Background: Neural stem cells generate all the cell types of the central nervous system.
Results: Transcription factor, PRDM4, recruits protein arginine methyltransferase 5 (PRMT5) to control the timing of neurogenesis.
Conclusion: PRDM4- and PRMT5-mediated histone arginine methylation controls neural stem cell proliferation and differentiation.
Significance: Histone arginine methylation is a novel epigenetic mechanism that regulates neural stem cell reprogramming.
Keywords: Epigenetics, Histone Methylation, Neural Stem Cell, Neurodevelopment, Protein Methylation, PRDM, PRMT5, Protein Arginine Methylation
Abstract
During development of the cerebral cortex, neural stem cells (NSCs) undergo a temporal switch from proliferative (symmetric) to neuron-generating (asymmetric) divisions. We investigated the role of Schwann cell factor 1 (SC1/PRDM4), a member of the PRDM family of transcription factors, in this critical transition. We discovered that SC1 recruits the chromatin modifier PRMT5, an arginine methyltransferase that catalyzes symmetric dimethylation of histone H4 arginine 3 (H4R3me2s) and that this modification is preferentially associated with undifferentiated cortical NSCs. Overexpressing SC1 in embryonic NSCs led to an increase in the number of Nestin-expressing precursors; mutational analysis of SC1 showed that this was dependent on recruitment of PRMT5. We found that SC1 protein levels are down-regulated at the onset of neurogenesis and that experimental knockdown of SC1 in primary NSCs triggers precocious neuronal differentiation. We propose that SC1 and PRMT5 are components of an epigenetic regulatory complex that maintains the “stem-like” cellular state of the NSC by preserving their proliferative capacity and modulating their cell cycle progression. Our findings provide evidence that histone arginine methylation regulates NSC differentiation.
Introduction
During CNS development, embryonic neural stem cells (NSCs)2 in the ventricular zone of the brain and spinal cord first proliferate symmetrically to increase NSC numbers and expand the ventricular zone, and then they switch to an asymmetric mode of division to generate postmitotic neurons while maintaining the NSC pool (1–4). After neurogenesis is complete, the NSCs switch to production of glial cells (astrocytes and oligodendrocytes). The mechanisms that control the temporal transition from proliferation to differentiation are poorly understood. Aberrations in the timing of this transition can lead to abnormalities in CNS development (5, 6).
In the developing cerebral cortex NSCs generate a series of neuronal subtypes that populate different cortical layers in sequence and then switch to glial cell production (7, 8). This program of neurogenesis can be recapitulated by individual mouse cortical NSCs isolated at embryonic day 10 (E10) and cultured at low density in vitro (2, 3). This suggests that the temporal program of NSC division and cell fate specification is cell intrinsic, but the molecular nature of the program is not known. The transitions in NSC fate are likely to be governed by cell lineage-specific transcription factors acting in concert with epigenetic mechanisms (9–14). The latter include post-translational modifications of histones associated with regulatory elements of genes as well as DNA methylation at CpG dinucleotides, which together affect the accessibility of chromatin to the general transcriptional machinery. The details of the epigenetic regulation of NSC differentiation are still poorly understood. In addition to cell lineage-specific transcription factors, cell cycle parameters such as the length of specific cell cycle stages play an important role in controlling NSC proliferation and differentiation (5, 6, 15–17), and these parameters change during cortical development (18). Lineage-specific transcription factors (5) can fine-tune the expression of cell cycle genes and in this way influence the cell fates and division modes of NSCs and consequently their decision either to proliferate or differentiate (16, 19, 20).
Schwann cell factor 1 (SC1) is a protein that was first identified as a binding partner of the p75 neurotrophin receptor (21). SC1, also known as PRDM4, belongs to the PRDM family of proteins, of which 17 members have been identified in the human genome (22). All of the PRDM family members are characterized by the presence of a positive regulatory (PR) domain and multiple zinc finger domains. The PR domains are similar to but distinct from the SET domains found in many histone lysine methyltransferases (MTases) (23). PRDM proteins are either epigenetic modifiers in their own right or else they recruit third party chromatin modifiers—e.g., histone deacetylases (HDACs), histone lysine MTases, or histone arginine MTases—to regulate cell type-specific gene expression in various tissues (24–33). Our previous work identified SC1/PRDM4 as an HDAC-associated transcriptional repressor that modulates cell cycle progression (33). SC1 is highly expressed in the developing mouse cerebral cortex (34), so we set out to understand its role in the development of cortical NSCs as they switch from proliferative to neuron-generating divisions. We report that SC1 recruits a type II arginine MTase, PRMT5, that catalyzes histone H4R3 symmetric dimethylation (H4R3me2s), a modification that we recently showed to be present in undifferentiated NSCs in the murine cortex prior to the onset of neurogenesis (35). We now show that both SC1 and PRMT5 are highly expressed in the preneurogenic cortex and provide evidence that the interaction between SC1 and PRMT5 regulates the proliferative capacity of cultured cortical NSCs. Our findings suggest an important role for histone arginine methylation in epigenetic programming of NSCs during cortical development.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfections
HEK293T cells were cultured in DMEM (Invitrogen) supplemented with 10% (v/v) FCS and glutamine; P19 cells were cultured in α-minimum essential medium (Invitrogen) supplemented with 5% FCS and glutamine; PC12 cells were cultured in DMEM supplemented with 5% FCS, 10% horse serum and glutamine. Transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The cells were harvested and processed 48 h post-transfection unless indicated otherwise.
Immunoprecipitation and Methylation Assays
HEK293T cells were transfected with described plasmids and harvested in immunoprecipitation (IP) buffer (50 mm Tris-HCl, pH 7.4, 0.5% (v/v) Nonidet P-40, 300 mm NaCl) supplemented with a protease inhibitor mixture (Sigma) and phosphatase inhibitor cocktails 1 and 2 (Sigma). The cells were lysed for 20 min on ice in IP buffer, and the insoluble material was sonicated for 10 s on ice. Lysates were centrifuged, and the supernatants were precleared using protein A/G beads (Santa Cruz) and then immunoprecipitated overnight at 4 °C using anti-Myc (Upstate), anti-PRMT5 (Upstate), or anti-HA antibodies (Covance). The complexes were collected on protein A/G beads and washed five times with IP buffer, followed by a wash with cold PBS at 4 °C. Proteins were boiled at 95 °C for 5 min in Laemmli buffer (60 mm Tris-HCl, pH 6.8, 10% (v/v) glycerol, 2% (w/v) SDS, 5% (v/v) β-mercaptoethanol, 0.01% (w/v) bromphenol blue) and separated by SDS-PAGE. After separation, proteins were transferred onto PVDF membranes (Millipore), and Western blots were performed with specified antibodies in TBST buffer (100 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.1% (v/v) Tween 20) containing 5% (w/v) skimmed milk powder (Tesco) and detected using ECL (GE Healthcare). IPs from E10.5 mouse cortices were performed using the same buffers as above. At least 20 embryos were used/IP experiment.
For methylation assays the immunoprecipitates on the beads were washed as described above and then rinsed twice in methylation buffer (50 mm Tris-HCl, pH 8.5, 5 mm MgCl2, 4 mm DTT, 2 μl of S-adenosyl-l-([3H]methyl) methionine (Amersham Biosciences and GE Healthcare) and 1 μg of histone mixture (Roche Applied Science) were added to the reaction in a total volume of 30 μl. Methylation reaction was allowed to proceed for 2 h at 30 °C and stopped by adding Laemmli buffer and boiling the samples for 5 min. Products of the methylation reactions were separated using 15% SDS-PAGE, transferred onto PVDF membranes, and visualized by Coomassie Brilliant Blue staining and fluorography. To examine H4-specific methylation, histones were incubated with immunoprecipitated Myc-tagged SC1 (mycSC1) complex and S-adenosyl-l-([3H]methyl) methionine as described above and analyzed on Western blots using anti-H4R3me2s antibodies (Abcam).
Primary Neural Stem Cell Cultures
Primary neural stem cells were isolated from mouse E10.5 cerebral cortices according to published procedures (36). Briefly, the cortices were harvested in EBSS (Invitrogen), and the meninges were removed. The cells were dissociated by incubation in trypsin at 37 °C for 40–50 min. Trypsinization was stopped by adding DMEM with 10% FCS. The cells were then further dissociated using preseparation filters (Milteneyi Biotec), centrifuged gently, resuspended in a small volume of DMEM with 10% FCS, and plated at a density of 2.5 × 105 cells/13-mm poly-d-lysine-coated coverslip. The cells were cultured in DMEM supplemented with 10 ng/ml basic FGF (PeproTech), 0.25% FCS, B27 supplement, sodium pyruvate, and glutamine (all from Invitrogen) (2). The cultures were routinely immunolabeled to monitor their ability to generate neurons, astrocytes, and oligodendrocytes.
Antibodies and Immunofluorescence Microscopy
Cultured cells on coverslips were fixed in 4% (w/v) paraformaldehyde for 10 min at 20–25 °C and permeabilized with cold methanol for 2–3 min at −20 °C. They were incubated for 1 h at 20–25 °C in blocking solution (10% normal goat serum, 0.1% (v/v) Triton X-100 in PBS). The following antibodies were used: anti-TuJ1 (Sigma, 1:500), anti-GFAP (Sigma, 1:1000), anti-Nestin (Santa Cruz Biotechnologies, 1:400), anti-O4 (kind gift from Nigel Pringle, 1:10), anti-SC1/PRDM4 (our own antibody, 1:100; Abcam 1:100; and a gift from P. Perez and M. V. Chao, 1:40 (33)), anti-PRMT5 (Upstate Biotech, 1:100), anti-H4R3me2s (Abcam, 1:1000), anti-EGFP (Fine Chemical Products Ltd., 1:3000), anti-FLAG (Sigma, 1:1000), anti-BrdU (American Type Culture Collection, Manassas, VA, 1:10), anti-cycB1 (GNS1, Santa Cruz Biotechnology, Inc, 1:500), and anti-Pan methyl Lysine (Abcam, 1:1000). For immunolabeling with antibody O4, methanol treatment and Triton X-100 were not used. When staining for BrdU and another antigen, the cells were stained sequentially, first for an antigen of interest other than BrdU, then rinsed, and treated as follows to visualize BrdU. First, the cells were fixed with 70% ethanol, 20% glacial acetic acid mixture at room temperature and then in 70% ethanol at −20 °C. The cells were then rinsed in PBS, 1% Triton X-100 at room temperature and denatured in PBS, 1% Triton X-100, 2 m HCl for 30 min at 37 °C. After washing, anti-BrdU antibody was added overnight at 4 °C. The rest of the staining was as described above. The coverslips were mounted in DAKO mounting medium. The following secondary antibodies were used: goat anti-mouse Alexa 488, goat anti-rabbit Alexa 568, goat anti-mouse Alexa 647 (Invitrogen, 1:1000), and goat anti-rat Alexa 488 (Invitrogen, 1:500). The fluorescent images were taken with a Leica Microsystems SPE confocal microscope.
E10.5 embryos were collected from timed-mated C57B/6 mice (Harlan), rinsed in PBS, and fixed in 4% paraformaldehyde at 4 °C for 1–2 h. The embryos were cryoprotected in 30% (w/v) sucrose in PBS and subsequently mounted in OCT (Tissue-Tek) on dry ice. The mounted embryos were sectioned at 10 μm using a Leica cryostat and air-dried for at least 1 h. The sections were permeabilized for 3 min with −20 °C methanol, rinsed three times in PBS, incubated in sodium citrate buffer (10 mm sodium citrate, 0.05% Tween 20, pH 6.0) at 95 °C for 30 min for antigen retrieval, allowed to cool to room temperature, rinsed three times in PBS, and then incubated for 1 h at 20–25 °C in blocking solution, following incubation with the primary antibody in blocking solution overnight at 4 °C. The sections were washed three times in PBS at 20–25 °C and incubated with fluorescent secondary antibodies and Hoechst dye (to visualize cell nuclei) for 1 h at 20–25 °C, rinsed three times in PBS and once in water, and mounted using DAKO mounting medium. The fluorescence images were made using a Leica Microsystems SPE confocal microscope.
siRNA Transfections
siRNA oligonucleotides against rat SC1 were synthesized by Thermo Scientific and assessed either by applying siRNA to rat NSCs or PC12 cells and monitoring the expression of endogenous SC1 protein by Western blotting and RT-PCR or by co-transfecting SC1-specific or scrambled sequence siRNA with an EGFP expression vector into cultured rat NSCs followed by immunolabeling with anti-EGFP and anti-SC1. We used DharmaFECT Duo Transfection reagent (Thermo Scientific) to introduce siRNA along with DNA according to the manufacturer's instructions. The cells were transfected with the siRNA oligonucleotides on 2 consecutive days 24 h after plating and processed for immunocytochemistry and Western blot analysis or semiquantitative RT-PCR 48 h after the second application of the siRNA reagent. The following siRNA oligonucleotides were used: siRNA-1 Pool, ACAAUUUGGUGCACGCUUU and GGAUGAUGUUUGUGCGCAA; and siRNA-2 Pool, UAAUGAUGGCCACGAAGUA and GUUCCUAUUCAGAGUUCAA. Scrambled sequence siRNAs were used as controls.
RT-PCR
RNA was isolated from P19 cells or PC12 cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. 1 μg of total RNA was used for RT-PCR. Total RNA was treated with RNase-free DNase (Ambion), and cDNA was synthesized using random hexamer primers (Invitrogen) and Moloney murine leukemia virus reverse transcriptase (USB). After incubation at 42 °C for 1.5 h, the enzyme was inactivated at 75 °C, and the cDNA was used for PCR. The following gene-specific primers were used: rat SC1, forward 5′-AAAGCCAGGAACCGTGAA-3′, and reverse 5′-ATGACCCATAAAGTGAACGTG-3′; mouse cycB, forward 5′-TCCCTCTCCAAGCCCGATGG-3′, and reverse 5′-TGGCCGTTACACCGACCAGC-3′; mouse Bub1b, forward 5′-AAGGGATTGAACGCAAGGCTG-3′, and reverse 5′-CATCAAAAACGGTGATCCTGCG-3′; mouse and rat GAPDH, forward 5′-ACAACTTTGGCATTGTGGAA-3′, and reverse 5′-GATGCAGGGATGATGTTCTG-3′; rat cycB, forward 5′-TGGACAAGGTGCCAGTGTGCG-3′, and reverse 5′-GGTCTCCTGCAGCAGCCGAAA-3′; and rat Bub1b, forward 5′-GCCAGGCCCGTGGAACACAG-3′, and reverse 5′-CAGGACGGAGGCACTCCCGA-3′.
RESULTS
Expression of SC1 in Embryonic Cortical NSCs
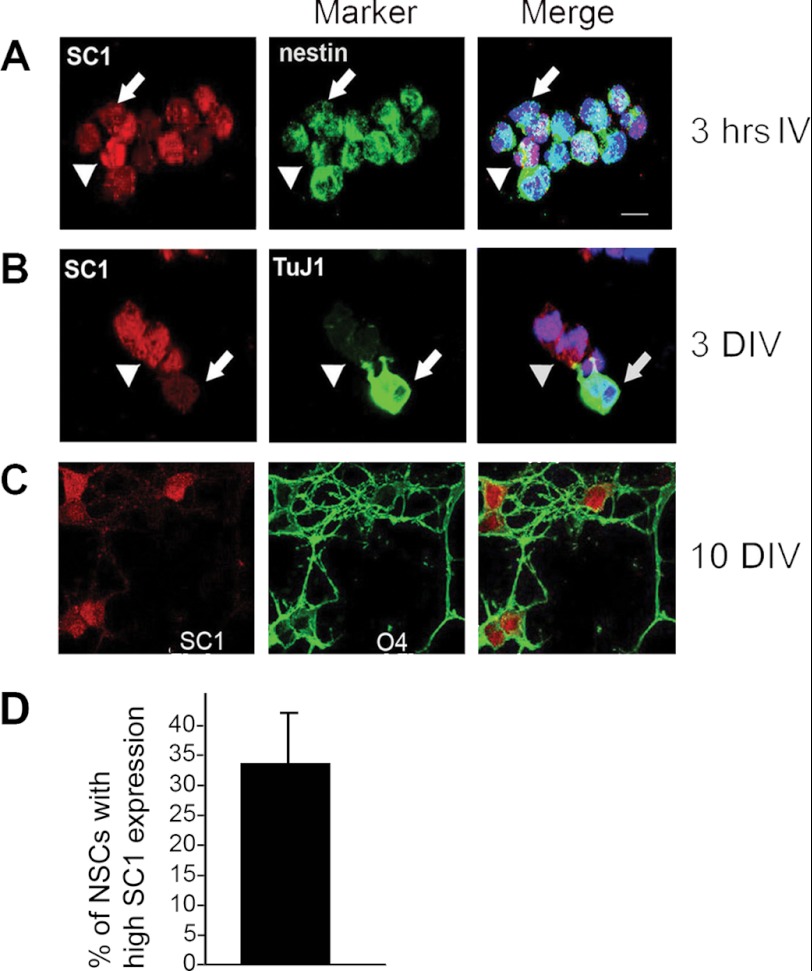
SC1 mRNA is highly expressed in the developing cortex (34). We investigated the expression of SC1 protein in dissociated primary mouse cortical NSCs isolated at E10.5 and cultured for up to 10 days in vitro (10DIV). We identified cells in these cultures by immunolabeling for Nestin (NSCs), TuJ1 (neurons), GFAP (astrocytes), or O4 (oligodendrocyte precursors (OLPs)). The different cell types were generated in the appropriate temporal order (2, 37): Nestin+ precursors were present from the outset, followed by TuJ1+ neurons, GFAP+ astrocytes, and O4+ OLPs at progressively longer culture periods.3 We found that immediately after plating, Nestin+ NSCs could be characterized as either strongly or weakly SC1-positive (Fig. 1A, arrowhead and arrow, respectively, quantified in Fig. 1D). Co-immunolabeling with anti-TuJ1 and anti-SC1 at 3DIV revealed that TuJ1 negative NSCs co-expressed SC1 strongly, whereas more mature neurons with high levels of TuJ1 expression expressed low levels of SC1 (Fig. 1B). After the onset of glial differentiation at 10DIV, we detected high levels of SC1 expression in all proliferating O4-positive OLPs (Fig. 1C), but not in GFAP-expressing differentiated astrocytes.3 Thus, high levels of SC1 appeared to be expressed preferentially in mitotically active cells (NSCs and OLPs) and down-regulated in differentiated neurons and astroglia. This suggested that down-regulation of SC1 might be involved in, and possibly required for, the switch from cell proliferation to differentiation.
FIGURE 1.
Dynamic expression of SC1 in developing NSCs. A–C, NSC cultures immunolabeled with anti-SC1 (left panels), anti-Nestin, anti-TuJ1, or O4 (center panels) and merged with Hoechst DNA stain (right panels). A, 3 h after plating, NSCs show different levels of expression of SC1 in Nestin-expressing precursor cells. Arrowheads, cells with high SC1 expression; arrows, cells with low SC1 expression. B, at 3 DIV, differentiating neurons activate TuJ1 expression and down-regulate SC1. Arrowhead, high SC1-expressing cell; arrow, Tuj1+ neuron with low level SC1 expression. C, after 10 DIV, high levels of SC1 expression are detected in O4+ oligodendrocyte precursors. D, quantification of the percentage of NSCs with high SC1 expression levels. Scale bar, 10 μm.
Knockdown of SC1 in NSCs Leads to Precocious Neuronal Differentiation
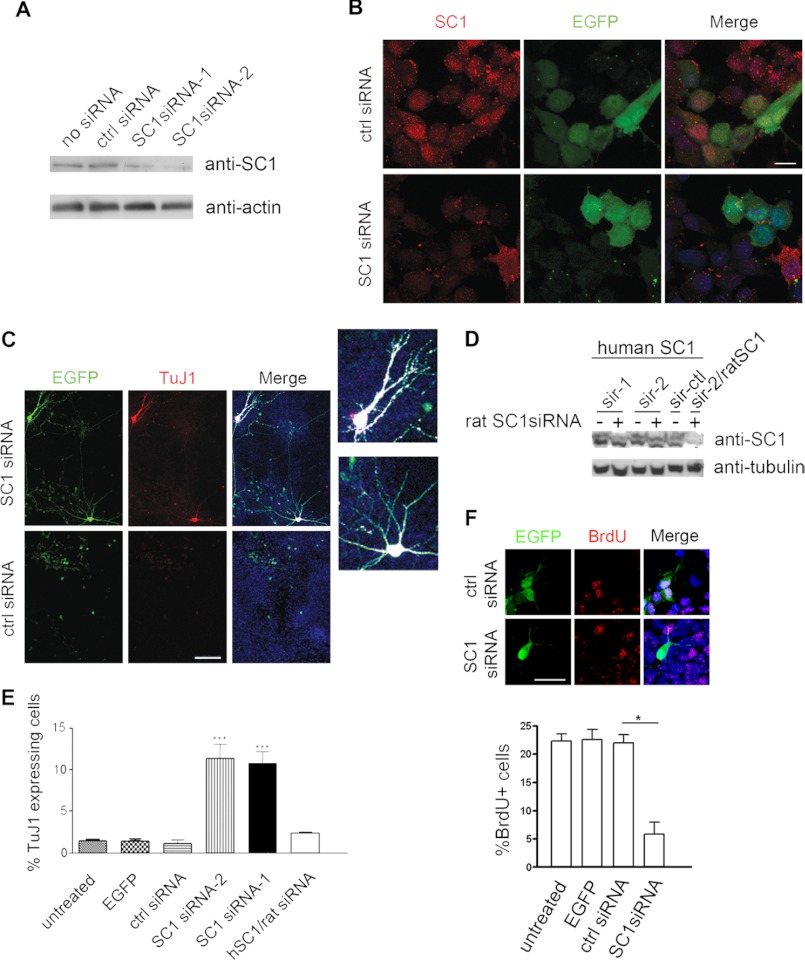
To test whether SC1 down-regulation is sufficient to trigger NSC differentiation, we examined the effects of SC1 knockdown in primary rat NSCs isolated from E11.5 cortex. We used rat NSCs in our experiment because we could knock down rat SC1 protein expression very efficiently. Transfecting two independent sets of rat SC1-specific siRNA oligonucleotides (SC1siRNA-1 and SC1siRNA-2) into cultured rat NSCs markedly decreased the expression of endogenous SC1 protein, as detected on Western blots (Fig. 2A). Moreover, SC1 immunoreactivity was low or undetectable in siRNA-transfected NSCs, compared with control siRNA-transfected NSCs (Fig. 2B). Successfully transfected cells were identified in this experiment by co-transfection of an enhanced green fluorescent protein (EGFP) expression vector. Both SC1siRNA-1 and SC1siRNA-2 gave similar results and are referred to below simply as SC1siRNA (Fig. 2).4 To investigate the effect of SC1 depletion on NSC differentiation, SC1-specific or scrambled sequence siRNAs were applied twice to cultured E11.5 rat NSCs, 24 and 48 h after plating. At 96 h after plating, the treated NSCs were fixed and immunolabeled with anti-EGFP to identify transfected cells and anti-TuJ1 to visualize differentiating neurons. NSCs treated with SC1siRNA consistently gave rise to ∼10% of TuJ1-expressing cells with the process-bearing morphology of neurons (Fig. 2C, top panels). We did not detect a change in the number of astrocytes or oligodendrocytes after the application of SC1siRNA in these experiments within the time frame of investigation.4 Very few TuJ1-expressing cells were observed when NSCs were transfected with control, scrambled sequence siRNA (Fig. 2C, bottom panels). To confirm the specificity of these knockdown experiments, we transfected rat NSCs with SC1siRNA together with a human SC1 cDNA that was insensitive to inhibition by the rat-specific SC1siRNAs (Fig. 2D). This “rescued” SC1 expression in the presence of rat SC1siRNA and resulted in a reduction in numbers of TuJ1-positive neurons to control levels (i.e., as observed in NSC cultures without added siRNA or with scrambled sequence SC1siRNA) (Fig. 2E). Consistent with the observation that a fraction of NSCs treated with SC1siRNA induced precocious neurogenesis, we found that a similar fraction of NSCs treated as above showed reduced levels of BrdU incorporation (Fig. 2F). Thus, we concluded that knockdown of SC1 in NSCs leads to precocious neuronal differentiation of a subset of the NSCs and controls their proliferative capacity, consistent with our observation that newly differentiating neurons express low levels of SC1 protein.
FIGURE 2.
siRNA knockdown of SC1 leads to precocious differentiation of NSCs into neurons. A, Western blots of protein samples from rat NSCs transfected with no siRNA (first lane), control (second lane, scrambled sequence, ctrl) siRNA, SC1 siRNA-1 (third lane), and SC1 siRNA-2 (fourth lane), probed for expression of endogenous SC1. Bottom panel, same blots probed for actin demonstrate similar protein levels in the designated lanes. B, NSCs co-transfected with an EGFP expression vector together with either control siRNA (top row) or SC1-specific siRNA (bottom row) were immunolabeled with anti-SC1 and anti-EGFP to identify transfected cells. C, expression of TuJ1 in NSCs transfected with EGFP and siRNA-1 or EGFP and control siRNA. Right two panels, merged images with Hoechst-stained DNA. Two of the neurite-bearing EGFP+ cells from the siRNA-1-treated cultures are magnified to visualize the extensive arborization. D, human or rat SC1 was overexpressed in HEK293T cells with or without rat-specific SC1siRNA-1 or SC1siRNA-2 (sir-1 and sir-2, respectively), and its expression was monitored by probing Western blots of transfected cell lysates with anti-SC1. Neither of the applied siRNAs affected the expression of human SC1 (top panel), attesting to the target specificity of the siRNAs. The blots were also probed with anti-tubulin antibodies to control for gel loadings (bottom panel). Co-transfection of ratSC1 cDNA with rat SC1siRNA-2 resulted in the expected reduction in the levels of rat SC1 protein (sir-2/ratSC1 lane). E, quantification of TuJ1 expression in NSCs transfected with either control siRNA, siRNA-1, or siRNA-2 or the indicated control siRNA (means ± S.D., n = 3, t test, p < 0.0005, *). At least 300 transfected cells were counted per coverslip. F, quantification of BrdU incorporation into rat NSCs after transfection with control siRNA, siRNA-2, EGFP alone, or nothing (bottom panel). Cells expressing EGFP and indicated siRNAs were immunolabeled to detect BrdU incorporation and EGFP expression (top panel). Scale bars, 100 μm (C) and 10 μm (B and F).
SC1 Is Associated with Histone Methyltransferase Activity
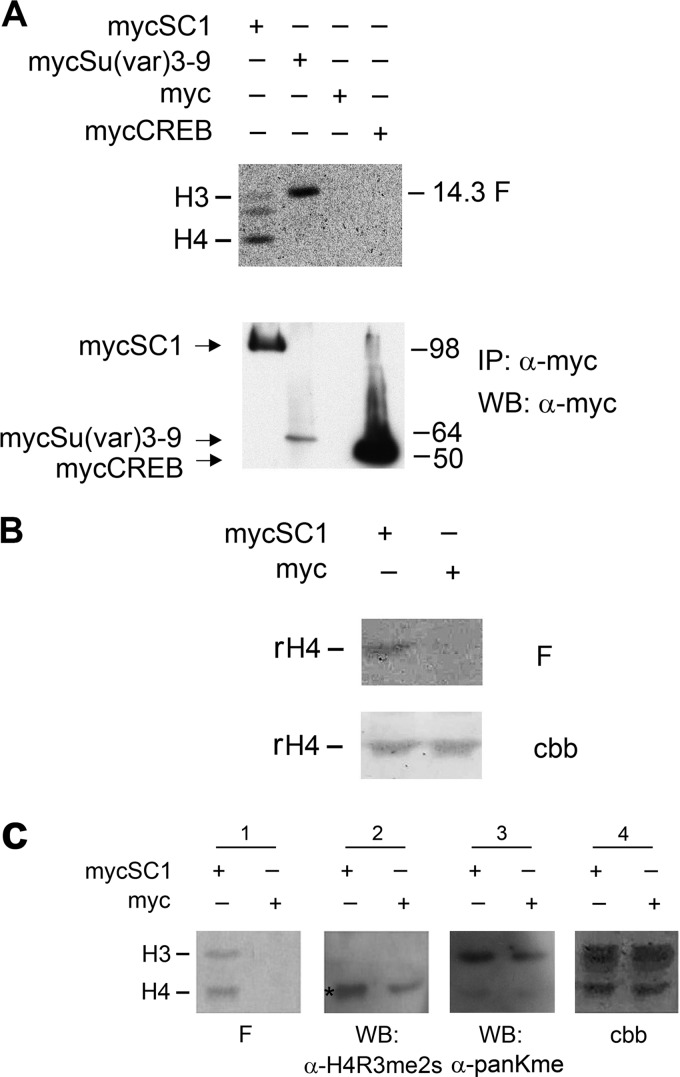
Like other PRDM proteins, SC1 possesses a PR/SET domain—a hallmark of lysine histone MTases (HMTases)—that can regulate gene expression by modifying histones in chromatin (23). Therefore we tested the possibility that SC1 methylates histones as part of its transcriptional repressor function. We transiently expressed mycSC1 in HEK293T cells and immunoprecipitated mycSC1 protein from cell lysates using an anti-Myc antibody. Purified histones from calf thymus were incubated with immunoprecipitated mycSC1 and subjected to an in vitro radioactive HMTase assay (38). We detected methylated histones, the preferred substrate being histone H4 (Fig. 3A). As a positive control, we immunoprecipitated Myc-Su(var)3–9, an H3K9 HMTase, and showed that this preferentially methylated histone H3 (Fig. 3A). As negative controls, we immunoprecipitated Myc-tagged CREB or empty Myc vector and showed that neither of these expressed histone methylase activity (Fig. 3A). Immunoprecipitated mycSC1 also methylated recombinant histone H4 (Fig. 3B). We concluded that SC1 exhibits an HMTase activity, preferentially toward histone H4.
FIGURE 3.
Immunoprecipitated SC1 complex exhibits an H4 HMTase activity. A, Myc-tagged SC1 or indicated controls were expressed in HEK293T cells and immunoprecipitated (IPed) using an anti-Myc antibody (bottom panel). The same IPs were used for an in vitro HMTase assay with purified calf thymus histone mix; a fluorogram of the in vitro methylation reaction is shown (above, F). B, cells were transfected with mycSC1 expression vector or empty Myc vector, and cell lysates were immunoprecipitated with anti-Myc antibody. The mycSC1 immunoprecipitate methylates recombinant H4 in an in vitro methylation reaction. F, fluorogram of the methylation reaction products; cbb, Coomassie Brilliant Blue-stained membrane showing the histones used for the in vitro methylation reaction. C, mycSC1-mediated histone methylation increases the levels of H4R3me2s. The same procedure was carried out as in A, and the blots of methylated histones were probed with antibodies against lysine and arginine modifications. Panel 1, fluorogram of methylated histones in the indicated IPs. Panel 2, the same blot probed with anti-H4R3me2s antibody. Panel 3, the same blot probed with anti-pan lysine antibody. Panel 4, the same membrane stained with Coomassie Brilliant Blue. WB, Western blot
Histone methylation can occur on a variety of lysine and arginine residues leading to repression or activation of gene transcription, depending on the precise modification. To identify the histone modification mediated by mycSC1, the products of in vitro methylation reactions were analyzed by Western blotting using antibodies directed against different H4 modifications. We detected an increased level of H4R3me2s in the sample containing immunoprecipitated mycSC1 (Fig. 3C, highlighted by an asterisk), although no change in overall lysine methylation was observed using an antibody directed against pan-methyl-lysine (Fig. 3C, panel 3). We concluded that mycSC1 mediates symmetric arginine dimethylation on H4R3.
SC1 Recruits the Histone Arginine Methyltransferase PRMT5
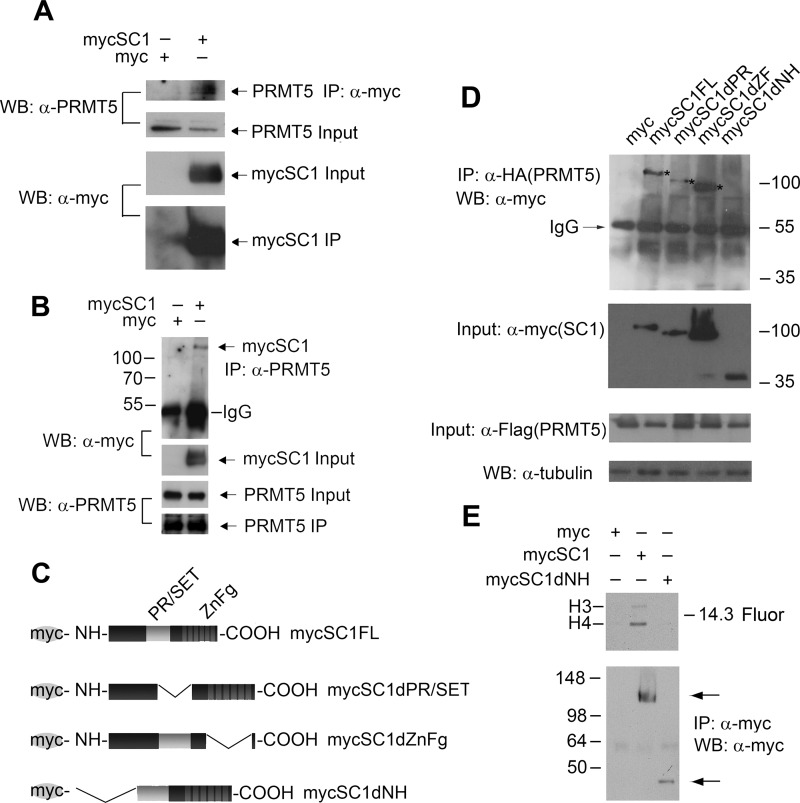
Given that PR/SET domains are found in lysine HMTases but that we detected increased arginine methylation, we considered the possibility that SC1 might bind to and recruit a third party arginine HMTase. In our methylation assays, we detected an increase in H4R3me2s, a product of a type II protein arginine MTase (PRMT). Therefore, we asked whether PRMT5, a type II PRMT that is known to catalyze H4R3me2s (39, 40), might interact with SC1 protein. mycSC1 expression vector was transfected into HEK293T cells, and lysates were immunoprecipitated 48 h later with anti-Myc antibody (Fig. 4A). Western blotting with anti-PRMT5 showed that endogenous PRMT5 co-immunoprecipitated with mycSC1 (Fig. 4A). HEK293T cells transfected with empty Myc vector served as a negative control. In a complementary approach, lysates of HEK293T cells transfected with mycSC1 or empty vector were immunoprecipitated with anti-PRMT5, and the precipitates were analyzed on Western blots with anti-Myc. These experiments revealed that mycSC1 co-immunoprecipitated with endogenous PRMT5 (Fig. 4B).
FIGURE 4.
SC1 recruits PRMT5 to direct H4 methylation. A, mycSC1 or empty vector was expressed in HEK293T cells as indicated on the panel. Anti-Myc IPs from transfected cells were analyzed using anti-PRMT5 antibody. Endogenous PRMT5 is found in the complex (arrow, top panel) with IPed mycSC1. Total protein inputs and IPed mycSC1 are shown (two middle and bottom panels, respectively). B, endogenous PRMT5 was IPed from HEK293T cells transfected with mycSC1 or empty vector, and the IPs were analyzed by Western blot (WB) with anti-Myc (arrow, mycSC1 protein); the two middle and bottom panels show the input mycSC1 and PRMT5 and IPed PRMT5, respectively. C, diagram of the deletion constructs of Myc-tagged SC1 used to map the domains of interaction with PRMT5. D, the N terminus and to a lesser extent PR/SET domain of SC1 bind PRMT5. Indicated Myc-tagged SC1 full-length or deleted constructs and HA/FLAG-tagged PRMT5 were co-expressed in HEK293T cells. Anti-HA IPs (PRMT5) were probed on Western blots with anti-Myc antibody to detect co-IPed Myc-tagged SC1 constructs. The inputs were analyzed using anti-FLAG antibody for PRMT5 and anti-Myc antibody for SC1. Anti-tubulin antibody was used as a loading control. mycSC1 proteins that co-IP with PRMT5 are highlighted by asterisks on the top panel of the Western blot. E, mycSC1FL, mycSC1dNH, or empty vector was transfected into HEK293T cells. Anti-Myc IPs were used for in vitro HMTase assays. Top panel, fluorogram (Fluor) of histones methylated by the indicated IPed complexes. Bottom panel, Western blot of IPed proteins used for the in vitro methylation reactions, probed with anti-Myc.
The N Terminus and the PR/SET Domain of SC1 Are Necessary to Recruit PRMT5 and Mediate Histone Methylation
SC1 contains PR/SET and zinc finger domains characteristic of the PRDM family of proteins. To map the domain of interaction between PRMT5 and SC1, we generated a series of SC1 mutants with deletions of the PR/SET domain (mycSC1dPR), zinc finger domain (mycSC1dZF), or the N terminus up to the PR/SET domain (mycSC1dNH) (Fig. 4C). Full-length or truncated mycSC1 constructs were transfected together with HA/FLAG-tagged PRMT5 into HEK293T cells, and binding between pairs of expressed proteins was detected by co-immunoprecipitation assays, using anti-HA to immunoprecipitate PRMT5 followed by Western blotting with anti-Myc to detect SC1. Co-transfection of PRMT5 and full-length mycSC1 or empty vector acted as positive and negative controls, respectively. According to this assay, mycSC1dPR bound only very weakly to PRMT5, whereas mycSC1dZF retained strong PRMT5 binding (Fig. 4D). mycSC1dNH did not bind detectably to PRMT5 (Fig. 4D). We conclude that SC1 recruits PRMT5 mainly via its N terminus but partly via the PR/SET domain.
Our data suggest that SC1-mediated histone arginine methylation depends on recruitment of PRMT5. To test this further we transfected mycSC1dNH (which cannot bind PRMT5) into HEK293T cells, immunoprecipitated cell lysates with anti-Myc, and assayed the precipitate for HMTase activity in vitro. HEK293T cells transfected with full-length mycSC1 or empty vector served as positive and negative controls. We detected HMTase activity in cells transfected with full-length mycSC1 but not in cells transfected with either mycSC1dNH or empty vector (Fig. 4E). We conclude that SC1 and PRMT5 interaction is necessary for histone methylation.
SC1 and PRMT5 Are Co-expressed and Found in a Complex in Embryonic Cortical NSCs
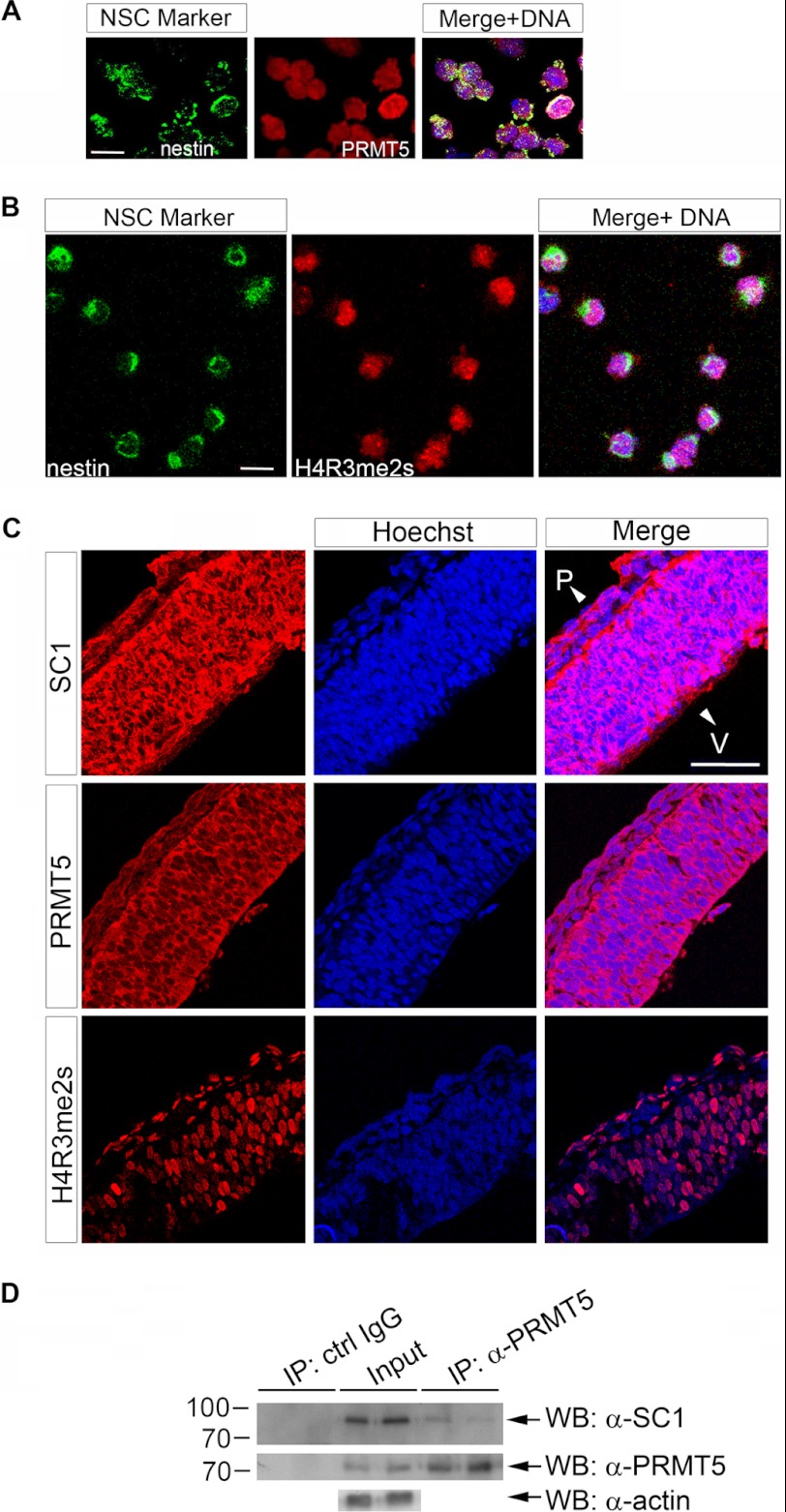
In light of the above experiments, we asked whether PRMT5 is co-expressed with SC1 in cultured cortical NSCs. A strong PRMT5 immunofluorescence signal was detected in all Nestin-expressing NSCs isolated from E10.5 cerebral cortex (Fig. 5A). Consistent with this, we detected high levels of H4R3me2s immunoreactivity in cultured E10.5 NSCs, suggesting that there are high levels of PRMT5 enzyme activity in these cells (Fig. 5B). Moreover, we found high levels of both PRMT5 and H4R3me2s immunoreactivity in sections through the developing cortical neuroepithelium at E10.5, as well as SC1 immunoreactivity (Fig. 5C). SC1 and PRMT5 were found in the nucleus and cytoplasm of neuroepithelial cells, suggesting that part of their mode of action might be through methylation of a cytoplasmic pool of newly synthesized histones (41). To investigate whether we can detect a complex between the endogenous SC1 and PRMT5 in the developing cortex, we performed an immunoprecipitation using mouse E10.5 cortices as the source of endogenous PRMT5 and SC1 proteins. PRMT5 was immunoprecipitated from the cortical homogenates and the precipitates analyzed on Western blots using anti-SC1 antibodies. Endogenous SC1 co-precipitated with endogenous PRMT5, but not with a control IgG (Fig. 5D; as indicated on the figure panels, two independent co-immunoprecipitations were performed). Taken together, these data suggest that SC1 in complex with PRMT5 directs H4R3me2s modifications in proliferating cortical NSCs in vivo.
FIGURE 5.
PRMT5, SC1, and H4R3me2s are expressed in developing NSCs and cortical neuroepithelium and can be co-immunoprecipitated from E10.5 cortex. A, expression of PRMT5 in E10.5 Nestin+ NSCs, 3 h after plating, was detected by immunolabeling with PRMT5-specific antibodies. Right panels, merged images with Hoechst DNA stain. B, H4R3me2s modification is detected in Nestin+ NSCs 3 h after plating. C, both SC1 and PRMT5 are expressed in the developing mouse cortex at E10.5, and high levels of H4R3me2s modifications are detected in the cortical neuroepithelium at this stage. Expression of the relevant proteins or modification in A–C was detected by immunolabeling with anti-SC1, anti-PRMT5, or anti-H4R3me2s. V, ventricular zone; P, pial surface. D, endogenous PRMT5 was IPed from E10.5 cortices, and the presence of endogenous SC1 in the co-IPed complex was analyzed by using anti-SC1 antibodies. Nonspecific IgG was used to control for the specificity of IP reactions. Input and IPed PRMT5 is shown on the middle panel, and anti-actin antibody was used as a loading control. Scale bars, 10 μm (A and B) and 50 μm (C).
SC1 and PRMT5 Interaction Is Required to Control the Timing of NSC Differentiation
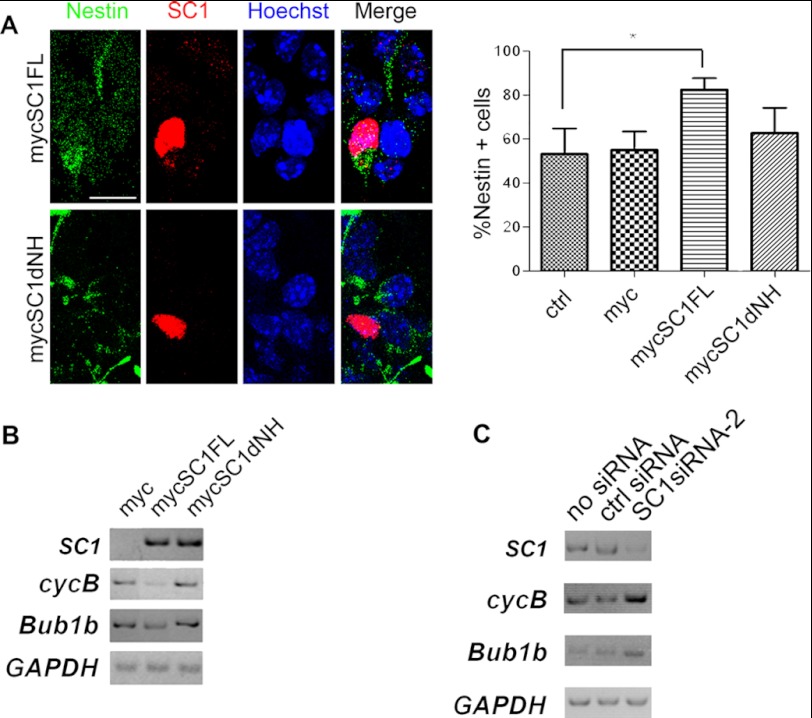
Because SC1, PRMT5, and high levels of H4R3me2s are found in the early proliferating neuroepithelium and knockdown of SC1 in NSCs leads to precocious neuronal differentiation, it seems possible that SC1 overexpression might delay the timing of neurogenesis and that the SC1-PRMT5 complex might be required for this function. To test this we transiently overexpressed either full-length mycSC1 or mycSC1dNH mutant (which cannot recruit PRMT5) in cultured NSCs isolated from E10.5 mouse cerebral cortex. The cells were transfected 24 h postplating, fixed, and double-immunolabeled 48 h after transfection with anti-Nestin to identify the NSCs and anti-Myc to identify cells that expressed SC1. We found that overexpression of full-length mycSC1 led to a moderate increase in the number of Nestin+ NSCs (Fig. 6A). This increase was not observed with mycSC1dNH, suggesting that SC1-PRMT5 interaction is necessary to control the timing of neuronal differentiation of the NSCs (Fig. 6A).
FIGURE 6.
SC1 and PRMT5 complex increases the number of Nestin-expressing neural precursors and regulates expression of promitotic genes. A, overexpressed mycSC1 increases the number of Nestin+ NSCs. mycSC1FL and mycSC1dNH proteins were detected by immunolabeling with anti-Myc antibodies and NSCs by the presence of Nestin immunoreactivity. Quantification of Nestin+/mycSC1 expressing NSCs is shown in the graph on the right. At least 300 cells were counted per transfection, and the data are shown as the means ± S.D. (n = 3, p < 0.05, *). B, semiquantitative RT-PCR was used to estimate the relative levels of cycB and Bub1b mRNA in P19 cells transfected with mycSC1FL, mycSC1dNH, or empty vector. C, semiquantitative RT-PCR was used to estimate the relative levels of SC1, cycB, and Bub1b mRNA in PC12 cells transfected with SC1siRNA, control siRNA, or no siRNA. Levels of mRNA were normalized to GAPDH mRNA. Scale bar, 5 μm (A). ctrl, control.
Previously, we showed that SC1 controls cellular proliferation by down-regulating promitotic genes, e.g., cycB was one of the transcriptional targets of SC1-mediated repression (33). Therefore, we considered the possibility that the SC1-PRMT5 complex might regulate the timing of neurogenesis in developing NSCs in part by regulating their cell cycle parameters. We investigated whether SC1-PRMT5 might regulate the transcription of genes that control mitotic progression. To address this, we overexpressed full-length mycSC1 and mycSC1dNH proteins in P19 embryonal carcinoma cells. P19 cells were chosen because they can be differentiated into neural lineages under defined culture conditions (42) and are easily transfected to high efficiency. 48 h after transfection, we performed RT-PCR on mRNA isolated from P19 cells transfected with mycSC1, mycSC1dNH, or Myc vector alone and measured the expression of mitotic genes, cycB and Bub1b. We found that overexpression of full-length mycSC1, but not mycSC1dNH, led to a repression of both cycB and Bub1b (Fig. 6B). We conclude that SC1 in complex with PRMT5 down-regulates expression of certain promitotic genes, e.g., cycB and Bub1b. To test whether knockdown of SC1 leads to an increase in the mRNA expression of these genes, we performed RT-PCR using total RNA isolated from PC12 cells transfected with SC1siRNA or control siRNA. PC12 cells were used because they can be transfected to a high level easily, differentiate into neurons upon treatment with NGF and are of rat origin allowing the use of SC1siRNA used in previous experiments with the rat NSCs. We detected an increase in the levels of cycB and Bub1b mRNA after SC1 knockdown but not when control siRNA was used (Fig. 6C), indicating that SC1 is involved in negatively regulating the expression of these genes.
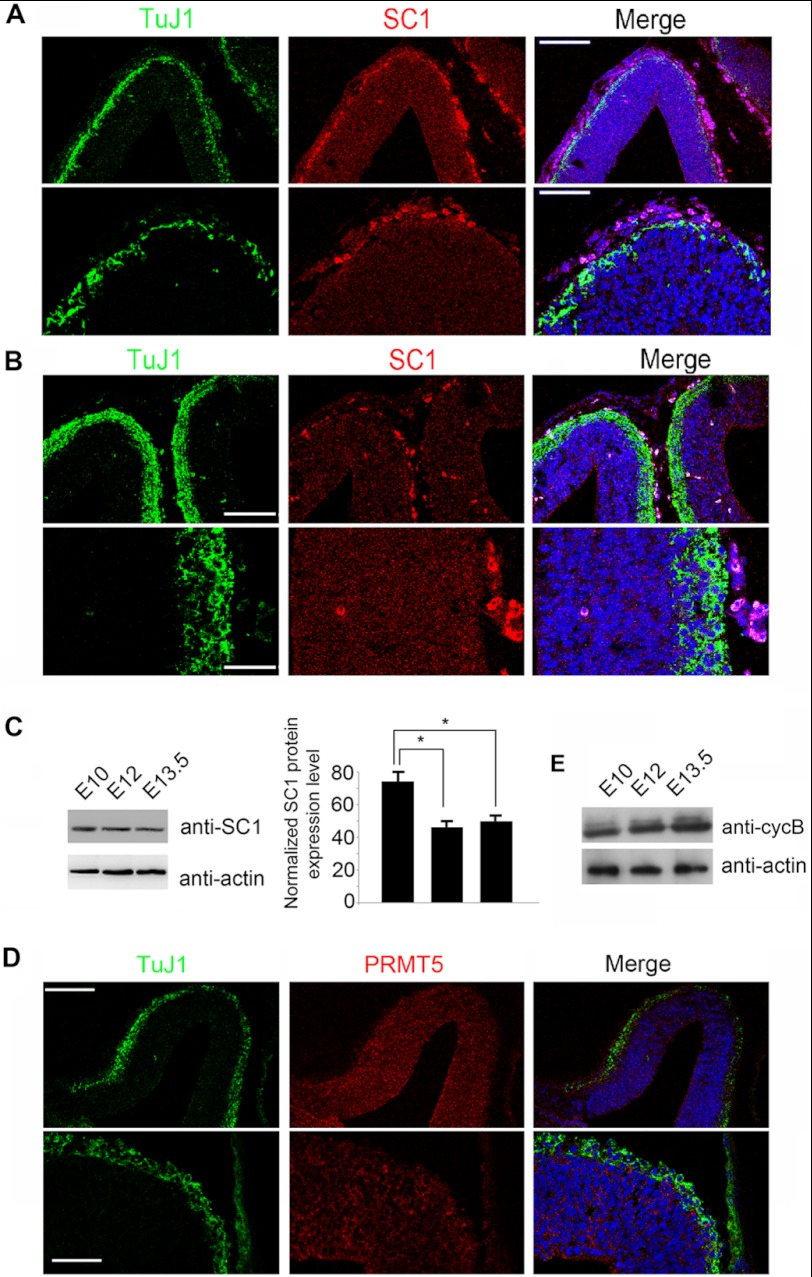
It was previously reported that a high level and activity of the cell cycle regulator cdc2, in association with cycB, is necessary for the asymmetric partitioning of cell fate determinants in neuroblasts of Drosophila melanogaster (20). These observations suggest that the levels and activity of promitotic genes might be involved in influencing the mode of cell division adopted by the NSCs. Our observations that the SC1-PRMT5 complex down-regulates the expression of cycB and that SC1 protein levels are down-regulated in the newly differentiated neurons suggest a possibility that varying amounts of SC1 protein regulate the levels of expression of promitotic genes during cortical development and in this way may indirectly influence the mode of NSC division. We therefore investigated whether we can detect the down-regulation of SC1 and PRMT5 protein levels in the developing cortex at the time when the NSCs switch from proliferative to neuron-generating divisions. We observed that SC1 protein levels and to a lesser extent PRMT5 protein levels were reduced in the developing cortex at E12 and E13.5 when symmetric proliferative divisions give way to asymmetric neurogenic divisions (Fig. 7, A–D). Moreover, we detected a moderate increase in the levels of cycB1 protein in the developing cortex at these developmental stages (Fig. 7E). We conclude that the expression level of SC1 and PRMT5 proteins is down-regulated during the transition from proliferation to neurogenesis of the cortical NSCs concomitant with the elevation of cycB1 protein expression.
FIGURE 7.
SC1 and PRMT5 protein levels are reduced in the developing cortex at E12 and E13.5. A and B, mouse cortices from E12 (A) and E13.5 (B) embryos were immunolabeled for TuJ1 and SC1. SC1 protein levels are reduced compared with those detected at E10.5 prior to the onset of neurogenesis (see Fig. 5). The bright red signal in the tissue represents nonspecific labeling of blood vessels after antigen retrieval by heating with citrate buffer. C, Western blot analysis of SC1 protein expression in the developing cortex. Protein homogenates from embryonic cortices of indicated ages were analyzed by probing with anti-SC1 antibodies (top panel) and anti-actin (bottom panel) antibodies to control for protein loading. Normalized protein levels of SC1 are shown in the graph. D, mouse cortex from E13.5 embryos was immunolabeled for TuJ1 and PRMT5. Moderate levels of PRMT5 protein were detected in the cortex at E13.5. TuJ1 staining is toward the pial surface in all panels. E, Western blot analysis of cycB1 protein expression in the developing cortex. Protein homogenates from embryonic cortices of indicated ages were analyzed by probing with anti-cycB1 antibodies (top panel) and anti-actin antibodies (bottom panel) to control for protein loading. The data (in C) are shown as the means ± S.D. from three independent Western blot quantifications (n = 3, p < 0.05, *). Scale bars, 75 μm (top panels) and 25 μm (bottom panels).
DISCUSSION
In this study we investigated the role of the transcription factor SC1 in neural development. We demonstrated that SC1 expression is dynamically regulated in developing NSCs, being strongly expressed in proliferating NSCs but down-regulated at the onset of neurogenesis. Moreover, experimental knockdown of SC1 in NSCs led to precocious neurogenesis. Notably, we demonstrated that SC1 recruits an epigenetic modifier, the histone arginine methyltransferase PRMT5, and that high levels of SC1-PRMT5 complex are required to maintain the proliferative capacity and “stem-like” cellular state of the NSCs. Furthermore, we showed that SC1 in complex with PRMT5 directs H4R3me2s, a modification that is prevalent in the developing neuroepithelium during the expansion phase of cortical development (35). In addition, we demonstrated that the SC1-PRMT5 complex modulates the levels of expression of promitotic genes that regulate the G2/M transition and mitotic progression.
Our findings suggest that SC1 in complex with the epigenetic modifier, PRMT5, plays an important role in the control of timing of neurogenesis in developing cortical NSCs. Previous work showed that SC1 mRNA is highly expressed in the developing mouse cerebral cortex (34). We found that in E10.5 mouse cortical cell cultures Nestin-positive NSCs expressed variable amounts of SC1 protein. It is not clear whether this reflects heterogeneity within the NSC population, different developmental stages of the NSC lineage(s) or varying levels of SC1 expression during the stages of the cell cycle. Nevertheless, the facts that SC1 expression is low in early born neurons and that SC1 knockdown in NSCs triggers precocious neuronal differentiation of a subset of the NSCs suggest that NSC differentiation depends on down-regulation of SC1. Consistent with this, we see high levels of SC1, PRMT5, and H4R3me2s in the early proliferative neuroepithelium at E10.5, whereas both SC1 and to a lesser extent PRMT5 protein levels are diminished in the neuroepithelium at E13.5 at the onset of neurogenesis. Moreover, we also find that overexpression of SC1 in NSCs isolated from E10.5 mouse cortices leads to a moderate increase in the number of Nestin-expressing NSCs, suggesting that high levels of SC1 prevent differentiation. It is noteworthy in this respect that in the mouse embryonic stem (ES) cells, one of the essential regulators of “stemness,” Nanog, exhibits fluctuating levels of expression and that ES cells expressing low levels of Nanog are predisposed to differentiate, whereas those with high Nanog levels retain their pluripotency (43). Perhaps, the low and high SC1-expressing NSCs that we observe within the NSC population reflect a similar heterogeneity of these cells with respect to their predisposition to differentiate.
The dynamic nature of expression of both SC1 and PRMT5 is evident at E15.5 in the developing cortex when both proteins become up-regulated in postmitotic neurons (34).3 Similarly, another PRDM family member, Blimp1/PRDM1, has been reported to undergo temporally dynamic expression during development of primordial germ cells and various other tissues (44, 45) and has also been shown to recruit PRMT5 during primordial germ cell development (25). Importantly, various levels of Blimp appear to be necessary to direct differentiation of different tissues, reflecting precise dose dependence of different cell types on Blimp1 requirement (45). These observations highlight the general principle of utilizing the same transcriptional regulators during development in various tissues in a graded manner to specify different cell fates, possibly through recruitment of different partner proteins and downstream choice of gene targets. The similarities between the utilization of both Blimp1 and SC1 proteins during development presumably reflect analogous functions of these proteins in different cell lineage precursors during development. For example, the changing cell cycle parameters and kinetics during precursor cell differentiation may be a common mechanism that contributes to the developmental decisions made by these cells (16–19). Precise control of cell cycle progression is one of the critical components of precursor cell differentiation, and changes in the cell cycle parameters are likely to regulate various aspects of the responsiveness of these cells to extracellular signals. Moreover, the observation that both SC1 and PRMT5 are found at high levels in the developing OLPs where PRMT5 has been shown to be necessary for OLP differentiation (this paper and Ref. 17) further underscores the principle of utilizing the same transcription factors in a graded manned throughout development to direct different developmental outcomes.
We observe that SC1 and PRMT5 complex is involved in the down-regulation of cycB and Bub1b genes. The observation is of interest because previous investigation of the mechanisms responsible for asymmetric partitioning of cell fate determinants during neuroblast divisions in D. melanogaster has identified the cdc2-cycB complex as instrumental in regulating this process. High levels and activity of these classical regulators of mitosis were found to be necessary for asymmetric division of the neuroblasts during development (19, 20). In this respect, our finding of diminished expression of both SC1 and to a lesser extent PRMT5 in the developing cortex at E12 and E13.5, when the NSCs switch from symmetric proliferative divisions to asymmetric neuron-generating ones, is important. It suggests the possibility that the SC1-PRMT5 complex might regulate the timing of neurogenesis in the cortical NSCs by fine-tuning the expression levels of promitotic genes. High levels of SC1-PRMT5 complex found in the preneurogenic cortex at E10.5 may keep the expression of promitotic genes cycB and Bub1b low and might therefore favor symmetric divisions (20), indirectly inhibiting neuronal differentiation, a possibility that should be tested in future investigations. That this might be a more general role of PRDM family members is suggested by the fact that Blimp1, a critical regulator of primordial germ cell development, has also been shown to down-regulate promitotic genes (46). Blimp1, like SC1, favors the preservation of the undifferentiated cellular state in primordial germ cells and, also like SC1, exerts its action through recruitment of PRMT5 (25).
PRMT5 is emerging as a critical regulator of cellular stemness. Its role in preserving the less differentiated cellular state has been demonstrated in PGCs, erythrocyte progenitors, and ES cells (25, 41, 46, 47). We now provide evidence that PRMT5 is also expressed in developing NSCs during their stem-like proliferative stage of development. Together, these observations suggest a fundamental role for PRMT5 and its cognate modifications, H4R3me2s and H2AR3, in maintaining the less differentiated state of stem/progenitor cells in a variety of cell lineages. An intriguing aspect of PRMT5 biology is its dynamic subcellular localization and the recent observation that during ES cell development PRMT5 mediates methylation of R3 on cytoplasmic histone H2A, thereby preserving ES cell stemness (41). We also detect high levels of both SC1 and PRMT5 in the cytoplasm of neuroepithelial cells in the developing cerebral cortex, suggesting that SC1-PRMT5 might methylate newly synthesized cytosolic histones during the S phase, prior to the association of new histones with replicated DNA. Moreover, we find that overexpression of SC1 protein lacking its zinc finger domain, which is exclusively cytosolic and binds PRMT5 very strongly, induces the highest increase in the number of Nestin-expressing NSCs,3 further supporting the importance of cytosolic localization of both proteins in the preservation of cellular stemness. A recent report also highlighted the role of PRMT5 in modulating the responsiveness of different cell types to differentiation- or proliferation-inducing growth factors. Intriguingly, high PRMT5 activity was sustained by proliferation-inducing growth factors that favor symmetric divisions, whereas differentiation-inducing growth factors dampened PRMT5 activity, leading to cellular differentiation (48).
Regarding the role of histone arginine methylation during cellular development, we recently showed that postmitotic neurons are marked by a different modification—asymmetric dimethylation—of precisely the same arginine residue (H4R3) that is targeted for symmetric dimethylation by PRMT5 (35). The asymmetric modification (H4R3me2a) is mediated by PRMT1, a type I arginine methyltransferase (39). Although the symmetric dimethylation of arginine by PRMT5 is mainly associated with transcriptional repression, the asymmetric dimethylation mediated by PRMT1 leads to transcriptional activation (39). Because both PRMT5 and SC1 have been found in association with HDAC1 and HDAC2 (33, 39, 40), it is possible that SC1 might be a common component of the repressive chromatin remodeling complexes during early neural development and that the principal role of SC1 in such complexes might be to provide targeting specificity via its sequence-specific DNA binding properties. It is therefore conceivable that down-regulation of SC1 at the onset of neurogenesis has the effect of vacating sites in chromatin that were previously targets of symmetric methylation (repression) by SC1-PRMT5, making them accessible for asymmetric methylation (activation) by PRMT1. This is consistent with our observation that knockdown of SC1 in developing NSCs induces precocious neurogenesis, because it might allow PRMT1-mediated deposition of H4R3me2a modifications, leading to the activation of genes necessary for neuronal differentiation. It is also noteworthy in this respect that previous work has identified a protein, Tis21/Btg2, a known stimulator of PRMT1 activity, as a marker of NSCs that are undergoing their final mitosis on their way to becoming postmitotic neurons (18). Moreover, it was previously shown that in PC12 cells, which can respond to NGF by differentiating into sympathetic-like neurons, application of NGF increases asymmetric arginine dimethylation of proteins mediated by PRMT1 (49, 50). Taken together, these observations suggest that NSC division and neurogenesis is at least partly regulated by the sequential activation of PRMT5 and PRMT1; high levels of SC1-PRMT5 protein complex during the proliferative stage of cortical development might control the onset of neurogenesis by controlling the cell cycle parameters of the developing NSCs, possibly by maintaining symmetric proliferative divisions of the NSCs during the early phase of cortical development, whereas the progression to asymmetric division and neuronal differentiation depends on PRMT1.
In conclusion, our study identifies SC1 as a modulator of the NSC developmental program that acts through recruitment of a histone arginine methyltransferase, PRMT5. Given that SC1 is a p75 neurotrophin receptor-interacting protein (21), it will be important to determine whether neurotrophins or other signaling molecules can trigger modifications of SC1 that regulate its ability to recruit PRMT5 and thereby transmit extracellular information to the nuclear interior. Perhaps such differentiation-inducing factors as the neurotrophins regulate which epigenetic modifiers will be recruited by SC1 at different stages of cortical development and regulate the activity of PRMTs involved in the process of neuronal differentiation. Together, our findings uncover a novel role for histone arginine methylation in the control of cortical NSC proliferation and differentiation.
Acknowledgments
We thank our colleagues in the Wolfson Institute for Biomedical Research, especially Joana Paes de Faria, Ingvar Ferby, Sarah Hopkins, Huiliang Li, Andrei Okorokov, Nigel Pringle, and Kaylene Young for discussions and help with NSC cultures and staining procedures.
This work was supported by Wellcome Trust Career Re-entry Fellowship WT076656MA (to A. C.) and by funds from the United Kingdom Medical Research Council (to W. D. R.).
A. Chittka, unpublished observations.
A. Chittka, J. Nitarska, U. Grazini, and W. D. Richardson, unpublished observations.
- NSC
- neural stem cell
- PRMT
- protein arginine methyltransferase
- SC1
- Schwann cell factor 1
- En
- embryonic day n
- PR
- positive regulatory
- MTase
- methyltransferase
- HDAC
- histone deacetylase
- IP
- immunoprecipitation
- IPed
- immunoprecipitated
- mycSC1
- Myc-tagged SC1
- OLP
- oligodendrocyte precursor
- EGFP
- enhanced green fluorescent protein
- ES
- embryonic stem
- DIV
- days in vitro
- SET
- Su(var)3–9, Enhancer of zeste, Trithorax.
REFERENCES
- 1. Okano H., Temple S. (2009) Cell types to order. Temporal specification of CNS stem cells. Curr. Opin. Neurobiol. 19, 112–119 [DOI] [PubMed] [Google Scholar]
- 2. Qian X., Shen Q., Goderie S. K., He W., Capela A., Davis A. A., Temple S. (2000) Timing of CNS cell generation. A programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 28, 69–80 [DOI] [PubMed] [Google Scholar]
- 3. Shen Q., Wang Y., Dimos J. T., Fasano C. A., Phoenix T. N., Lemischka I. R., Ivanova N. B., Stifani S., Morrisey E. E., Temple S. (2006) The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat. Neurosci. 9, 743–751 [DOI] [PubMed] [Google Scholar]
- 4. Guillemot F. (2007) Cell fate specification in the mammalian telencephalon. Prog. Neurobiol. 83, 37–52 [DOI] [PubMed] [Google Scholar]
- 5. Ohnuma S., Philpott A., Harris W. A. (2001) Cell cycle and cell fate in the nervous system. Curr. Opin. Neurobiol. 11, 66–73 [DOI] [PubMed] [Google Scholar]
- 6. Ohnuma S., Harris W. A. (2003) Neurogenesis and the cell cycle. Neuron 40, 199–208 [DOI] [PubMed] [Google Scholar]
- 7. Hirabayashi Y., Gotoh Y. (2005) Stage-dependent fate determination of neural precursor cells in mouse forebrain. Neurosci. Res. 51, 331–336 [DOI] [PubMed] [Google Scholar]
- 8. Miller F. D., Gauthier A. S. (2007) Timing is everything. Making neurons versus glia in the developing cortex. Neuron 54, 357–369 [DOI] [PubMed] [Google Scholar]
- 9. Hsieh J., Gage F. H. (2004) Epigenetic control of neural stem cell fate. Curr. Opin. Genet. Dev. 14, 461–469 [DOI] [PubMed] [Google Scholar]
- 10. Jepsen K., Solum D., Zhou T., McEvilly R. J., Kim H. J., Glass C. K., Hermanson O., Rosenfeld M. G. (2007) SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature 450, 415–419 [DOI] [PubMed] [Google Scholar]
- 11. Song M. R., Ghosh A. (2004) FGF2-induced chromatin remodeling regulates CNTF-mediated gene expression and astrocyte differentiation. Nat. Neurosci. 7, 229–235 [DOI] [PubMed] [Google Scholar]
- 12. Matsumoto S., Banine F., Struve J., Xing R., Adams C., Liu Y., Metzger D., Chambon P., Rao M. S., Sherman L. S. (2006) Brg1 is required for murine neural stem cell maintenance and gliogenesis. Dev. Biol. 289, 372–383 [DOI] [PubMed] [Google Scholar]
- 13. Ballas N., Grunseich C., Lu D. D., Speh J. C., Mandel G. (2005) REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 121, 645–657 [DOI] [PubMed] [Google Scholar]
- 14. Hermanson O., Jepsen K., Rosenfeld M. G. (2002) N-CoR controls differentiation of neural stem cells into astrocytes. Nature 419, 934–939 [DOI] [PubMed] [Google Scholar]
- 15. Lukaszewicz A., Savatier P., Cortay V., Giroud P., Huissoud C., Berland M., Kennedy H., Dehay C. (2005) G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron 47, 353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dirks P. B. (2010) Brain tumor stem cells. The cancer stem cell hypothesis writ large. Mol. Oncol. 4, 420–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang J., Vogel G., Yu Z., Almazan G., Richard S. (2011) Type II arginine methyltransferase PRMT5 regulates gene expression of inhibitors of differentiation/DNA binding Id2 and Id4 during glial cell differentiation. J. Biol. Chem. 286, 44424–44432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iacopetti P., Michelini M., Stuckmann I., Oback B., Aaku-Saraste E., Huttner W. B. (1999) Expression of the antiproliferative gene TIS21 at the onset of neurogenesis identifies single neuroepithelial cells that switch from proliferative to neuron-generating division. Proc. Natl. Acad. Sci. U.S.A. 96, 4639–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tedeschi A., Di Giovanni S. (2009) The non-apoptotic role of p53 in neuronal biology. Enlightening the dark side of the moon. EMBO Rep. 10, 576–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tio M., Udolph G., Yang X., Chia W. (2001) cdc2 links the Drosophila cell cycle and asymmetric division machineries. Nature 409, 1063–1067 [DOI] [PubMed] [Google Scholar]
- 21. Chittka A., Chao M. V. (1999) Identification of a zinc finger protein whose subcellular distribution is regulated by serum and nerve growth factor. Proc. Natl. Acad. Sci. U.S.A. 96, 10705–10710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fumasoni I., Meani N., Rambaldi D., Scafetta G., Alcalay M., Ciccarelli F. D. (2007) Family expansion and gene rearrangements contributed to the functional specialization of PRDM genes in vertebrates. BMC Evol. Biol. 7, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schneider R., Bannister A. J., Kouzarides T. (2002) Unsafe SETs. Histone lysine methyltransferases and cancer. Trends Biochem. Sci. 27, 396–402 [DOI] [PubMed] [Google Scholar]
- 24. Yu J., Angelin-Duclos C., Greenwood J., Liao J., Calame K. (2000) Transcriptional repression by blimp-1 (PRDI-BF1) involves recruitment of histone deacetylase. Mol. Cell. Biol. 20, 2592–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ancelin K., Lange U. C., Hajkova P., Schneider R., Bannister A. J., Kouzarides T., Surani M. A. (2006) Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat. Cell Biol. 8, 623–630 [DOI] [PubMed] [Google Scholar]
- 26. Bikoff E. K., Morgan M. A., Robertson E. J. (2009) An expanding job description for Blimp-1/PRDM1. Curr. Opin. Genet. Dev. 19, 379–385 [DOI] [PubMed] [Google Scholar]
- 27. Hayashi K., Matsui Y. (2006) Meisetz, a novel histone tri-methyltransferase, regulates meiosis-specific epigenesis. Cell Cycle 5, 615–620 [DOI] [PubMed] [Google Scholar]
- 28. Eom G. H., Kim K., Kim S. M., Kee H. J., Kim J. Y., Jin H. M., Kim J. R., Kim J. H., Choe N., Kim K. B., Lee J., Kook H., Kim N., Seo S. B. (2009) Histone methyltransferase PRDM8 regulates mouse testis steroidogenesis. Biochem. Biophys. Res. Commun. 388, 131–136 [DOI] [PubMed] [Google Scholar]
- 29. Derunes C., Briknarová K., Geng L., Li S., Gessner C. R., Hewitt K., Wu S., Huang S., Woods V. I., Jr., Ely K. R. (2005) Characterization of the PR domain of RIZ1 histone methyltransferase. Biochem. Biophys. Res. Commun. 333, 925–934 [DOI] [PubMed] [Google Scholar]
- 30. John S. A., Garrett-Sinha L. A. (2009) Blimp1. A conserved transcriptional repressor critical for differentiation of many tissues. Exp. Cell Res. 315, 1077–1084 [DOI] [PubMed] [Google Scholar]
- 31. Gyory I., Wu J., Fejér G., Seto E., Wright K. L. (2004) PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat. Immunol. 5, 299–308 [DOI] [PubMed] [Google Scholar]
- 32. Davis C. A., Haberland M., Arnold M. A., Sutherland L. B., McDonald O. G., Richardson J. A., Childs G., Harris S., Owens G. K., Olson E. N. (2006) PRISM/PRDM6, a transcriptional repressor that promotes the proliferative gene program in smooth muscle cells. Mol. Cell. Biol. 26, 2626–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chittka A., Arevalo J. C., Rodriguez-Guzman M., Pérez P., Chao M. V., Sendtner M. (2004) The p75NTR-interacting protein SC1 inhibits cell cycle progression by transcriptional repression of cyclin E. J. Cell Biol. 164, 985–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kendall S. E., Ryczko M. C., Mehan M., Verdi J. M. (2003) Characterization of NADE, NRIF and SC-1 gene expression during mouse neurogenesis. Brain Res. Dev. Brain Res. 144, 151–158 [DOI] [PubMed] [Google Scholar]
- 35. Chittka A. (2010) Dynamic distribution of histone H4 arginine 3 methylation marks in the developing murine cortex. PLoS ONE 5, e13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kessaris N., Jamen F., Rubin L. L., Richardson W. D. (2004) Cooperation between sonic hedgehog and fibroblast growth factor/MAPK signalling pathways in neocortical precursors. Development 131, 1289–1298 [DOI] [PubMed] [Google Scholar]
- 37. Qian X., Davis A. A., Goderie S. K., Temple S. (1997) FGF2 concentration regulates the generation of neurons and glia from multipotent cortical stem cells. Neuron 18, 81–93 [DOI] [PubMed] [Google Scholar]
- 38. Nishioka K., Reinberg D. (2003) Methods and tips for the purification of human histone methyltransferases. Methods 31, 49–58 [DOI] [PubMed] [Google Scholar]
- 39. Bedford M. T., Clarke S. G. (2009) Protein arginine methylation in mammals. Who, what, and why. Mol. Cell 33, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pal S., Sif S. (2007) Interplay between chromatin remodelers and protein arginine methyltransferases. J. Cell. Physiol. 213, 306–315 [DOI] [PubMed] [Google Scholar]
- 41. Xu X., Hoang S., Mayo M. W., Bekiranov S. (2010) Application of machine learning methods to histone methylation ChIP-Seq data reveals H4R3me2 globally represses gene expression. BMC Bioinformatics 11, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McBurney M. W. (1993) P19 embryonal carcinoma cells. Int. J. Dev. Biol. 37, 135–140 [PubMed] [Google Scholar]
- 43. Chambers I., Silva J., Colby D., Nichols J., Nijmeijer B., Robertson M., Vrana J., Jones K., Grotewold L., Smith A. (2007) Nanog safeguards pluripotency and mediates germline development. Nature 450, 1230–1234 [DOI] [PubMed] [Google Scholar]
- 44. Hayashi K., de Sousa Lopes S. M., Surani M. A. (2007) Germ cell specification in mice. Science 316, 394–396 [DOI] [PubMed] [Google Scholar]
- 45. Robertson E. J., Charatsi I., Joyner C. J., Koonce C. H., Morgan M., Islam A., Paterson C., Lejsek E., Arnold S. J., Kallies A., Nutt S. L., Bikoff E. K. (2007) Blimp1 regulates development of the posterior forelimb, caudal pharyngeal arches, heart and sensory vibrissae in mice. Development 134, 4335–4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saitou M. (2009) Germ cell specification in mice. Curr. Opin. Genet. Dev. 19, 386–395 [DOI] [PubMed] [Google Scholar]
- 47. Zhao Q., Rank G., Tan Y. T., Li H., Moritz R. L., Simpson R. J., Cerruti L., Curtis D. J., Patel D. J., Allis C. D., Cunningham J. M., Jane S. M. (2009) PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat. Struct. Mol. Biol. 16, 304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Andreu-Pérez P., Esteve-Puig R., de Torre-Minguela C., López-Fauqued M., Bech-Serra J. J., Tenbaum S., García-Trevijano E. R., Canals F., Merlino G., Avila M. A., Recio J. A. (2011) Protein arginine methyltransferase 5 regulates ERK1/2 signal transduction amplitude and cell fate through CRAF. Sci. Signal. 4, ra58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cimato T. R., Ettinger M. J., Zhou X., Aletta J. M. (1997) Nerve growth factor-specific regulation of protein methylation during neuronal differentiation of PC12 cells. J. Cell Biol. 138, 1089–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cimato T. R., Tang J., Xu Y., Guarnaccia C., Herschman H. R., Pongor S., Aletta J. M. (2002) Nerve growth factor-mediated increases in protein methylation occur predominantly at type I arginine methylation sites and involve protein arginine methyltransferase 1. J. Neurosci. Res. 67, 435–442 [DOI] [PubMed] [Google Scholar]