Abstract
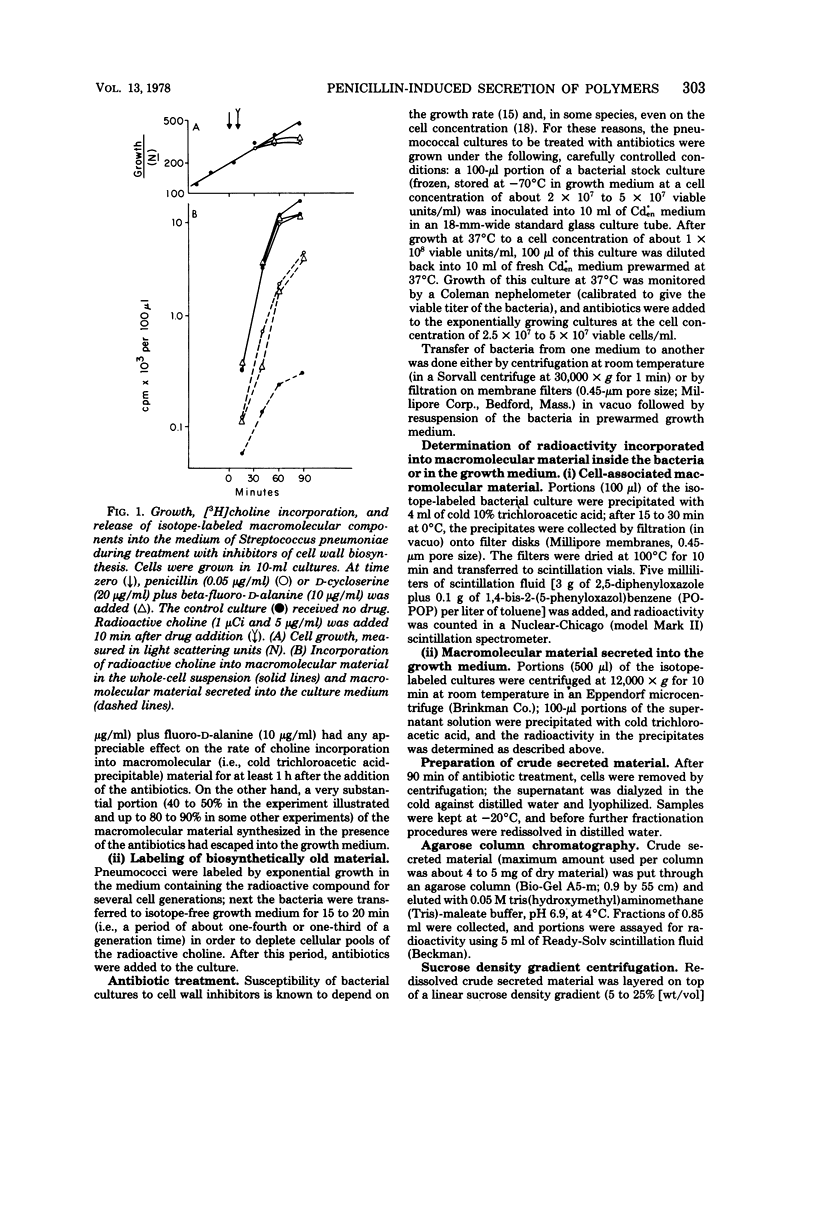
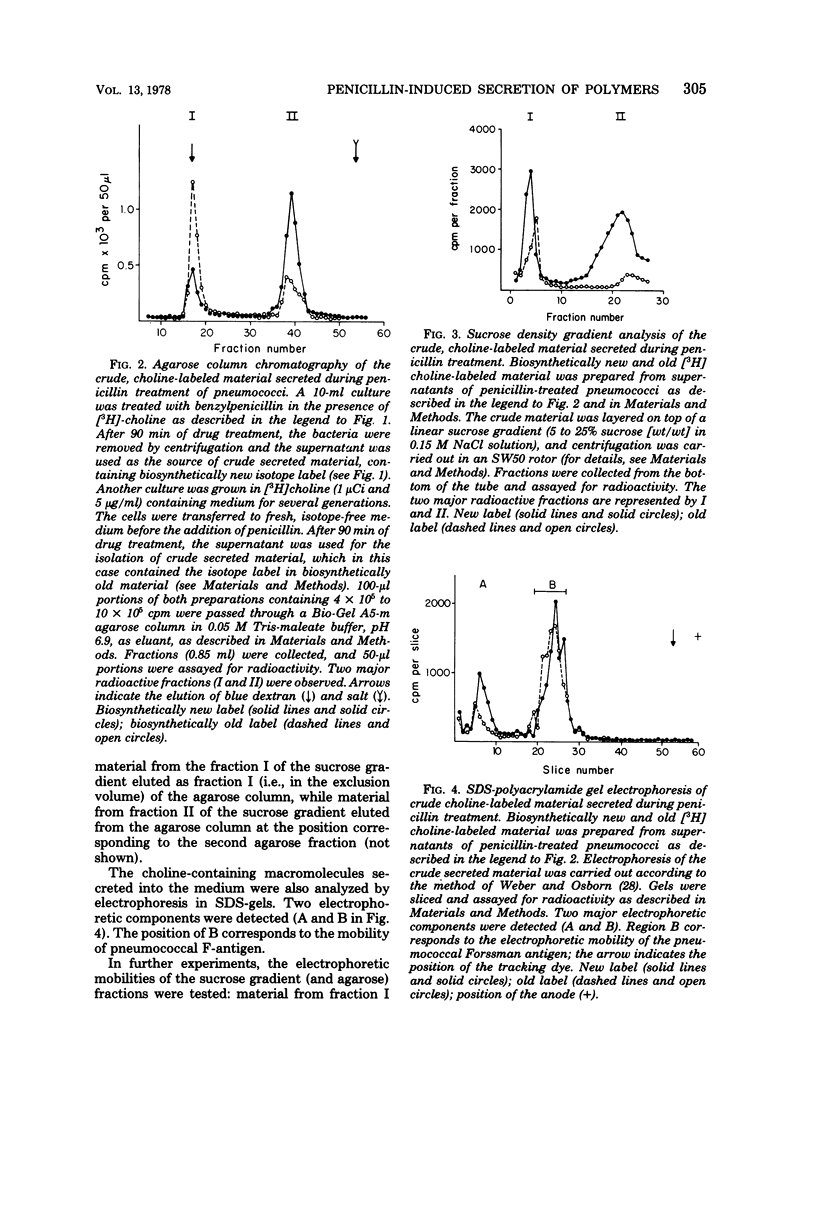
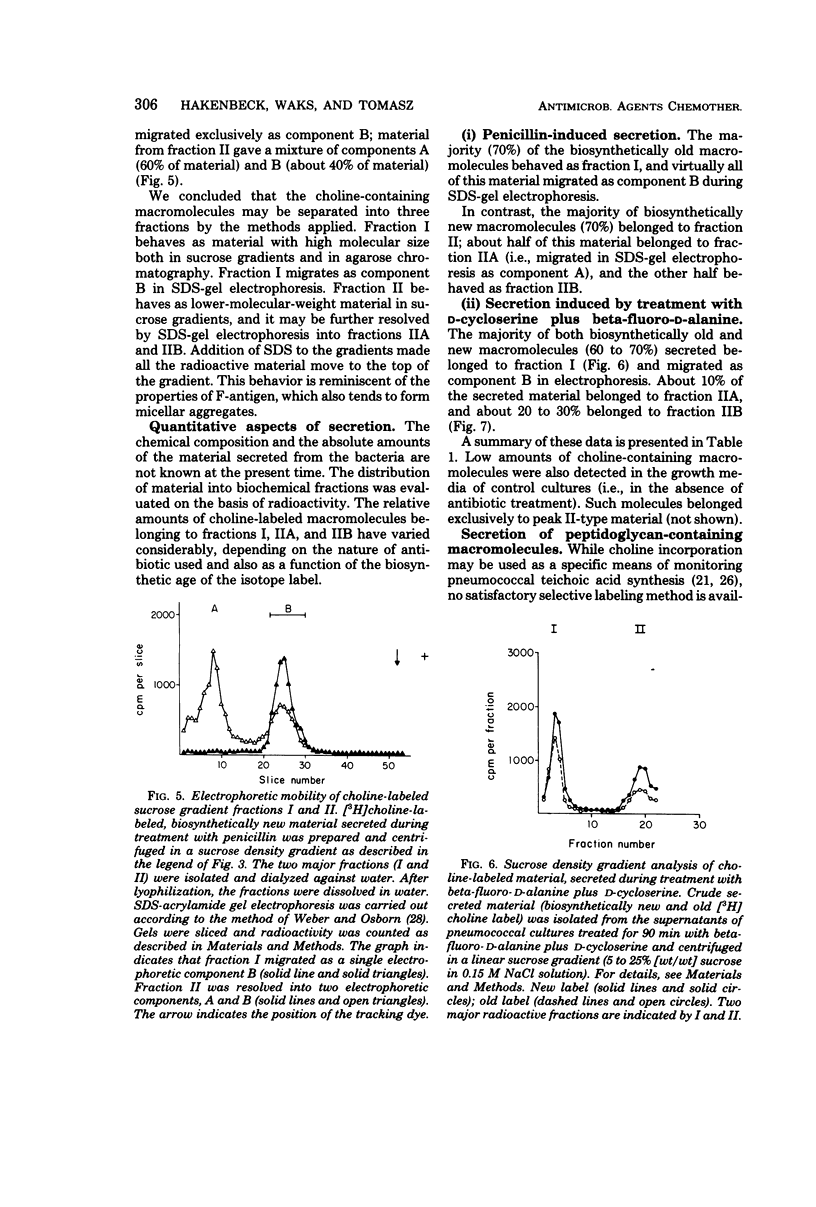
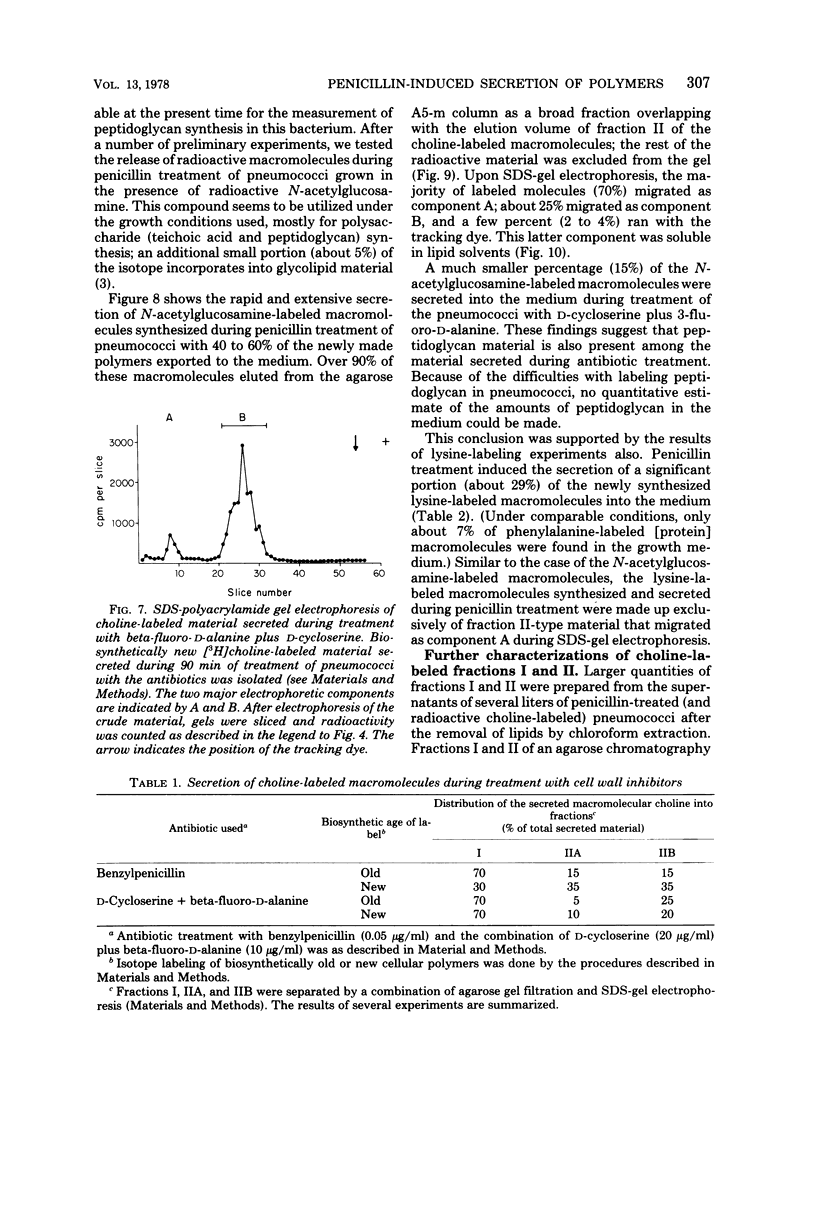
Autolysin-defective pneumococci secrete large quantities of choline-containing cell wall polymers into the growth medium during treatment with inhibitors of peptidoglycan synthesis. The secreted polymers were separated into three fractions by a combination of gel filtration on agarose and sodium dodecyl sulfate-gel electrophoresis. Fraction I had a high apparent molecular size and contained the Forssman antigen in complex with material exhibiting properties of cell wall teichoic acid. Choline-containing polymers of as yet uncharacterized structure were present in both fractions IIA and IIB, and fraction IIA also contained peptidoglycan components.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bracha R., Glaser L. In vitro system for the synthesis of teichoic acid linked to peptidoglycan. J Bacteriol. 1976 Mar;125(3):872–879. doi: 10.1128/jb.125.3.872-879.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Pneumococcal Forssman antigen. A choline-containing lipoteichoic acid. J Biol Chem. 1973 Sep 25;248(18):6394–6397. [PubMed] [Google Scholar]
- Brundish D. E., Baddiley J. Pneumococcal C-substance, a ribitol teichoic acid containing choline phosphate. Biochem J. 1968 Dec;110(3):573–582. doi: 10.1042/bj1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundish D. E., Shaw N., Baddiley J. The glycolipids from the non-capsulated strain of Pneumococcus I-192R, A.T.C.C. 12213. Biochem J. 1965 Oct;97(1):158–165. doi: 10.1042/bj0970158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T. S., Ward J. B., Rogers H. J. Formation of cell wall polymers by reverting protoplasts of Bacillus licheniformis. J Bacteriol. 1975 Nov;124(2):623–632. doi: 10.1128/jb.124.2.623-632.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler F., Glaser L. The synthesis of polyribitol phosphate. I. Purification of polyribitol phosphate polymerase and lipoteichoic acid carrier. J Biol Chem. 1974 May 10;249(9):2684–2689. [PubMed] [Google Scholar]
- Fiedler F., Glaser L. The synthesis of polyribitol phosphate. II. On the mechanism of polyribitol phosphate polymerase. J Biol Chem. 1974 May 10;249(9):2690–2695. [PubMed] [Google Scholar]
- Fuchs-Cleveland E., Gilvarg C. Oligomeric intermediate in peptidoglycan biosynthesis in Bacillus megaterium. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4200–4204. doi: 10.1073/pnas.73.11.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y. Structural and immunological studies on the pneumococcal C polysaccharide. J Biol Chem. 1967 Feb 10;242(3):463–470. [PubMed] [Google Scholar]
- Hancock I., Baddiley J. In vitro synthesis of the unit that links teichoic acid to peptidoglycan. J Bacteriol. 1976 Mar;125(3):880–886. doi: 10.1128/jb.125.3.880-886.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Purification of the pneumococcal N-acetylmuramyl-L-alanine amidase to biochemical homogeneity. J Biol Chem. 1976 Jul 25;251(14):4199–4207. [PubMed] [Google Scholar]
- JAWETZ E., GUNNISON J. B., SPECK R. S., COLEMAN V. R. Studies on antibiotic synergism and antagonism; the interference of chloramphenicol with the action of penicillin. AMA Arch Intern Med. 1951 Mar;87(3):349–359. doi: 10.1001/archinte.1951.03810030022002. [DOI] [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez R., Ronda-Lain C., Tapia A., Waks S. B., Tomasz A. Suppression of the lytic and bactericidal effects of cell wallinhibitory antibiotics. Antimicrob Agents Chemother. 1976 Oct;10(4):697–706. doi: 10.1128/aac.10.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda G., Tomioka S., Uchida H., Hasegawa M. Bacteriostatic and bactericidal activities of selected beta-lactam antibiotics studied on agar plates. Antimicrob Agents Chemother. 1977 Mar;11(3):376–382. doi: 10.1128/aac.11.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Penicillin-induced secretion of soluble, uncross-linked peptidoglycan by Micrococcus luteus cells. Biochemistry. 1974 Nov 19;13(24):5045–5053. doi: 10.1021/bi00721a028. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Role of the penicillin-sensitive transpeptidation reaction in attachment of newly synthesized peptidoglycan to cell walls of Micrococcus luteus. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3355–3359. doi: 10.1073/pnas.69.11.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Tomasz A. Cellular metabolism in genetic transformation of pneumococci: requirement for protein synthesis during induction of competence. J Bacteriol. 1970 Mar;101(3):860–871. doi: 10.1128/jb.101.3.860-871.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., McDonnell M., Westphal M., Zanati E. Coordinated incorporation of nascent peptidoglycan and teichoic acid into pneumococcal cell walls and conservation of peptidoglycan during growth. J Biol Chem. 1975 Jan 10;250(1):337–341. [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Ward J. B. Peptidoglycan synthesis in Bacillus licheniformis. The inhibition of cross-linking by benzylpenicillin and cephaloridine in vivo accompanied by the formation of soluble peptidoglycan. Biochem J. 1975 Jan;146(1):253–267. doi: 10.1042/bj1460253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waks S., Tomasz A. Secretion of cell wall polymers into the growth medium of lysis-defective pneumococci during treatment with penicillin and other inhibitors of cell wall synthesis. Antimicrob Agents Chemother. 1978 Feb;13(2):293–301. doi: 10.1128/aac.13.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B. The synthesis of peptidoglycan in an autolysin-deficient mutant of Bacillus licheniformis N.C.T.C. 6346 and the effect of beta-lactam antibiotics, bacitracin and vancomycin. Biochem J. 1974 Jul;141(1):227–241. doi: 10.1042/bj1410227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Winkelstein J. A., Tomasz A. Activation of the alternative pathway by pneumococcal cell walls. J Immunol. 1977 Feb;118(2):451–454. [PubMed] [Google Scholar]



