh (Pro-26) does not reduce palmitoylation (13). A murine Pro-27 mutant has reduced activity in cell-based assays, and mutation of Pro-26 has been identified in known human cases of holoprosencephaly (31, 32). Likewise, the G27A mutant of Shh is palmitoylated to the same extent as WT Shh (Fig. 3) but has reduced activity in the chick neural tube and is associated with holoprosencephaly in humans (31, 33). Taken together, these findings imply that palmitoylation is independent of ShhNp multimerization.
A Single Cysteine Following the Signal Sequence Is Not Sufficient for Palmitoylation
We showed that three of the highly conserved residues (GPG) in the Shh N terminus can be mutated to Ala without affecting palmitoylation. This led us to explore whether the mere presence of a cysteine following the signal sequence would suffice for palmitoylation of a GFP fusion protein. Within the context of Shh(1–24) EGFP, the signal sequence cleavage site is retained with Lys, Gly, or Ser at position 2 but is altered with Ala. Because Lys at position 2 interferes with palmitoylation (Fig. 2), we chose to analyze a Shh(1–24) EGFP construct with Ser at position 2. The data presented in Fig. 2 indicate that a single cysteine followed by a heterologous sequence is not sufficient for palmitoylation. Again, this statement is made with the caveat that other amino acids in the linker sequence may influence palmitoylation of Shh(1–24) EGFP.
A Difference in Substrate Specificity between Hhat and Rasp
At least three proteins have been reported to be O-acylated by a fatty acid attached via oxyester linkage to a Ser residue. Wnt proteins contain palmitoleic acid attached to Ser-209 (Wnt3a), ghrelin contains an octanoate moiety linked to Ser-3, and the N-methyl-d-aspartate receptor is acylated with palmitate and octanoate on a Ser (or Thr) residue(s) (27, 34, 35). We found that an N-terminal Ser can also serve as a substrate for Hhat-mediated palmitoylation in cells, albeit at reduced efficiency compared with an N-terminal Cys (Fig. 5). This was particularly evident when C24S, C24A, and wild-type Shh were compared side by side. Incorporation of [125I]iodopalmitate was observed with C24S mutants of Shh EGFP constructs of varying lengths, from 1–34 to 1–197 (equivalent to ShhNp), as well as with C24S ShhNp. Several studies have shown that murine C25S Shh retains residual signaling activity in mice. Misexpression of wild-type Shh induces polydactyly in the mouse limb bud, and C24S Shh exhibits a similar phenotype, albeit less severe (19). Likewise, although homozygous ShhC25S embryos display multiple phenotypic defects, they lack the obvious skeletal defects observed with loss of Shh or Hhat (13). One potential explanation to account for the residual activity observed for C24S,C25S mutants is that a low but significant percentage of the mutant molecules are palmitoylated. Thus, a C24A mutant would be a more suitable negative control to assess the role of palmitoylation in Shh signaling in mammalian systems.
In contrast to Hhat, Rasp was unable to promote incorporation of [125I]iodopalmitate into proteins that contain Ser at the N terminus. This result was observed when Rasp was tested using Cys to Ser mutants of Shh, Hh or Spitz as substrates (Fig. 6). In vivo studies of Hh signaling in flies indicate that the C84S mutant is clearly inactive and cannot rescue the severe segment polarity phenotype induced by loss of Hh (19). These findings suggest that differences in either substrate recognition, binding and/or fatty acid transfer exists between the mammalian and insect enzymes.
Are There Other Substrates for Hhat in Mammalian Cells?
Palmitate incorporation into Hh and Spitz has been experimentally validated, and Gurken and Keren are likely to be palmitoylated by Rasp, based on sequence similarity to Spitz (10, 22). In mammals, Shh is the only Hh family member that has been shown to be palmitoylated by Hhat in cells and in vitro. The other two family members, Ihh and Dhh, are highly likely to be Hhat targets, based on sequence similarity and the finding that recombinant Ihh incorporates radiolabel when incubated with [3H]palmitate in vitro (16). None of the mammalian EGF-like ligands contains an N-terminal Cys. A bioinformatics search for other potential Hhat substrates encoded in the human genome yielded 87 proteins predicted to contain an N-terminal Cys following the signal sequence. Of these, 32 proteins have an N-terminal Cys documented to be involved in a disulfide bond. The reaction mechanism for Hhat-mediated palmitoylation of Shh is proposed to involve initial formation of a thioester bond between the N-terminal Cys thiol group and palmitate, followed by an S-to-N shift to generate amide-linked palmitate (8). Disulfide bonds are generally formed in the ER, which is also the site for Hhat-mediated palmitoylation. Under these circumstances, it is unlikely that a protein whose N-terminal Cys is disulfide-bonded could also be N-palmitoylated. However, our previous study (11) failed to detect a thioester catalytic intermediate for Hhat, suggesting that palmitate might be directly attached to the N-terminal amino group. Thus, it remains possible that the N-terminal Cys of some proteins could be involved in both an amide link to palmitate via the N-terminal amine and a disulfide bond via the thiol side chain. Five additional proteins were eliminated based on properties that are either not consistent with or not conducive to palmitoylation. Two potential substrates that contain Lys within the N-terminal region, HLA-C heavy chain (11 isoforms) and Slit3, were tested directly. We were unable to detect palmitoylation of Slit3 in a cell-based assay. A peptide containing the N-terminal HLA-C sequence exhibited a low level of palmitate incorporation, but this occurred in an Hhat-independent manner (data not shown). It remains possible that full-length HLA-C could be a substrate for Hhat, but this will require expression with β2 microglobulin and Hhat in a cell-based palmitoylation assay. Further testing of the remaining 38 protein sequences, particularly those containing basic residues in the N-terminal region or Pro or Gln at position 2, may assist in developing a more refined algorithm to define Hhat substrate specificity in greater detail.
Supplementary Material
Acknowledgments
We thank Elissaveta Petrova, John Buglino, Armine Matevossian, and Jessica Rios for critical reading of the manuscript, Dr. Jessica Treisman for Hh and Spitz constructs; Dr. Lorine Wilkinson and Dr. Melissa Little for providing the Slit3-GFP fusion construct; Mono Pirun for performing the bioinformatics search; and Raisa Louft-Nisenbaum for expert technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant GM57966.

This article contains supplemental references and Table S1.
- Hhat
- Hedgehog acyltransferase
- EGFP
- enhanced GFP.
REFERENCES
- 1. Jiang J., Hui C. C. (2008) Hedgehog signaling in development and cancer. Dev. Cell 15, 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ingham P. W., McMahon A. P. (2001) Hedgehog signaling in animal development. Paradigms and principles. Genes Dev. 15, 3059–3087 [DOI] [PubMed] [Google Scholar]
- 3. Chiang C., Litingtung Y., Lee E., Young K. E., Corden J. L., Westphal H., Beachy P. A. (1996) Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407–413 [DOI] [PubMed] [Google Scholar]
- 4. Roessler E., Belloni E., Gaudenz K., Jay P., Berta P., Scherer S. W., Tsui L. C., Muenke M. (1996) Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat. Genet. 14, 357–360 [DOI] [PubMed] [Google Scholar]
- 5. Odent S., Atti-Bitach T., Blayau M., Mathieu M., Aug J., Delezo de A. L., Gall J. Y., Le Marec B., Munnich A., David V., Vekemans M. (1999) Expression of the Sonic hedgehog (SHH) gene during early human development and phenotypic expression of new mutations causing holoprosencephaly. Hum. Mol. Genet. 8, 1683–1689 [DOI] [PubMed] [Google Scholar]
- 6. Thayer S. P., di Magliano M. P., Heiser P. W., Nielsen C. M., Roberts D. J., Lauwers G. Y., Qi Y. P., Gysin S., Fernández-delCastillo C., Yajnik V., Antoniu B., McMahon M., Warshaw A. L., Hebrok M. (2003) Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 425, 851–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beachy P. A., Karhadkar S. S., Berman D. M. (2004) Tissue repair and stem cell renewal in carcinogenesis. Nature 432, 324–331 [DOI] [PubMed] [Google Scholar]
- 8. Mann R. K., Beachy P. A. (2004) Novel lipid modifications of secreted protein signals. Annu. Rev. Biochem. 73, 891–923 [DOI] [PubMed] [Google Scholar]
- 9. Porter J. A., Young K. E., Beachy P. A. (1996) Cholesterol modification of hedgehog signaling proteins in animal development. Science 274, 255–259 [DOI] [PubMed] [Google Scholar]
- 10. Chamoun Z., Mann R. K., Nellen D., von Kessler D. P., Bellotto M., Beachy P. A., Basler K. (2001) Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science 293, 2080–2084 [DOI] [PubMed] [Google Scholar]
- 11. Buglino J. A., Resh M. D. (2008) Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. J. Biol. Chem. 283, 22076–22088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee J. D., Treisman J. E. (2001) Sightless has homology to transmembrane acyltransferases and is required to generate active Hedgehog protein. Curr. Biol. 11, 1147–1152 [DOI] [PubMed] [Google Scholar]
- 13. Chen M. H., Li Y. J., Kawakami T., Xu S. M., Chuang P. T. (2004) Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev. 18, 641–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lewis P. M., Dunn M. P., McMahon J. A., Logan M., Martin J. F., St-Jacques B., McMahon A. P. (2001) Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell 105, 599–612 [DOI] [PubMed] [Google Scholar]
- 15. Li Y., Zhang H., Litingtung Y., Chiang C. (2006) Cholesterol modification restricts the spread of Shh gradient in the limb bud. Proc. Natl. Acad. Sci. U.S.A. 103, 6548–6553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pepinsky R. B., Zeng C., Wen D., Rayhorn P., Baker D. P., Williams K. P., Bixler S. A., Ambrose C. M., Garber E. A., Miatkowski K., Taylor F. R., Wang E. A., Galdes A. (1998) Identification of a palmitic acid-modified form of human Sonic hedgehog. J. Biol. Chem. 273, 14037–14045 [DOI] [PubMed] [Google Scholar]
- 17. Taylor F. R., Wen D., Garber E. A., Carmillo A. N., Baker D. P., Arduini R. M., Williams K. P., Weinreb P. H., Rayhorn P., Hronowski X., Whitty A., Day E. S., Boriack-Sjodin A., Shapiro R. I., Galdes A., Pepinsky R. B. (2001) Enhanced potency of human Sonic hedgehog by hydrophobic modification. Biochemistry 40, 4359–4371 [DOI] [PubMed] [Google Scholar]
- 18. Goetz J. A., Singh S., Suber L. M., Kull F. J., Robbins D. J. (2006) A highly conserved amino-terminal region of sonic hedgehog is required for the formation of its freely diffusible multimeric form. J. Biol. Chem. 281, 4087–4093 [DOI] [PubMed] [Google Scholar]
- 19. Lee J. D., Kraus P., Gaiano N., Nery S., Kohtz J., Fishell G., Loomis C. A., Treisman J. E. (2001) An acylatable residue of Hedgehog is differentially required in Drosophila and mouse limb development. Dev. Biol. 233, 122–136 [DOI] [PubMed] [Google Scholar]
- 20. Dawber R. J., Hebbes S., Herpers B., Docquier F., van den Heuvel M. (2005) Differential range and activity of various forms of the Hedgehog protein. BMC Dev. Biol. 5, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buglino J. A., Resh M. D. (2010) Identification of conserved regions and residues within Hedgehog acyltransferase critical for palmitoylation of Sonic Hedgehog. PLoS One 5, e11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miura G. I., Buglino J., Alvarado D., Lemmon M. A., Resh M. D., Treisman J. E. (2006) Palmitoylation of the EGFR ligand Spitz by Rasp increases Spitz activity by restricting its diffusion. Dev. Cell 10, 167–176 [DOI] [PubMed] [Google Scholar]
- 23. Berthiaume L., Peseckis S. M., Resh M. D. (1995) Synthesis and use of iodo-fatty acid analogs. Methods Enzymol. 250, 454–466 [DOI] [PubMed] [Google Scholar]
- 24. Peseckis S. M., Deichaite I., Resh M. D. (1993) Iodinated fatty acids as probes for myristate processing and function. Incorporation into pp60v-src. J. Biol. Chem. 268, 5107–5114 [PubMed] [Google Scholar]
- 25. Alland L., Peseckis S. M., Atherton R. E., Berthiaume L., Resh M. D. (1994) Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J. Biol. Chem. 269, 16701–16705 [PubMed] [Google Scholar]
- 26. Pathi S., Pagan-Westphal S., Baker D. P., Garber E. A., Rayhorn P., Bumcrot D., Tabin C. J., Blake Pepinsky R., Williams K. P. (2001) Comparative biological responses to human Sonic, Indian, and Desert hedgehog. Mech. Dev. 106, 107–117 [DOI] [PubMed] [Google Scholar]
- 27. Takada R., Satomi Y., Kurata T., Ueno N., Norioka S., Kondoh H., Takao T., Takada S. (2006) Monounsaturated fatty acid modification of Wnt protein. Its role in Wnt secretion. Dev. Cell 11, 791–801 [DOI] [PubMed] [Google Scholar]
- 28. Chen Y., Zhang Y., Yin Y., Gao G., Li S., Jiang Y., Gu X., Luo J. (2005) SPD. A web-based secreted protein database. Nucleic Acids Res. 33, D169–D173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maurer-Stroh S., Eisenhaber B., Eisenhaber F. (2002) N-terminal N-myristoylation of proteins. Refinement of the sequence motif and its taxon-specific differences. J. Mol. Biol. 317, 523–540 [DOI] [PubMed] [Google Scholar]
- 30. Zeng X., Goetz J. A., Suber L. M., Scott W. J., Jr., Schreiner C. M., Robbins D. J. (2001) A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 411, 716–720 [DOI] [PubMed] [Google Scholar]
- 31. Roessler E., El-Jaick K. B., Dubourg C., Vélez J. I., Solomon B. D., Pineda-Alvarez D. E., Lacbawan F., Zhou N., Ouspenskaia M., Paulussen A., Smeets H. J., Hehr U., Bendavid C., Bale S., Odent S., David V., Muenke M. (2009) The mutational spectrum of holoprosencephaly-associated changes within the SHH gene in humans predicts loss-of-function through either key structural alterations of the ligand or its altered synthesis. Hum. Mutat. 30, E921–E935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hehr U., Gross C., Diebold U., Wahl D., Beudt U., Heidemann P., Hehr A., Mueller D. (2004) Wide phenotypic variability in families with holoprosencephaly and a sonic hedgehog mutation. Eur. J. Pediatr. 163, 347–352 [DOI] [PubMed] [Google Scholar]
- 33. Singh S., Tokhunts R., Baubet V., Goetz J. A., Huang Z. J., Schilling N. S., Black K. E., MacKenzie T. A., Dahmane N., Robbins D.J. (2009) Hum. Genet. 125, 95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang J., Brown M. S., Liang G., Grishin N. V., Goldstein J. L. (2008) Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132, 387–396 [DOI] [PubMed] [Google Scholar]
- 35. Balan L., Foltyn V. N., Zehl M., Dumin E., Dikopoltsev E., Knoh D., Ohno Y., Kihara A., Jensen O. N., Radzishevsky I. S., Wolosker H. (2009) Feedback inactivation of d-serine synthesis by NMDA receptor-elicited translocation of serine racemase to the membrane. Proc. Natl. Acad. Sci. U.S.A. 106, 7589–7594 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.