Background: Only few substrates of the essential membrane-anchored protease FtsH are known.
Results: New cytoplasmic and membrane-bound substrates of FtsH were trapped in vivo.
Conclusion: FtsH is involved in the sulfatation of molecules, d-amino acid metabolism, and adaptation to anaerobiosis and stress conditions.
Significance: The novel FtsH substrates significantly expand our knowledge on the biological functions of this fundamentally important protease.
Keywords: ATP-dependent Protease, Bacteria, Enzyme Turnover, Escherichia coli, Iron-Sulfur Protein, Lipopolysaccharide (LPS)
Abstract
Proteolysis is a universal strategy to rapidly adjust the amount of regulatory and metabolic proteins to cellular demand. FtsH is the only membrane-anchored and essential ATP-dependent protease in Escherichia coli. Among the known functions of FtsH are the control of the heat shock response by proteolysis of the transcription factor RpoH (σ32) and its essential role in lipopolysaccharide biosynthesis by degradation of the two key enzymes LpxC and KdtA. Here, we identified new FtsH substrates by using a proteomic-based substrate trapping approach. An FtsH variant (FtsHtrap) carrying a single amino acid exchange in the proteolytic center was expressed and purified in E. coli. FtsHtrap is devoid of its proteolytic activity but fully retains ATPase activity allowing for unfolding and translocation of substrates into the inactivated proteolytic chamber. Proteins associated with FtsHtrap and wild-type FtsH (FtsHWT) were purified, separated by two-dimensional PAGE, and subjected to mass spectrometry. Over-representation of LpxC in the FtsHtrap preparation validated the trapping strategy. Four novel FtsH substrates were identified. The sulfur delivery protein IscS and the d-amino acid dehydrogenase DadA were degraded under all tested conditions. The formate dehydrogenase subunit FdoH and the yet uncharacterized YfgM protein were subject to growth condition-dependent regulated proteolysis. Several lines of evidence suggest that YfgM serves as negative regulator of the RcsB-dependent stress response pathway, which must be degraded under stress conditions. The proteins captured by FtsHtrap revealed previously unknown biological functions of the physiologically most important AAA+ protease in E. coli.
Introduction
The cellular protein pool varies with changing conditions. Protein degradation is a common but costly mechanism to shape the cellular proteome. As it acts on already synthesized proteins, proteolysis is more efficient than delayed transcriptional or translational control mechanisms because it removes undesired proteins rapidly and irreversibly. In Escherichia coli, the five AAA+ (ATPases associated with various cellular activities) proteases ClpAP/XP, HslUV, Lon, and FtsH play important roles in diverse regulatory networks (1, 2). Among these, FtsH is the only essential and the only membrane-bound protease in E. coli (3, 4). It forms a homo-hexameric barrel-like structure (5). AAA+ proteases commonly consist of an ATPase and a protease domain, which can reside in a single polypeptide or in two separate polypeptides. The ATPase domain of FtsH contains the Walker A/B motifs and the second region of homology, which allow the binding and hydrolysis of ATP (6). In general, the ATPase domain is solely responsible for unfolding and translocation of the substrate into the proteolytic chamber, which is driven by conformational changes after hydrolysis of ATP (7, 8). The subsequent degradation reaction is carried out by the protease domain. FtsH is a metalloprotease that requires catalytic Zn2+ ions for functionality. The histidines in the 417HEXXH421 motif and a glutamic acid at position 495 are responsible for zinc binding. The imidazoles of these histidines are positioned by Glu479 in the correct conformation (9, 10). Substitution of either histidine against a tyrosine retained ATPase activity but impaired proteolytic activity (11).
The FtsH protease is known to control several important physiological processes (12–14) and plays a major role in the quality control of membrane proteins (15). The essential function of FtsH in E. coli is to degrade the lipopolysaccharide (LPS) biosynthesis enzymes LpxC and KdtA, thereby controlling production of lipid A, the hydrophobic anchor of LPS (3, 16). Altered LPS amounts are lethal because LPS in the outer leaflet of the outer membrane of Gram-negative bacteria form a crucial permeability barrier. FtsH guarantees a strict balance between LPS and phospholipids. An ftsH deletion mutant is viable only in the presence of a suppressor mutation within the fabZ gene, resulting in a balance of LPS to phospholipids (3).
FtsH also controls heat shock gene expression by degrading the heat shock σ factor RpoH (σ32) thereby adapting the cellular amount of the transcription factor to different temperatures (17, 18). Together with the Lon protease, FtsH participates in the shutoff of the superoxide stress response (19, 20). Furthermore, FtsH degrades a number of phage proteins and thereby takes part in the lysis/lysogeny decision of the phage λ (21–23).
Despite the importance of FtsH for bacterial survival, only a limited number of substrates are known to date as compared with other proteolytic machineries. Furthermore, the substrate recognition logics of FtsH are poorly understood and require further investigation. For example, proteolysis of LpxC depends on a length- and sequence-specific C-terminal degradation signal (24, 25). Although the replication inhibitor CspD carries a similar C-terminal sequence, it is not an FtsH but a Lon substrate (26). Degradation of RpoH requires an internal structural element composed of an α-helix and the chaperone systems DnaK/J and GroEL/ES (17, 27–30).
To fully comprehend the biological functions of FtsH and its substrate selection principles, we set out to identify new substrates of the protease by an experimental approach. Entirely unrelated recognition motifs in LpxC and RpoH and unknown degradation signals in other known substrates excluded in silico searches. Instead, we employed a substrate trapping approach as has been used for the AAA+ protease ClpXP (31). Here, we present the construction of FtsHtrap, an ATPase-competent protease variant with an active-site mutation in the Zn2+-binding motif (H417Y). Proteomic-based identification of proteins co-purified with FtsHtrap revealed 14 potential new FtsH substrates. Among these, four proteins were proven to be degraded by FtsH using in vivo degradation experiments. This study gives new insights into the extent of FtsH-dependent proteolysis in E. coli and provides a basis to further characterize the cellular functions and substrate selection of this unique protease.
EXPERIMENTAL PROCEDURES
Bacteria and Growth Conditions
E. coli strains used in this study are listed in supplemental Table S1. Except for the ΔftsH strain, E. coli cells were grown aerobically in LB medium at 37 °C. ΔftsH cells were routinely cultivated at 30 °C. When needed, antibiotics were used as follows: ampicillin, 100 μg ml−1; chloramphenicol, 200 μg ml−1; kanamycin, 50 μg ml−1; tetracycline, 12.5 μg ml−1.
Construction of Plasmids
Plasmids used in this study are listed in supplemental Table S1. For mutagenesis of ftsH, a fragment of the gene (1024 bp) was cloned from pGST-FtsH into a pBCSK(+) derivative (pBO966) using the Acc65I and NdeI sites. The resulting plasmid was used as a template for QuikChange® PCR (oligonucleotides: H417Y.fw and H417Y.rv; E479D.fw and E479D.rv; supplemental Table S2) to generate the amino acid substitutions H417Y and E479D. Success of the QuikChange® PCR was analyzed by sequencing, and the mutated fragment was used to substitute the equivalent wild-type fragment in pMAL-C-FtsH. For construction of expression plasmids coding for putative FtsH substrates, the respective genes were amplified by PCR using E. coli K12 genomic DNA as a template. The coding regions were inserted into the backbone of pBO1199. The resulting expression plasmids code for proteins with an N-terminal hexahistidine (His6) sequence under the control of an inducible anhydrotetracycline promoter. For construction of a plasmid allowing constitutive expression of YfgM, a yfgM PCR product was inserted in pBO1750 using the SmaI and Bsp1407I sites.
Protein Purification
His6-MBP-FtsHWT, His6-MBP-FtsHH417Y, and His6-MBP-FtsHE479D were produced in E. coli ΔftsH. Cells were grown in 2× 500 ml of LB broth at 30 °C to an A580 nm of 0.5. The overexpression of the protein variants was induced by 1 mm isopropyl 1-thio-β-d-galactopyranoside for 18 h at 20 °C. Cells were harvested and resuspended in 12 ml of 140 mm NaCl, 2.7 mm KCl, 1.8 mm KH2HPO4, 10 mm Na2HPO4, pH 7.3, 0.2 mg ml−1 DNase, and 0.2 mg ml−1 RNase. Cells were disrupted by a French press, and FtsH variants were purified using nickel-nitrilotriacetic acid columns (Qiagen) equilibrated with 0.5 m KCl and 20 mm Tris-HCl, pH 7.9. For washing (W) and elution (E) of the column, increasing amounts of imidazole were used (W, 5–50 mm; E, 150 mm to 1 m). The elution fractions were stored in 0.2 m NaCl, 0.5% (v/v) Nonidet P-40, 20 mm monoethanolamine, 20% (v/v) glycerol, and 1 mm DTT at −80 °C. Protein concentrations were determined by Bradford assays (32).
ATPase Activity Assay
To measure ATPase activity, 1 μg of purified FtsH was mixed with 1 mm ATP, 50 mm Tris-HCl, pH 7.5, 5 mm MgCl2, 25 μm zinc acetate, 2.5 mm C2H3KO2, 1 mm DTT, and 0.1% (v/v) Nonidet P-40 in a total volume of 500 μl. The reaction was incubated at 37 °C. After 0, 30, 60, and 90 min, 120 μl of this mixture was added to 480 μl of detection solution (0.027% (w/v) malachite green, 0.1% (v/v) Triton X-100, 0.95% (w/v) ammonium molybdate) and 40 μl of 34% C6H5Na3O7 solution. After 40 min of incubation at room temperature, the absorption was measured at 660 nm. Increasing concentrations of KH2PO4 were used as reference to calculate the amount of free phosphate. As controls, the assay was performed without addition of ATP or FtsH.
In Vitro Degradation of β-Casein
In vitro degradation of β-casein by purified FtsH variants was performed as described previously (10) with minor modifications. In a 100-μl reaction mix, 20 μg of His6-MBP-FtsHWT or His6-MBP-FtsHH417Y and 5 μg of β-casein were used and incubated at 42 °C. The reaction was started with 10 mm ATP, and samples were taken at different time points. Degradation of β-casein was quantified using the AlphaEaseFC (Alpha Innotec) software after SDS-PAGE and Coomassie staining.
Two-dimensional PAGE and Mass Spectrometry
Elution fractions of His6-MBP-FtsHWT and His6-MBP-FtsHH417Y were concentrated by chloroform/methanol precipitation (33) up to 600 μg μl−1. Isoelectric focusing and SDS-PAGE were performed as described previously (34). Protein solutions were loaded on Immobiline DryStrip pH 4–7, 24 cm (GE Healthcare). After isoelectric focusing, proteins were subjected to 12.5% SDS-PAGE, and the spots were visualized using RuBPS (C72H42N6Na4O18RuS6) staining. Protein spots were scanned using a Typhoon TRIO (GE Healthcare) and were quantified with the Delta two-dimensional software (version 4.0, Decodon). Selected protein spots were excised from the gel, and protein identification using mass spectrometry was performed by MALDI-TOF mass spectrometry as described previously (35).
In Vivo Degradation Experiments in E. coli
To analyze the stability of proteins, cells containing plasmids encoding for the proteins of interest were grown at 37 °C (ΔftsH at 30 °C) in a water bath shaker (180 rpm) to different growth phases. Protein expression was induced by the addition of 100–200 ng ml−1 anhydrotetracycline for 30 min. Then translation was blocked using 300 μg ml−1 spectinomycin. Samples were taken at different time points and were frozen in liquid nitrogen. To measure the effects of oxygen on degradation of His6-FdoH, anaerobic conditions were generated by incubating the cultures in hungate tubes without oxygen and shaking. To analyze whether degradation of His6-YfgM is regulated by osmotic conditions, cells in exponential phase of a growth curve were shocked with 15% (w/v) sucrose.
Preparation of Protein Extracts and Immunodetection
Depending on their absorbance, cell pellets were resuspended in TE buffer (10 mm Tris-HCl, pH 8, 1 mm EDTA, 100 μl of TE buffer per A580 nm of 1.0) and mixed with protein sample buffer (final concentrations of 2% SDS (w/v), 0.1% (w/v) bromophenol blue, 10% glycerol (v/v), 1% (v/v) β-mercaptoethanol, 50 mm Tris-HCl, pH 6.8). After incubation for 10 min at 100 °C, samples were centrifuged (1 min, 16,000 × g), and the protein extract was subjected to SDS gel electrophoresis and Western transfer using standard protocols (36). Proteins containing His tag fusions were detected using a Penta-His-HRP conjugate (Qiagen). FtsH and RpoH were detected using a respective polyclonal antiserum derived from rabbit as the primary antibody. A goat anti-rabbit immunoglobulin G(H+L)-HRP conjugate (Bio-Rad) served as a secondary antibody. Protein signals were visualized using Luminata Forte Western HRP substrate (Millipore) and the ChemiImager Ready (Alpha Innotec). Half-lives of proteins were calculated with the AlphaEaseFC software (version 4.0.0, Alpha Innotec).
Survival after Acidic Stress
The survival rate after an acidic shock was determined as described previously (37). E. coli cells containing a plasmid mediating constitutive expression of YfgM as well as cells carrying the empty vector were grown to an A580 nm of 0.6 in M9 minimal medium with glucose (22.2 mm) and casamino acids (0.2% w/v), buffered with MOPS and MES. The cultures were centrifuged, and the pellets were resuspended in 15 ml of M9 medium (+ MOPS; pH 5.7) and incubated for 70 min in a water bath shaker at 37 °C. After centrifugation, cells were resuspended in 15 ml of M9 medium, pH 2.4, and incubated for 2 h in a water bath shaker at 37 °C. Cells were sedimented and resuspended in 15 ml M9 medium (+ MOPS; pH 7). 100 μl of serial dilutions (10−2, 10−4, 10−6, and 10−8) were plated on LB agar plates. After incubation overnight at 37 °C, colonies were counted, and the survival rate was calculated. Serial dilutions of untreated cells were used as references. The survival rate of cells carrying the empty vector incubated in M9 medium (+ MOPS; pH 2.4) was set to 100%.
RESULTS
Validation of FtsHtrap for Substrate Identification
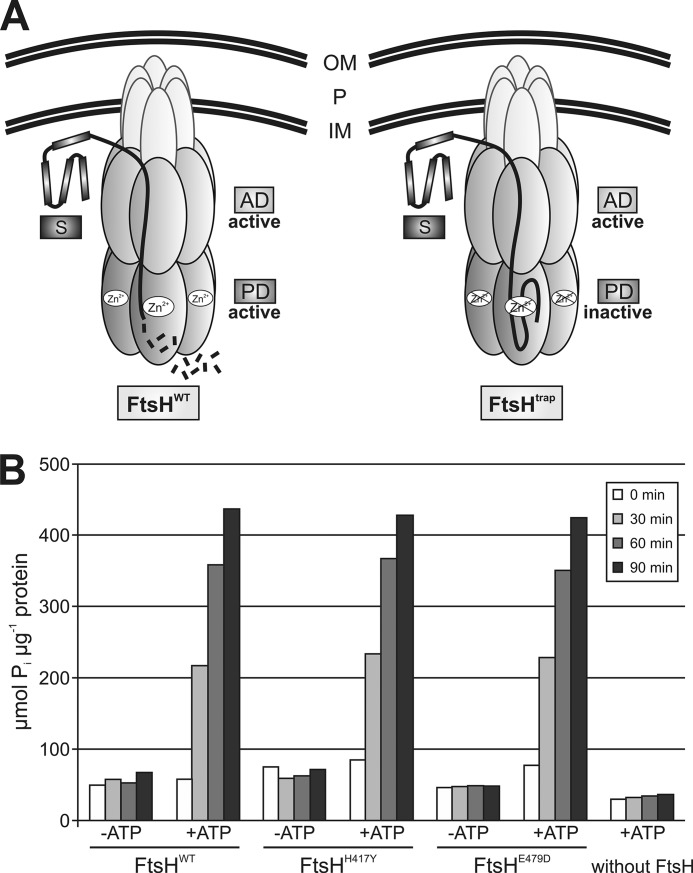
A promising strategy for identification of new protease substrates was to capture proteins by an inactive protease that accepts and retains substrates but is unable to degrade them (31). To trap FtsH substrates, we constructed variants expected to have normal ATPase activity that promotes translocation of substrates into the proteolytic chamber but were deficient in proteolysis (Fig. 1A). The catalytic activity should be destroyed by a single point mutation exchanging the first histidine in the zinc-binding motif (HEXXH) to a tyrosine or by exchanging the glutamic acid at position 479 to an aspartic acid. To verify that the ATPase activity was not affected by the H417Y or the E479D exchange, we purified His6-MBP-FtsHWT, His6-MBP-FtsHH417Y, and His6-MBP-FtsHE479D (from now on called FtsHWT, FtsHH417Y, and FtsHE479D) by nickel-nitrilotriacetic acid chromatography. Both proteins were subjected to an in vitro ATPase assay based on the photometric detection of free phosphate (Pi) from ATP hydrolysis (Fig. 1B). FtsHWT, FtsHH417Y, and FtsHE479D were able to hydrolyze ATP in a time-dependent manner, although there was no significant increase of free Pi in the absence of ATP or without FtsH. Full ATPase activity of FtsHH417Y and FtsHE479D suggested that they should be competent in substrate translocation.
FIGURE 1.
Principle of the FtsHtrap-based substrate identification approach. A, substrates (S) bind to both FtsHWT and FtsHtrap and are translocated by their active ATPase domains (AD). FtsHWT degrades the substrate in its protease domain (PD). In contrast, substrates are trapped in FtsHtrap due to an amino acid substitution that renders the protease domain of this protease inactive. IM and OM, inner and outer membrane, respectively; P, periplasm. B, ATPase domain of the FtsHH417Y and FtsHE479D protein is active. ATPase activity of FtsHH417Y, FtsHE479D, and FtsHWT was monitored using an in vitro ATP hydrolysis assay. Purified proteins were incubated with ATP, and the time-dependent accumulation of free Pi was measured by the malachite green assay. Control reactions incubated without ATP (−ATP) or FtsH showed no accumulation of Pi.
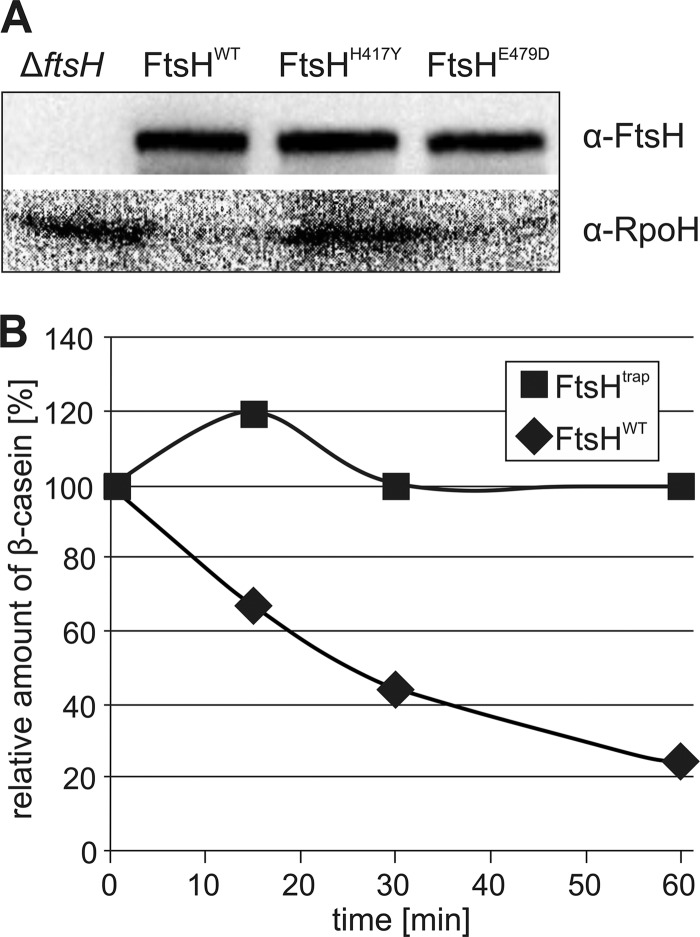
Two independent assays were used to examine the proteolytic activity of these proteins. In an in vivo approach, we analyzed whether the heat shock σ factor RpoH could be degraded by FtsHWT, FtsHH417Y, and FtsHE479D. Consistent with previous studies (11), RpoH accumulated in the ΔftsH strain (Fig. 2A). In contrast, the σ factor was not detectable by immunoblot analysis in the presence of plasmid-encoded FtsHWT or FtsHE479D. Equal amounts of FtsHH417Y (from now on called FtsHtrap), however, were unable to degrade the σ factor suggesting that the mutated protein is impaired in proteolysis. A complementary in vitro approach showed that FtsHtrap was unable to degrade the standard protease substrate β-casein (Fig. 2B). Both assays thus confirmed the suitability of FtsHtrap for the trapping approach.
FIGURE 2.
FtsHH417Y ( = FtsHtrap) is protease-deficient in vivo and in vitro. A, σ factor RpoH is not degraded by FtsHtrap in vivo. Production of plasmid-encoded FtsHWT, FtsHtrap, or FtsHE479D and the presence of chromosomally encoded RpoH in E. coli ΔftsH cells were monitored by Western blot analysis. B, purified FtsHWT or FtsHtrap was incubated with the model substrate β-casein, and proteolysis was monitored for 60 min using SDS-PAGE and densitometry.
Identification of Co-purified Proteins
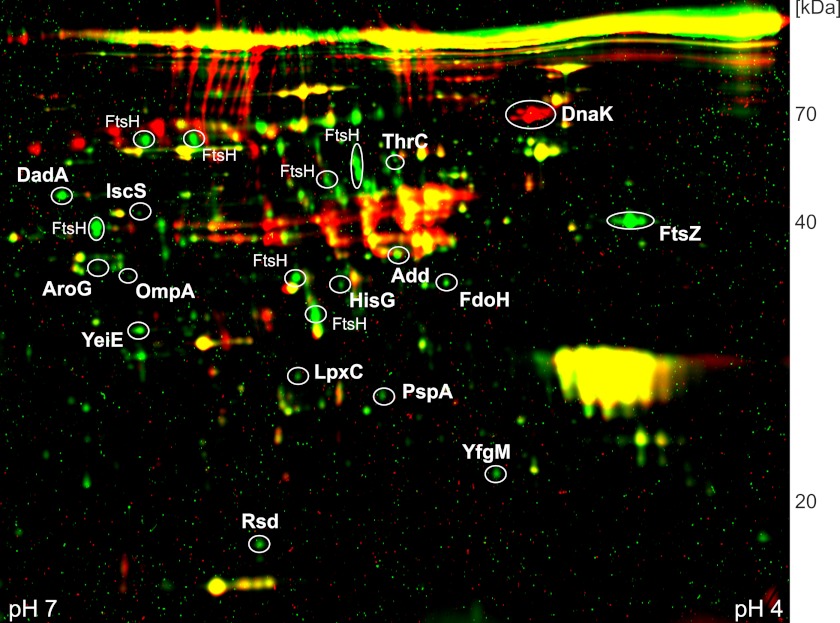
To identify putative substrates of FtsH, plasmid-encoded FtsHWT and FtsHtrap were expressed in a ΔftsH strain. The FtsH preparations were subjected to two-dimensional PAGE. Dual-channel images of two-dimensional gels of representative elution fractions from FtsHWT and FtsHtrap (Fig. 3) show proteins present in both preparations (yellow spots), and proteins that were over-represented in elution fractions of FtsHWT (red spots; DnaK) or FtsHtrap (green spots). After data analysis of three replicates, 15 putative FtsH substrates were selected and identified by mass spectrometry. The presence of the known substrate LpxC among them supported the value of this approach. The other proteins that co-eluted with FtsHtrap fall into a wide variety of cellular functions (Table 1); for example, HisG, a histidine biosynthesis enzyme (38). PspA is a protein involved in phage shock and stress response (39, 40). Rsd, the anti-σ factor of the housekeeping σ factor RpoD (41) was not only trapped with FtsH but also with ClpXP (31).
FIGURE 3.
Comparative proteome analysis of proteins purified with FtsHWT and FtsHtrap. Elution fractions of FtsHWT and FtsHtrap were subjected to two-dimensional gel electrophoresis. Proteins over-represented in FtsHWT or FtsHtrap preparations are shown in red or green, respectively. Proteins present in equal amounts in both preparations appear in yellow.
TABLE 1.
List of putative FtsH substrates that were co-purified by FtsHtrap
| Trapped protein | Function | Degradation |
|---|---|---|
| Known substrates | ||
| LpxC | Key enzyme in LPS biosynthesis | Yes |
| Putative substrates | ||
| OmpA | Outer membrane protein | Not determined (ND) |
| FtsZ | Cell division protein | No (70) |
| ThrC | Threonine synthase | ND |
| PurA | De novo synthesis of purine nucleotides | ND |
| PspA | Regulator of phage shock and stress response | No |
| Rsd | Anti-σ factor of RpoD (σ70) | No |
| AroG | Biosynthesis of 3-deoxy-d-arabino-heptulosonate 7-phosphate | No |
| Add | Deamination of adenosine | No |
| YeiE | Putative transcription activator | No |
| HisG | Biosynthesis of histidine | No |
| DadA | Oxidative deamination of d-amino acids | Yes |
| IscS | Sulfatation of molecules | Yes |
| YfgM | Unknown function | Yes (growth phase-dependent) |
| FdoH | Adaptation to anaerobic conditions | Yes (oxygen-dependent) |
Not All Proteins Co-purified with FtsHtrap Are Protease Substrates
To verify whether the trapped proteins are subject to FtsH-dependent proteolysis, we constructed expression plasmids coding for N-terminal His6 tag fusions of a selection of these proteins. Because degradation of many protease substrates depends on the actual growth conditions, stability of the putative FtsH substrates was assayed by in vivo degradation experiments at different time points along a growth curve of E. coli at 37 and 20 °C. PspA, Rsd, HisG, Add, AroG, and YeiE were stable under all tested conditions (Fig. 4). Consistent with this, PspA has recently been shown not to be an FtsH substrate (42). Rsd, the anti-σ factor of the housekeeping σ factor RpoD, was also stable after a sudden shift from stationary phase to log phase, when inhibition of RpoD is not needed anymore (data not shown).
FIGURE 4.
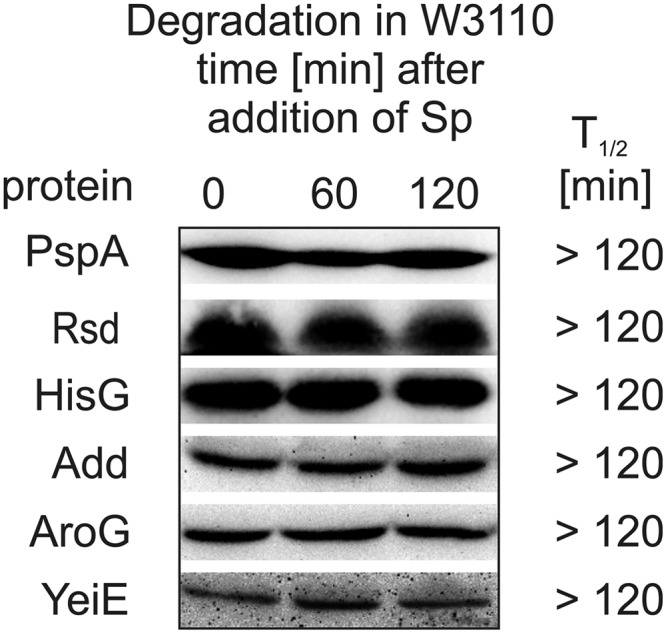
In vivo stability of selected putative FtsH substrates. Stability of plasmid-encoded His6-PspA, -Rsd, -HisG, -Add, -AroG, or -YeiE was analyzed in E. coli W3110. After synthesis of the putative substrate, translation was blocked by addition of spectinomycin, and samples were taken at different time points. Protein half-lives were calculated after SDS-PAGE, Western transfer, and immunodetection. The results are based on three independent experiments.
Validation of Four New FtsH Substrates
In contrast to the six proteins that did not seem to be prone to proteolytic control, the cytosolic protein IscS was subject to proteolysis (Fig. 5A). In an E. coli W3110 (WT) strain, IscS decayed in all growth phases with half-lives of about 40 min. IscS is an FtsH substrate as it was stabilized in the ΔftsH background.
FIGURE 5.
FtsH-dependent proteolysis of IscS and DadA. E. coli W3110 or ΔftsH cultures carrying the respective expression plasmids were grown in LB broth. Subcultures for in vivo degradation experiments were taken in different growth phases at the indicated absorbances (A580 nm). Half-lives of IscS (A) or DadA (B) from three independent experiments were calculated as in Fig. 4.
Another novel FtsH substrate is DadA, which is an inner membrane protein able to deaminate d-amino acids. Like IscS, DadA was degraded in all growth phases but with shorter half-lives around 25 min (Fig. 5B). Stabilization in the ΔftsH strain indicated FtsH-dependent degradation of DadA.
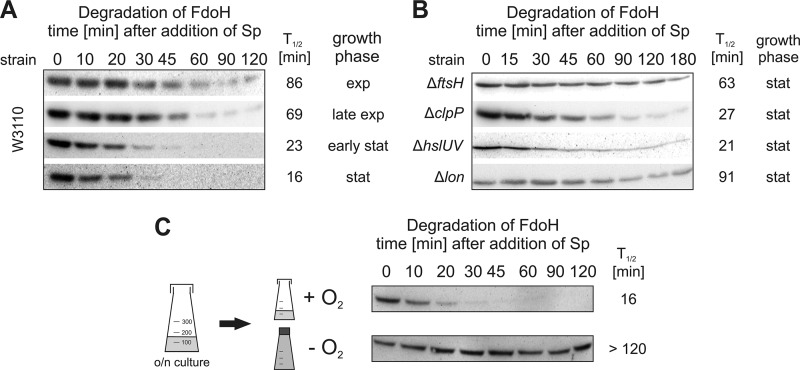
FdoH and YfgM are also inner membrane proteins, which are directed to proteolysis. FdoH is part of the formate dehydrogenase complex FDH-O that is responsible for the adaptation to a sudden shift from aerobic to anaerobic conditions. The protein was slowly degraded with half-lives of about 70 min in the early, middle, and late logarithmic phase (Fig. 6A). Turnover increased significantly in the stationary phase. Here, FdoH was degraded with a half-life of 15 min. Slow degradation of FdoH in stationary phase cultures of ΔftsH and Δlon strains suggested an involvement of both proteases (Fig. 6B). To address whether degradation of FdoH was affected by the availability of oxygen, stationary overnight cultures were split into two new cultures and subjected to either aerobic and anaerobic conditions (Fig. 6C). In an aerated shaken culture, FdoH was degraded with a half-life of 15 min. In the absence of oxygen, however, FdoH was stable indicating an important role of O2 for regulation of FdoH proteolysis. Consistent with these findings under two extreme situations, intermediate oxygen levels correlated with intermediate FdoH stabilities (data not shown). A general decrease in FtsH activity under oxygen-limited conditions can be excluded because the YfgM protein was degraded by FtsH under both aerobic and anaerobic conditions (data not shown).
FIGURE 6.
Proteolysis of FdoH depends on growth phase and oxygen status. The half-life of FdoH in E. coli W3110 (A) or different protease knock-out strains (ΔftsH, ΔclpP, ΔhslUV, Δlon) (B) was monitored as described above. C, after splitting a stationary phase overnight (o/n) culture into two subcultures, one well aerated and the other filled to the top and sealed, the stability of FdoH was monitored. The half-lives of FdoH were calculated as described above. All results were reproduced in three independent experiments.
As the Y designation indicates, the exact function of the FtsH substrate YfgM is unknown. Similar to FdoH, YfgM was poorly degraded during exponential growth and showed the shortest half-lives in stationary phase (t½ ≈10 min; Fig. 7A). Degradation of YfgM was mediated by FtsH as the protein was stabilized in all growth phases in the ΔftsH strain (Fig. 7B; note that the ftsH mutant reaches lower optical densities in stationary phase than the WT strain). Moreover, turnover of YfgM was influenced by osmotic conditions, because an osmotic shock with 15% sucrose (v/v) induced a 4-fold faster degradation in the exponential phase (Fig. 7C).
FIGURE 7.
YfgM is rapidly degraded in stationary phase and under osmotic stress in an FtsH-dependent manner. The half-life of YfgM in E. coli W3110 (A) and in an ftsH knock-out strains (ΔftsH; B) was monitored as described above. C, after splitting an exponential phase (A580 nm = 0.5) culture into two subcultures, one was shocked with sucrose (+ Suc) and the other served as a control (− Suc). The stability of YfgM was monitored, and the half-lives of YfgM were calculated as described above. All results were reproduced in three independent experiments.
Phenotypes Caused by YfgM Overproduction
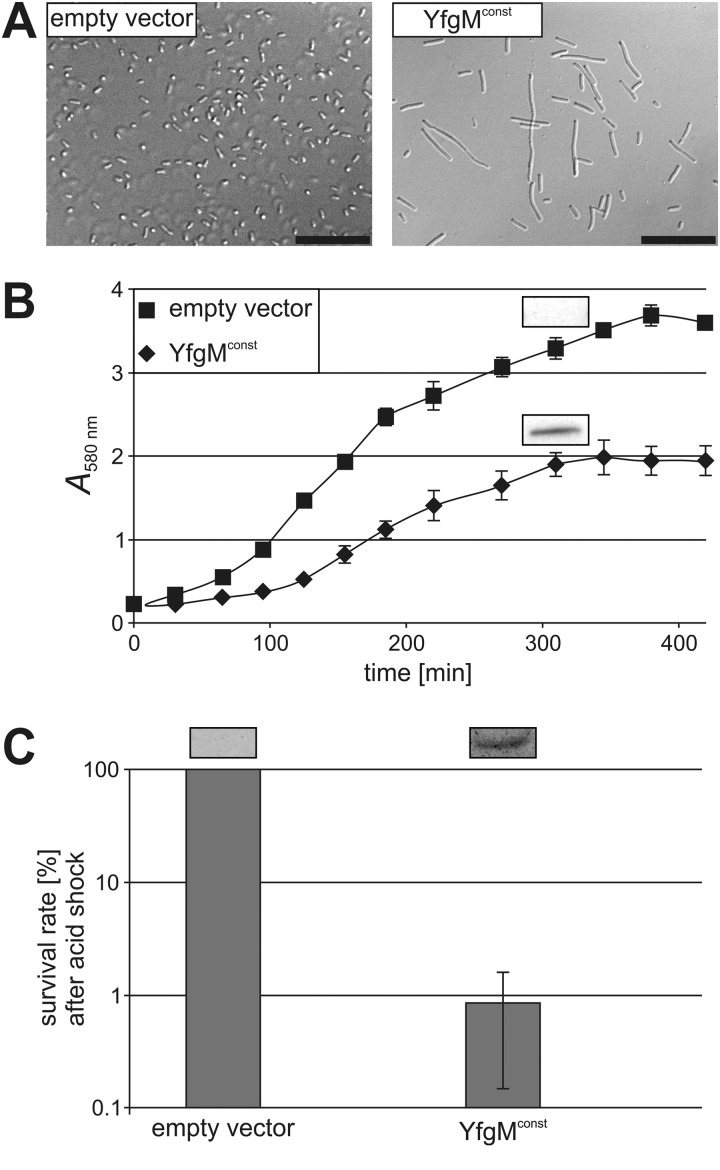
To approach a possible biological function of YfgM, we analyzed the effects of constitutive YfgM expression and observed several striking phenotypes. For example, elevated amounts of YfgM led to a drastic defect in cell division resulting in filamentous cells (Fig. 8A). The cells showed an elongated lag phase and a much earlier entry into the stationary phase compared with cells without constitutive YfgM expression (Fig. 8B). Moreover, the resistance against acidic stress was affected by YfgM. Overproduction of the protein decreased survival after an acidic shock to a pH of 2.4 about 100 times (Fig. 8C).
FIGURE 8.
Overproduction of YfgM is toxic and impairs survival after an acidic shock. In contrast to cells carrying the empty vector (control), constitutive expression of yfgM (YfgMconst) resulted in a filamentous cell shape (A; scale bar, 20 μm), a general growth defect (B), and reduced survival when cells were shocked with acid (pH 2.4). Production of YfgM was determined by immunoblot analysis with anti-His antisera (shown in boxes; B and C).
DISCUSSION
To extend our knowledge on the physiological functions of FtsH, the only essential protease in E. coli, we established a substrate trapping approach based on an ATPase-competent but protease-deficient FtsH variant. Differential proteomics of protein preparations associated with FtsHWT and FtsHtrap revealed four proteins that were authentic substrates of the E. coli FtsH protease. This expands the collection of known targets from 11 to 15 (Fig. 9). Compared with other proteases, this seems like a limited number. Many more substrates have been reported for ClpXP (31, 43) and the Lon protease (44). Nonetheless, it is unlikely that the number of undiscovered FtsH substrates is going to increase substantially. The previously reported poor unfoldase activity of the protease (45) might severely restrict the repertoire of proteins susceptible to degradation by FtsH.
FIGURE 9.
Diverse functions of the FtsH protease. Previously known functions (boxes) and substrates (ellipses) of FtsH are shown in white. Novel substrates and their known or putative biological functions revealed by this study are in gray. IM and OM = inner and outer membrane.
Among the newly identified FtsH substrates were two that have also been trapped with ClpXP, namely IscS and DadA (31). Fusion of the 11 N-terminal amino acids of IscS to a stable reporter protein rendered it unstable in vitro with a half-life of 13 min suggesting that the N-terminal end of IscS carries a recognition motif for the ClpX ATPase. DadA was proposed to carry a similar N-terminal motif. It is quite common that bacterial proteins are subject to proteolysis by several proteases (19). How and when they are directed to one or the other protease is unknown. IscS is the only newly identified FtsH target that resides in the cytosol. It is a cysteine desulfurase responsible for iron-sulfur cluster formation and sulfatation of thiamine and biotin (46). IscS was degraded by FtsH in all growth phases with a half-life of about 40 min. In addition to proteolytic control, activity of the IscS enzyme is gradually inhibited as the amount of bound iron and sulfide increase (47). The need for tight control of iron-sulfur cluster assembly is further emphasized by the finding that the transcription factor IscR, which serves as the intracellular sensor of the demand for FeS cluster biosynthesis, and the FeS cluster scaffolding protein IscU were captured with Clptrap (31).
Because of its unique location in the cytoplasmic membrane, FtsH is well suited for the quality control and regulated proteolysis of membrane proteins (15). We identified DadA as membrane-integrated FtsH substrate that was degraded in all growth phases with a moderate half-life of about 25 min. DadA has been implicated in the oxidative deamination of a broad range of d-amino acids into their corresponding ketoacid (48). Bacterial growth on d-amino acids should only be allowed when these non-proteinogenic amino acids are abundant. Otherwise, DadA would deplete the pool of certain d-amino acids required as building blocks for peptidoglycan biosynthesis, in particular in stationary phase when d-amino acids play a major role in cell wall remodeling (49). This suggests that either the activity or the amount of DadA in the cell needs to be under control. The flavin-dependent enzyme is predicted to form a heterodimeric complex with an unidentified partner protein (50). As we expressed epitope-tagged DadA to facilitate its immunodetection, it is possible that DadA is directed to proteolysis whenever it is not bound to its partner protein. Similar turnover in the absence of interaction partners is known for other membrane substrates like PspC, F0α, and SecY (40, 51, 52).
The final two novel FtsH substrates were degraded in a strictly condition-dependent manner. To our knowledge, this is the first report of growth phase-regulated proteolysis of membrane-bound FtsH substrates. FdoH is likely degraded by FtsH and the Lon protease, as it has been described for SoxS (19, 20). FdoH is a subunit of one of two formate dehydrogenases (FDH)2 in E. coli. FDH enzymes catalyze the proton-translocating oxidation of formate at the expense of nitrate reduction to nitrite. FDH-N, the more effective enzyme, is induced by nitrate (53, 54). FDH-O, consisting of FdoG, FdoH, and FdoI, is constitutively expressed and only slightly induced by nitrate. This enzyme complex permits adaptation to a shift from aerobiosis to anaerobiosis until a sufficient level of the inducible FDH-N pathway is reached (55–58). Assuming that FdoH might be stabilized in cells challenged with sudden anaerobiosis, we indeed found that the exposure of a stationary overnight culture to oxygen-limited conditions completely prevented proteolysis of FdoH. When growing cultures reach stationary phase, bacteria are adapted to oxygen deprivation by synthesis of FDH-N. In that case, FdoH was removed from the cell probably to avoid competition between FDH-N and the much less effective FDH-O complex.
YfgM has been reported to interact with PpiD and RcsB (59, 60). Interestingly, both proteins reside in separate compartments of the cell. PpiD is a periplasmic chaperone (61) suggesting that YfgM might be involved in the extracytoplasmic stress response. RcsB as interaction partner suggests that YfgM is involved in the general stress response. RcsB is the response regulator of the RCS phosphorelay system. Phosphorylated RcsB induces the expression of genes to survive an acidic or osmotic shock, to adapt to stationary phase, and to induce capsule biosynthesis (37, 62–64). In stationary phase, RcsB activates expression of the small RNA RprA, which promotes translation of the rpoS mRNA leading to increased amounts of RpoS ensuring adaptation to starvation (65). Based on the interaction of YfgM with RcsB, we speculated that it might act as an inhibitor of the RcsB pathway. Fully consistent with a functional link between RcsB and YfgM, an osmotic shock induced the RCS phosphorelay system (64) as well as a faster degradation of YfgM (Fig. 7C). As one might expect for an inhibitor of a stress response pathway, an yfgM deletion strain did not reveal any obvious phenotypic defects (data not shown). In contrast, elevated levels of YfgM drastically decreased the acid resistance (Fig. 8C) much like the lack of RcsB (37). Our findings might explain why YfgM is proteolytically removed from the cell upon entry into stationary phase and under certain stress conditions to release the response regulator RcsB, which activates expression of RcsB-dependent genes.
The proteins degraded by FtsH are surprisingly diverse in their cellular localization, topology, and biological function (Fig. 9). It is unlikely that they are recognized and degraded by a unifying concept. In fact, individual principles seem to apply for different substrates. Although degradation of LpxC depends on conserved non-polar residues in the C-terminal tail (25), the mechanism of KdtA degradation, another FtsH substrate acting in the very same biosynthesis pathway as LpxC, is different and still unknown (16). Proteolysis follows another route for the heat shock σ factor RpoH, which bears internal recognition sites and requires the contribution of chaperones for degradation (66). Misfolded membrane proteins destined for degradation are recognized via exposed N or C termini (67). Intriguingly, the newly discovered FtsH substrates YfgM and FdoH differ entirely in their topology. Both span the cytoplasmic membrane by one transmembrane helix, but YfgM contains a short N terminus (20 amino acids) in the cytoplasm and more than 200 amino acids in the periplasm (60), whereas more than 250 N-terminal amino acids of FdoH reside in the cytoplasm and 20 C-terminal amino acids are exposed to the periplasm (68). Differences in topology and the fact that FdoH but not YfgM was stabilized under anaerobic conditions suggest that they are degraded by unrelated pathways.
Diverse recognition mechanisms for FtsH substrates are contrasted by more unifying concepts in the case of other proteases. Lon prefers degrons rich in aromatic and hydrophobic residues (68). Proteolysis mediated by ClpXP tends to use N- or C-terminal motifs that can be divided in five distinct classes (31). Many ClpAP substrates follow the N-end rule with N-terminal Phe, Leu, Trp, or Tyr residues (69). The fact that the known FtsH substrates share no obvious structural features emphasizes the need for unbiased global strategies to identify new substrates. Our trapping approach has proven successful to elucidate previously unknown substrates and biological functions of FtsH. Exploiting the FtsHtrap protein as bait under different growth conditions might help reveal even more substrates of this versatile protease.
Supplementary Material
Acknowledgments
We thank Regine Hengge (Berlin, Germany), Eberhard Klauck (Berlin, Germany), and Axel Mogk (Heidelberg, Germany) for providing E. coli strains; Eliora Ron (Tel Aviv, Israel) for strains and the pMAL-C-FtsH expression plasmid; Philippe Bouloc (Paris, France) for antisera against FtsH; Julia Bandow for sharing proteomic infrastructure and expertise; Knut Büttner (Greifswald, Germany) for MALDI-MS analysis, and Michael Schäkermann and Nicole Frankenberg-Dinkel for critical reading of the manuscript.
This work was supported by German Research Foundation Grant SFB 642 for GTP- and ATP-dependent membrane processes (to F. N.).

This article contains supplemental Tables S1 and S2 and additional references.
- FDH
- formate dehydrogenase.
REFERENCES
- 1. Gur E., Biran D., Ron E. Z. (2011) Regulated proteolysis in Gram-negative bacteria-how and when? Nat. Rev. Microbiol. 9, 839–848 [DOI] [PubMed] [Google Scholar]
- 2. Sauer R. T., Baker T. A. (2011) AAA+ proteases: ATP-fueled machines of protein destruction. Annu. Rev. Biochem. 80, 587–612 [DOI] [PubMed] [Google Scholar]
- 3. Ogura T., Inoue K., Tatsuta T., Suzaki T., Karata K., Young K., Su L. H., Fierke C. A., Jackman J. E., Raetz C. R., Coleman J., Tomoyasu T., Matsuzawa H. (1999) Balanced biosynthesis of major membrane components through regulated degradation of the committed enzyme of lipid A biosynthesis by the AAA protease FtsH (HflB) in Escherichia coli. Mol. Microbiol. 31, 833–844 [DOI] [PubMed] [Google Scholar]
- 4. Tomoyasu T., Yamanaka K., Murata K., Suzaki T., Bouloc P., Kato A., Niki H., Hiraga S., Ogura T. (1993) Topology and subcellular localization of FtsH protein in Escherichia coli. J. Bacteriol. 175, 1352–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krzywda S., Brzozowski A. M., Verma C., Karata K., Ogura T., Wilkinson A. J. (2002) The crystal structure of the AAA domain of the ATP-dependent protease FtsH of Escherichia coli at 1.5 Å resolution. Structure 10, 1073–1083 [DOI] [PubMed] [Google Scholar]
- 6. Ogura T., Wilkinson A. J. (2001) AAA+ superfamily ATPases. Common structure-diverse function. Genes Cells 6, 575–597 [DOI] [PubMed] [Google Scholar]
- 7. Okuno T., Yamanaka K., Ogura T. (2006) Characterization of mutants of the Escherichia coli AAA protease, FtsH, carrying a mutation in the central pore region. J. Struct. Biol. 156, 109–114 [DOI] [PubMed] [Google Scholar]
- 8. Yamada-Inagawa T., Okuno T., Karata K., Yamanaka K., Ogura T. (2003) Conserved pore residues in the AAA protease FtsH are important for proteolysis and its coupling to ATP hydrolysis. J. Biol. Chem. 278, 50182–50187 [DOI] [PubMed] [Google Scholar]
- 9. Bieniossek C., Schalch T., Bumann M., Meister M., Meier R., Baumann U. (2006) The molecular architecture of the metalloprotease FtsH. Proc. Natl. Acad. Sci. U.S.A. 103, 3066–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tomoyasu T., Gamer J., Bukau B., Kanemori M., Mori H., Rutman A. J., Oppenheim A. B., Yura T., Yamanaka K., Niki H., et al. (1995) Escherichia coli FtsH is a membrane-bound, ATP-dependent protease that degrades the heat-shock transcription factor σ32. EMBO J. 14, 2551–2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saikawa N., Ito K., Akiyama Y. (2002) Identification of glutamic acid 479 as the gluzincin coordinator of zinc in FtsH (HflB). Biochemistry 41, 1861–1868 [DOI] [PubMed] [Google Scholar]
- 12. Ito K., Akiyama Y. (2005) Cellular functions, mechanism of action, and regulation of FtsH protease. Annu. Rev. Microbiol. 59, 211–231 [DOI] [PubMed] [Google Scholar]
- 13. Langklotz S., Baumann U., Narberhaus F. (2012) Structure and function of the bacterial AAA protease FtsH. Biochim. Biophys. Acta 1823, 40–48 [DOI] [PubMed] [Google Scholar]
- 14. Narberhaus F., Obrist M., Führer F., Langklotz S. (2009) Degradation of cytoplasmic substrates by FtsH, a membrane-anchored protease with many talents. Res. Microbiol. 160, 652–659 [DOI] [PubMed] [Google Scholar]
- 15. Akiyama Y. (2009) Quality control of cytoplasmic membrane proteins in Escherichia coli. J. Biochem. 146, 449–454 [DOI] [PubMed] [Google Scholar]
- 16. Katz C., Ron E. Z. (2008) Dual role of FtsH in regulating lipopolysaccharide biosynthesis in Escherichia coli. J. Bacteriol. 190, 7117–7122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gamer J., Multhaup G., Tomoyasu T., McCarty J. S., Rüdiger S., Schönfeld H. J., Schirra C., Bujard H., Bukau B. (1996) A cycle of binding and release of the DnaK, DnaJ, and GrpE chaperones regulates activity of the Escherichia coli heat shock transcription factor σ32. EMBO J. 15, 607–617 [PMC free article] [PubMed] [Google Scholar]
- 18. Herman C., Thévenet D., D'Ari R., Bouloc P. (1995) Degradation of σ32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc. Natl. Acad. Sci. U.S.A. 92, 3516–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Griffith K. L., Shah I. M., Wolf R. E., Jr. (2004) Proteolytic degradation of Escherichia coli transcription activators SoxS and MarA as the mechanism for reversing the induction of the superoxide (SoxRS) and multiple antibiotic resistance (Mar) regulons. Mol. Microbiol. 51, 1801–1816 [DOI] [PubMed] [Google Scholar]
- 20. Shah I. M., Wolf R. E., Jr. (2006) Sequence requirements for Lon-dependent degradation of the Escherichia coli transcription activator SoxS. Identification of the SoxS residues critical to proteolysis and specific inhibition of in vitro degradation by a peptide comprised of the N-terminal 21 amino acid residues. J. Mol. Biol. 357, 718–731 [DOI] [PubMed] [Google Scholar]
- 21. Herman C., Thévenet D., D'Ari R., Bouloc P. (1997) The HflB protease of Escherichia coli degrades its inhibitor λ cIII. J. Bacteriol. 179, 358–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kihara A., Akiyama Y., Ito K. (1997) Host regulation of lysogenic decision in bacteriophage λ. Transmembrane modulation of FtsH (HflB), the cII degrading protease, by HflKC (HflA). Proc. Natl. Acad. Sci. U.S.A. 94, 5544–5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leffers G. G., Jr., Gottesman S. (1998) λ Xis degradation in vivo by Lon and FtsH. J. Bacteriol. 180, 1573–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Führer F., Langklotz S., Narberhaus F. (2006) The C-terminal end of LpxC is required for degradation by the FtsH protease. Mol. Microbiol. 59, 1025–1036 [DOI] [PubMed] [Google Scholar]
- 25. Führer F., Müller A., Baumann H., Langklotz S., Kutscher B., Narberhaus F. (2007) Sequence and length recognition of the C-terminal turnover element of LpxC, a soluble substrate of the membrane-bound FtsH protease. J. Mol. Biol. 372, 485–496 [DOI] [PubMed] [Google Scholar]
- 26. Langklotz S., Narberhaus F. (2011) The Escherichia coli replication inhibitor CspD is subject to growth-regulated degradation by the Lon protease. Mol. Microbiol. 80, 1313–1325 [DOI] [PubMed] [Google Scholar]
- 27. Guisbert E., Herman C., Lu C. Z., Gross C. A. (2004) A chaperone network controls the heat shock response in E. coli. Genes Dev. 18, 2812–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Obrist M., Langklotz S., Milek S., Führer F., Narberhaus F. (2009) Region C of the Escherichia coli heat shock σ factor RpoH (σ32) contains a turnover element for proteolysis by the FtsH protease. FEMS Microbiol. Lett. 290, 199–208 [DOI] [PubMed] [Google Scholar]
- 29. Obrist M., Narberhaus F. (2005) Identification of a turnover element in region 2.1 of Escherichia coli σ32 by a bacterial one-hybrid approach. J. Bacteriol. 187, 3807–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horikoshi M., Yura T., Tsuchimoto S., Fukumori Y., Kanemori M. (2004) Conserved region 2.1 of Escherichia coli heat shock transcription factor σ32 is required for modulating both metabolic stability and transcriptional activity. J. Bacteriol. 186, 7474–7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flynn J. M., Neher S. B., Kim Y. I., Sauer R. T., Baker T. A. (2003) Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol. Cell 11, 671–683 [DOI] [PubMed] [Google Scholar]
- 32. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 33. Wessel D., Flügge U. I. (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138, 141–143 [DOI] [PubMed] [Google Scholar]
- 34. Bandow J. E., Baker J. D., Berth M., Painter C., Sepulveda O. J., Clark K. A., Kilty I., VanBogelen R. A. (2008) Improved image analysis workflow for 2-D gels enables large-scale 2-D gel-based proteomics studies–COPD biomarker discovery study. Proteomics 8, 3030–3041 [DOI] [PubMed] [Google Scholar]
- 35. Klüsener S., Hacker S., Tsai Y. L., Bandow J. E., Gust R., Lai E. M., Narberhaus F. (2010) Proteomic and transcriptomic characterization of a virulence-deficient phosphatidylcholine-negative Agrobacterium tumefaciens mutant. Mol. Genet. Genomics 283, 575–589 [DOI] [PubMed] [Google Scholar]
- 36. Sambrook J., Russell D. W. (2001) Molecular Cloning: A Laboratory Manual, 3rd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 37. Johnson M. D., Burton N. A., Gutiérrez B., Painter K., Lund P. A. (2011) RcsB is required for inducible acid resistance in Escherichia coli and acts at gadE-dependent and -independent promoters. J. Bacteriol. 193, 3653–3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuroda A., Tanaka S., Ikeda T., Kato J., Takiguchi N., Ohtake H. (1999) Inorganic polyphosphate kinase is required to stimulate protein degradation and for adaptation to amino acid starvation in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 96, 14264–14269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weiner L., Brissette J. L., Model P. (1991) Stress-induced expression of the Escherichia coli phage shock protein operon is dependent on σ54 and modulated by positive and negative feedback mechanisms. Genes Dev. 5, 1912–1923 [DOI] [PubMed] [Google Scholar]
- 40. Joly N., Engl C., Jovanovic G., Huvet M., Toni T., Sheng X., Stumpf M. P., Buck M. (2010) Managing membrane stress. The phage shock protein (Psp) response, from molecular mechanisms to physiology. FEMS Microbiol. Rev. 34, 797–827 [DOI] [PubMed] [Google Scholar]
- 41. Jishage M., Ishihama A. (1998) A stationary phase protein in Escherichia coli with binding activity to the major σ subunit of RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 95, 4953–4958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Singh S., Darwin A. J. (2011) FtsH-dependent degradation of phage shock protein C in Yersinia enterocolitica and Escherichia coli. J. Bacteriol. 193, 6436–6442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Neher S. B., Villén J., Oakes E. C., Bakalarski C. E., Sauer R. T., Gygi S. P., Baker T. A. (2006) Proteomic profiling of ClpXP substrates after DNA damage reveals extensive instability within SOS regulon. Mol. Cell 22, 193–204 [DOI] [PubMed] [Google Scholar]
- 44. Van Melderen L., Aertsen A. (2009) Regulation and quality control by Lon-dependent proteolysis. Res. Microbiol. 160, 645–651 [DOI] [PubMed] [Google Scholar]
- 45. Herman C., Prakash S., Lu C. Z., Matouschek A., Gross C. A. (2003) Lack of a robust unfoldase activity confers a unique level of substrate specificity to the universal AAA protease FtsH. Mol. Cell 11, 659–669 [DOI] [PubMed] [Google Scholar]
- 46. Johnson D. C., Dean D. R., Smith A. D., Johnson M. K. (2005) Structure, function, and formation of biological iron-sulfur clusters. Annu. Rev. Biochem. 74, 247–281 [DOI] [PubMed] [Google Scholar]
- 47. Wu G., Li P., Wu X. (2008) Regulation of Escherichia coli IscS desulfurase activity by ferrous iron and cysteine. Biochem. Biophys. Res. Commun. 374, 399–404 [DOI] [PubMed] [Google Scholar]
- 48. Olsiewski P. J., Kaczorowski G. J., Walsh C. (1980) Purification and properties of d-amino acid dehydrogenase, an inducible membrane-bound iron-sulfur flavoenzyme from Escherichia coli B. J. Biol. Chem. 255, 4487–4494 [PubMed] [Google Scholar]
- 49. Lam H., Oh D. C., Cava F., Takacs C. N., Clardy J., de Pedro M. A., Waldor M. K. (2009) d-Amino acids govern stationary phase cell wall remodeling in bacteria. Science 325, 1552–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lobocka M., Hennig J., Wild J., Kłopotowski T. (1994) Organization and expression of the Escherichia coli K-12 dad operon encoding the smaller subunit of d-amino acid dehydrogenase and the catabolic alanine racemase. J. Bacteriol. 176, 1500–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Akiyama Y., Kihara A., Ito K. (1996) Subunit a of proton ATPase F0 sector is a substrate of the FtsH protease in Escherichia coli. FEBS Lett. 399, 26–28 [DOI] [PubMed] [Google Scholar]
- 52. Akiyama Y., Kihara A., Tokuda H., Ito K. (1996) FtsH (HflB) is an ATP-dependent protease selectively acting on SecY and some other membrane proteins. J. Biol. Chem. 271, 31196–31201 [DOI] [PubMed] [Google Scholar]
- 53. Benoit S., Abaibou H., Mandrand-Berthelot M. A. (1998) Topological analysis of the aerobic membrane-bound formate dehydrogenase of Escherichia coli. J. Bacteriol. 180, 6625–6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Enoch H. G., Lester R. L. (1975) The purification and properties of formate dehydrogenase and nitrate reductase from Escherichia coli. J. Biol. Chem. 250, 6693–6705 [PubMed] [Google Scholar]
- 55. Abaibou H., Pommier J., Benoit S., Giordano G., Mandrand-Berthelot M. A. (1995) Expression and characterization of the Escherichia coli fdo locus and a possible physiological role for aerobic formate dehydrogenase. J. Bacteriol. 177, 7141–7149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Iobbi-Nivol C., Santini C. L., Blasco F., Giordano G. (1990) Purification and further characterization of the second nitrate reductase of Escherichia coli K12. Eur. J. Biochem. 188, 679–687 [DOI] [PubMed] [Google Scholar]
- 57. Plunkett G., 3rd, Burland V., Daniels D. L., Blattner F. R. (1993) Analysis of the Escherichia coli genome. III. DNA sequence of the region from 87.2 to 89.2 minutes. Nucleic Acids Res. 21, 3391–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pommier J., Mandrand M. A., Holt S. E., Boxer D. H., Giordano G. (1992) A second phenazine methosulphate-linked formate dehydrogenase isoenzyme in Escherichia coli. Biochim. Biophys. Acta 1107, 305–313 [DOI] [PubMed] [Google Scholar]
- 59. Lasserre J. P., Beyne E., Pyndiah S., Lapaillerie D., Claverol S., Bonneu M. (2006) A complexomic study of Escherichia coli using two-dimensional blue native/SDS-polyacrylamide gel electrophoresis. Electrophoresis 27, 3306–3321 [DOI] [PubMed] [Google Scholar]
- 60. Maddalo G., Stenberg-Bruzell F., Götzke H., Toddo S., Björkholm P., Eriksson H., Chovanec P., Genevaux P., Lehtiö J., Ilag L. L., Daley D. O. (2011) Systematic analysis of native membrane protein complexes in Escherichia coli. J. Proteome Res. 10, 1848–1859 [DOI] [PubMed] [Google Scholar]
- 61. Matern Y., Barion B., Behrens-Kneip S. (2010) PpiD is a player in the network of periplasmic chaperones in Escherichia coli. BMC Microbiol. 10, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hagiwara D., Sugiura M., Oshima T., Mori H., Aiba H., Yamashino T., Mizuno T. (2003) Genome-wide analyses revealing a signaling network of the RcsC-YojN-RcsB phosphorelay system in Escherichia coli. J. Bacteriol. 185, 5735–5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Majdalani N., Hernandez D., Gottesman S. (2002) Regulation and mode of action of the second small RNA activator of RpoS translation, RprA. Mol. Microbiol. 46, 813–826 [DOI] [PubMed] [Google Scholar]
- 64. Sledjeski D. D., Gottesman S. (1996) Osmotic shock induction of capsule synthesis in Escherichia coli K-12. J. Bacteriol. 178, 1204–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Peterson C. N., Carabetta V. J., Chowdhury T., Silhavy T. J. (2006) LrhA regulates rpoS translation in response to the Rcs phosphorelay system in Escherichia coli. J. Bacteriol. 188, 3175–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rodriguez F., Arsène-Ploetze F., Rist W., Rüdiger S., Schneider-Mergener J., Mayer M. P., Bukau B. (2008) Molecular basis for regulation of the heat shock transcription factor σ32 by the DnaK and DnaJ chaperones. Mol. Cell 32, 347–358 [DOI] [PubMed] [Google Scholar]
- 67. Chiba S., Akiyama Y., Ito K. (2002) Membrane protein degradation by FtsH can be initiated from either end. J. Bacteriol. 184, 4775–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gonzalez M., Frank E. G., Levine A. S., Woodgate R. (1998) Lon-mediated proteolysis of the Escherichia coli UmuD mutagenesis protein. In vitro degradation and identification of residues required for proteolysis. Genes Dev. 12, 3889–3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Erbse A., Schmidt R., Bornemann T., Schneider-Mergener J., Mogk A., Zahn R., Dougan D. A., Bukau B. (2006) ClpS is an essential component of the N-end rule pathway in Escherichia coli. Nature 439, 753–756 [DOI] [PubMed] [Google Scholar]
- 70. Srinivasan R., Ajitkumar P. (2007) Bacterial cell division protein FtsZ is stable against degradation by AAA family protease FtsH in Escherichia coli cells. J. Basic Microbiol. 47, 251–259 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.